Influence of Cement Replacement with Sewage Sludge Ash (SSA) on the Heat of Hydration of Cement Mortar
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. The Chemical Composition and the Physical Properties of the Raw Materials (OPC, SSA)
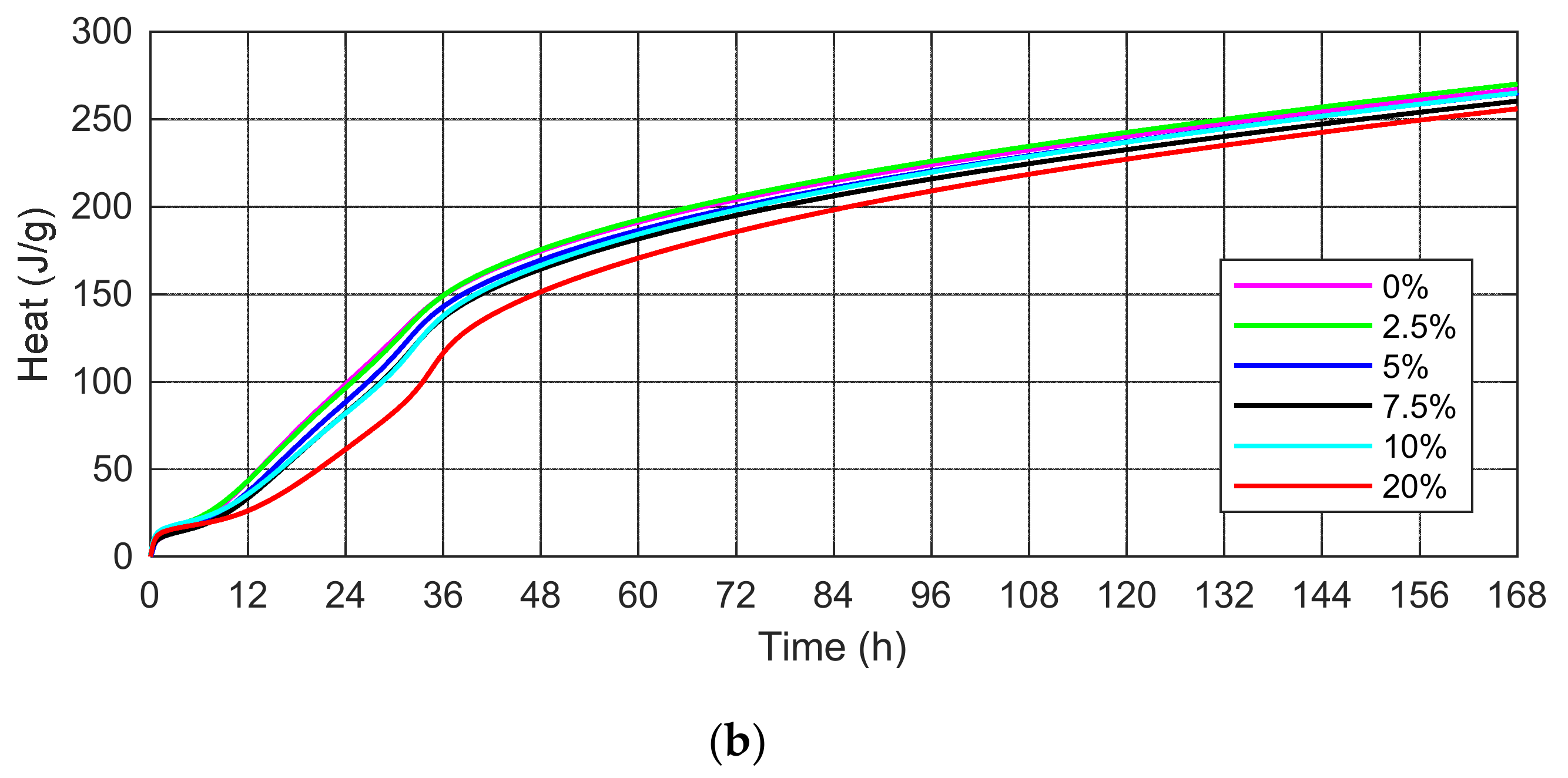
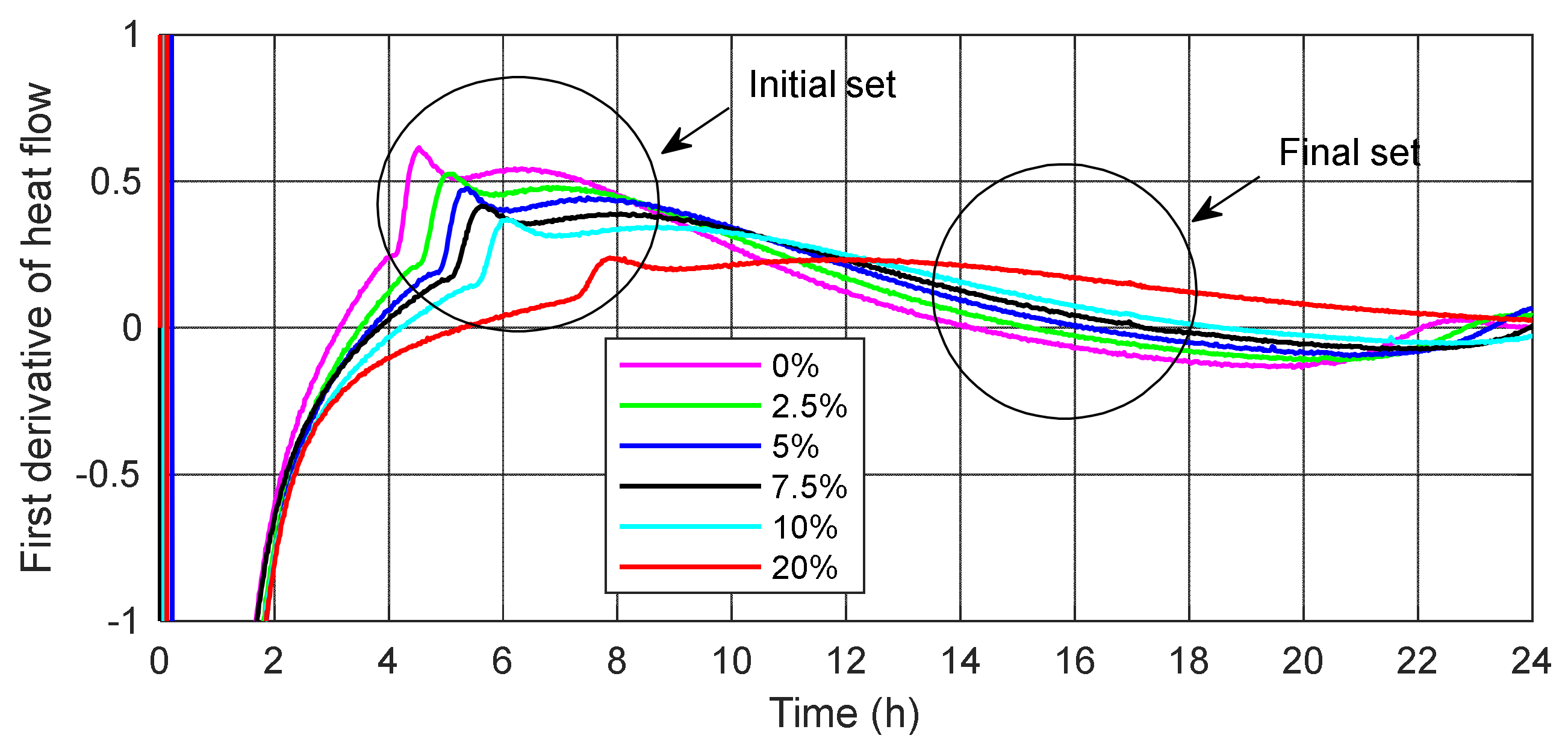
3.2. Heat of Hydration
3.3. The Pozzolanic Activity of Sewage Sludge Ash
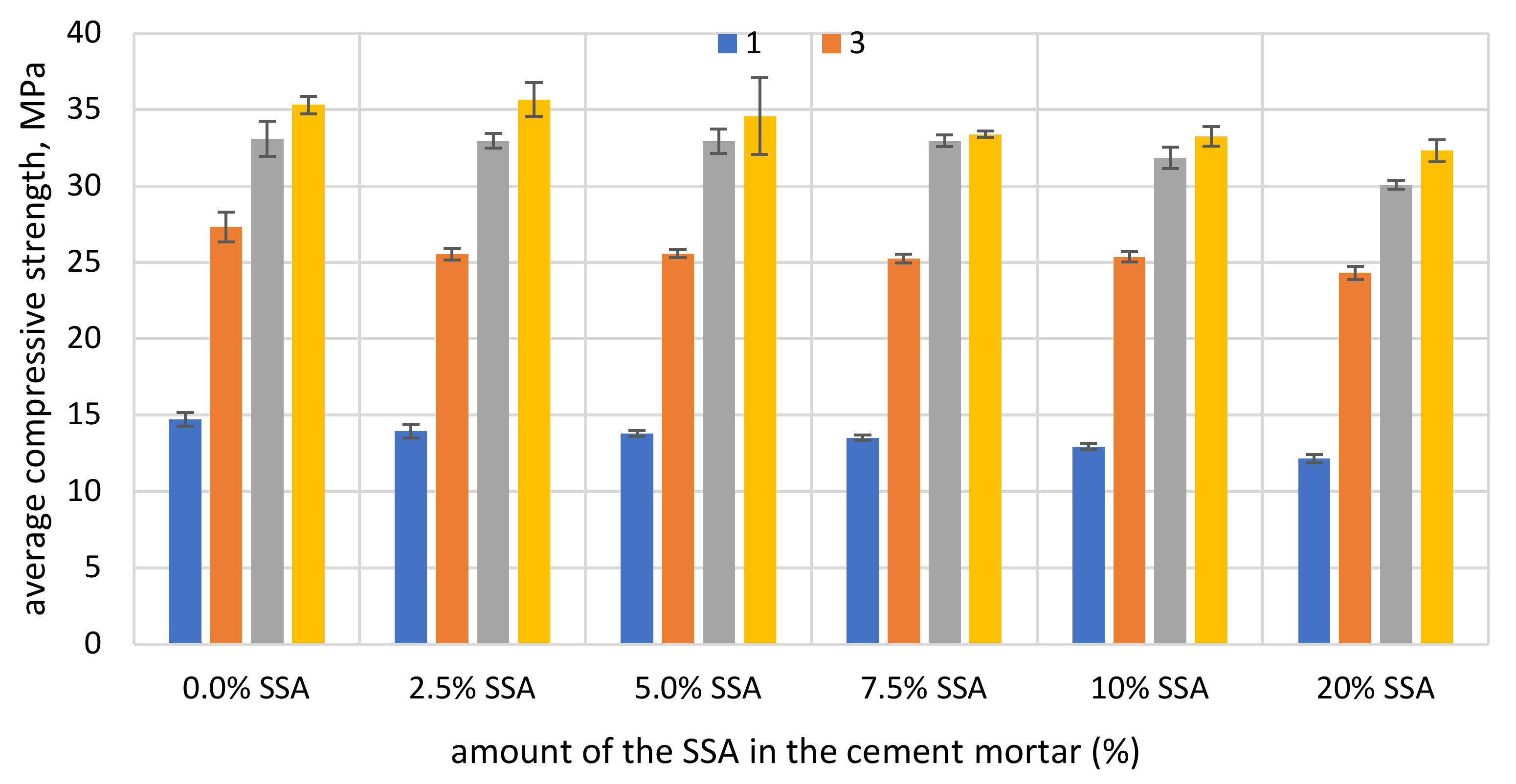
3.4. The Compressive Strength
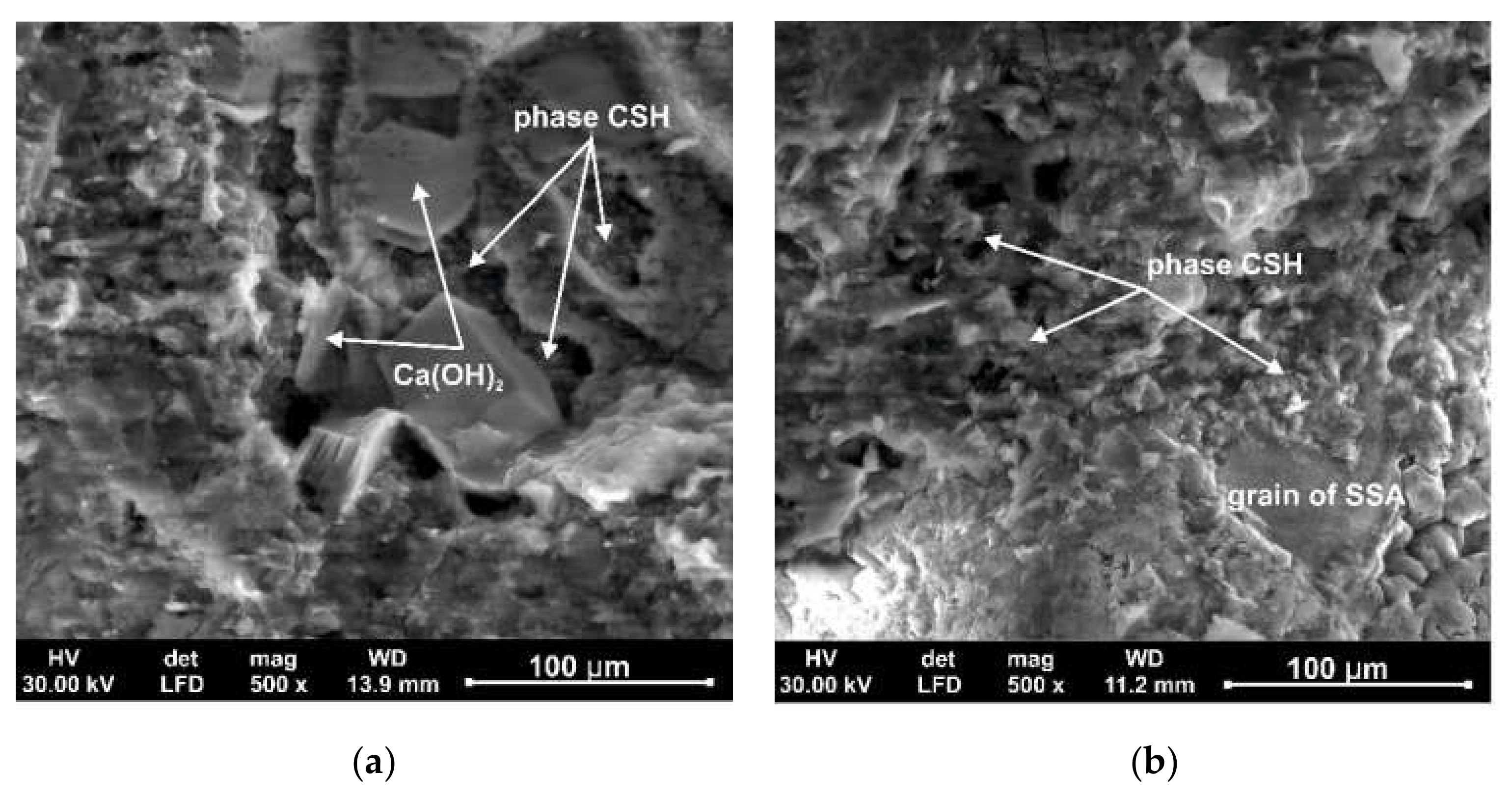
3.5. The Scanning Electron Microscopy
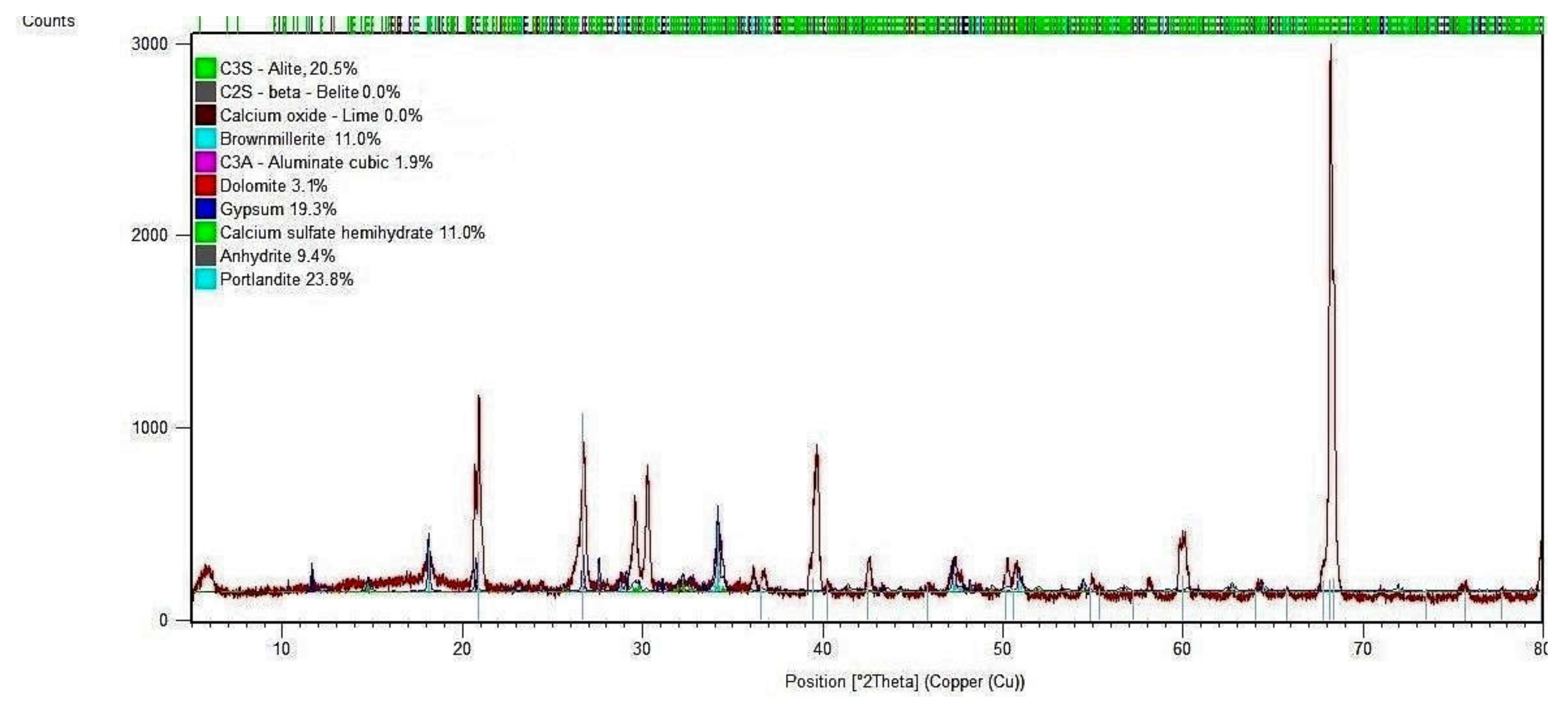
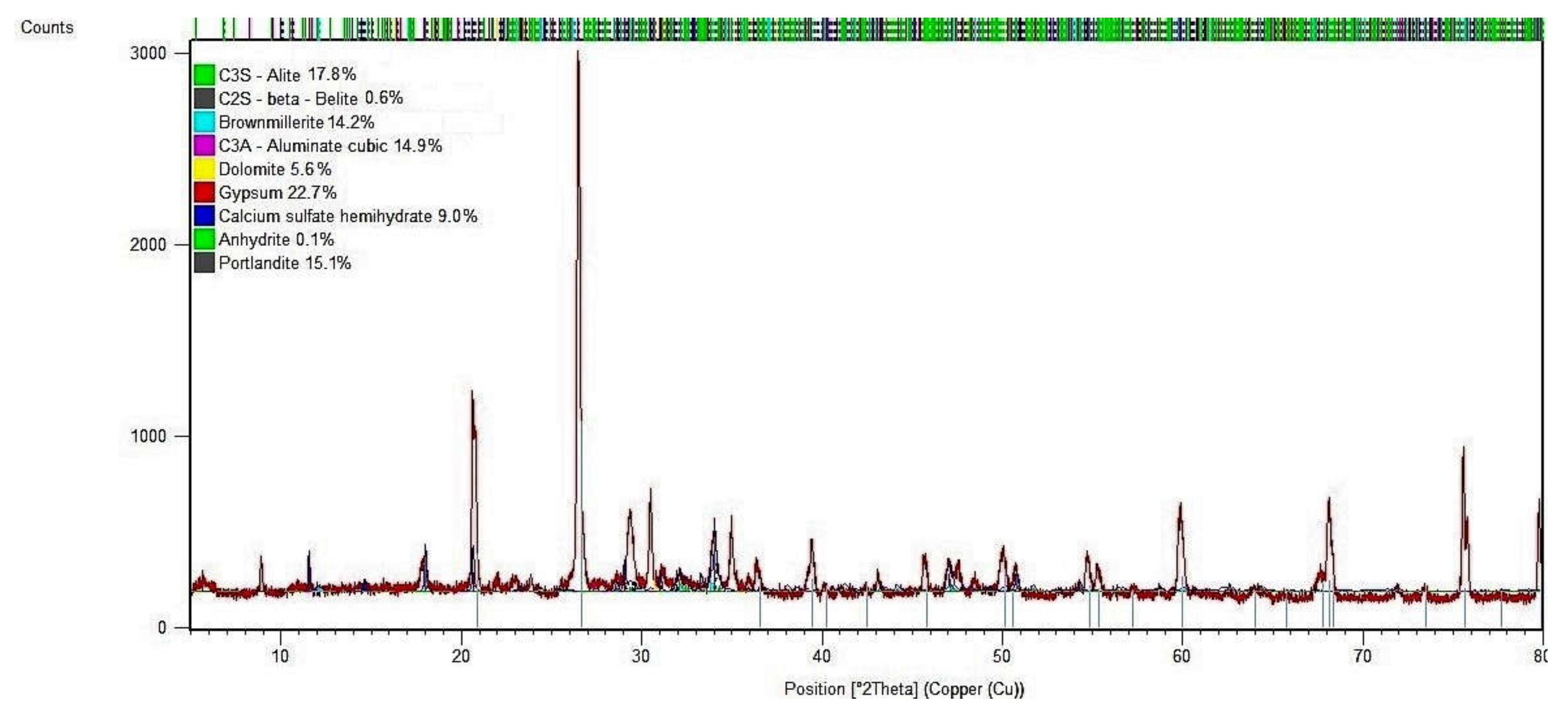
3.6. X-ray Diffraction (XRD) Analysis
4. Conclusions
- 1
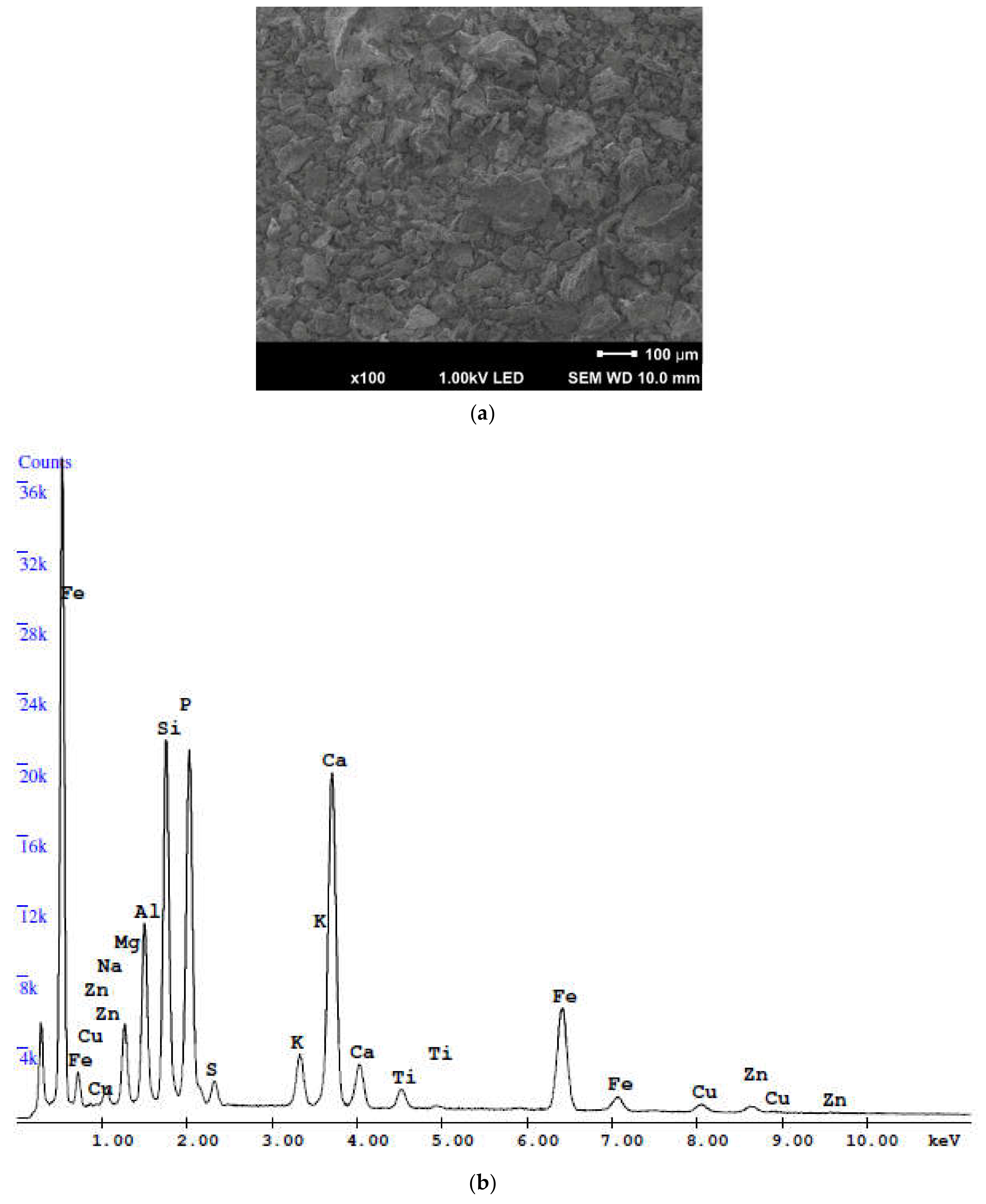
- The main components of the SSA samples are SiO2, CaO and P2O5. However, other oxides, such as Al2O3, Fe2O3, MgO, K2O and adsorbed SO3, are also present, but in a smaller amount. The phosphorus content is particularly significant compared to other components due to the characteristic of sewage sludge being incinerated and the influence of the mentioned phosphorus species on the final mortar-based products. The sum of silica, aluminum and iron oxides does not meet the requirements of the standards (ASTM C618 in the USA and EN 450 in Europe) dedicated to fly ash from conventional coal combustion.
- 2
- The use of this type of ash in concrete technology should be preceded by obtaining European technical approvals. According to EN 450-1 there is a possibility of obtaining fly ash from the co-combustion of sewage sludge with coal under proper conditions. However, it cannot be done within the wastewater treatment plant, where the fluidized bed furnace is considered BAT (best available technology).
- 3
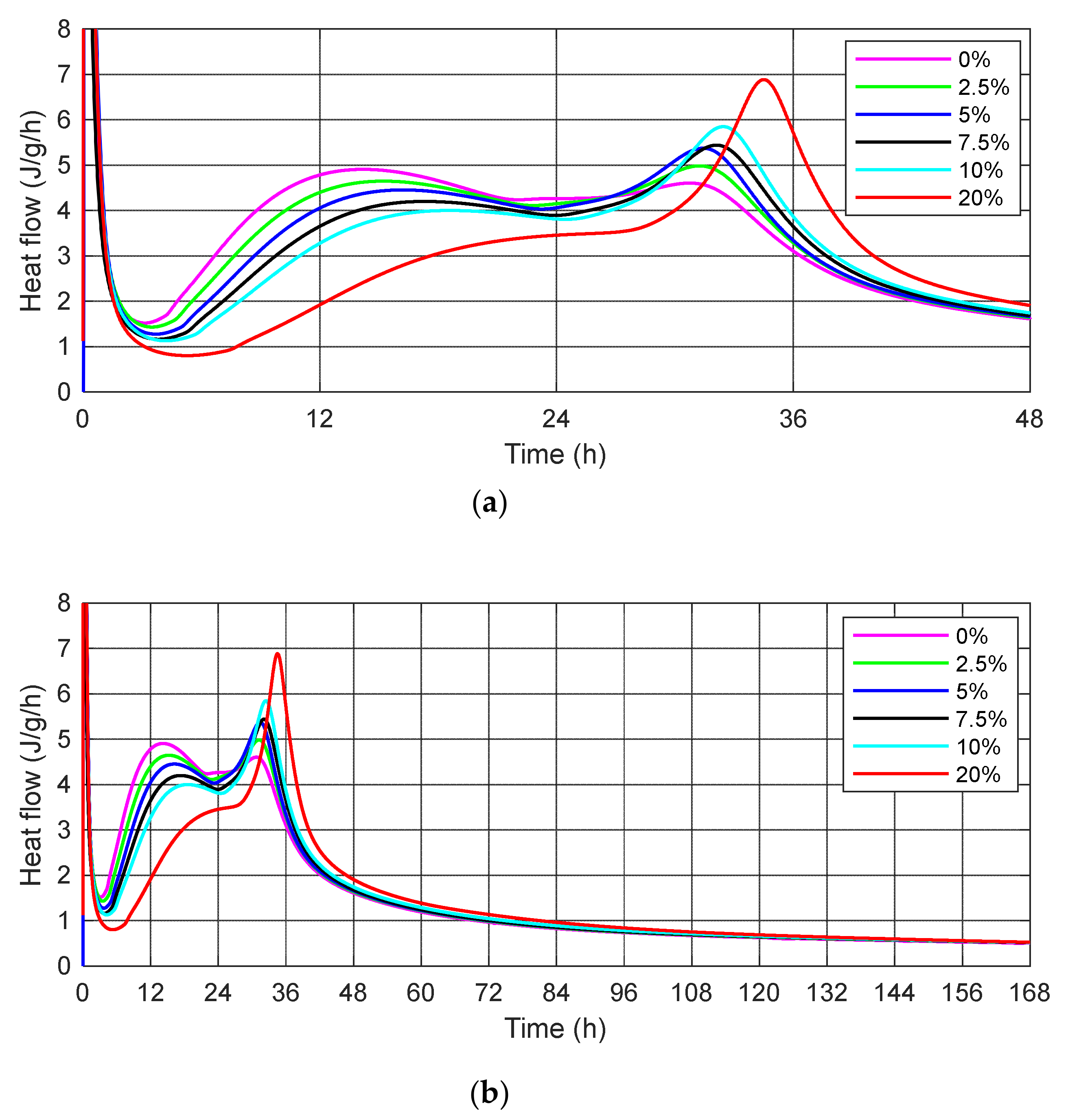
- The substitution of cement by SSA delays the evolution of the hydration process compared to the control cement mortar. SSA samples are characterized by a high content of calcium and phosphorus, which reduces the rate of heat hydration. The increasing amount of ash causes a lower value of the maximum first peak of heat flow and delays its occurrence.
- 4
- The impact on the binding time of slurries, apart from the water demand, is mainly the phosphorus oxide content in the binder. A small change in the mineral supplement rich in phosphorus can cause a sharp decline in the initiation or elongation of hydration, creating a critical interval that occurs at a dosage of 5–10% ash.
- 5
- The initial and final setting times of the tested mortars are longer with increasing SSA content, which is caused by a slower rate of SSA pozzolanic reaction and slower development of the microstructure. The heavy metals in SSA probably affected the hydration of the cement and therefore the initial and final setting time of the mortars.
- 6
- The pozzolanic activity of SSA does not meet the requirements of the standard EN 450-1 after 28 days (≥75%) and 90 days (≥85%) of curing. The addition of SSA reduces the compressive strength of mortar samples in the early stages of development.
- 7
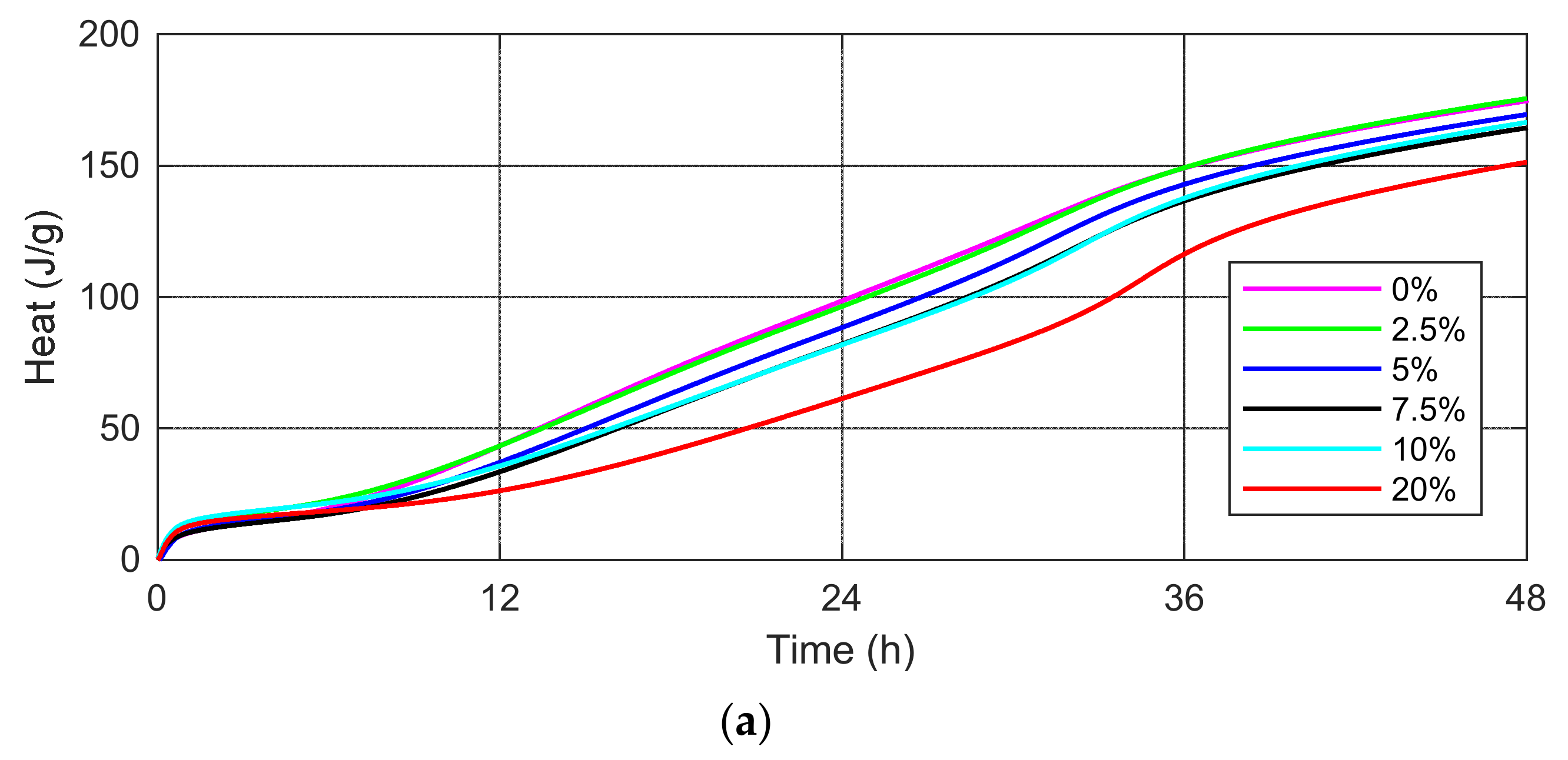
- The higher SSA content reduces the cumulative heat of hydration, thus favorably affecting the hardening process of massive structures due to the reduction of the risk of concrete microcracks at an early age.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The European Commission. EU/2014/955 Commission Decision of 18 December 2014 Amending Decision 2000/532/EC on the List of Waste Pursuant to Directive 2008/98/EC of the European Parliament and the Council. Off. J. Eur. Union 2014, 1–43. [Google Scholar]
- Haustein, E.; Kuryłowicz-Cudowska, A. The Effect of Fly Ash Microspheres on the Pore Structure of Concrete. Minerals 2020, 10, 58. [Google Scholar] [CrossRef] [Green Version]
- Kurpińska, M.; Ferenc, T. Experimental and Numerical Investigation of Mechanical Properties of Lightweight Concretes (LWCs) with Various Aggregates. Materials 2020, 13, 3474. [Google Scholar] [CrossRef]
- Kurpińska, M.; Ferenc, T. Application of lightweight cement composite with foamed glass aggregate in shell structures. In Shell Structures: Theory and Applications; CRC Press: London, UK, 2018; Volume 4, pp. 549–552. [Google Scholar] [CrossRef]
- Yusuf, R.O.; Noor, Z.Z.; Din, M.D.F.M.D.; Abba, A.H. Use of sewage sludge ash (SSA) in the production of cement and concrete–a review. Int. J. Glob. Environ. Issues 2012, 12, 214–228. [Google Scholar] [CrossRef]
- Vouk, D.; Nakic, D.; Stirmer, N.; Cheeseman, C.R. Use of sewage sludge ash in cementitious materials. Rev. Adv. Mater. Sci. 2017, 49, 158–170. [Google Scholar]
- Cieślik, B.; Konieczka, P. A review of phosphorus recovery methods at various steps of wastewater treatment and sewage sludge management. The concept of “no solid waste generation” and analytical methods. J. Clean. Prod. 2017, 142, 1728–1740. [Google Scholar] [CrossRef]
- Chen, M.; Blanc, D.; Gautier, M.; Mehu, J.; Gourdon, R. Environmental and technical assessments of the potential utilization of sewage sludge ashes (SSAs) as secondary raw material in construction. Waste Manag. 2013, 33, 1268–1275. [Google Scholar] [CrossRef]
- Donatello, S.; Tyrer, M.; Cheeseman, C.R. EU landfill waste acceptance criteria and EU Hazardous Waste Directive compliance testing of incinerated sewage sludge ash. Waste Manag. 2009, 30, 63–71. [Google Scholar] [CrossRef] [PubMed]
- EN 15863; Characterization of Waste—Leaching BEHAVIOUR test for Basic Characterization—Dynamic Monolithic Leaching Test with Periodic Leachant Renewal, under Fixed Conditions. European Standards; European Committee for Standardization: Brussels, Belgium, 2015.
- Coutand, M.; Cyr, M.; Clastres, P. Use of sewage sludge ash as mineral admixture in mortars. Constr. Mater. 2006, 159, 153–162. [Google Scholar] [CrossRef]
- Cenni, R.; Janisch, B.; Spliethoff, H.; Hein, K.R.G. Legislative and environmental issues on the use of ash from coal and municipal sewage sludge co-firing as construction materials. Waste Manag. 2001, 21, 17–31. [Google Scholar] [CrossRef]
- Latosińska, J.; Lech, M.; Paluch, A.; Galarczyk, Ł. Effect of the addition of ash from the incineration of sewage sludge on the properties of cement slurries. Struct. Environ. 2016, 8, 253–259. [Google Scholar]
- Wichowski, P.; Rutkowska, G.; Nowak, P. Elution of selected heavy metals from concretes containing ashes produced in thermal conversion of sludge. Acta Sci. Pol. Arch. 2017, 16, 43–51. [Google Scholar] [CrossRef]
- Vouk, D.; Nakic, D.; Stirmer, N. Reuse of sewage sludge—Problems and possibilities. In Proceedings of the International Conference IWWATV, Athens, Greece, 21–23 May 2015. [Google Scholar]
- Monzó, J.; Payá, J.; Borrachero, M.V.; Bellver, A.; Peris-Mora, E. Study of cement-based mortars containing Spanish ground sewage sludge ash. Stud. Environ. Sci. 1997, 71, 349–354. [Google Scholar]
- Baeza-Brotons, F.; Garces, P.; Payá, J.; Saval, J.M. Portland cement systems with addition of sewage sludge ash. Application in concretes for the manufacture of blocks. J. Clean. Prod. 2014, 82, 112–124. [Google Scholar] [CrossRef] [Green Version]
- Jamshidi, A.; Jamshid, M.; Mehrdadi, N.; Shasavandi, A.; Pacheco-Torgal, F. Mechanical performance of concrete with partial replacement of sand by sewage sludge ash. Mater. Sci. Forum 2012, 730–732, 462–467. [Google Scholar] [CrossRef] [Green Version]
- Fontes, C.M.A.; Barbosa, M.C.; Toledo Filho, R.D.; Goncalves, J.P. Potentiality of sewage sludge ash as mineral additive in cement mortar and high performance concrete. In Proceedings of the International RILEM Conference on the Use of Recycled Materials in Buildings and Structures, Barcelona, Spain, 8–11 November 2004. [Google Scholar]
- Monzó, J.; Paya, J.; Borrachero, M.V.; Girbe, I. Reuse of sewage sludge ashes (SSA) in cement mixtures: The effect of SSA on the workability of cement mortars. Waste Manag. 2003, 23, 373–381. [Google Scholar] [CrossRef]
- Cyr, M.; Coutand, M.; Clastres, P. Technological and environmental behavior of sewage sludge ash (SSA) in cement-based materials. Cem. Concr. Res. 2007, 37, 1278–1289. [Google Scholar] [CrossRef]
- Lin, K.L.; Chang, W.C.; Lin, D.F.; Luo, H.L.; Tsai, M.C. Effects of nano-SiO2 and different ash particle sizes on sludge ash—Cement mortar. J. Environ. Manag. 2008, 88, 708–714. [Google Scholar] [CrossRef]
- Garcés, P.; Perez-Carrión, M.; García-Alcocel, E.; Payá, J.; Monzó, J.; Borrachero, M.V. Mechanical and physical properties of cement blended with sewage sludge ash. Waste Manag. 2008, 28, 2495–2502. [Google Scholar] [CrossRef]
- Cusidó, J.A.; Cremades, L.V. Environmental effects of using clay bricks produced with sewage sludge: Leachability and toxicity studies. Waste Manag. 2012, 32, 1202–1208. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, D.; Hinge, P.; Waghe, U.P.; Raut, S.P. Utilization of industrial waste in construction material—A review. Int. J. Innov. Res. Sci. Eng. Technol. 2014, 3, 8390–8397. [Google Scholar]
- Lynn, C.J.; Dhir, R.K.; Ghataora, G.S.; West, R.P. Sewage sludge ash characteristics and potential for use in concrete. Constr. Build. Mater. 2015, 98, 767–779. [Google Scholar] [CrossRef] [Green Version]
- Cieślik, B.M.; Zając, M.; Gałuszka, A.; Konieczka, P. Comprehensive stabilization of all streams of solid residues formed during sewage sludge thermal treatment–Case study. J. Clean. Prod. 2018, 178, 757–767. [Google Scholar] [CrossRef]
- Lin, K.L.; Lin, C.Y. Hydration characteristics of waste sludge ash utilized as raw cement material. Cem. Concr. Res. 2005, 35, 1999–2007. [Google Scholar] [CrossRef]
- Monzó, J.; Paya, J.; Borrachero, M.V.; Córcoles, A. Use of sewage sludge ash (SSA)-cement admixtures in mortars. Cem. Concr. Res. 1996, 26, 1389–1398. [Google Scholar] [CrossRef]
- Chin, S.C.; Ing, D.S.; Kusbiantoro, A.; Wong, Y.K.; Ahmad, S.W. Characterization of sewage sludge ash (SSA) in cement mortar. J. Eng. Appl. Sci. 2016, 11, 2042–2047. [Google Scholar]
- Ingunza, M.D.P.D.; Camarini, G.; Silva da Costa, F.M. Performance of mortars with the addition of septic tank sludge ash. Constr. Build. Mater. 2018, 160, 308–315. [Google Scholar] [CrossRef]
- Pan, S.C.; Tseng, D.H.; Lee, C.C.; Lee, C. Influence of the fineness of sewage sludge ash on the mortar properties. Cem. Concr. Res. 2003, 33, 1749–1754. [Google Scholar] [CrossRef]
- Donatello, S.; Freeman-Pask, A.; Tyrer, M.; Cheeseman, C.R. Effect of milling and acid washing on the pozzolanic activity of incinerator sewage sludge ash. Cem. Concr. Compos. 2010, 32, 54–61. [Google Scholar] [CrossRef]
- Kappel, A.; Ottosen, L.M.; Kirkelund, G.M. Colour, compressive strength and workability of mortars with an iron rich sewage sludge ash. Constr. Build. Mater. 2017, 157, 1199–1205. [Google Scholar] [CrossRef] [Green Version]
- Piasta, W.; Lukawska, M. The effect of sewage sludge ash on properties of cement composites. Procedia Eng. 2016, 161, 1018–1024. [Google Scholar] [CrossRef] [Green Version]
- Dyer, T.D.; Halliday, J.E.; Dhir, R.K. Hydration chemistry of sewage sludge ash used as a cement component. J. Mater. Civil Eng. 2011, 23, 648–655. [Google Scholar] [CrossRef]
- Dyer, T.D.; Halliday, J.E.; Dhir, R.K. Hydration reaction of sewage sludge ash for use as a cement component in concrete production, recycling and reuse of sewage sludge. In Proceedings of the International Symposium, Dundee, UK, 19–20 March 2001. [Google Scholar]
- EN 197-1; Cement. Part 1: Composition, Specifications and Conformity for Common Cements. European Standards; European Committee for Standardization: Brussels, Belgium, 2012.
- EN 196-2; Methods of Testing Cement. Part. 2: Chemical Analysis of Cement. European Standards; European Committee for Standardization: Brussels, Belgium, 2013.
- Ambroziak, A.; Haustein, E.; Kondrat, J. Chemical and mechanical properties of 70-year-old concrete. J. Mater. Civ. Eng. 2019, 31, 04019159. [Google Scholar] [CrossRef]
- Ambroziak, A.; Haustein, E.; Niedostatkiewicz, M. Chemical, physical, and mechanical properties of 95-year-old concrete built-in arch bridge. Materials 2020, 14, 20. [Google Scholar] [CrossRef]
- Ambroziak, A.; Haustein, E. Properties of Old Concrete Built-In Former Leipziger Palace. Materials 2022, 15, 673. [Google Scholar] [CrossRef] [PubMed]
- ISO 13320:2009; Particle Size Analysis-Laser Diffraction Methods. Part. 1: General Principles. International Organization for Standardization ISO: Geneva, Switzerland, 2009.
- ASTM C188-17; Standard Test Method for Density of Hydraulic Cement. ASTM International (American Society for Testing and Materials): West Conshohocken, PA, USA, 2017.
- EN 451-2; Method of Testing Fly Ash. Determination of Fineness by Wet Sieving. European Standards; European Committee for Standardization: Brussels, Belgium, 2017.
- ASTM C1679-17; Standard Practice for Measuring Hydration Kinetics of Hydraulic Cementitious Mixtures Using Isothermal Calorimetry. ASTM International (American Society for Testing and Materials): West Conshohocken, PA, USA, 2017.
- Kuryłowicz-Cudowska, A. Determination of thermophysical parameters involved in the numerical model to predict the temperature field of cast-in-place concrete bridge deck. Materials 2019, 12, 3089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuryłowicz-Cudowska, A.; Wilde, K.; Chróścielewski, J. Prediction of cast-in–place concrete strength of the extradosed bridge deck based on temperature monitoring and numerical simulations. Constr. Build. Mater. 2020, 254, 119224. [Google Scholar] [CrossRef]
- Kuryłowicz-Cudowska, A.; Haustein, E. Isothermal Calorimetry and Compressive Strength Tests of Mortar Specimens for Determination of Apparent Activation Energy. J. Mater. Civ. Eng. 2021, 33, 1–14. [Google Scholar] [CrossRef]
- TA Instruments. TAM Air Calorimeter Operator’s Manual; New Cable; TA Instruments: New Castle, UK, 2008; pp. 1–64. [Google Scholar]
- EN 450–1; Fly Ash for Concrete. Part. 1: Definition, Specifications and Conformity Criteria. European Standards; European Committee for Standardization: Brussels, Belgium, 2012.
- ASTM C109/C109M; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50-mm] Cube Specimens). ASTM International (American Society for Testing and Materials): West Conshohocken, PA, USA, 2019.
- Yu, T.Y.; Ing, D.S.; Choo, C.S.; Thong, Y.H. The Potential use of SSA and ISSA in construction field. A Review. IJISET Int. J. Innov. Sci. Eng. Technol. 2016, 3, 171–179. [Google Scholar]
- ASTM C618–19; Standard Specification for Coal Fly Ash and Raw or Calcined natural Pozzolan for Use in Concrete. ASTM International (American Society for Testing and Materials): West Conshohocken, PA, USA, 2019.
- Werther, J.; Ogada, T. Sewage sludge combustion. Prog. Energy Combust. Sci. 1999, 25, 55–116. [Google Scholar] [CrossRef]
- Rutkowska, G.; Wichowski, P.; Franus, M.; Mendryk, M.; Fronczyk, J. Modification of Ordinary Concrete Using Fly Ash from Combustion of Municipal Sewage Sludge. Minerals 2020, 13, 487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mejdia, M.; Saillioa, M.; Chaussadenta, T.; Diveta, L.; Tagnit-Hamou, A. Hydration mechanisms of sewage sludge ashes used as cement replacement. Cem. Concr. Res. 2020, 135, 106115. [Google Scholar] [CrossRef]
- Hu, J.; Ge, Z.; Wang, K. Influence of cement fineness and water-to-cement ratio on mortar early-age heat of hydration and set times. Constr. Build. Mater. 2014, 5, 657–663. [Google Scholar] [CrossRef]

| SSA Substitution Rate (%) | Components of Cement Mortar, kg/m3 | w/b (-) | |||
|---|---|---|---|---|---|
| OPC 42.5 R | SSA | Sand | Water | ||
| 0.00 | 390.00 | 0.00 | 1085.0 | 214.0 | 0.55 |
| 2.50 | 380.25 | 9.75 | 1085.0 | 214.0 | 0.55 |
| 5.00 | 370.50 | 19.50 | 1085.0 | 214.0 | 0.55 |
| 7.50 | 360.75 | 29.25 | 1085.0 | 214.0 | 0.55 |
| 10.00 | 351.00 | 39.00 | 1085.0 | 214.0 | 0.55 |
| 20.00 | 312.00 | 78.00 | 1085.0 | 214.0 | 0.55 |
| Determined Species and Parameters | OPC 42.5 R | Sewage Sludge Ash (SSA) | Admissible Content acc. to EN 450-1 [51] |
|---|---|---|---|
| SiO2 (%) | 21.2 | 18.9 | ≤25% mass |
| Fe2O3 (%) | 2.2 | 13.8 | - |
| Al2O3 (%) | 5.0 | 9.6 | - |
| The sum of oxide content (SiO2 + Fe2O3 + Al2O3) | 28.4 | 42.3 | ≥70% mass |
| CaO (%) | 64.1 | 18.2 | ≤10% mass 1 |
| CaOfree (%) | - | - | - |
| MgO (%) | 2.5 | 4.4 | ≤4.0% mass |
| SO3 (%) | 3.0 | 2.1 | ≤3.0% mass |
| Na2O (%) | 0.13 | 1.1 | - |
| K2O (%) | 0.92 | 2.2 | - |
| Na2Oeq (Na2O + 0.658K2O) | 0.74 | 2.6 | ≤5.0% mass |
| P2O5 (%) | - | 25.1 | ≤5.0 mg/kg |
| TiO2 (%) | - | 1.5 | - |
| Chloride content (%) | 0.03 | - | ≤0.10% mass |
| Loss on ignition LOI (%) | 3.1 | 2.7 | Cat.: A ≤ 5%; B 2 ÷ 7% C 4 ÷ 9% mass |
| Type of Sample | Type of Determined Element, (mg/kg) Dry Weight | |||||
|---|---|---|---|---|---|---|
| Cadmium (Cd) | Copper (Cu) | Nickel (Ni) | Lead (Pb) | Zinc (Zn) | Chrome (Cr) | |
| SSA | 3.3 ± 1.32 | 750 ± 3.54 | 68.3 ± 0.05 | 88.7 ± 2.01 | 2015 ± 0.09 | 54.2 ± 1.03 |
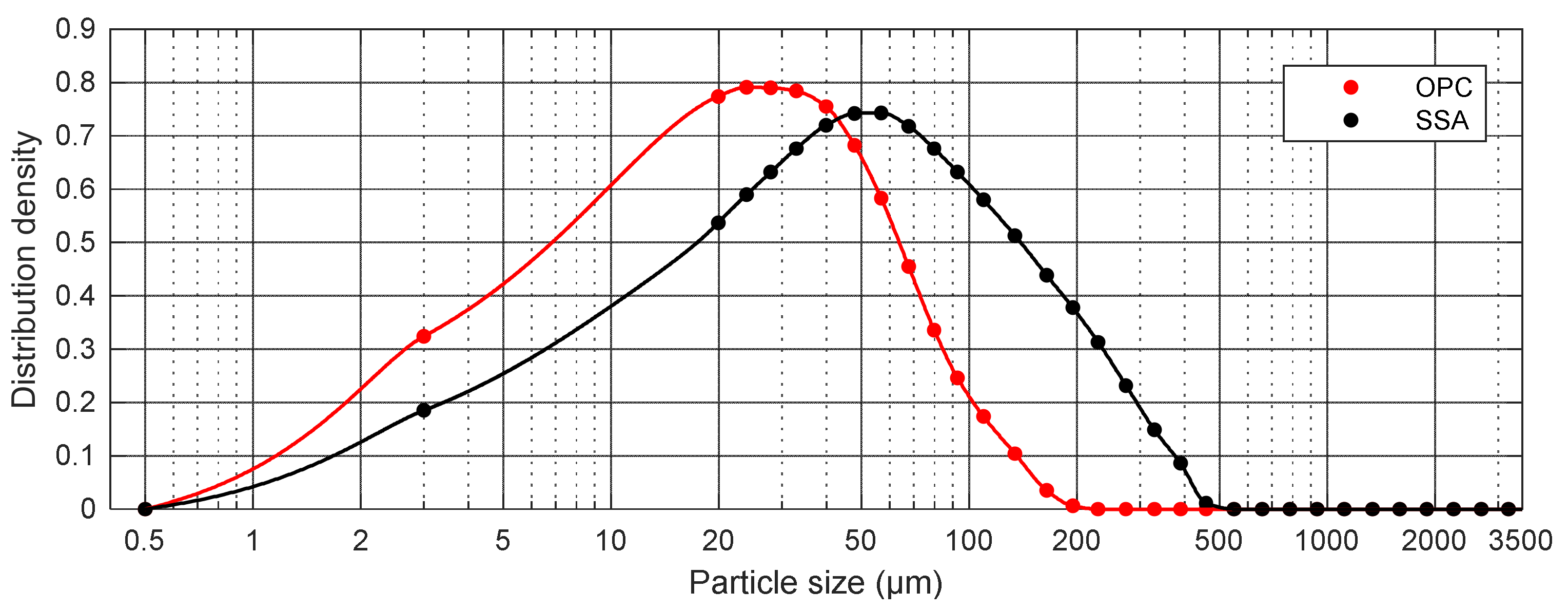
| Samples | OPC | SSA |
|---|---|---|
| D10 (µm) | 3.97 | 6.59 |
| D50 (µm) | 17.86 | 39.86 |
| D90 (µm) | 62.04 | 167.75 |
| VMD (Dmean) (µm) | 27.41 | 66.42 |
| Specific density (g/cm3) | 3.05 | 2.45 |
| Fineness *, % | 19.40 | 46.80 |
| Peak and Time Value of the Heat Flow | Content of the Sewage Sludge Ash in the Cement Mortar | |||||||
|---|---|---|---|---|---|---|---|---|
| 0% | 2.5% | 5.0% | 7.5% | 10% | 20% | |||
| first | dQ/dt | (J/g/h) | 4.91 | 4.65 | 4.45 | 4.20 | 4.00 | - |
| t | (h) | 14.20 | 15.28 | 16.25 | 17.28 | 18.55 | - | |
| second | dQ/dt | (J/g/h) | 4.60 | 4.98 | 5.37 | 5.44 | 5.85 | 6.88 |
| t | (h) | 30.77 | 31.25 | 31.45 | 32.13 | 32.48 | 34.52 | |
| Mortar Type | Time (h) | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 7 | 24 | 48 | 72 | 120 | 168 | |
| 0.0% SSA (J/g) | 9.91 | 22.58 | 98.54 | 174.6 | 203.99 | 240.24 | 267.1 |
| 2.5% SSA (J/g) | 13.31 | 24.97 | 96.45 | 175.5 | 205.49 | 242.49 | 265.0 |
| 5.0% SSA (J/g) | 10.36 | 20.78 | 88.40 | 169.4 | 199.73 | 237.18 | 264.9 |
| 7.5% SSA (J/g) | 10.03 | 19.16 | 82.10 | 164.4 | 195.12 | 232.69 | 260.3 |
| 10% SSA (J/g) | 14.14 | 23.17 | 81.87 | 160.5 | 198.18 | 236.87 | 258.0 |
| 20% SSA (J/g) | 12.51 | 19.44 | 61.34 | 151.3 | 185.58 | 227.03 | 255.9 |
| Setting Time (h) | Content of Sewage Sludge Ash in the Cement Mortar | |||||
|---|---|---|---|---|---|---|
| 0% | 2.5% | 5.0% | 7.5% | 10% | 20% | |
| initial | 4.53 | 5.12 | 5.38 | 5.63 | 6.07 | 7.87 |
| final | 14.22 | 15.17 | 16.22 | 17.18 | 18.57 | 25.10 |
| Days | Mortar Type | Average Compressive Strength, MPa | SD *, MPa | Strength Activity Index (SAI) |
|---|---|---|---|---|
| 28 | 100% OPC 42.5 R | 56.49 | 3.56 | 72.38% |
| 28 | 75% OPC + 25% SSA | 40.89 | 1.42 | |
| 90 | 100% OPC 42.5 R | 59.48 | 2.67 | 73.30% |
| 90 | 75% OPC + 25% SSA | 43.60 | 1.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haustein, E.; Kuryłowicz-Cudowska, A.; Łuczkiewicz, A.; Fudala-Książek, S.; Cieślik, B.M. Influence of Cement Replacement with Sewage Sludge Ash (SSA) on the Heat of Hydration of Cement Mortar. Materials 2022, 15, 1547. https://doi.org/10.3390/ma15041547
Haustein E, Kuryłowicz-Cudowska A, Łuczkiewicz A, Fudala-Książek S, Cieślik BM. Influence of Cement Replacement with Sewage Sludge Ash (SSA) on the Heat of Hydration of Cement Mortar. Materials. 2022; 15(4):1547. https://doi.org/10.3390/ma15041547
Chicago/Turabian StyleHaustein, Elżbieta, Aleksandra Kuryłowicz-Cudowska, Aneta Łuczkiewicz, Sylwia Fudala-Książek, and Bartłomiej Michał Cieślik. 2022. "Influence of Cement Replacement with Sewage Sludge Ash (SSA) on the Heat of Hydration of Cement Mortar" Materials 15, no. 4: 1547. https://doi.org/10.3390/ma15041547
APA StyleHaustein, E., Kuryłowicz-Cudowska, A., Łuczkiewicz, A., Fudala-Książek, S., & Cieślik, B. M. (2022). Influence of Cement Replacement with Sewage Sludge Ash (SSA) on the Heat of Hydration of Cement Mortar. Materials, 15(4), 1547. https://doi.org/10.3390/ma15041547






