Comparative Study on Biodegradation of Pure Iron Prepared by Microwave Sintering and Laser Melting
Abstract
:1. Introduction
2. Experiments
3. Results and Discussion
3.1. Microstructure and Hardness
3.2. Biodegradation
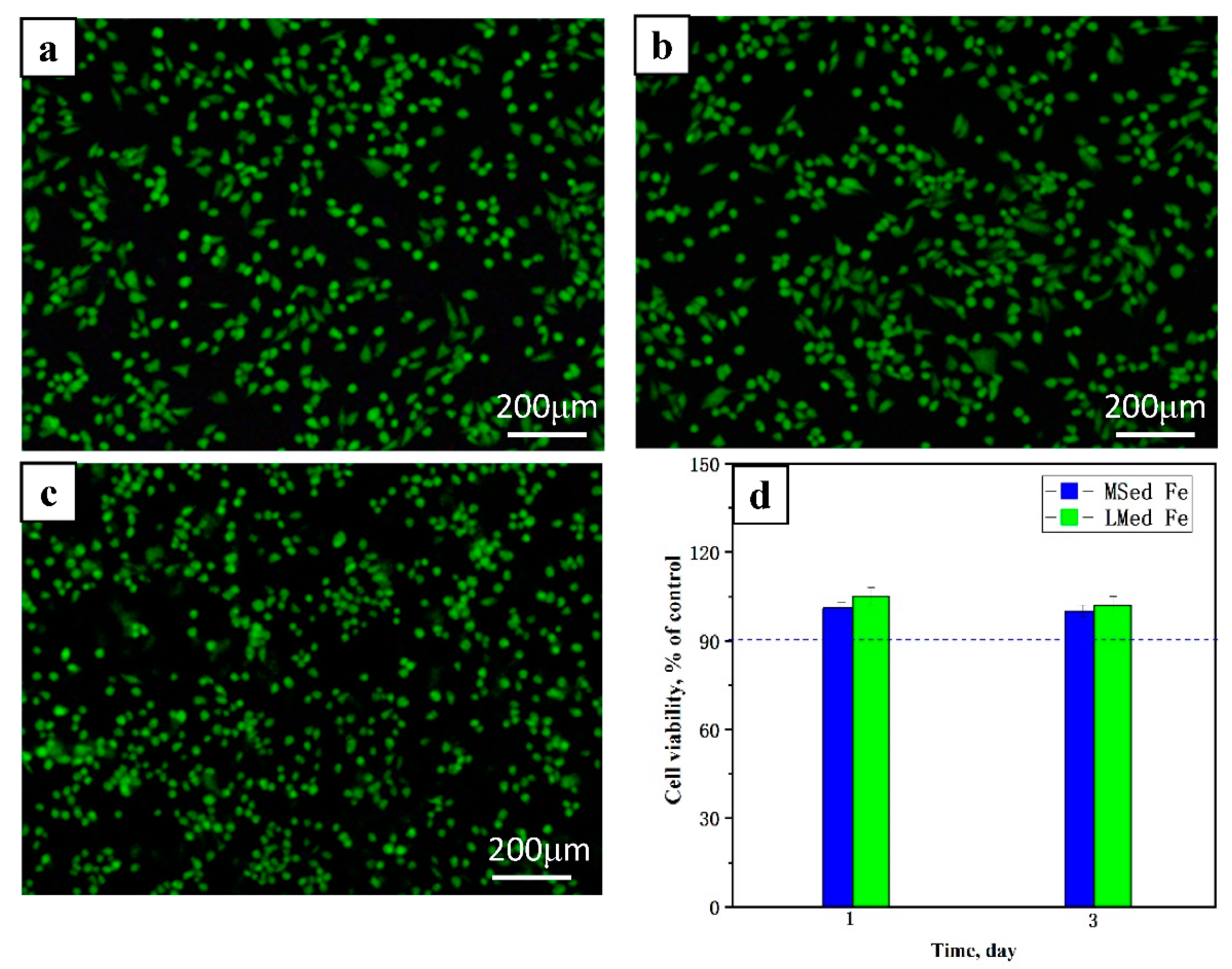
3.3. Biocompatibility
4. Conclusions
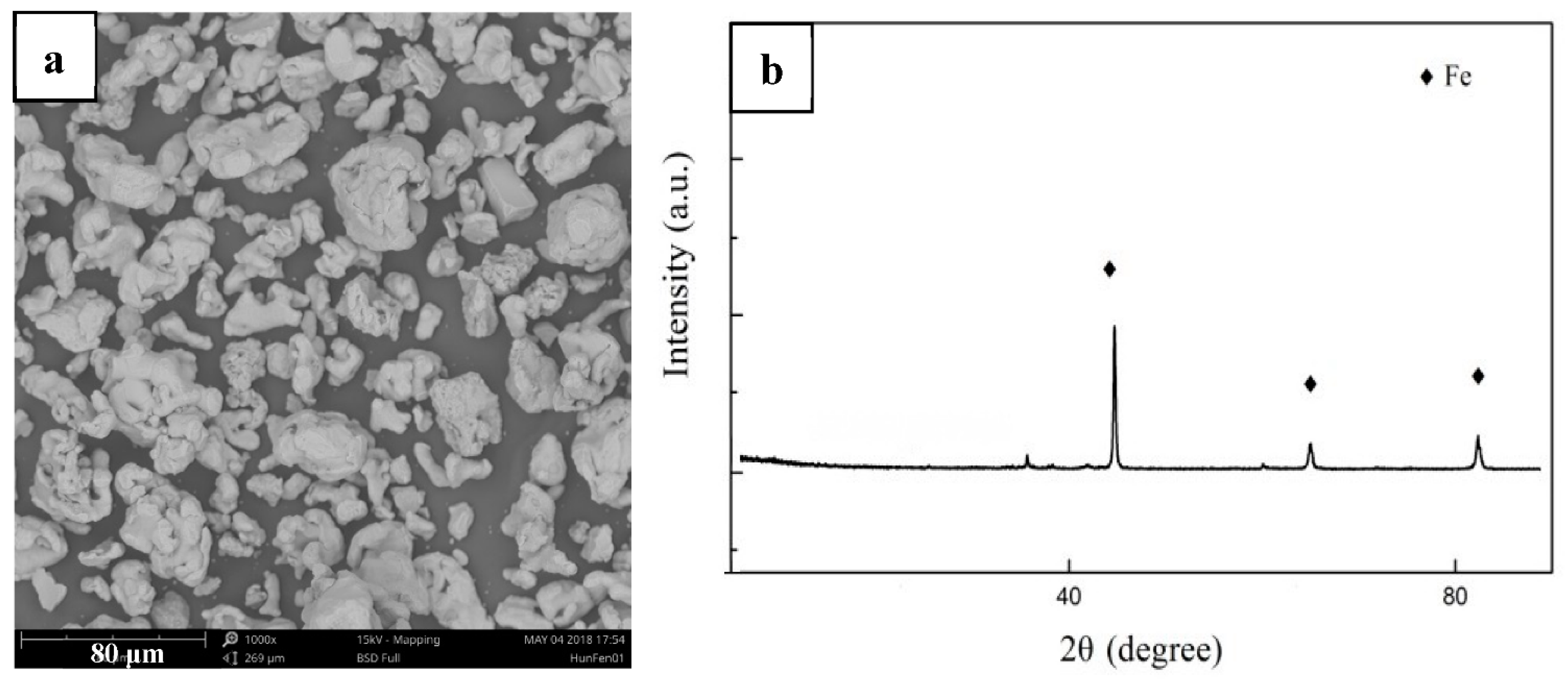
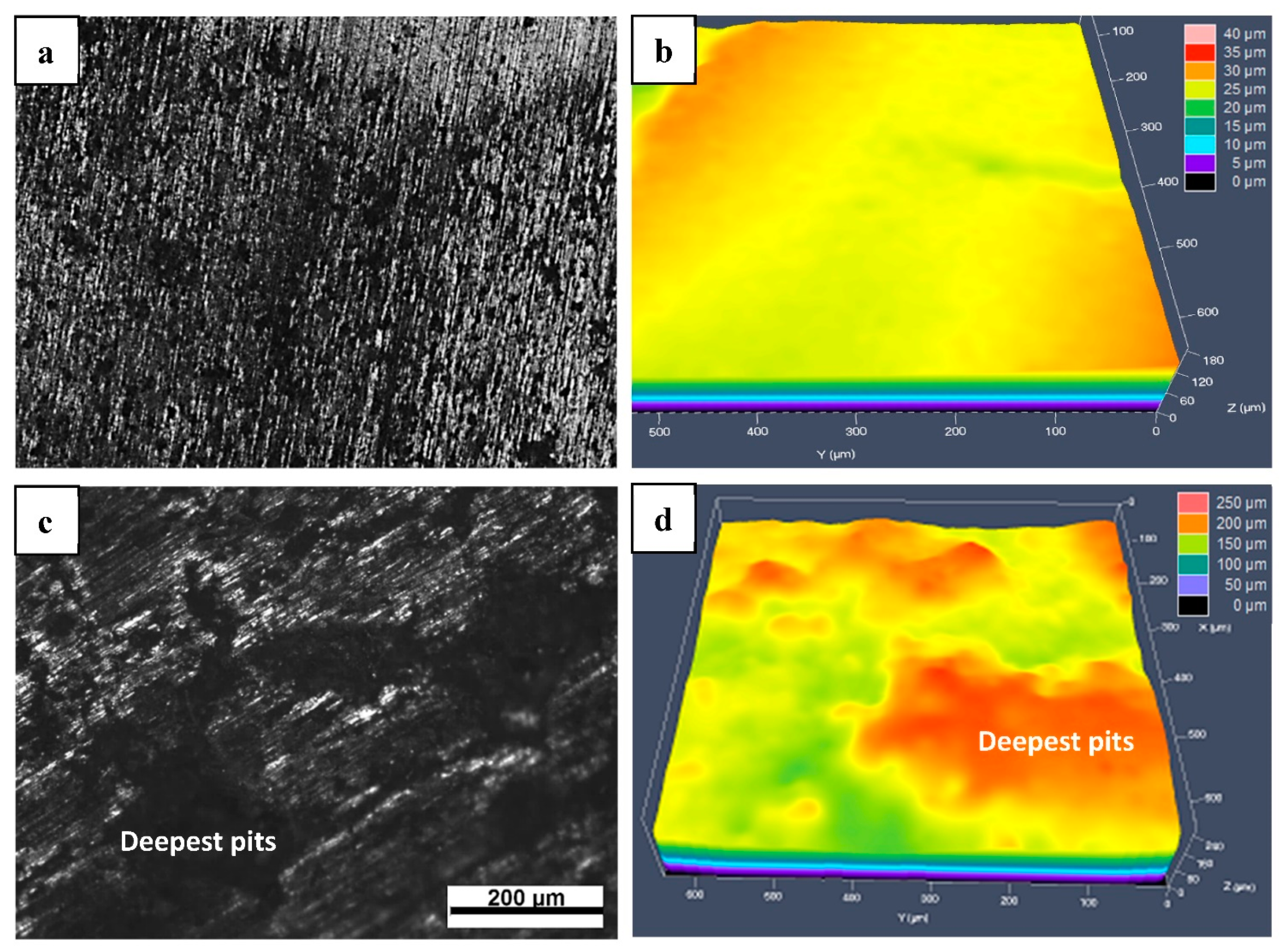
- MSed Fe presented a distinct porous structure, while LMed Fe presented a relatively compact structure without any obvious pores.
- MSed Fe had lower a density and hardness than LMed Fe, and both had lower density, but higher hardness than the as-cast Fe.
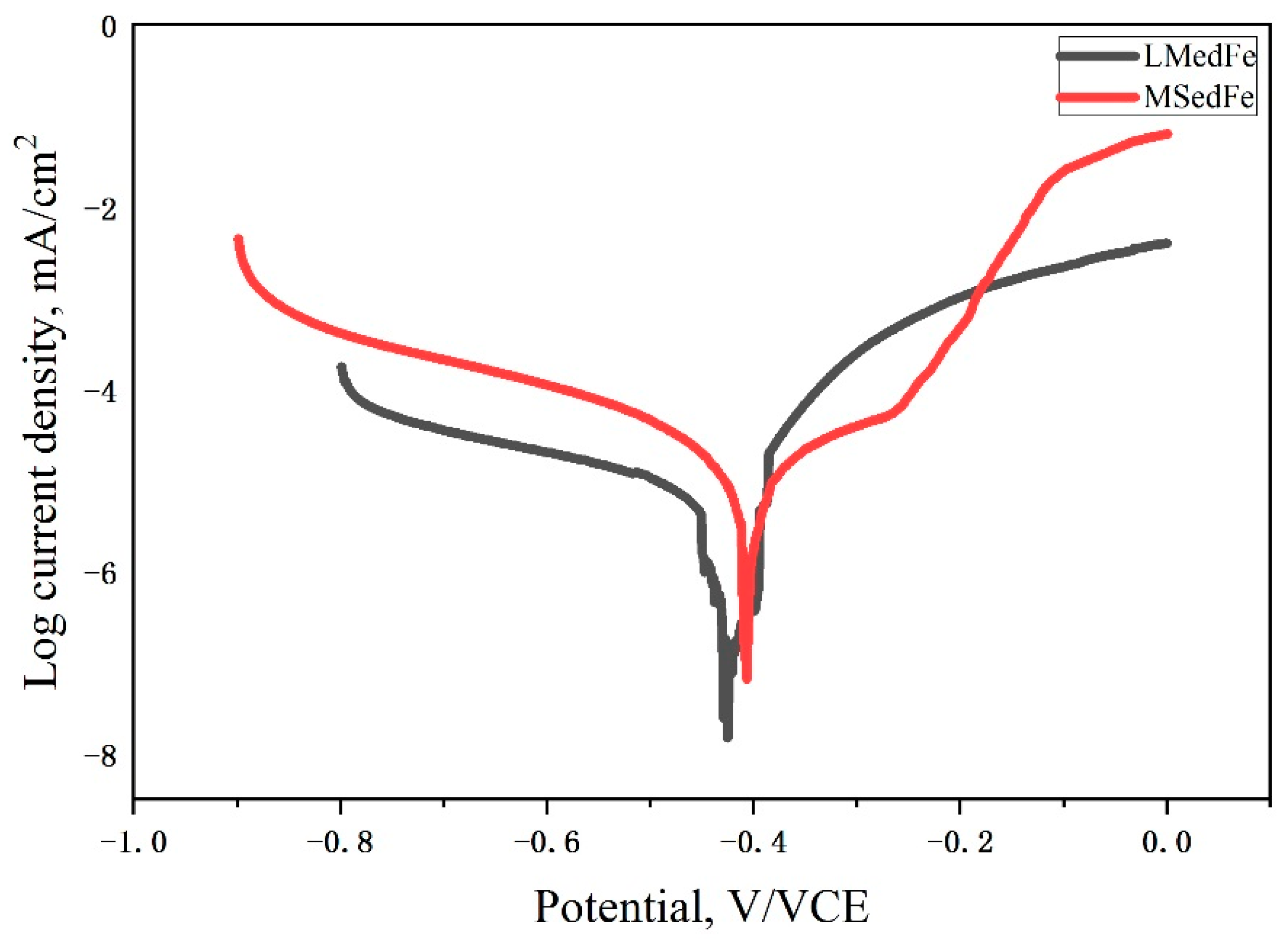
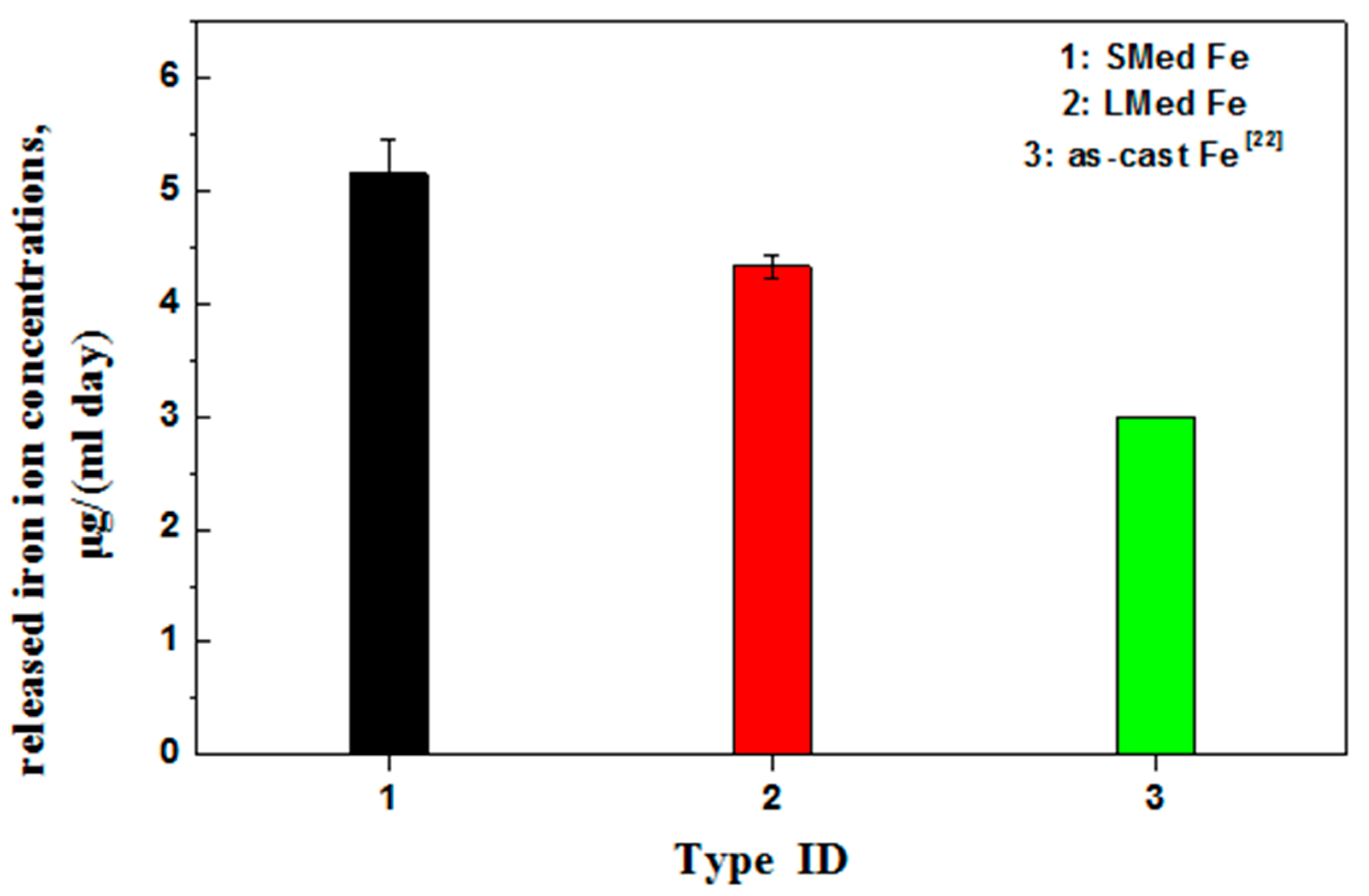
- The order of the biodegradation rate was as follows: MSed Fe > LMed Fe > as-cast Fe. That is, the biodegradation rates of MSed Fe and LMed Fe were approximately 44 and 13 times higher than that of as-cast Fe, respectively.
- Microwave sintering and laser melting were effective methods of increasing the biodegradation rate. The biodegradation behavior was closely related to the microstructure compactness and grain size.
- MSed Fe and LMed Fe had satisfactory biocompatibility.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gorejová, R.; Haverová, L.; Oriňaková, R.; Oriňak, A.; Oriňak, M. Recent advancements in Fe-based biodegradable materials for bone repair. J. Mater. Sci. 2019, 54, 1913–1947. [Google Scholar] [CrossRef]
- Lee, M.K.; Lee, H.; Park, C.; Kang, I.G.; Kim, J.; Kim, H.E.; Jung, H.D.; Jang, T.S. Accelerated biodegradation of iron-based implants via tantalum-implanted surface nanostructures. Bioact. Mater. 2022, 9, 239–250. [Google Scholar] [CrossRef]
- Guo, Y.X.; Zhao, M.C.; Xie, B.; Zhao, Y.C.; Yin, D.; Gao, C.; Shuai, C.; Atrens, A. In vitro corrosion resistance and antibacterial performance of novel fe-xcu biomedical alloys prepared by selective laser melting. Adv. Eng. Mater. 2020, 23, 2001000. [Google Scholar] [CrossRef]
- Carluccio, D.; Bermingham, M.; Kent, D.; Demir, A.G.; Previtali, B.; Dargusch, M.S. Comparative study of pure iron manufactured by selective laser melting, laser metal deposition, and casting processes. Adv. Eng. Mater. 2018, 21, 1900049. [Google Scholar] [CrossRef]
- Gasior, G.; Szczepanski, J.; Radtke, A. Biodegradable iron-based materials-what was done and what more can be done. Materials 2021, 14, 3381. [Google Scholar] [CrossRef] [PubMed]
- Peuster, M.; Hesse, C.; Schloo, T.; Fink, C.; Beerbaum, P.; von Schna-Kenburg, C. Long-term biocompatibility of a corrodible peripheral iron stent in the porcine descending aorta. Biomaterials 2006, 27, 4955–4962. [Google Scholar] [CrossRef]
- Kraus, T.; Moszner, F.; Fischerauer, S.; Fiedler, M.; Martinelli, E.; Eichler, J.; Witte, F.; Willbold, E.; Schinhammer, M.; Meischel, M.; et al. Biodegradable Fe-based alloys for use in osteosynthesis: Out-come of an in vivo study after 52 weeks. Acta Biomater. 2014, 10, 3346–3353. [Google Scholar] [CrossRef]
- Moravej, M.; Prima, F.; Fiset, M.; Mantovani, D. Electroformed iron as new biomaterial for degradable stents: Development process and structure-properties relationship. Biomaterials 2010, 6, 1726–1735. [Google Scholar] [CrossRef]
- Li, Y.; Jahr, H.; Lietaert, K.; Pavanram, P.; Yilmaz, A.; Fockaert, L.I.; Leeflang, M.A.; Pouran, B.; Gonzalez-Garcia, Y.; Weinans, H.; et al. Additively manufactured biodegradable porous iron. Acta Biomater. 2018, 77, 380–393. [Google Scholar] [CrossRef]
- Oghbaei, M.; Mirzaee, O. Microwave versus conventional sintering: A review of fundamentals, advantages and applications. J. Alloy. Compd. 2010, 494, 175–189. [Google Scholar] [CrossRef]
- Xie, B.; Zhao, M.C.; Xu, R.; Zhao, Y.C.; Yin, D.F.; Gao, C.; Atrens, A. Biodegradation, Antibacterial Performance, and Cytocompatibility of a Novel ZK30-Cu-Mn Biomedical Alloy Produced by Selective Laser Melting. Int. J. Bioprinting 2021, 7, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Zhao, M.; Tao, J.; Zhao, Y.; Yin, D.; Gao, C.; Shuai, C.; Atrens, A. Comparison of the biodegradation of ZK30 subjected to solid solution treating and selective laser melting. J. Mater. Res. Technol.-JMR&T 2021, 10, 722–729. [Google Scholar]
- Deng, B.; Guo, Y.X.; Zhao, M.C.; Li, Q.F.; Ma, B.; Duan, B.H.; Yin, D.F.; Atrens, A. Study on a Novel Biodegradable and Antibacterial Fe-Based Alloy Prepared by Microwave Sintering. Materials 2021, 14, 3784. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, Y.; Zhao, M.; Liu, C.; Liu, L.; Gao, C.; Shuai, C.; Atrens, A. Study on Fe-xGO Composites Prepared by Selective Laser Melting: Microstructure, Hardness, Biodegradation and Cytocompatibility. JOM 2020, 72, 1163–1174. [Google Scholar] [CrossRef]
- Tolouei, R.; Harrison, J.; Paternoster, C.; Turgeon, S.; Chevallier, P.; Mantovani, D. The use of multiple pseudo-physiological solutions to simulate the degradation behavior of pure iron as a metallic resorbable implant: A surface-characterization study. Phys. Chem. Chem. Phys. 2016, 18, 19637–19646. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.S. Determining Methods for Aperture and Aperture Distribution of Porous Materials. Titan. Ind. Prog. 2006, 2, 29–34. [Google Scholar]
- GB/T 3850-2015; Impermeable Sintered Metal Materials and Hardmetals—Determination of Density. China Standards Press: Beijing, China, 2016.
- Tao, S.C.; Xu, J.L.; Yuan, L.; Luo, J.M.; Zheng, Y.F. Microstructure, mechanical properties and antibacterial properties of the microwave sintered porous Ti-3Cu alloys. J. Alloys Compd. 2020, 812, 152142. [Google Scholar] [CrossRef]
- Kato, H.; Todaka, Y.; Umemoto, M.; Haga, M.; Sentoku, E. Sliding wear behavior of sub-microcrystalline pure iron produced by high-pressure torsion straining. Wear 2015, 336, 58–68. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, M.C.; Wang, Z.B.; Tan, L.; Qi, Y.; Yin, D.F.; Yang, K.; Atrens, A. Enhanced initial biodegradation resistance of the biomedical Mg-Cu alloy by surface nanomodification. J. Magnes. Alloy. 2022, 10. in Press. [Google Scholar] [CrossRef]
- Yan, X.; Zhao, M.; Yang, Y.; Tan, L.; Zhao, Y.; Yin, D.; Yang, K.; Atrens, A. Improvement of biodegradable and antibacte-rial properties by solution treatment and micro-arc oxidation (MAO) of a magnesium alloy with a trace of copper. Corros. Sci. 2019, 156, 125–138. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, Y.; Zhao, M.; Liu, L.; Gao, C.; Shuai, C.; Zeng, R.; Atrens, A.; Lin, Y. Graphene oxide reinforced iron matrix composite with enhanced biodegradation rate prepared by selective laser melting. Adv. Eng. Mater. 2019, 21, 1900314. [Google Scholar] [CrossRef]
- Cheng, J.; Zheng, Y.F. In vitro study on newly designed biodegradable Fe-X composites (X=W, CNT) prepared by spark plasma sintering. J. Biomed. Mater. Res. B 2013, 101B, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Zhao, M.; Zhao, Y.; Yin, D.; Liu, L.; Gao, C.; Shuai, C.; Atrens, A. Influence of graphene oxide (GO) on microstructure and biodegradation of ZK30-xGO composites prepared by selective laser melting. J. Magnes. Alloy. 2020, 8, 952–962. [Google Scholar] [CrossRef]
- Ji, H.; Zhao, M.C.; Xie, B.; Zhao, Y.C.; Yin, D.; Gao, C.; Shuai, C.; Andrej, A. Corrosion and antibacterial performance of novel selective-laser-melted (SLMed) Ti-xCu biomedical alloys. J. Alloys Compd. 2021, 864, 158415. [Google Scholar] [CrossRef]
- Annamalai, R.; Upadhyaya, A.; Agrawal, D. An investigation on microwave sintering of Fe, Fe-Cu and Fe-Cu-C alloys. Bull. Mater. Sci. 2013, 36, 447–456. [Google Scholar] [CrossRef]
- Peuster, M.; Wohlsein, P.; Brűgmann, M.; Ehlerding, M.; Seidler, K.; Fink, C.; Brauer, H.; Fischer, A.; Hausdorf, G. A novel approach to temporary stenting: Degradable cardiovascular stents produced from corrodible metal-results 6–18 months after implantation into New Zealand white rabbits. Heart 2001, 86, 563–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duck, K.A.; Connor, J.R. Iron uptake and transport across physiological barriers. Biometals 2016, 29, 573–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, S.; Huang, N.; Xu, L.; Zhang, Y.; Liu, H.; Sun, H.; Leng, Y. Biocompatibility of pure iron: In vitro assessment of degradation kinetics and cytotoxicity on endothelial cells. Mater. Sci. Eng. C 2009, 29, 1589–1592. [Google Scholar] [CrossRef]
- Yamamoto, A.; Honma, R.; Sumita, M. Cytotoxicity evaluation of 43 metal salts using murine fibroblasts and osteoblastic cells. J. Biomed. Mater. Res. 1998, 39, 331–340. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Feng, J.; Yu, H.; Lin, W.; Li, X.; Tian, Y.; Zhao, M. Comparative Study on Biodegradation of Pure Iron Prepared by Microwave Sintering and Laser Melting. Materials 2022, 15, 1604. https://doi.org/10.3390/ma15041604
Zhao Y, Feng J, Yu H, Lin W, Li X, Tian Y, Zhao M. Comparative Study on Biodegradation of Pure Iron Prepared by Microwave Sintering and Laser Melting. Materials. 2022; 15(4):1604. https://doi.org/10.3390/ma15041604
Chicago/Turabian StyleZhao, Yingchao, Jun Feng, Hui Yu, Wangyang Lin, Xin Li, Yan Tian, and Mingchun Zhao. 2022. "Comparative Study on Biodegradation of Pure Iron Prepared by Microwave Sintering and Laser Melting" Materials 15, no. 4: 1604. https://doi.org/10.3390/ma15041604
APA StyleZhao, Y., Feng, J., Yu, H., Lin, W., Li, X., Tian, Y., & Zhao, M. (2022). Comparative Study on Biodegradation of Pure Iron Prepared by Microwave Sintering and Laser Melting. Materials, 15(4), 1604. https://doi.org/10.3390/ma15041604







