Porosity of a Fast-Setting Mortar with Crystallization Admixture and Effect of a SA-PA Modification
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
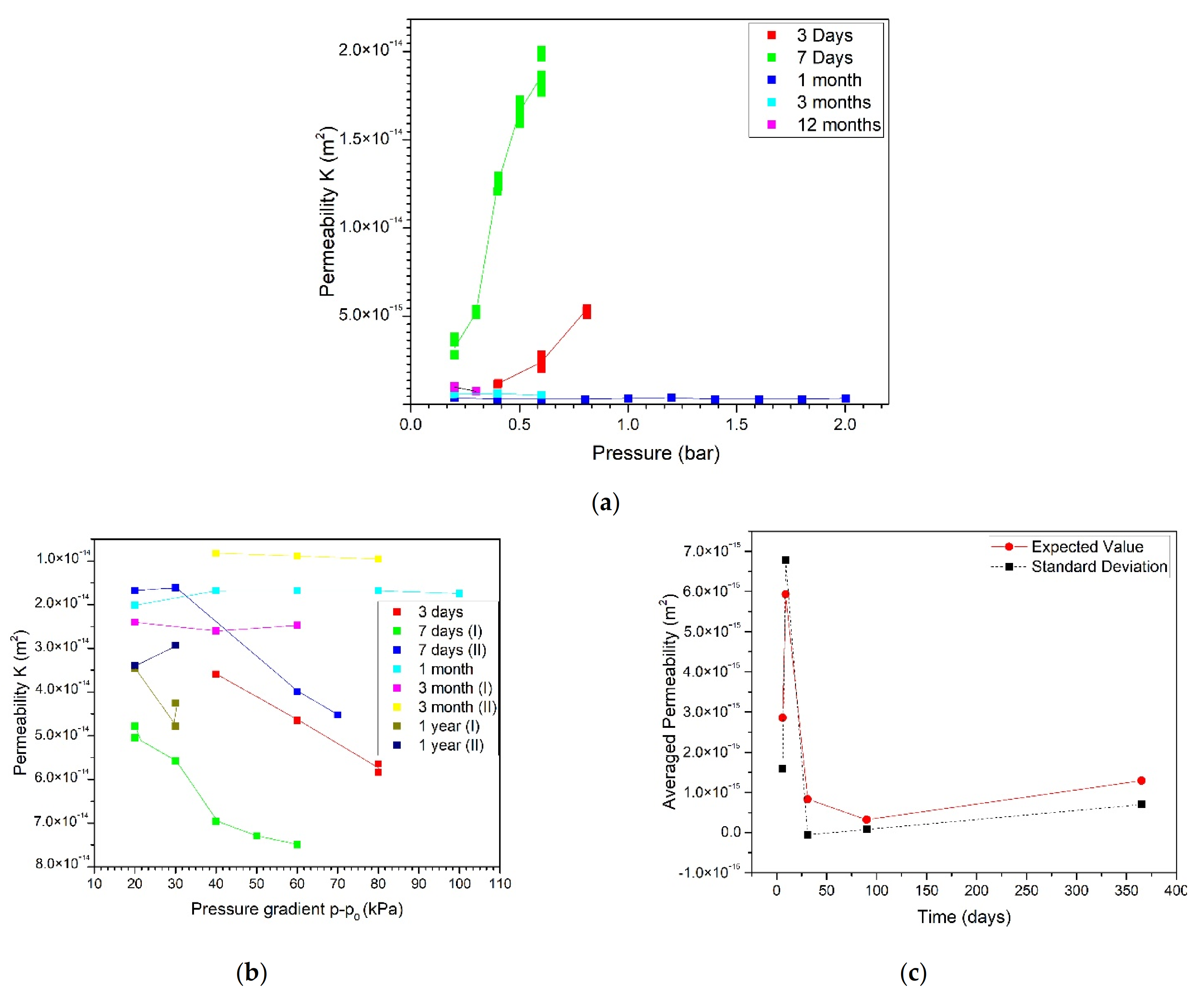
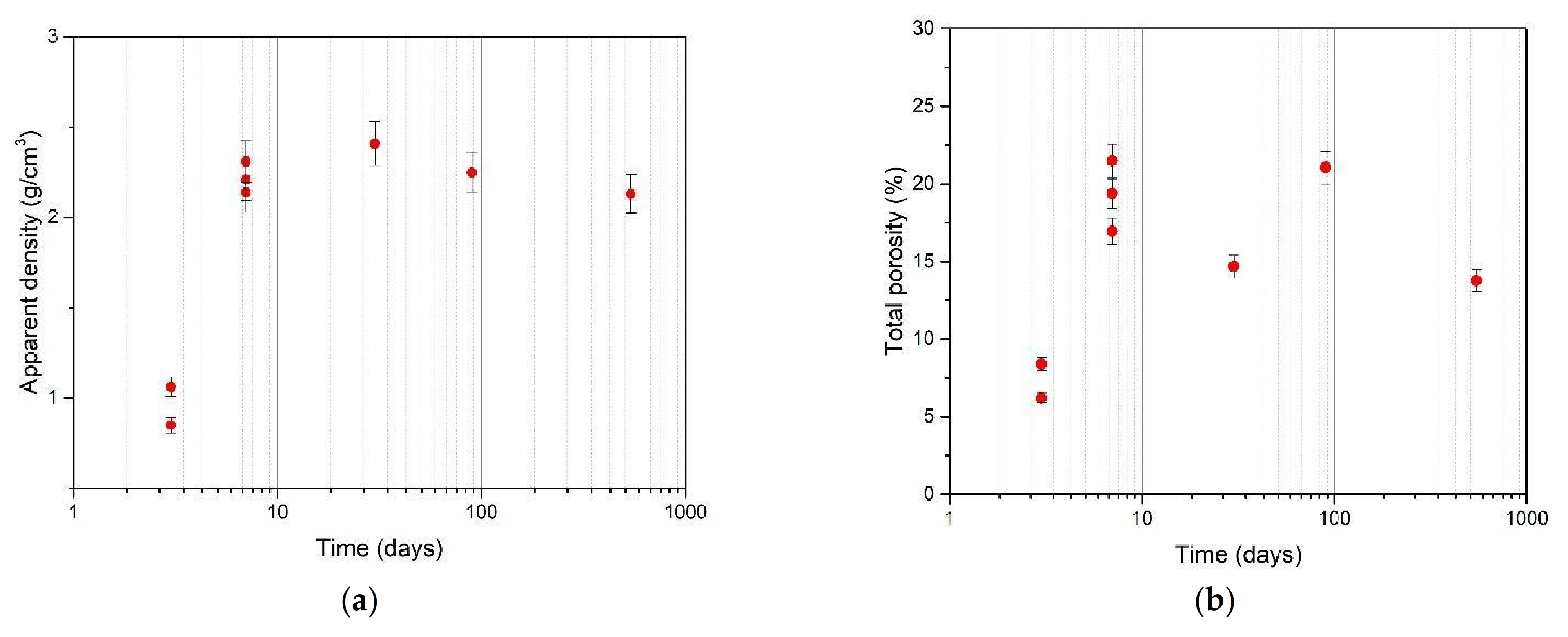
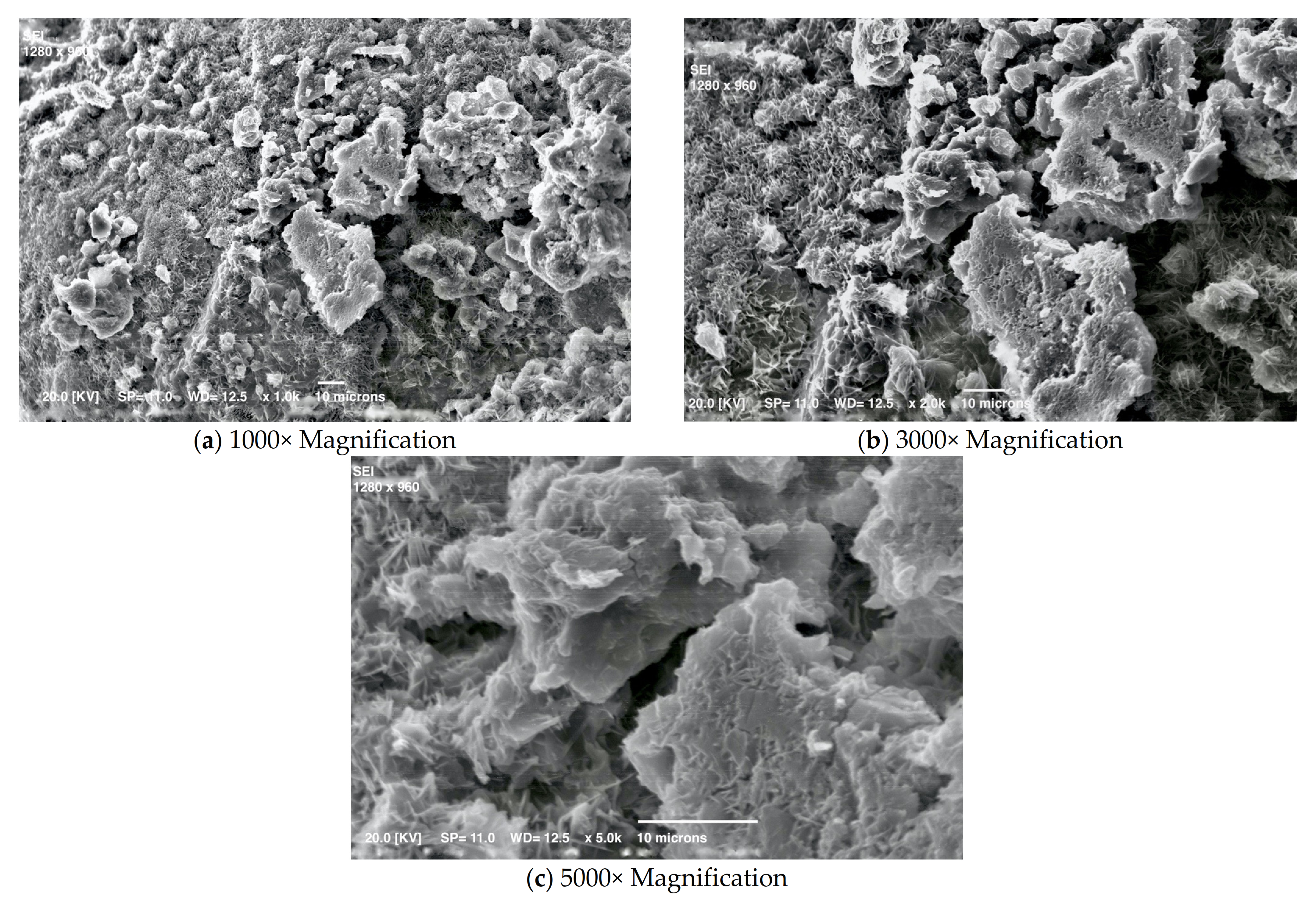
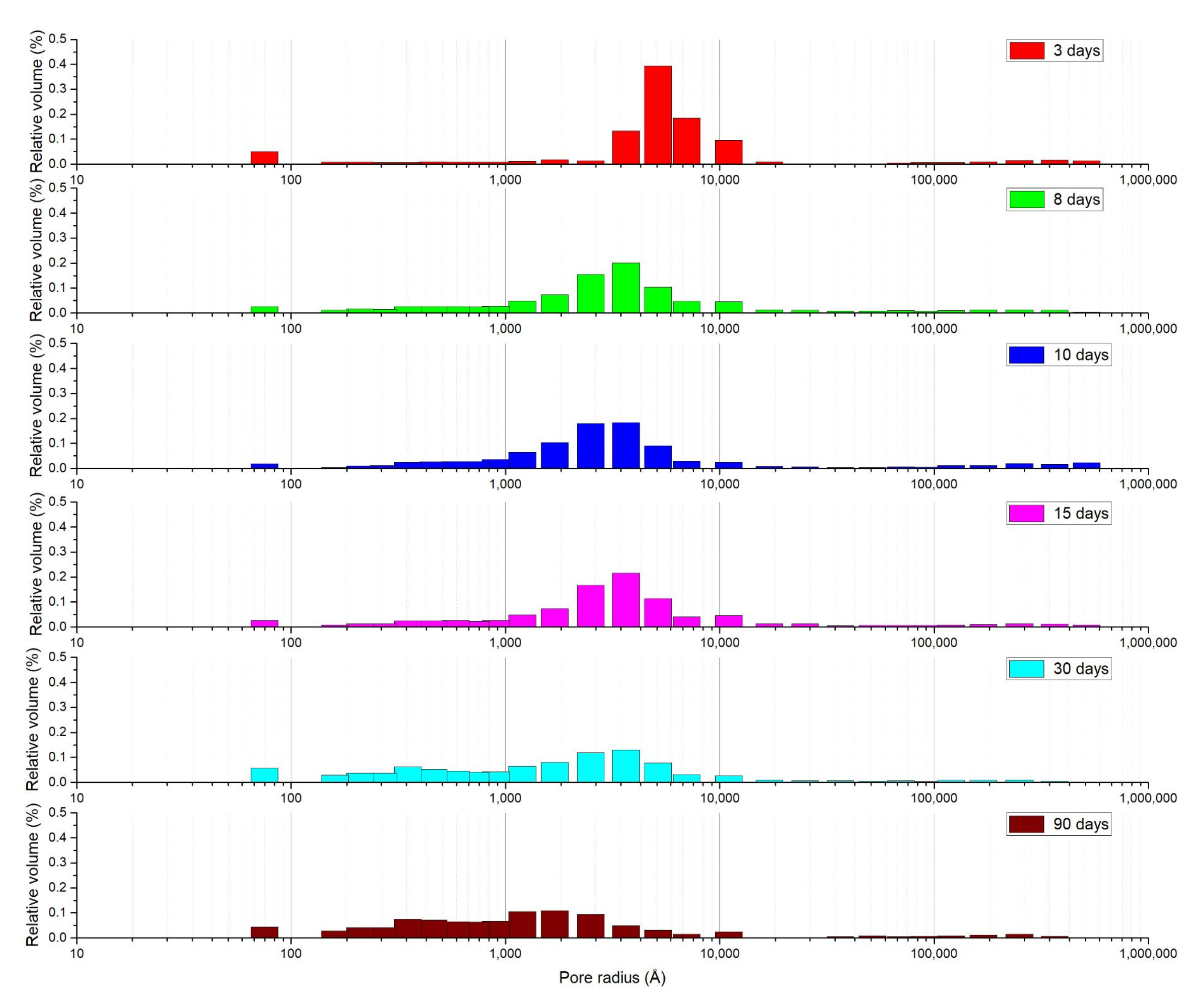
3.1. Mortar with a Crystallization Admixture
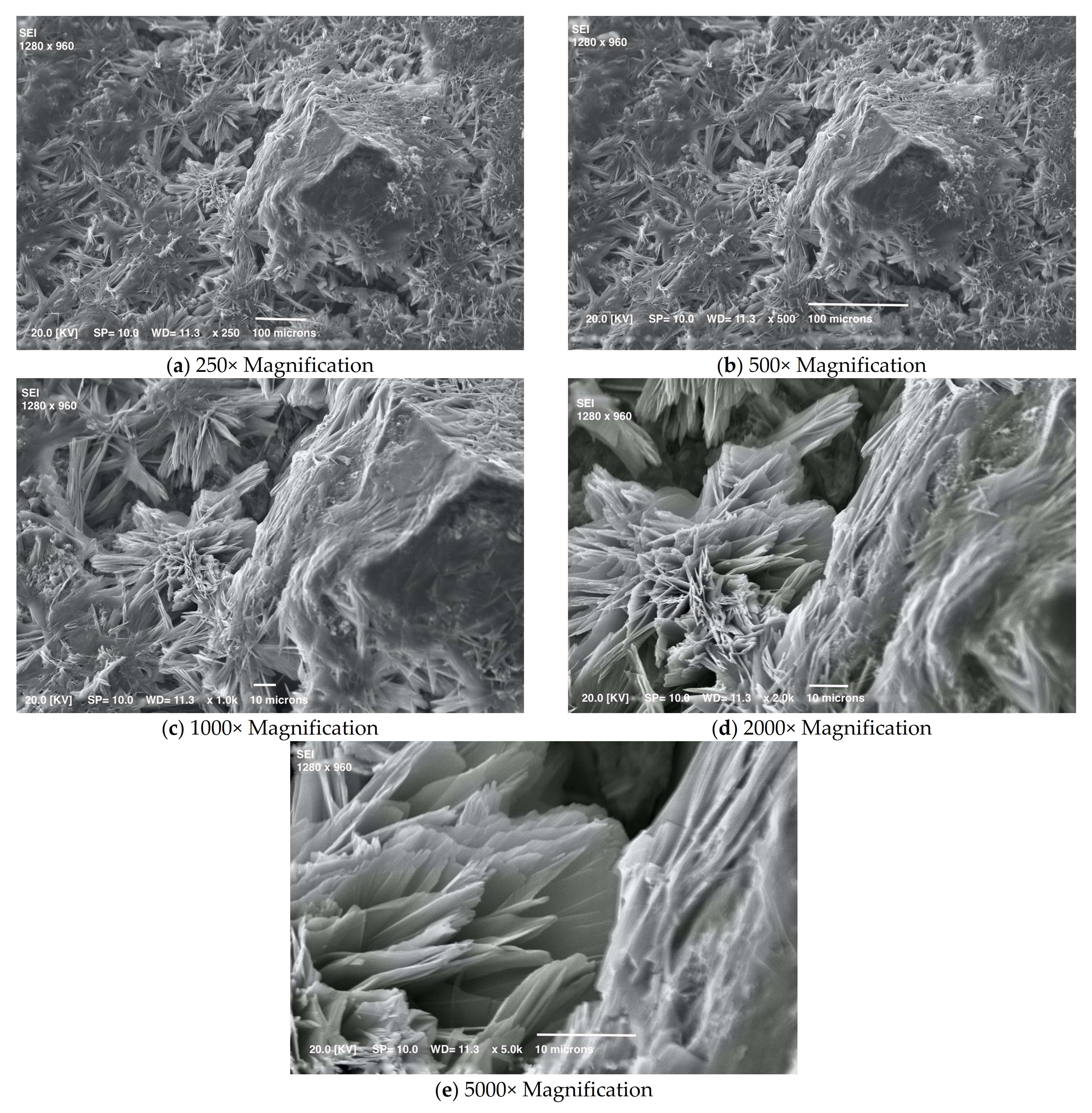
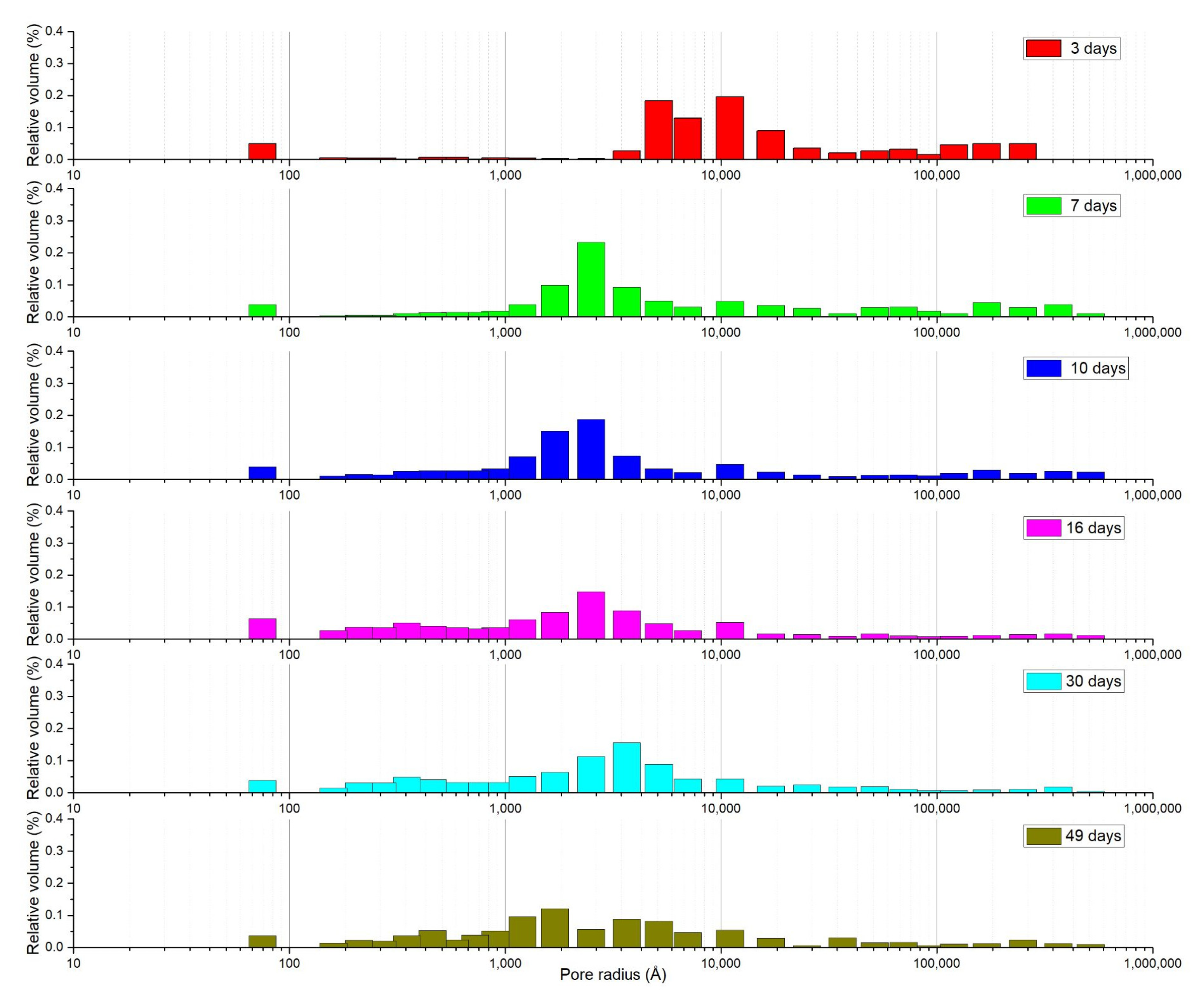
3.2. Mortar with a Crystallization Admixture: Effect of SA-PA Modification
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Longhi, F.; Surico, F. Effect of a Crystallizing Admixtures on Concrete Properties: Italian Concrete Days. In Proceedings of the Conference on Italian Concrete Days, Naples, Italy, 14–17 April 2021; Springer: Berlin, Germany, 2018; pp. 312–324. [Google Scholar]
- Coppola, L.; Coffetti, D.; Crotti, E. Innovative carboxylic acid waterproofing admixture for self-healing watertight concretes. Const. Build. Mater. 2018, 171, 817–824. [Google Scholar] [CrossRef]
- Cui, L.; Cahyadi, J.H. Permeability and pore structure of OPC paste. Cem. Conc. Res. 2001, 31, 277–282. [Google Scholar] [CrossRef]
- Muhammad, N.Z.; Keyvanafar, A.; Majid, M.Z.A.; Shafaghat, A.; Mirza, J. Waterproof performance of concrete: A critical review on implemented approaches. Constr. Build. Mater. 2015, 101, 80–90. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Wang, S.; Yao, D.; Wang, X.; Yu, X.; Zhang, Y. Fabrication of all-dimensional superhydrophobic mortar with enhanced waterproof ability and freeze-thaw resistance. Constr. Build. Mater. 2020, 238, 117626. [Google Scholar] [CrossRef]
- Li, C.; Wu, M.; Chen, Q.; Jiang, Z. Chemical and mineralogical alterations of concrete subjected to chemical attacks in complex underground tunnel environments during 20–36 years. Cem. Concr. Compos. 2018, 86, 139–159. [Google Scholar] [CrossRef]
- Li, J.; Jin, Q.; Zhang, W.; Li, C.; Monteiro, P.J.M. Microstructure and durability performance of sustainable cementitious composites containing high-volume regenerative biosilica. Resour. Conserv. Recycl. 2022, 178, 106038. [Google Scholar] [CrossRef]
- Nemes, R. Surface Properties of Lightweight Aggregate Concrete and its Correlation with Durability. In Materials Science Forum; Trans Tech. Publications: Freinbach, Switzerland, 2015; pp. 207–212. [Google Scholar]
- Sercombe, J.; Vidal, R.; Gallé, C.; Adenot, F. Experimental study of gas diffusion in cement paste. Cem. Conc. Res. 2007, 37, 579–588. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Stroeven, P. Local porosity analysis of pore structure in cement paste. Cem. Conc. Res. 2005, 35, 233–242. [Google Scholar] [CrossRef]
- Zdravkov, B.D.; Cermàk, J.J.; Sefara, M.; Janku, J. Pore classification in the characterization of porous materials: A perspective. Centr. Eur. J. Chem. 2007, 2, 385–395. [Google Scholar]
- Rouquerol, J.; Avnir, D.; Fairbridge, C.W.; Everett, D.H.; Haynes, J.H.; Pernicone, N.; Ramsay, J.D.F.; Sing, K.S.W.; Unger, K.K. Recommendations for the characterization of porous solids. Pure Appl. Chem. 1994, 8, 1739–1758. [Google Scholar] [CrossRef]
- Reinhardt, H.W.; Gaber, K. From pore size distribution to an equivalent pore size of cement mortar. Mater. Struct. 1990, 23, 3–15. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, P.; Ding, H.; Le, C. Experimental Study on the Permeability of SAP Modified Concrete. Materials 2020, 13, 3368. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.A. Water movement in unsaturated porous materials. RILEM Bull. 1965, 2912, 119–124. [Google Scholar]
- Basheer, L.; Kropp, J.; Cleland, D.J. Assessment of the durability of concrete from its permeation properties: A review. Constr. Build. Mater. 2001, 15, 93–103. [Google Scholar] [CrossRef]
- Calcestruzzo—Determinazione della Permeabilità All’ossigeno; UNI 1116400:2005; Ente Nazionale Italiano di Unificazione (UNI): Milano, Italy, 2005.
- Testing Hardened Concrete—Part 8: Depth of Penetration of Water under Pressure; BS EN 12390-8:2009; British Standard Institution: London, UK, 2019.
- Products and Systems for the Protection and Repair of Concrete Structures—Test Methods—Determination of Resistance of Capillary Absorption; UNI EN 13057:2002; European Committee for Standardization: Brussels, Belgium, 2002.
- Dunant, C.F.; Granja, J.; Muller, A.; Azenha, M.; Scrivener, K.L. Microstructural simulation and measurement of elastic modulus evolution of hydrating cement pastes. Cem. Conc. Res. 2020, 130, 106007. [Google Scholar] [CrossRef]
- Jensen, O.M.; Hansen, P.F. Water-entrained cement-based materials: I. Principles and theoretical background. Cem. Concr. Res. 2001, 31, 647–654. [Google Scholar] [CrossRef]
- Stutzman, P. Scanning electron microscopy imaging of hydraulic cement and microstructure. Cem. Conc. Comp. 2004, 26, 957–966. [Google Scholar] [CrossRef]
- Goldstein, J.I.; Newbury, D.E.; Joy, D.C.; Lyman, C.E.; Echlin, P.; Lifshin, E.; Sawyer, L.; Michael, J.R. Scanning Electron Microscopy and X-Ray Microanalysis; Springer: Berlin, Germany, 2017. [Google Scholar]
- Cook, R.A.; Hover, K.C. Mercury porosimetry of hardened cement pastes. Cem. Conc. Res. 1999, 29, 933–943. [Google Scholar] [CrossRef]
- Zhou, J.; Ye, G.; Breugel, K.V. Characterization of pore structure in cement-based materials using pressurization—depressurization cycling mercury intrusion porosimetry. Cem. Conc. Res. 2010, 40, 1120–1128. [Google Scholar] [CrossRef]
- Lange, D.A.; Jennings, H.M.; Surenda, S.P. Image analysis techniques for characterization of pore structure of cement-based materials. Cem. Conc. Res. 1994, 24, 841–853. [Google Scholar] [CrossRef]
- Yu, Y.G.; Angel, R.J.; Ross, N.L. Pressure impact on the structure, elasticity, and electron density distribution of CaSi2O5. Phys. Rev. B Condens. Matter Mater.Phys. 2013, 87, 184112. [Google Scholar] [CrossRef] [Green Version]
- Schoenitz, M.; Navrotsky, A.; Ross, N. Enthalpy of formation of CaSi2O5, a quenched high-pressure phase with pentacoordinate silicon. Phys. Chem. Miner. 2001, 1, 57–60. [Google Scholar] [CrossRef]
- Artioli, G.; Bullard, J.W. Cement hydration: The role of adsorption and crystal growth. Cryst. Res. Technol. 2013, 48, 903–918. [Google Scholar] [CrossRef]
- Vocka, R.; Gallè, C.; Dubois, M.; Lovera, P. Mercury intrusion porosimetry and hierarchical structure of cement pastes: Theory and experiment. Cem. Conc. Res. 2000, 30, 521–527. [Google Scholar] [CrossRef]
- Li, J.; Zhang, W.; Xu, K.; Monteiro, P.J.M. Fibrillar calcium silicate hydrate seeds from hydrated tricalcium silicate lower cement demand. Cem. Concr. Res. 2020, 137, 106195. [Google Scholar] [CrossRef]
- Bullard, J.W.; Jennings, H.M.; Livingston, R.A.; Nonat, A.; Scherer, G.W.; Schweitzer, J.S.; Scrivener, K.L.; Thomas, J.J. Mechanisms of cement hydration. Cem. Conc. Res. 2011, 41, 1208–1223. [Google Scholar] [CrossRef]
- Thomas, J.J.; Biernacki, J.J.; Bullard, J.W.; Shashank, B.; Dolado, J.S.; Scherer, G.W.; Luttge, A. Modeling and simulation of cement hydration kinetics and microstructure development. Cem. Conc. Res. 2011, 41, 1257–1278. [Google Scholar] [CrossRef]
- Pham, S.T. Modifications on Microporosity and Physical Properties of Cement Mortar Caused by Carbonation: Comparison of Experimental Methods. Adv. Mater. Sci. Eng. 2013, 2013, 672325. [Google Scholar] [CrossRef] [Green Version]
- Diamond, S. The microstructure of cement paste and concrete. Cem. Concr. Comp. 2004, 26, 919–933. [Google Scholar] [CrossRef]
- Ren, F.; Zhou, C.; Zeng, Q.; Ding, Z.; Xing, F.; Wang, W. The dependence of capillary sorptivity and gas permeability on initial water content for unsaturated cement mortars. Cem. Concr. Comp. 2019, 104, 103356. [Google Scholar] [CrossRef]
- Jensen, O.M.; Hansen, P.F. Water-entrained cement-based materials: II. Experimental observations. Cem. Concr. Res. 2002, 32, 973–978. [Google Scholar] [CrossRef]
- Shen, D.; Feng, Z.; Kang, J.; Wen, C.; Shi, H. Eect of Barchip fiber on stress relaxation and cracking potential of concrete internally cured with super absorbent polymers. Constr. Build. Mater. 2020, 249, 1–13. [Google Scholar] [CrossRef]
- Maggio, R.D.; Dirè, S.; Callone, E.; Bergamonti, L.; Lottici, P.P.; Albatici, R.; Rigon, R.; Ataollahi, N. Super-adsorbent polyacrylate under swelling in water for passive solar control of building envelope. SN Appl. Sci. 2020, 2, 45–58. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Tan, K.H. Effect of various polymer fibers on spalling mitigation of ultrahigh performance concrete at high temperature. Cem. Concr. Comp. 2020, 114, 103815. [Google Scholar] [CrossRef]
- Ozger, O.B.; Girardi, F.; Giannuzzi, G.M.; Salomoni, V.A.; Majorana, C.E.; Fambri, L.; Baldassino, N.; Maggio, R.D. Effect of nylon fibres on mechanical and thermaò properties of hardened concrete for energy storage systems. Mater. Des. 2013, 51, 989–997. [Google Scholar] [CrossRef]






| Phase | % | Error |
|---|---|---|
| Quartz | 11 | 1 |
| Portlandite | 13 | 1 |
| CS2 | 14 | 1 |
| Hartrurite | 26 | 1 |
| C3A | 2.8 | 0.1 |
| C2S | 11 | 1 |
| Gypsum | 2 | 1 |
| Brucite | 5 | 0.3 |
| Calcite | 5 | 1 |
| Kyanite | 3 | 1 |
| Arcanite | 2 | 0.3 |
| Brownmillerite | 3 | 0.3 |
| Anhydrite | 2 | 1 |
| Crystalline Index * | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cotini, O.; Di Maggio, R.; Tonelli, D.; Nascimben, R.; Ataollahi, N. Porosity of a Fast-Setting Mortar with Crystallization Admixture and Effect of a SA-PA Modification. Materials 2022, 15, 1542. https://doi.org/10.3390/ma15041542
Cotini O, Di Maggio R, Tonelli D, Nascimben R, Ataollahi N. Porosity of a Fast-Setting Mortar with Crystallization Admixture and Effect of a SA-PA Modification. Materials. 2022; 15(4):1542. https://doi.org/10.3390/ma15041542
Chicago/Turabian StyleCotini, Oscar, Rosa Di Maggio, Daniel Tonelli, Roger Nascimben, and Narges Ataollahi. 2022. "Porosity of a Fast-Setting Mortar with Crystallization Admixture and Effect of a SA-PA Modification" Materials 15, no. 4: 1542. https://doi.org/10.3390/ma15041542







