Photothermal and Photodynamic Therapy of Tumors with Plasmonic Nanoparticles: Challenges and Prospects
Abstract
:1. Plasmonic Tuning of Au Nanoparticles for Plasmonic Photothermal Therapy
1.1. Optical Cross-Sections and Plasmonic Tuning
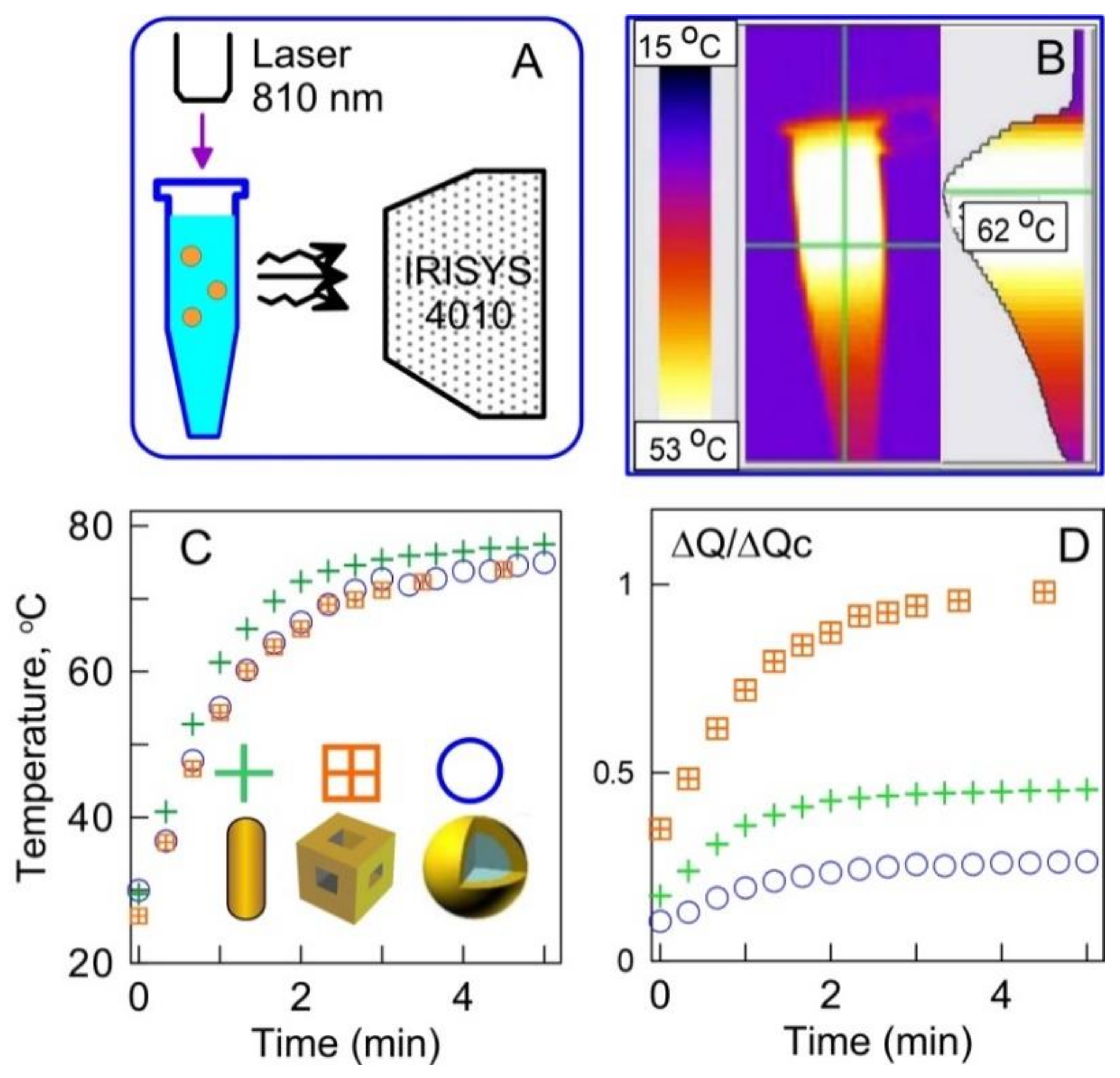
1.2. Generating Heat with Plasmonic Particles
2. The Main Types of Plasmonic Particles in PPT
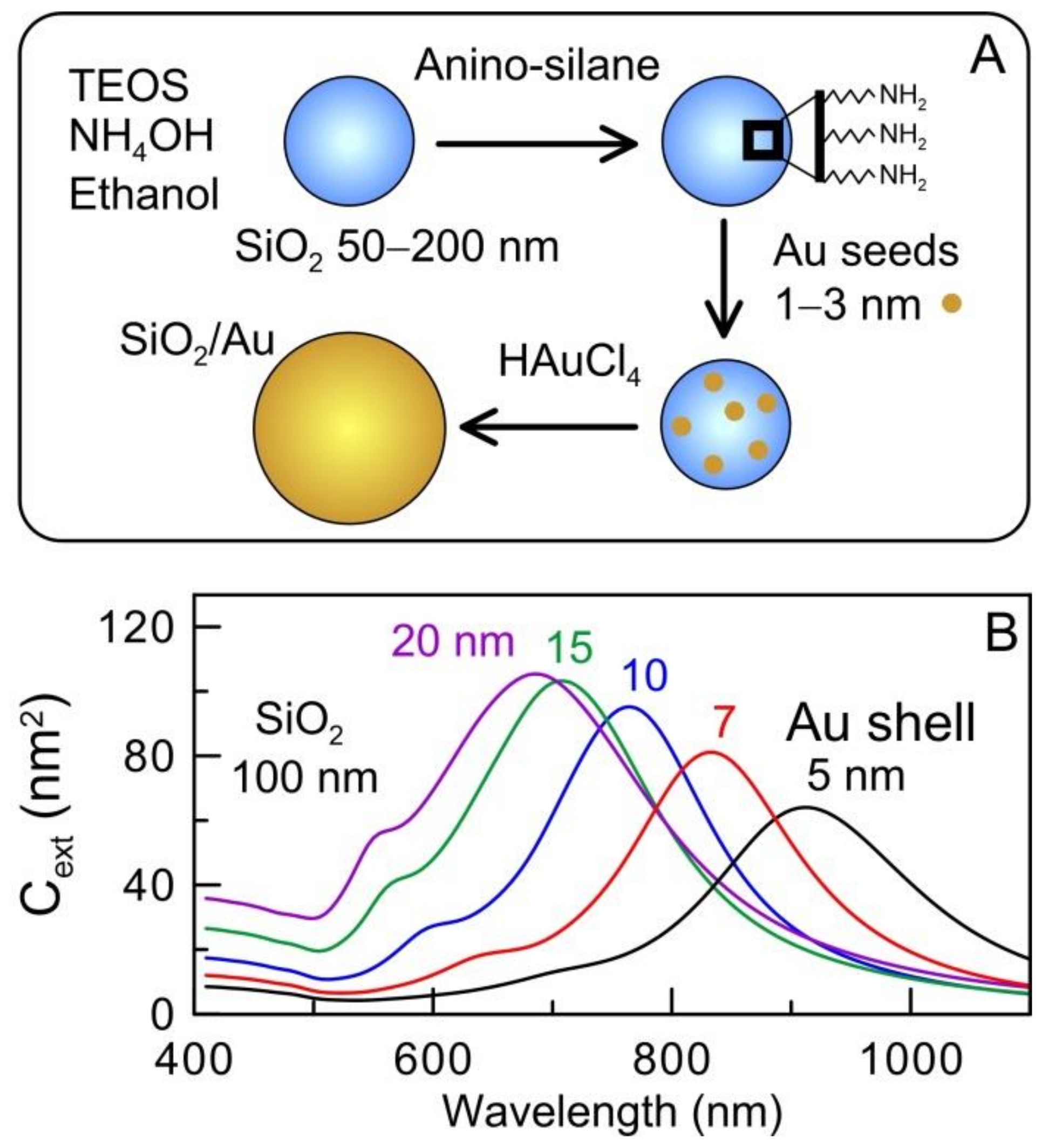
2.1. Silica/Gold Nanoshells (AuNSHs)
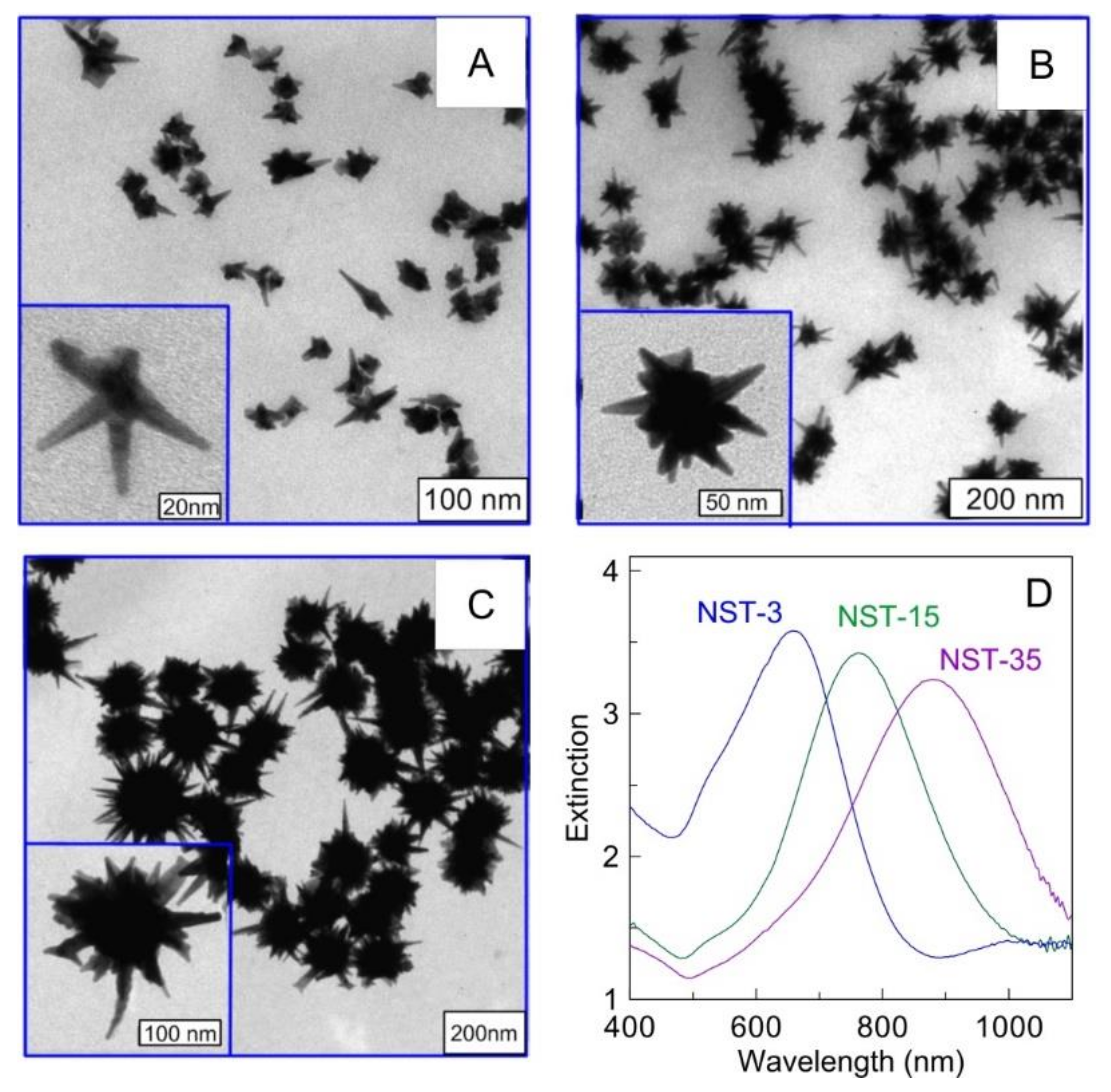
2.2. Gold Nanostars (AuNSTs)
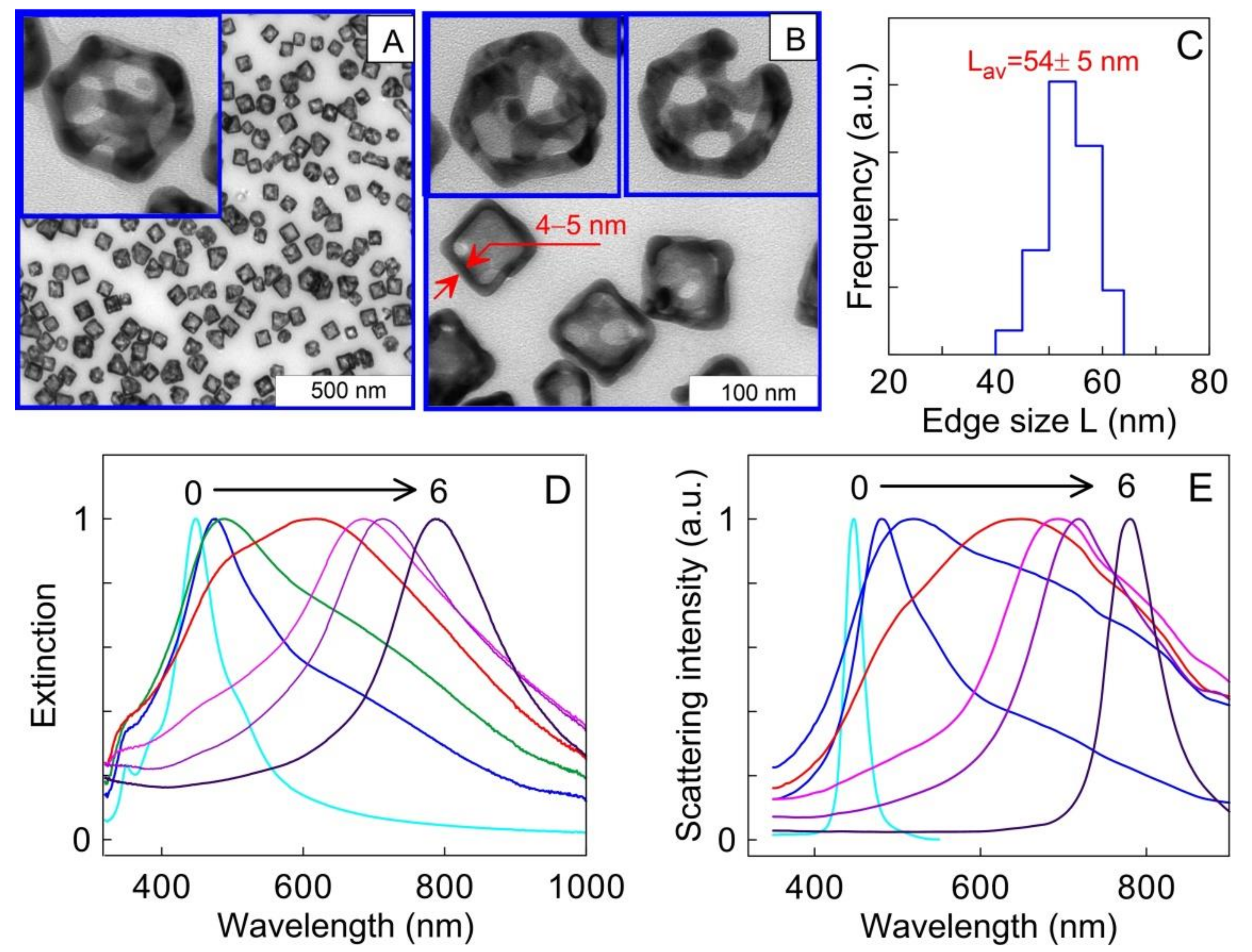
2.3. Au-Ag Nanocages (AuNCGs)
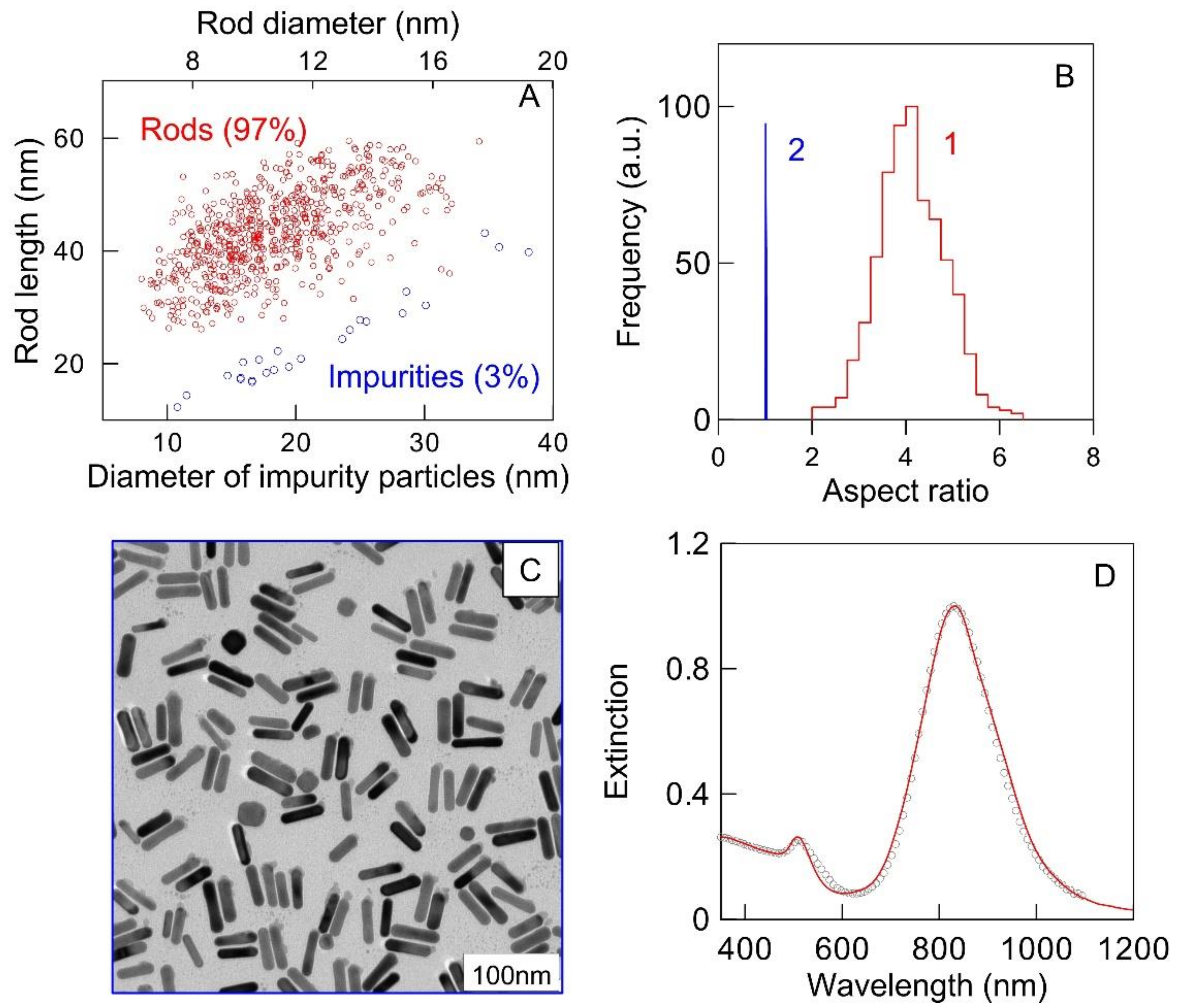
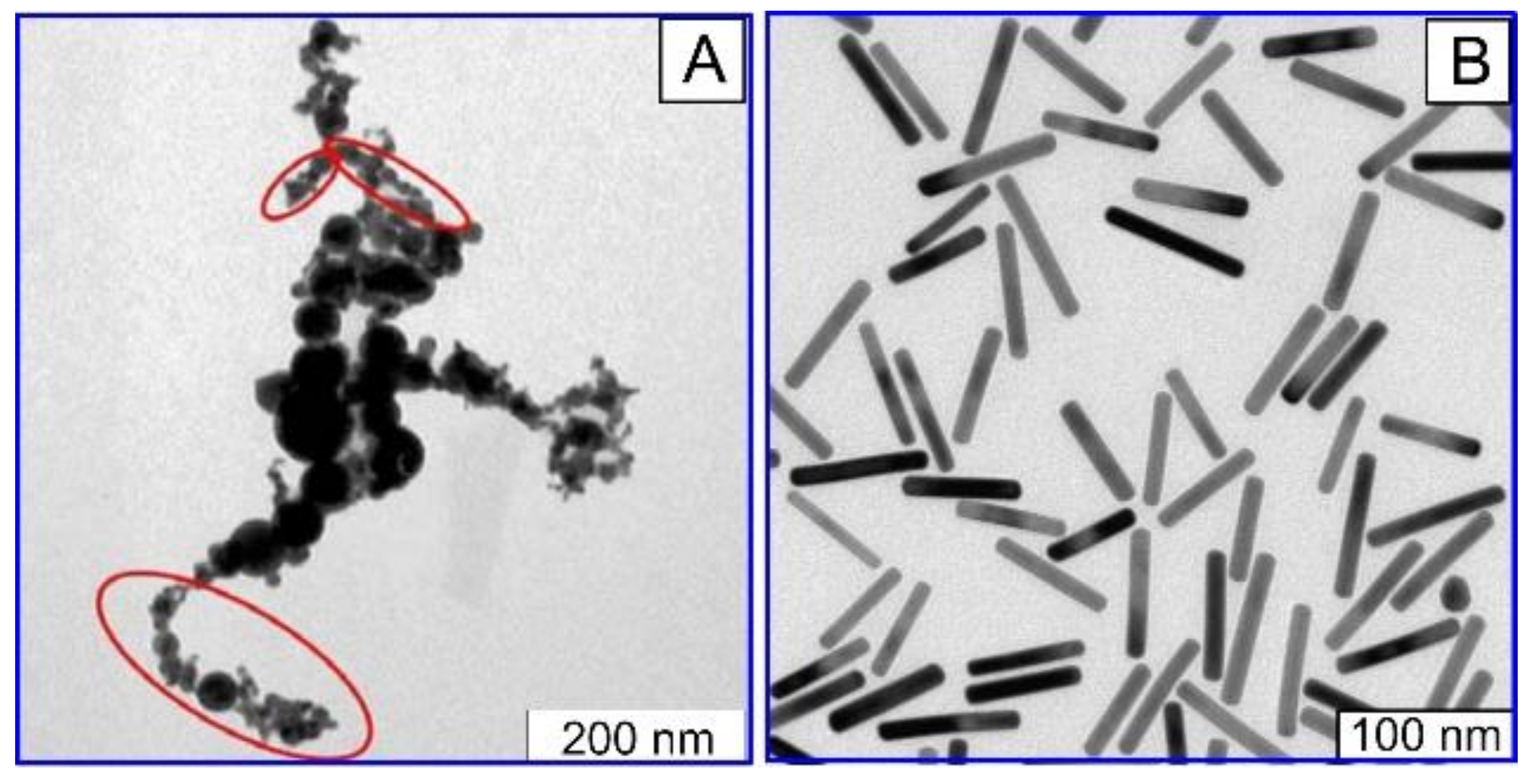
2.4. Au Nanorods (AuNRs)
2.5. Comparison of PTT Efficiency of Different Plasmonic Nanoparticles
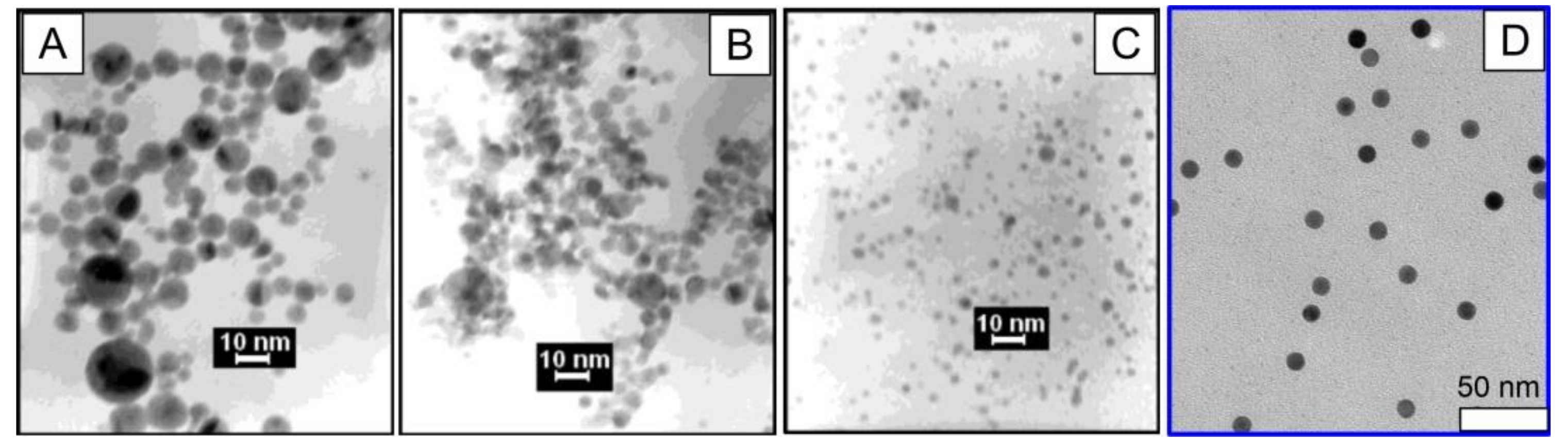
2.6. Fabrication of Plasmonic Nanoparticles by Pulsed Laser Ablation in Liquid (PLAL)
3. The Nanoparticle Application for Antitumor Therapy
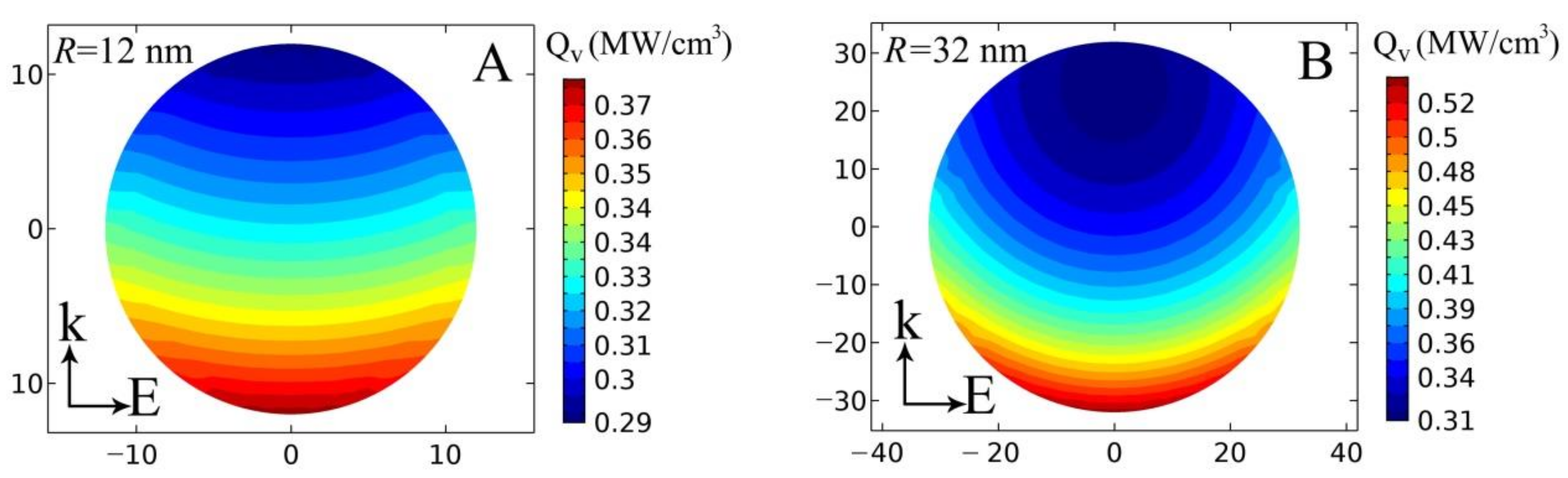
4. Evaluation of Optical Properties of Tumors and the Propagation of Laser Radiation and Heat in Models Sensitized with AuNPs
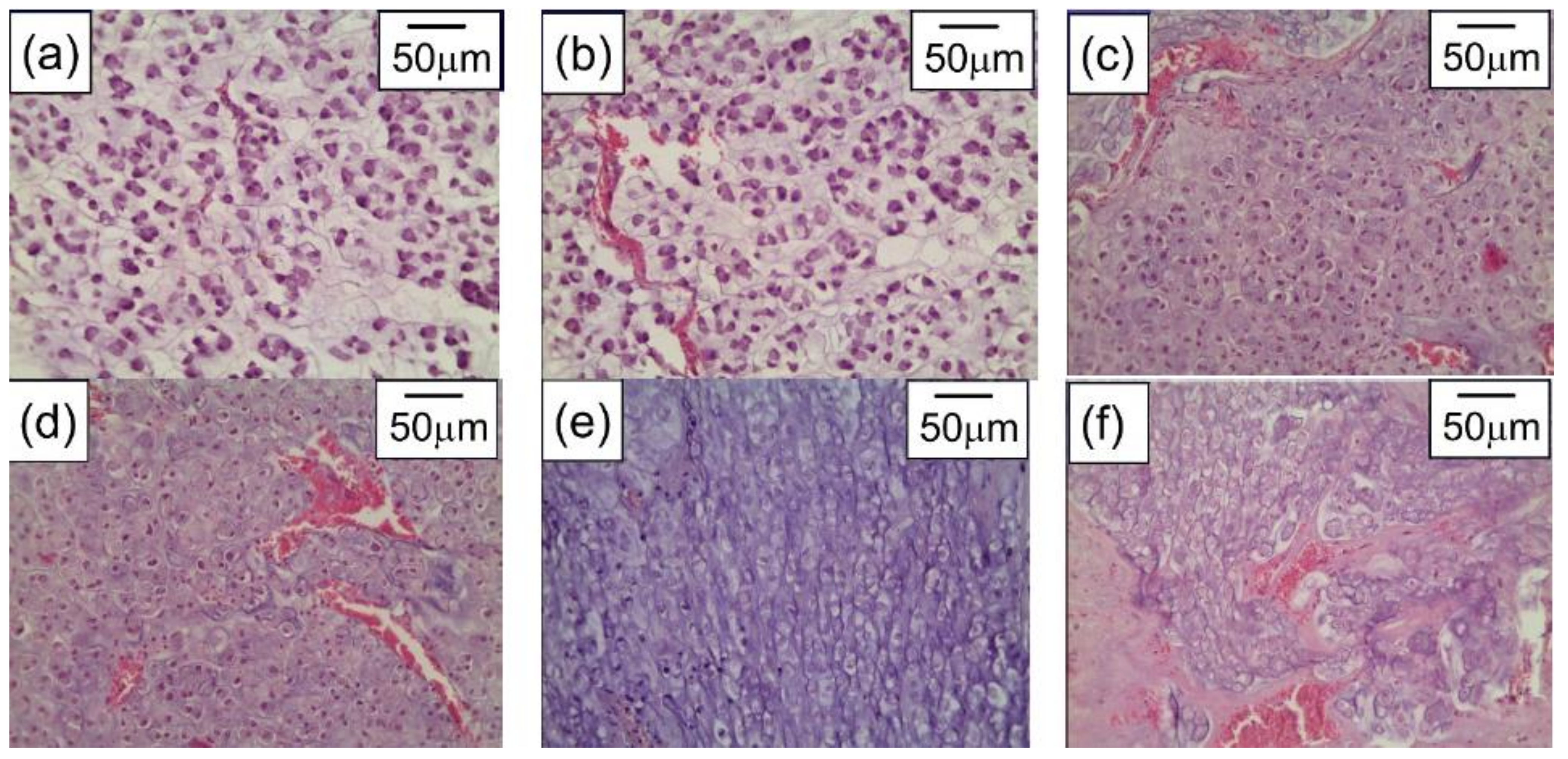
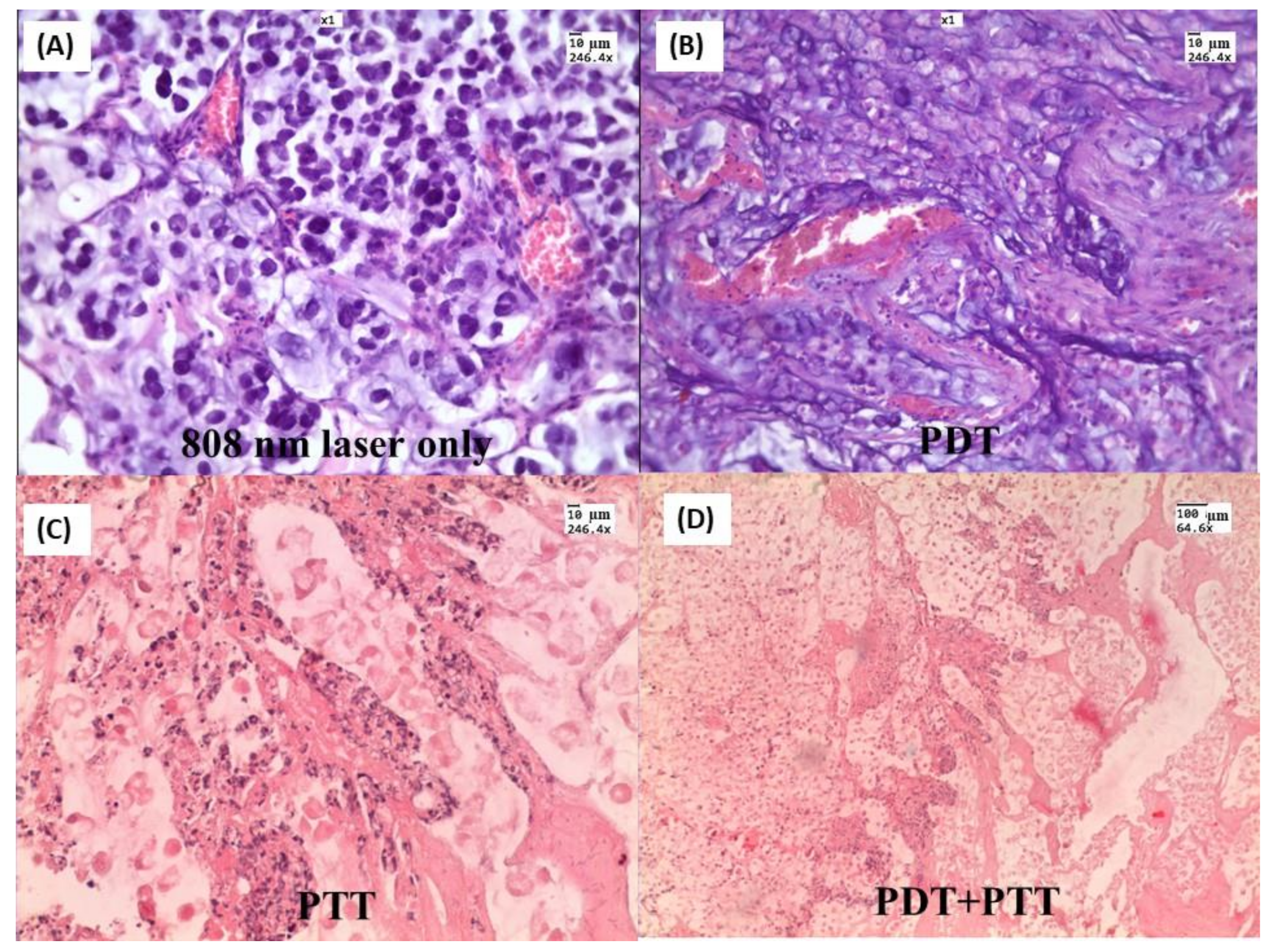
5. Combining PPT with Other Therapies to Achieve Synergic Efficiency
6. Future Perspective and Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- Bohren, C.F.; Huffman, D.R. Absorption and Scattering of Light by Small Particles; John and Wiley and Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Khlebtsov, N.; Dykman, L.A. Optical Properties and Biomedical Applications of Plasmonic Nanoparticles. J. Quant. Spectrosc. Radiat. Transf. 2010, 111, 1–35. [Google Scholar] [CrossRef]
- Khlebtsov, N.G. T-Matrix Method in Plasmonics: An Overview. J. Quant. Spectrosc. Radiat. Transf. 2013, 123, 184–217. [Google Scholar] [CrossRef]
- Yu, R.; Liz-Marzán, L.M.; de Abajo, F.J.G. Universal Analytical Modeling of Plasmonic Nanoparticles. Chem. Soc. Rev. 2017, 46, 6710–6724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klimov, V. Nanoplasmonics; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar] [CrossRef]
- Rasskazov, I.L.; Zakomirnyi, V.I.; Utyushev, A.D.; Carney, P.S.; Moroz, A. Remarkable Predictive Power of the Modified Long Wavelength Approximation. J. Phys. Chem. C 2021, 125, 1963–1971. [Google Scholar] [CrossRef]
- Khlebtsov, N.G. Extinction and Scattering of Light by Nonspherical Plasmonic Particles in Absorbing Media. J. Quant. Spectrosc. Radiat. Transf. 2022, 280, 108069. [Google Scholar] [CrossRef]
- Majic, M.; Pratley, L.; Schebarchov, D.; Somerville, W.R.C.; Auguié, B.; Le Ru, E.C. Approximate T Matrix and Optical Properties of Spheroidal Particles to Third Order with Respect to Size Parameter. Phys. Rev. A 2019, 99, 013853. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.-S.; El-Sayed, M.A. Dependence of the Enhanced Optical Scattering Efficiency Relative to That of Absorption for Gold Metal Nanorods on Aspect Ratio, Size, End-Cap Shape, and Medium Refractive Index. J. Phys. Chem. B 2005, 109, 20331–20338. [Google Scholar] [CrossRef]
- Mackey, M.A.; Ali, M.R.K.; Austin, L.; Near, R.D.; El-Sayed, M.A. The Most Effective Gold Nanorod Size for Plasmonic Photothermal Therapy: Theory and In Vitro Experiments. J. Phys. Chem. B 2014, 118, 1319–1326. [Google Scholar] [CrossRef]
- Baffou, G.; Quidant, R.; Girard, C. Heat Generation in Plasmonic Nanostructures: Influence of Morphology. Appl. Phys. Lett. 2009, 94, 153109. [Google Scholar] [CrossRef] [Green Version]
- Pustovalov, V.K. Light-to-Heat Conversion and Heating of Single Nanoparticles, Their Assemblies, and the Surrounding Medium under Laser Pulses. RSC Adv. 2016, 6, 81266–81289. [Google Scholar] [CrossRef]
- Baffou, G.; Rigneault, H. Femtosecond-Pulsed Optical Heating of Gold Nanoparticles. Phys. Rev. B 2011, 84, 035415. [Google Scholar] [CrossRef] [Green Version]
- Baffou, G.; Quidant, R. Thermo-Plasmonics: Using Metallic Nanostructures as Nano-Sources of Heat. Laser Photon-Rev. 2012, 7, 171–187. [Google Scholar] [CrossRef]
- Baffou, G.; Quidant, R.; de Abajo, F.J.G. Nanoscale Control of Optical Heating in Complex Plasmonic Systems. ACS Nano 2010, 4, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Govorov, A.O.; Richardson, H. Generating Heat with Metal Nanoparticles. Nano Today 2007, 2, 30–38. [Google Scholar] [CrossRef]
- Keblinski, P.; Cahill, D.G.; Bodapati, A.; Sullivan, C.R.; Taton, T.A. Limits of Localized Heating by Electromagnetically Excited Nanoparticles. J. Appl. Phys. 2006, 100, 054305. [Google Scholar] [CrossRef]
- Manrique-Bedoya, S.; Abdul-Moqueet, M.; Lopez, P.; Gray, T.; Disiena, M.; Locker, A.; Kwee, S.; Tang, L.; Hood, R.L.; Feng, Y.; et al. Multiphysics Modeling of Plasmonic Photothermal Heating Effects in Gold Nanoparticles and Nanoparticle Arrays. J. Phys. Chem. C 2020, 124, 17172–17182. [Google Scholar] [CrossRef]
- Ge, Z.; Kang, Y.; Taton, T.A.; Braun, A.P.V.; Cahill, D.G. Thermal Transport in Au-Core Polymer-Shell Nanoparticles. Nano Lett. 2005, 5, 531–535. [Google Scholar] [CrossRef]
- Ge, Z.; Cahill, A.D.G.; Braun, P.V. AuPd Metal Nanoparticles as Probes of Nanoscale Thermal Transport in Aqueous Solution. J. Phys. Chem. B 2004, 108, 18870–18875. [Google Scholar] [CrossRef]
- Akchurin, G.; Khlebtsov, B.; Akchurin, G.; Tuchin, V.; Zharov, V.; Khlebtsov, N. Gold Nanoshell Photomodification under a Single-Nanosecond Laser Pulse Accompanied by Color-Shifting and Bubble Formation Phenomena. Nanotechnology 2007, 19, 015701. [Google Scholar] [CrossRef]
- Fedorenko, S.G.; Romanishkin, I.D.; Vanetsev, A.S.; Sildos, I.; Ryabova, A.V.; Loschenov, V.B.; Orlovskii, Y.V. Heating and Cooling Transients in the DyPO4 Nanocrystals under Femtosecond Laser Irradiation in the NIR Spectral Range. Phys. Wave Phenom. 2018, 26, 198–206. [Google Scholar] [CrossRef]
- Orlovskii, Y.V.; Vanetsev, A.S.; Romanishkin, I.D.; Ryabova, A.V.; Pukhov, K.K.; Baranchikov, A.E.; Samsonova, E.V.; Keevend, K.; Sildos, I.; Loschenov, V.B. Laser Heating of the Y_1-xDy_xPO_4 Nanocrystals. Opt. Mater. Express 2015, 5, 1230–1239. [Google Scholar] [CrossRef]
- Ge, Z.; Cahill, D.G.; Braun, P.V. Thermal Conductance of Hydrophilic and Hydrophobic Interfaces. Phys. Rev. Lett. 2006, 96, 186101. [Google Scholar] [CrossRef] [PubMed]
- Dykman, L.A.; Khlebtsov, N.G. Methods for Chemical Synthesis of Colloidal Gold. Russ. Chem. Rev. 2019, 88, 229–247. [Google Scholar] [CrossRef]
- Dykman, L.; Khlebtsov, N. Gold Nanoparticles in Biomedical Applications: Recent Advances and Perspectives. Chem. Soc. Rev. 2011, 41, 2256–2282. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, J.; Li, H.; Zhao, W.; Zhao, T. Preparation and Progress in Application of Gold Nanorods. J. Nanomater. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.-H.; Nam, J.M. Plasmonic Photothermal Nanoparticles for Biomedical Applications. Adv. Sci. 2019, 6, 1900471. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Yang, M.; Pang, B.; Vara, M.; Xia, Y. Gold Nanomaterials at Work in Biomedicine. Chem. Rev. 2015, 115, 10410–10488. [Google Scholar] [CrossRef]
- Sharifi, M.; Attar, F.; Saboury, A.A.; Akhtari, K.; Hooshmand, N.; Hasan, A.; El-Sayed, M.A.; Falahati, M. Plasmonic Gold Nanoparticles: Optical Manipulation, Imaging, Drug Delivery and Therapy. J. Control. Release 2019, 311–312, 170–189. [Google Scholar] [CrossRef]
- Yaseen, M.; Humayun, M.; Khan, A.; Usman, M.; Ullah, H.; Tahir, A.; Ullah, H. Preparation, Functionalization, Modification, and Applications of Nanostructured Gold: A Critical Review. Energies 2021, 14, 1278. [Google Scholar] [CrossRef]
- Park, J.-E.; Kim, M.; Hwang, J.-H.; Nam, J.-M. Golden Opportunities: Plasmonic Gold Nanostructures for Biomedical Applications Based on the Second Near-Infrared Window. Small Methods 2017, 1, 1600032. [Google Scholar] [CrossRef] [Green Version]
- Vijayaraghavan, P.; Vankayala, R.; Chiang, C.-S.; Sung, H.-W.; Hwang, K.C. Complete Destruction of Deep-Tissue Buried Tumors via Combination of Gene Silencing and Gold Nanoechinus-Mediated Photodynamic Therapy. Biomaterials 2015, 62, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Liow, C.H.; Zhang, M.; Huang, R.; Li, C.; Shen, H.; Liu, M.; Zou, Y.; Gao, N.; Zhang, Z.; et al. Surface Plasmon Resonance Enhanced Light Absorption and Photothermal Therapy in the Second Near-Infrared Window. J. Am. Chem. Soc. 2014, 136, 15684–15693. [Google Scholar] [CrossRef] [PubMed]
- Khlebtsov, N.G.; Zarkov, S.V.; Khanadeev, V.A.; Avetisyan, Y.A. A Novel Concept of Two-Component Dielectric Function for Gold Nanostars: Theoretical Modelling and Experimental Verification. Nanoscale 2020, 12, 19963–19981. [Google Scholar] [CrossRef] [PubMed]
- Khoury, C.G.; Vo-Dinh, T. Gold Nanostars for Surface-Enhanced Raman Scattering: Synthesis, Characterization and Optimization. J. Phys. Chem. C 2008, 112, 18849–18859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stöber, W.; Fink, A.; Bohn, E. Controlled Growth of Monodisperse Silica Spheres in the Micron Size Range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Kalele, S.; Gosavi, S.W.; Urban, J.; Kulkarni, S.K. Nanoshell Particles: Synthesis, Properties and Applications. Curr. Sci. 2006, 91, 1038–1052. [Google Scholar]
- Westcott, S.L.; Oldenburg, S.J.; Lee, A.T.R.; Halas, N.J. Formation and Adsorption of Clusters of Gold Nanoparticles onto Functionalized Silica Nanoparticle Surfaces. Langmuir 1998, 14, 5396–5401. [Google Scholar] [CrossRef]
- Guan, Y.; Xue, Z.; Liang, J.; Huang, Z.; Yang, W. One-Pot Synthesis of Size-Tunable Hollow Gold Nanoshells via APTES-in-Water Suspension. Colloids Surf. A Physicochem. Eng. Asp. 2016, 502, 6–12. [Google Scholar] [CrossRef]
- Abadeer, N.S.; Murphy, C.J. Recent Progress in Cancer Thermal Therapy Using Gold Nanoparticles. J. Phys. Chem. C 2016, 120, 4691–4716. [Google Scholar] [CrossRef]
- Ali, M.R.K.; Wu, Y.; El-Sayed, M.A. Gold-Nanoparticle-Assisted Plasmonic Photothermal Therapy Advances Toward Clinical Application. J. Phys. Chem. C 2019, 123, 15375–15393. [Google Scholar] [CrossRef]
- Vilches, C.; Quidant, R. Targeted Hyperthermia with Plasmonic Nanoparticles. Front. Nanosci. 2020, 16, 307–352. [Google Scholar] [CrossRef]
- Rastinehad, A.R.; Anastos, H.; Wajswol, E.; Winoker, J.S.; Sfakianos, J.P.; Doppalapudi, S.K.; Carrick, M.R.; Knauer, C.J.; Taouli, B.; Lewis, S.C.; et al. Gold Nanoshell-Localized Photothermal Ablation of Prostate Tumors in a Clinical Pilot Device Study. Proc. Natl. Acad. Sci. USA 2019, 116, 18590–18596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, X.; Ying, E.; Dong, S. Seed-Mediated Synthesis of Branched Gold Nanoparticles with the Assistance of Citrate and Their Surface-Enhanced Raman Scattering Properties. Nanotechnology 2006, 17, 4758–4764. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.S.; Pastoriza-Santos, I.; Rodriguez-Gonzalez, B.; De Abajo, F.J.G.; Liz-Marzán, L.M. High-Yield Synthesis and Optical Response of Gold Nanostars. Nanotechnology 2007, 19, 015606. [Google Scholar] [CrossRef]
- Jeong, G.H.; Lee, Y.W.; Kim, M.; Han, S.W. High-Yield Synthesis of Multi-Branched Gold Nanoparticles and Their Surface-Enhanced Raman Scattering Properties. J. Colloid Interface Sci. 2008, 329, 97–102. [Google Scholar] [CrossRef]
- Xie, J.; Lee, J.Y.; Wang, D.I.C. Seedless, Surfactantless, High-Yield Synthesis of Branched Gold Nanocrystals in HEPES Buffer Solution. Chem. Mater. 2007, 19, 2823–2830. [Google Scholar] [CrossRef]
- Guerrero-Martínez, A.; Barbosa, S.; Pastoriza-Santos, I.; Liz-Marzán, L.M. Nanostars Shine Bright for You: Colloidal Synthesis, Properties and Applications of Branched Metallic Nanoparticles. Curr. Opin. Colloid Interface Sci. 2011, 16, 118–127. [Google Scholar] [CrossRef]
- Yuan, H.; Khoury, C.G.; Hwang, H.; Wilson, C.M.; Grant, G.A.; Vo-Dinh, T. Gold Nanostars: Surfactant-Free Synthesis, 3D Modelling, and Two-Photon Photoluminescence Imaging. Nanotechnology 2012, 23, 075102. [Google Scholar] [CrossRef] [Green Version]
- Khlebtsov, B.; Panfilova, E.; Khanadeev, V.; Khlebtsov, N. Improved Size-Tunable Synthesis and SERS Properties of Au Nanostars. J. Nanoparticle Res. 2014, 16, 1–12. [Google Scholar] [CrossRef]
- Indrasekara, A.S.D.S.; Johnson, S.; Odion, R.A.; Vo-Dinh, T. Manipulation of the Geometry and Modulation of the Optical Response of Surfactant-Free Gold Nanostars: A Systematic Bottom-Up Synthesis. ACS Omega 2018, 3, 2202–2210. [Google Scholar] [CrossRef] [Green Version]
- Duff, D.G.; Baiker, A.; Edwards, P.P. A New Hydrosol of Gold Clusters. J. Chem. Soc. Chem. Commun. 1993, 9, 96–98. [Google Scholar] [CrossRef]
- Frens, G. Controlled Nucleation for the Regulation of the Particle Size in Monodisperse Gold Suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Siegel, A.L.; Baker, G.A. Bespoke Nanostars: Synthetic Strategies, Tactics, and Uses of Tailored Branched Gold Nanoparticles. Nanoscale Adv. 2021, 3, 3980–4004. [Google Scholar] [CrossRef]
- Chirico, G.; Borzenkov, M.; Pallavicini, P. Gold Nanostars. Synthesis, Properties and Biomedical Application; Springer: Cham, Switzerland, 2015; pp. 1–74. [Google Scholar]
- Crawford, B.M.; Shammas, R.; Fales, A.M.; Brown, D.A.; Hollenbeck, S.T.; Vo-Dinh, T.; Devi, G.R. Photothermal Ablation of Inflammatory Breast Cancer Tumor Emboli Using Plasmonic Gold Nanostars. Int. J. Nanomed. 2017, 12, 6259–6272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espinosa, A.; Silva, A.K.A.; Sánchez-Iglesias, A.; Grzelczak, M.; Péchoux, C.; Desboeufs, K.; Liz-Marzán, L.M.; Wilhelm, C. Cancer Cell Internalization of Gold Nanostars Impacts Their Photothermal Efficiency In Vitro and In Vivo: Toward a Plasmonic Thermal Fingerprint in Tumoral Environment. Adv. Healthc. Mater. 2016, 5, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Vo-Dinh, T. Shining Gold Nanostars: From Cancer Diagnostics to Photothermal Treatment and Immunotherapy. J. Immunol. Sci. 2018, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Han, R.; Liu, Q.; Lu, Y.; Peng, J.; Pan, M.; Wang, G.; Chen, W.; Xiao, Y.; Yang, C.; Qian, Z. Tumor Microenvironment-Responsive Ag2S-PAsp(DOX)-cRGD Nanoparticles-Mediated Photochemotherapy Enhances the Immune Response to Tumor Therapy. Biomaterials 2022, 281, 121328. [Google Scholar] [CrossRef]
- Khlebtsov, B.; Khanadeev, V.A.; Maksimova, I.L.; Terentyuk, G.S.; Khlebtsov, N.G. Silver Nanocubes and Gold Nanocages: Fabrication and Optical and Photothermal Properties. Nanotechnol. Russ. 2010, 5, 454–468. [Google Scholar] [CrossRef]
- Sun, Y.; Xia, Y. Alloying and Dealloying Processes Involved in the Preparation of Metal Nanoshells through a Galvanic Replacement Reaction. Nano Lett. 2003, 3, 1569–1572. [Google Scholar] [CrossRef]
- Sun, Y.; Xia, Y. Mechanistic Study on the Replacement Reaction between Silver Nanostructures and Chloroauric Acid in Aqueous Medium. J. Am. Chem. Soc. 2004, 126, 3892–3901. [Google Scholar] [CrossRef]
- Chen, J.; Saeki, F.; Wiley, B.; Cang, H.; Cobb, M.J.; Li, Z.-Y.; Au, L.; Zhang, H.; Kimmey, M.B.; Li, X.; et al. Gold Nanocages: Bioconjugation and Their Potential Use as Optical Imaging Contrast Agents. Nano Lett. 2005, 5, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Khlebtsov, N.; Bogatyrev, V.; Melnikov, A.; Dykman, L.; Khlebtsov, B.; Krasnov, Y.M. Differential Light-Scattering Spectroscopy: A New Approach to Studying of Colloidal Gold Nanosensors. J. Quant. Spectrosc. Radiat. Transf. 2004, 89, 133–142. [Google Scholar] [CrossRef]
- Panfilova, E.V.; Khlebtsov, B.N.; Burov, A.M.; Khlebtsov, N.G. Study of Polyol Synthesis Reaction Parameters Controlling High Yield of Silver Nanocubes. Colloid J. 2012, 74, 99–109. [Google Scholar] [CrossRef]
- Maksimova, E.A.; Barmin, R.A.; Rudakovskaya, P.G.; Sindeeva, O.A.; Prikhozhdenko, E.S.; Yashchenok, A.M.; Khlebtsov, B.N.; Solovev, A.A.; Huang, G.; Mei, Y.; et al. Air-Filled Microbubbles Based on Albumin Functionalized with Gold Nanocages and Zinc Phthalocyanine for Multimodal Imaging. Micromachines 2021, 12, 1161. [Google Scholar] [CrossRef]
- Chen, J.; Wang, D.; Xi, J.; Au, L.; Siekkinen, A.; Warsen, A.; Li, Z.-Y.; Zhang, H.; Xia, Y.; Li, X. Immuno Gold Nanocages with Tailored Optical Properties for Targeted Photothermal Destruction of Cancer Cells. Nano Lett. 2007, 7, 1318–1322. [Google Scholar] [CrossRef] [Green Version]
- Qiu, J.; Xie, M.; Wu, T.; Qin, D.; Xia, Y. Gold Nanocages for Effective Photothermal Conversion and Related Applications. Chem. Sci. 2020, 11, 12955–12973. [Google Scholar] [CrossRef]
- Pakravan, A.; Azizi, M.; Rahimi, F.; Bani, F.; Mahmoudzadeh, F.; Salehi, R.; Mahkam, M. Comparative Effect of Thermo/pH-Responsive Polymer-Coated Gold Nanocages and Hollow Nanostars on Chemo-Photothermal Therapy of Breast Cancer Cells. Cancer Nanotechnol. 2021, 12, 1–26. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, S.; Zhao, X.; Chang, L.; Ren, X.; Mei, X.; Chen, Z. Preparation of Photothermal Responsive and ROS Generative Gold Nanocages for Cancer Therapy. Chem. Eng. J. 2021, 421, 129744. [Google Scholar] [CrossRef]
- Alimardani, V.; Farahavar, G.; Salehi, S.; Taghizadeh, S.; Ghiasi, M.R.; Abolmaali, S.S. Gold Nanocages in Cancer Diagnosis, Therapy, and Theranostics: A Brief Review. Front. Mater. Sci. 2021, 15, 494–511. [Google Scholar] [CrossRef]
- Huang, S.; Liu, Y.; Xu, X.; Ji, M.; Li, Y.; Song, C.; Duan, S.; Hu, Y. Triple Therapy of Hepatocellular Carcinoma with MicroRNA-122 and Doxorubicin Co-Loaded Functionalized Gold Nanocages. J. Mater. Chem. B 2018, 6, 2217–2229. [Google Scholar] [CrossRef]
- Pang, B.; Yang, X.; Xia, Y. Putting Gold Nanocages to Work for Optical Imaging, Controlled Release and Cancer Theranostics. Nanomedicine 2016, 11, 1715–1728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Song, Y.; Cao, K.; Zhang, L.; Fang, X.; Chen, F.; Feng, S.; Yan, F. Photothermal Therapy Mediated by Gold Nanocages Composed of Anti-PDL1 and Galunisertib for Improved Synergistic Immunotherapy in Colorectal Cancer. Acta Biomater. 2021, 134, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wang, Z.; Yang, J.; Xu, R.; Deng, H.; Ma, S.; Fang, T.; Zhang, J.; Shen, Q. Reactive Oxygen Species/Photothermal Therapy Dual-Triggered Biomimetic Gold Nanocages Nanoplatform for Combination Cancer Therapy via Ferroptosis and Tumor-Associated Macrophage Repolarization Mechanism. J. Colloid Interface Sci. 2021, 606, 1950–1965. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Cheng, X.; Zhang, H.; Bai, X.; Ai, R.; Shao, L.; Wang, J. Gold Nanorods: The Most Versatile Plasmonic Nanoparticles. Chem. Rev. 2021, 121, 13342–13453. [Google Scholar] [CrossRef] [PubMed]
- Jana, N.R.; Gearheart, L.; Murphy, C.J. Wet Chemical Synthesis of High Aspect Ratio Cylindrical Gold Nanorods. J. Phys. Chem. B 2001, 105, 4065–4067. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and Growth Mechanism of Gold Nanorods (NRs) Using Seed-Mediated Growth Method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- Khlebtsov, B.; Khanadeev, V.; Pylaev, T.; Khlebtsov, N. A New T-Matrix Solvable Model for Nanorods: TEM-Based Ensemble Simulations Supported by Experiments. J. Phys. Chem. C 2011, 115, 6317–6323. [Google Scholar] [CrossRef]
- Ye, X.; Zheng, C.; Chen, J.; Gao, Y.; Murray, C.B. Using Binary Surfactant Mixtures to Simultaneously Improve the Dimensional Tunability and Monodispersity in the Seeded Growth of Gold Nanorods. Nano Lett. 2013, 13, 765–771. [Google Scholar] [CrossRef]
- Khlebtsov, B.N.; Khanadeev, V.; Ye, J.; Sukhorukov, G.B.; Khlebtsov, N. Overgrowth of Gold Nanorods by Using a Binary Surfactant Mixture. Langmuir 2014, 30, 1696–1703. [Google Scholar] [CrossRef]
- Vigderman, L.; Zubarev, E.R. High-Yield Synthesis of Gold Nanorods with Longitudinal SPR Peak Greater than 1200 nm Using Hydroquinone as a Reducing Agent. Chem. Mater. 2013, 25, 1450–1457. [Google Scholar] [CrossRef]
- Ali, M.R.K.; Snyder, B.; El-Sayed, M.A. Synthesis and Optical Properties of Small Au Nanorods Using a Seedless Growth Technique. Langmuir 2012, 28, 9807–9815. [Google Scholar] [CrossRef] [PubMed]
- Liopo, A.; Wang, S.; Derry, P.J.; Oraevsky, A.A.; Zubarev, E.R. Seedless Synthesis of Gold Nanorods Using Dopamine as a Reducing Agent. RSC Adv. 2015, 5, 91587–91593. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Q.; Huang, H.; Zhang, S.; Zhou, L.; Wang, Q. Three-Step Seedless Synthesis of Ultralong Gold Nanorods. Opt. Mater. 2021, 116, 111095. [Google Scholar] [CrossRef]
- Salavatov, N.A.; Dement′Eva, O.V.; Mikhailichenko, A.I.; Rudoy, V.M. Some Aspects of Seedless Synthesis of Gold Nanorods. Colloid J. 2018, 80, 541–549. [Google Scholar] [CrossRef]
- Khanadeev, V.; Khlebtsov, N.; Burov, A.; Khlebtsov, B.N. Tuning of Plasmon Resonance of Gold Nanorods by Controlled Etching. Colloid J. 2015, 77, 652–660. [Google Scholar] [CrossRef]
- Khlebtsov, B.N.; Khanadeev, V.A.; Burov, A.M.; Le Ru, E.C.; Khlebtsov, N.G. Reexamination of Surface-Enhanced Raman Scattering from Gold Nanorods as a Function of Aspect Ratio and Shape. J. Phys. Chem. C 2020, 124, 10647–10658. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, X.; Zhou, W.; Wu, Q.; Yan, J.; Xu, X.; Ghimire, B.; Rosenholm, J.M.; Feng, J.; Wang, D. Combination of Photothermal, Prodrug and Tumor Cell Camouflage Technologies for Triple-Negative Breast Cancer Treatment. Mater. Today Adv. 2022, 13, 100199. [Google Scholar] [CrossRef]
- Li, B.; Wang, Y.; He, J. Gold Nanorods-Based Smart Nanoplatforms for Synergic Thermotherapy and Chemotherapy of Tumor Metastasis. ACS Appl. Mater. Interfaces 2019, 11, 7800–7811. [Google Scholar] [CrossRef]
- Proshkina, G.M.; Shramova, E.I.; Shilova, M.V.; Zelepukin, I.V.; Shipunova, V.O.; Ryabova, A.V.; Deyev, S.M.; Kotlyar, A.B. DARPin_9–29-Targeted Gold Nanorods Selectively Suppress HER2-Positive Tumor Growth in Mice. Cancers 2021, 13, 5235. [Google Scholar] [CrossRef]
- Jin, X.; Yang, H.; Mao, Z.; Wang, B. Cathepsin B-Responsive Multifunctional Peptide Conjugated Gold Nanorods for Mitochondrial Targeting and Precise Photothermal Cancer Therapy. J. Colloid Interface Sci. 2021, 601, 714–726. [Google Scholar] [CrossRef]
- Liu, X.; Su, Q.; Song, H.; Shi, X.; Zhang, Y.; Zhang, C.; Huang, P.; Dong, A.; Kong, D.; Wang, W. PolyTLR7/8a-Conjugated, Antigen-Trapping Gold Nanorods Elicit Anticancer Immunity against Abscopal Tumors by Photothermal Therapy-Induced in Situ Vaccination. Biomaterials 2021, 275, 120921. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Wu, J.; Jin, L.; Hong, L.; Wang, F.; Mao, Z.; Wu, M. Cancer Cell Membrane-Coated Gold Nanorods for Photothermal Therapy and Radiotherapy on Oral Squamous Cancer. J. Mater. Chem. B 2020, 8, 7253–7263. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.; Lim, K.; Kim, S.S.; Oh, K.T.; Lee, E.S.; Choi, H.-G.; Shin, B.S.; Youn, Y.S. Small Gold Nanorods-Loaded Hybrid Albumin Nanoparticles with High Photothermal Efficacy for Tumor Ablation. Colloids Surf. B: Biointerfaces 2019, 179, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Feng, K.; Xie, N.; Zhao, W.; Ye, L.; Chen, B.; Tung, C.-H.; Wu, L.-Z. Mesoporous Silica-Coated Gold Nanorods with Designable Anchor Peptides for Chemo-Photothermal Cancer Therapy. ACS Appl. Nano Mater. 2020, 3, 5070–5078. [Google Scholar] [CrossRef]
- Liu, G.; Liang, H.; He, Y.; Lu, L.; Wang, L.; Liu, P.; Cai, K. A Nanoplatform Based on Mesoporous Silica-Coated Gold Nanorods for Cancer Triplex Therapy. J. Mater. Chem. B 2020, 8, 9686–9696. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.-C.; Cheng, C.-C.; Chen, P.-Y.; Lo, C.; Chen, Y.-N.; Shih, M.-H.; Chang, C.-W. Gold Nanorod-Encapsulated Biodegradable Polymeric Matrix for Combined Photothermal and Chemo-Cancer Therapy. Int. J. Nanomed. 2018, 14, 181–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, L.; Kuang, Z.; Song, P.; Li, W.; Gui, L.; Yang, K.; Ge, F.; Tao, Y.; Zhang, W. Gold Nanorod-Loaded Thermosensitive Liposomes Facilitate the Targeted Release of Ruthenium (II) Polypyridyl Complexes with Anti-Tumor Activity. Nanotechnology 2021, 32, 455103. [Google Scholar] [CrossRef]
- Liu, X.; Chen, B.; Wang, G.; Ma, S.; Cheng, L.; Liu, W.; Zhou, L.; Wang, Q. Controlled Growth of Hierarchical Bi 2 Se 3 /CdSe-Au Nanorods with Optimized Photothermal Conversion and Demonstrations in Photothermal Therapy. Adv. Funct. Mater. 2021, 31, 2104424. [Google Scholar] [CrossRef]
- Oladipo, A.O.; Lebepe, T.C.; Ncapayi, V.; Tsolekile, N.; Parani, S.; Songca, S.P.; Mori, S.; Kodama, T.; Oluwafemi, O.S. The Therapeutic Effect of Second Near-Infrared Absorbing Gold Nanorods on Metastatic Lymph Nodes via Lymphatic Delivery System. Pharmaceutics 2021, 13, 1359. [Google Scholar] [CrossRef]
- Ribera, J.; Vilches, C.; Sanz, V.; de Miguel, I.; Portolés, I.; Córdoba-Jover, B.; Prat, E.; Nunes, V.; Jiménez, W.; Quidant, R.; et al. Treatment of Hepatic Fibrosis in Mice Based on Targeted Plasmonic Hyperthermia. ACS Nano 2021, 15, 7547–7562. [Google Scholar] [CrossRef]
- Monem, A.S.; Sayed, F.A.-Z.; Rageh, M.M.; Mohamed, N. Cytotoxicity and Genotoxicity of Gold Nanorods Assisted Photothermal Therapy against Ehrlich Carcinoma in-Vivo. Life Sci. 2020, 257, 118108. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Gao, D.; Hu, D.; Zhou, H.; Ma, T.; Huang, L.; Liu, X.; Zheng, R.; Zheng, H.; Zhao, P.; et al. Activatable NIR-II Photoacoustic Imaging and Photochemical Synergistic Therapy of MRSA Infections Using Miniature Au/Ag Nanorods. Biomaterials 2020, 251, 120092. [Google Scholar] [CrossRef] [PubMed]
- Pulagam, K.R.; Henriksen-Lacey, M.; Uribe, K.B.; Renero-Lecuna, C.; Kumar, J.; Charalampopoulou, A.; Facoetti, A.; Protti, N.; Gómez-Vallejo, V.; Baz, Z.; et al. In Vivo Evaluation of Multifunctional Gold Nanorods for Boron Neutron Capture and Photothermal Therapies. ACS Appl. Mater. Interfaces 2021, 13, 49589–49601. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Wang, Y.; Lin, J.; Tian, Q.; Xie, Y.; Hu, J.; Yang, S.-P. Macrophages-Mediated Delivery of Small Gold Nanorods for Tumor Hypoxia Photoacoustic Imaging and Enhanced Photothermal Therapy. ACS Appl. Mater. Interfaces 2019, 11, 15251–15261. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, S.; Ma, R.; Zhu, L.; Yan, T.; Alimu, G.; Du, Z.; Alifu, N.; Zhang, X. NIR-Excitable PEG-Modified Au Nanorods for Photothermal Therapy of Cervical Cancer. ACS Appl. Nano Mater. 2021, 4, 13060–13070. [Google Scholar] [CrossRef]
- Zhang, W.; Cai, K.; Li, X.; Zhang, J.; Ma, Z.; Foda, M.F.; Mu, Y.; Dai, X.; Han, H. Au Hollow Nanorods-Chimeric Peptide Nanocarrier for NIR-II Photothermal Therapy and Real-time Apoptosis Imaging for Tumor Theranostics. Theranostics 2019, 9, 4971–4981. [Google Scholar] [CrossRef]
- Huang, X.; Neretina, S.; El-Sayed, M.A. Gold Nanorods: From Synthesis and Properties to Biological and Biomedical Applications. Adv. Mater. 2009, 21, 4880–4910. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, M.A. Plasmonic Photo-Thermal Therapy (PPTT). Alex. J. Med. 2011, 47, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The Golden Age: Gold Nanoparticles for Biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef] [Green Version]
- Liao, S.; Yue, W.; Cai, S.; Tang, Q.; Lu, W.; Huang, L.; Qi, T.; Liao, J. Improvement of Gold Nanorods in Photothermal Therapy: Recent Progress and Perspective. Front. Pharmacol. 2021, 12, 781. [Google Scholar] [CrossRef]
- Bromma, K.; Chithrani, D.B. Advances in Gold Nanoparticle-Based Combined Cancer Therapy. Nanomaterials 2020, 10, 1671. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shi, J.; Nie, W.; Wang, S.; Liu, G.; Cai, K. Recent Progress in the Development of Multifunctional Nanoplatform for Precise Tumor Phototherapy. Adv. Healthc. Mater. 2020, 10, e2001207. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.R.; Mirin, N.A.; Knight, M.W.; Goodrich, G.P.; Halas, N.J. Photothermal Efficiencies of Nanoshells and Nanorods for Clinical Therapeutic Applications. J. Phys. Chem. C 2009, 113, 12090–12094. [Google Scholar] [CrossRef]
- Roper, D.K.; Ahn, W.; Hoepfner, M. Microscale Heat Transfer Transduced by Surface Plasmon Resonant Gold Nanoparticles. J. Phys. Chem. C 2007, 111, 3636–3641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Maltzahn, G.; Park, J.-H.; Agrawal, A.; Bandaru, N.K.; Das, S.K.; Sailor, M.J.; Bhatia, S.N. Computationally Guided Photothermal Tumor Therapy Using Long-Circulating Gold Nanorod Antennas. Cancer Res. 2009, 69, 3892–3900. [Google Scholar] [CrossRef] [Green Version]
- Rengan, A.K.; Kundu, G.; Banerjee, R.; Srivastava, R. Gold Nanocages as Effective Photothermal Transducers in Killing Highly Tumorigenic Cancer Cells. Part. Part. Syst. Charact. 2013, 31, 398–405. [Google Scholar] [CrossRef]
- Pattani, V.P.; Tunnell, J.W. Nanoparticle-Mediated Photothermal Therapy: A Comparative Study of Heating for Different Particle Types. Lasers Surg. Med. 2012, 44, 675–684. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Xia, B.; Wang, L.; Ma, S.; Liang, H.; Wang, D.; Huang, J. Shape Effects of Gold Nanoparticles in Photothermal Cancer Therapy. Mater. Today Sustain. 2021, 13, 100078. [Google Scholar] [CrossRef]
- Pylaev, T.; Vanzha, E.; Avdeeva, E.; Khlebtsov, B.; Khlebtsov, N. A Novel Cell Transfection Platform Based on Laser Optoporation Mediated by Au Nanostar Layers. J. Biophotonics 2018, 12, e201800166. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Wang, J.; Sun, L.; Zhang, Y.; Huang, P.; Guo, J. Comparison of Gold Nanospheres, Nanorods, Nanocages and Nanoflowers for Combined Photothermal-Radiotherapy of Cancer. Nano 2021, 16, 2150037. [Google Scholar] [CrossRef]
- Robinson, R.; Gerlach, W.; Ghandehari, H. Comparative Effect of Gold Nanorods and Nanocages for Prostate Tumor Hyperthermia. J. Control. Release 2015, 220, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Black, K.C.L.; Luehmann, H.; Li, W.; Zhang, Y.S.; Cai, X.; Wan, D.; Liu, S.-Y.; Li, M.; Kim, P.; et al. Comparison Study of Gold Nanohexapods, Nanorods, and Nanocages for Photothermal Cancer Treatment. ACS Nano 2013, 7, 2068–2077. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Chang, Y.; Sun, X.; Cheng, Y.; Zheng, R.; Wu, X.; Wang, L.; Ma, X.; Li, X.; Zhang, H. Differential Photothermal and Photodynamic Performance Behaviors of Gold Nanorods, Nanoshells and Nanocages under Identical Energy Conditions. Biomater. Sci. 2019, 7, 1448–1462. [Google Scholar] [CrossRef] [PubMed]
- Neddersen, J.; Chumanov, G.; Cotton, T.M. Laser Ablation of Metals: A New Method for Preparing SERS Active Colloids. Appl. Spectrosc. 1993, 47, 1959–1964. [Google Scholar] [CrossRef]
- Kabashin, A.V.; Meunier, M.; Kingston, C.; Luong, J.H.T. Fabrication and Characterization of Gold Nanoparticles by Femtosecond Laser Ablation in an Aqueous Solution of Cyclodextrins. J. Phys. Chem. B 2003, 107, 4527–4531. [Google Scholar] [CrossRef]
- Burov, A.; Khlebtsov, B.; Ratto, F.; Khlebtsov, N.N. Citrate-Reduced Au Nanoparticles vs. Monodisperse Spheres: Extinction and Dynamic Light Scattering Measurements. In Proceedings of the Saratov Fall Meeting 2018: Computations and Data Analysis: From Nanoscale Tools to Brain Functions, Saratov, Russia, 23–27 September 2019; Volume 11067, pp. 272–278. [Google Scholar] [CrossRef]
- Sylvestre, J.-P.; Poulin, S.; Kabashin, A.V.; Sacher, E.; Meunier, A.M.; Luong, J.H.T. Surface Chemistry of Gold Nanoparticles Produced by Laser Ablation in Aqueous Media. J. Phys. Chem. B 2004, 108, 16864–16869. [Google Scholar] [CrossRef]
- Besner, S.; Kabashin, A.; Meunier, M. Two-Step Femtosecond Laser Ablation-Based Method for the Synthesis of Stable and Ultra-Pure Gold Nanoparticles in Water. Appl. Phys. A 2007, 88, 269–272. [Google Scholar] [CrossRef]
- Kubiliūtė, R.; Maximova, K.A.; Lajevardipour, A.; Yong, J.; Hartley, J.S.; Mohsin, A.S.M.; Blandin, P.; Chon, J.W.M.; Sentis, M.; Stoddart, P.R.; et al. Ultra-Pure, Water-Dispersed Au Nanoparticles Produced by Femtosecond Laser Ablation and Fragmentation. Inter. J. Nanomed. 2013, 8, 2601–2611. [Google Scholar]
- Ziefuß, A.R.; Reichenberger, S.; Rehbock, C.; Chakraborty, I.; Gharib, M.; Parak, W.J.; Barcikowski, S. Laser Fragmentation of Colloidal Gold Nanoparticles with High-Intensity Nanosecond Pulses is Driven by a Single-Step Fragmentation Mechanism with a Defined Educt Particle-Size Threshold. J. Phys. Chem. C 2018, 122, 22125–22136. [Google Scholar] [CrossRef]
- Bongiovanni, G.; Olshin, P.K.; Yan, C.; Voss, J.M.; Drabbels, M.; Lorenz, U.J. The Fragmentation Mechanism of Gold Nanoparticles in Water under Femtosecond Laser Irradiation. Nanoscale Adv. 2021, 3, 5277–5283. [Google Scholar] [CrossRef]
- Braguer, D.; Correard, F.; Maximova, K.; Villard, C.; Roy, M.; Al-Kattan, A.; Sentis, M.; Gingras, M.; Kabashin, A.; Esteve, M.-A. Gold Nanoparticles Prepared by Laser Ablation in Aqueous Biocompatible Solutions: Assessment of Safety and Biological Identity for Nanomedicine Applications. Int. J. Nanomed. 2014, 9, 5415–5430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailly, A.-L.; Correard, F.; Popov, A.; Tselikov, G.; Chaspoul, F.; Appay, R.; Al-Kattan, A.; Kabashin, A.V.; Braguer, D.; Esteve, M.-A. In Vivo Evaluation of Safety, Biodistribution and Pharmacokinetics of Laser-Synthesized Gold Nanoparticles. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Kattan, A.; Tselikov, G.; Metwally, K.; Popov, A.; Mensah, S.; Kabashin, A. Laser Ablation-Assisted Synthesis of Plasmonic Si@Au Core-Satellite Nanocomposites for Biomedical Applications. Nanomaterials 2021, 11, 592. [Google Scholar] [CrossRef]
- Khaniabadi, P.M.; Ahmed, N.M.; Dheyab, M.A.; Aziz, A.A.; Almessiere, M. Structure, Morphology and Absorption Characteristics of Gold Nanoparticles Produced via PLAL Method: Role of Low Energy X-Ray Dosage. Surf. Interfaces 2021, 24, 101139. [Google Scholar] [CrossRef]
- Waag, F.; Streubel, R.; Gökce, B.; Barcikowski, S. Synthesis of Gold, Platinum, and Gold-Platinum Alloy Nanoparticle Colloids with High-Power Megahertz-Repetition-Rate Lasers: The Importance of the Beam Guidance Method. Appl. Nanosci. 2021, 11, 1303–1312. [Google Scholar] [CrossRef]
- Izgaliev, A.T.; Simakin, A.V.; Shafeev, G.A. Formation of the Alloy of Au and Ag Nanoparticles Upon Laser Irradiation of the Mixture of Their Colloidal Solutions. Quantum Electron. 2004, 34, 47–50. [Google Scholar] [CrossRef]
- Kazakevich, P.; Simakin, A.; Shafeev, G. Laser Burning of a Gap in the Spectrum of Plasmon Resonance of Gold Nanoparticles. Chem. Phys. Lett. 2006, 421, 348–350. [Google Scholar] [CrossRef]
- Kirichenko, N.A.; Sukhov, I.A.; Shafeev, G.A.; Shcherbina, M.E. Evolution of the Distribution Function of Au Nanoparticles in a Liquid under the Action of Laser Radiation. Quantum Electron. 2012, 42, 175–180. [Google Scholar] [CrossRef]
- Barmina, E.V.; Shafeev, G.; Kuzmin, P.G.; Serkov, A.; Simakin, A.V.; Melnik, N. Laser-Assisted Generation of Gold Nanoparticles and Nanostructures in Liquid and Their Plasmonic Luminescence. Appl. Phys. A 2014, 115, 747–752. [Google Scholar] [CrossRef]
- Serkov, A.A.; Kuzmin, P.G.; Rakov, I.I.; Shafeev, G.A. Influence of Laser-Induced Breakdown on the Fragmentation of Gold Nanoparticles in Water. Quantum Electron. 2016, 46, 713–718. [Google Scholar] [CrossRef]
- Rakov, I.; Pridvorova, S.; Shafeev, G. Interaction of Gold and Phthalocyanines Nanoparticles Generated by Laser Radiation in Water. Colloids Surf. A Physicochem. Eng. Asp. 2021, 618, 126491. [Google Scholar] [CrossRef]
- Zhilnikova, M.I.; Barmina, E.V.; Shafeev, G.A. Laser-Assisted Generation of Elongated Au Nanoparticles and Analysis of Their Morphology under Pulsed Irradiation in Water and CaCl2 Solutions. J. Phys. Conf. Ser. 2020, 1439, 012026. [Google Scholar] [CrossRef]
- Zhilnikova, M.I.; Barmina, E.V.; Shafeev, G.A.; Pridvorova, S.M.; Uvarov, O.V. Laser-Assisted Generation of Elongated Au Nanoparticles in Aqueous Solutions of Divalent Ions. Gold Bull. 2020, 53, 129–134. [Google Scholar] [CrossRef]
- Kazakevich, P.; Simakin, A.; Shafeev, G.; Viau, G.; Soumare, Y.; Bozon-Verduraz, F. Laser-Assisted Shape Selective Fragmentation of Nanoparticles. Appl. Surf. Sci. 2007, 253, 7831–7834. [Google Scholar] [CrossRef]
- Blandin, P.; Maximova, K.A.; Gongalsky, M.B.; Sanchez-Royo, J.F.; Chirvony, V.S.; Sentis, M.; Timoshenko, V.Y.; Kabashin, A.V. Femtosecond Laser Fragmentation from Water-Dispersed Microcolloids: Toward Fast Controllable Growth of Ultrapure Si-Based Nanomaterials for Biological Applications. J. Mater. Chem. B 2013, 1, 2489–2495. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Altman, M.B.; Laszlo, A.; Straube, W.; Zoberi, I.; Hallahan, D.E.; Chen, H. Ultrasound Hyperthermia Technology for Radiosensitization. Ultrasound Med. Biol. 2019, 45, 1025–1043. [Google Scholar] [CrossRef] [Green Version]
- Kok, H.P.; Cressman, E.N.K.; Ceelen, W.; Brace, C.L.; Ivkov, R.; Grüll, H.; Ter Haar, G.; Wust, P.; Crezee, J. Heating Technology for Malignant Tumors: A Review. Int. J. Hyperth. 2020, 37, 711–741. [Google Scholar] [CrossRef]
- Yagawa, Y.; Tanigawa, K.; Kobayashi, Y.; Yamamoto, M. Cancer Immunity and Therapy Using Hyperthermia with Immunotherapy, Radiotherapy, Chemotherapy, and Surgery. J. Cancer Metastasis Treat. 2017, 3, 218. [Google Scholar] [CrossRef]
- Horsman, M.R. Tissue Physiology and the Response to Heat. Int. J. Hyperth. 2006, 22, 197–203. [Google Scholar] [CrossRef]
- Labavić, D.; Ladjimi, M.T.; Courtade, E.; Pfeuty, B.; Thommen, Q. Mammalian Cell Sensitivity to Hyperthermia in Various Cell Lines: A New Universal and Predictive Description. Int. J. Hyperth. 2020, 37, 506–516. [Google Scholar] [CrossRef]
- Habash, R.W.Y.; Bansal, R.; Krewski, D.; Alhafid, H.T. Thermal Therapy, Part III: Ablation Techniques. Crit. Rev. Biomed. Eng. 2007, 35, 37–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priester, M.I.; Curto, S.; van Rhoon, G.C.; Hagen, T.L.M.T. External Basic Hyperthermia Devices for Preclinical Studies in Small Animals. Cancers 2021, 13, 4628. [Google Scholar] [CrossRef]
- ElBialy, N.; Abdelhamid, M.; Youssef, T. Low Power Argon Laser-Induced Thermal Therapy for Subcutaneous Ehrlich Carcinoma in Mice Using Spherical Gold Nanoparticles. J. Biomed. Nanotechnol. 2010, 6, 687–693. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the Clinic. Bioeng. Transl. Med. 2016, 1, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, E.B.; Dreaden, E.C.; Huang, X.H.; El-Sayed, I.H.; Chu, H.H.; Pushpanketh, S.; McDonald, J.F.; El-Sayed, M.A. Gold Nanorod Assisted Near-Infrared Plasmonic Photothermal Therapy (PPTT) of Squamous Cell Carcinoma in Mice. Cancer Lett. 2008, 269, 57–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, S.; Xing, H.; Fu, X.; Yu, H.; Yang, Z.; Yang, Y.; Sun, W. The Effect of Photothermal Therapy on Osteosarcoma with Polyacrylic Acid–Coated Gold Nanorods. Dose-Response 2018, 16, 1559325818789841. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.-W.; Guo, W.-H.; Qi, Y.-F.; Wang, J.-Z.; Ma, X.-X.; Yu, D.-X. Synergistic Effects of Gold Nanocages in Hyperthermia and Radiotherapy Treatment. Nanoscale Res. Lett. 2016, 11, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vines, J.B.; Yoon, J.-H.; Ryu, N.-E.; Lim, D.-J.; Park, H. Gold Nanoparticles for Photothermal Cancer Therapy. Front. Chem. 2019, 7, 167. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonic Photothermal Therapy (PPTT) Using Gold Nanoparticles. Lasers Med. Sci. 2008, 23, 217–228. [Google Scholar] [CrossRef]
- Amendola, V.; Pilot, R.; Frasconi, M.; Marago, O.M.; Iatì, M.A. Surface Plasmon Resonance in Gold Nanoparticles: A Review. J. Physics Condens. Matter 2017, 29, 203002. [Google Scholar] [CrossRef]
- Terentyuk, G.S.; Maslyakova, G.; Suleymanova, L.V.; Khlebtsov, N.; Khlebtsov, B.; Akchurin, G.G.; Maksimova, I.L.; Tuchin, V. Laser-Induced Tissue Hyperthermia Mediated by Gold Nanoparticles: Toward Cancer Phototherapy. J. Biomed. Opt. 2009, 14, 021016. [Google Scholar] [CrossRef] [Green Version]
- Choi, W.I.; Sahu, A.; Kim, Y.H.; Tae, G. Photothermal Cancer Therapy and Imaging Based on Gold Nanorods. Ann. Biomed. Eng. 2011, 40, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Harris, N.; Ford, A.M.J.; Cortie, M.B. Optimization of Plasmonic Heating by Gold Nanospheres and Nanoshells. J. Phys. Chem. B 2006, 110, 10701–10707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hainfeld, J.F.; O′Connor, M.J.; Lin, P.; Qian, L.; Slatkin, D.N.; Smilowitz, H.M. Infrared-Transparent Gold Nanoparticles Converted by Tumors to Infrared Absorbers Cure Tumors in Mice by Photothermal Therapy. PLoS ONE 2014, 9, e88414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumura, Y.; Maeda, H. A New Concept for Macromolecular Therapeutics in Cancer Chemotherapy: Mechanism of Tu-moritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- Maeda, H. Toward a Full Understanding of the EPR Effect in Primary and Metastatic Tumors as Well as Issues Related to Its Heterogeneity. Adv. Drug Deliv. Rev. 2015, 91, 3–6. [Google Scholar] [CrossRef]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef]
- Sriraman, S.K.; Aryasomayajula, B.; Torchilin, V.P. Barriers to Drug Delivery in Solid Tumors. Tissue Barriers 2014, 2, e29528. [Google Scholar] [CrossRef] [Green Version]
- Khlebtsov, N.; Dykman, L. Biodistribution and Toxicity of Engineered Gold Nanoparticles: A Review of in Vitro and in Vivo Studies. Chem. Soc. Rev. 2011, 40, 1647–1671. [Google Scholar] [CrossRef]
- Bucharskaya, A.B.; Maslyakova, G.N.; Afanasyeva, G.A.; Terentyuk, G.S.; Navolokin, N.A.; Zlobina, O.V.; Chumakov, D.S.; Bashkatov, A.N.; Genina, E.; Khlebtsov, N.G.; et al. The Morpho-Functional Assessment of Plasmonic Photothermal Therapy Effects on Transplanted Liver Tumor. J. Innov. Opt. Health Sci. 2015, 8, 1541004. [Google Scholar] [CrossRef]
- Zhang, B.; Hu, Y.; Pang, Z. Modulating the Tumor Microenvironment to Enhance Tumor Nanomedicine Delivery. Front. Pharmacol. 2017, 8, 952. [Google Scholar] [CrossRef]
- Park, J.; Choi, Y.; Chang, H.; Um, W.; Ryu, J.H.; Kwon, I.C. Alliance with EPR Effect: Combined Strategies to Improve the EPR Effect in the Tumor Microenvironment. Theranostics 2019, 9, 8073–8090. [Google Scholar] [CrossRef]
- Durymanov, M.; Rosenkranz, A.; Sobolev, A.S. Current Approaches for Improving Intratumoral Accumulation and Distribution of Nanomedicines. Theranostics 2015, 5, 1007–1020. [Google Scholar] [CrossRef] [Green Version]
- Glomm, W. Functionalized Gold Nanoparticles for Applications in Bionanotechnology. J. Dispers. Sci. Technol. 2005, 26, 389–414. [Google Scholar] [CrossRef]
- Gholipourmalekabadi, M.; Mobaraki, M.; Ghaffari, M.; Zarebkohan, A.; Omrani, V.F.; Urbanska, A.M.; Seifalian, A. Targeted Drug Delivery Based on Gold Nanoparticle Derivatives. Curr. Pharm. Des. 2017, 23, 2918–2929. [Google Scholar] [CrossRef]
- Goddard, Z.R.; Marín, M.J.; Russell, D.A.; Searcey, M. Active Targeting of Gold Nanoparticles as Cancer Therapeutics. Chem. Soc. Rev. 2020, 49, 8774–8789. [Google Scholar] [CrossRef]
- O′Neal, D.; Hirsch, L.R.; Halas, N.; Payne, J.; West, J.L. Photo-Thermal Tumor Ablation in Mice Using Near Infrared-Absorbing Nanoparticles. Cancer Lett. 2004, 209, 171–176. [Google Scholar] [CrossRef]
- Gobin, A.M.; Lee, M.H.; Halas, N.J.; James, W.D.; Drezek, R.A.; West, J.L. Near-Infrared Resonant Nanoshells for Combined Optical Imaging and Photothermal Cancer Therapy. Nano Lett. 2007, 7, 1929–1934. [Google Scholar] [CrossRef]
- Chen, J.; Glaus, C.; Laforest, R.; Zhang, Q.; Yang, M.; Gidding, M.; Welch, M.J.; Xia, Y. Gold Nanocages as Photothermal Transducers for Cancer Treatment. Small 2010, 6, 811–817. [Google Scholar] [CrossRef] [Green Version]
- Choi, W.I.; Kim, J.-Y.; Kang, C.; Byeon, C.C.; Kim, Y.H.; Tae, G. Tumor Regression In Vivo by Photothermal Therapy Based on Gold-Nanorod-Loaded, Functional Nanocarriers. ACS Nano 2011, 5, 1995–2003. [Google Scholar] [CrossRef]
- El-Sayed, M.A.; Shabaka, A.A.; El-Shabrawy, O.A.; Yassin, N.A.; Mahmoud, S.S.; El-Shenawy, S.M.; Al-Ashqar, E.; Eisa, W.H.; Farag, N.M.; El-Shaer, M.A.; et al. Tissue Distribution and Efficacy of Gold Nanorods Coupled with Laser Induced Photoplasmonic Therapy in Ehrlich Carcinoma Solid Tumor Model. PLoS ONE 2013, 8, e76207. [Google Scholar] [CrossRef]
- Sirotkina, M.A. Visualization and Laser Hyperthermia of Biological Tissues Using Gold Plasmon Resonance Nanoparticles. Ph.D. Thesis, Institute of Theoretical and Experimental Biophysics RAS, Pushchino, Russia, 2014. [Google Scholar]
- Bucharskaya, A.B.; Maslyakova, G.N.; Dikht, N.I.; Navolokin, N.A.; Terentyuk, G.S.; Bashkatov, A.N.; Genina, E.; Khlebtsov, B.; Khlebtsov, N.G.; Tuchin, V. Plasmonic Photothermal Therapy of Transplanted Tumors in Rats at Multiple Intravenous Injection of Gold Nanorods. BioNanoScience 2016, 7, 216–221. [Google Scholar] [CrossRef]
- Bucharskaya, A.B.; Maslyakova, G.N.; Chekhonatskaya, M.L.; Terentyuk, G.S.; Navolokin, N.A.; Khlebtsov, B.N.; Khlebtsov, N.G.; Bashkatov, A.N.; Genina, E.A.; Tuchin, V.V. Plasmonic Photothermal Therapy: Approaches to Advanced Strategy. Lasers Surg. Med. 2018, 50, 1025–1033. [Google Scholar] [CrossRef]
- Lan, M.; Zhao, S.; Liu, W.; Lee, C.-S.; Zhang, W.; Wang, P. Photosensitizers for Photodynamic Therapy. Adv. Healthc. Mater. 2019, 8, e1900132. [Google Scholar] [CrossRef]
- Zhou, Z.; Song, J.; Nie, L.; Chen, X. Reactive Oxygen Species Generating Systems Meeting Challenges of Photodynamic Cancer Therapy. Chem. Soc. Rev. 2016, 45, 6597–6626. [Google Scholar] [CrossRef] [Green Version]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy—Current Limitations and Novel Approaches. Front. Chem. 2021, 9, 400. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, J.; Fan, J.; Chao, H.; Peng, X. Recent Progress in Photosensitizers for Overcoming the Challenges of Photodynamic Therapy: From Molecular Design to Application. Chem. Soc. Rev. 2021, 50, 4185–4219. [Google Scholar] [CrossRef]
- Mahajan, P.G.; Dige, N.C.; Vanjare, B.D.; Kim, C.-H.; Seo, S.-Y.; Lee, K.H. Design and Synthesis of New Porphyrin Analogues as Potent Photosensitizers for Photodynamic Therapy: Spectroscopic Approach. J. Fluoresc. 2020, 30, 397–406. [Google Scholar] [CrossRef]
- Kou, J.; Dou, D.; Yang, L. Porphyrin Photosensitizers in Photodynamic Therapy and Its Applications. Oncotarget 2017, 8, 81591–81603. [Google Scholar] [CrossRef] [Green Version]
- Mokhtari, R.B.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination Therapy in Combating Cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef] [Green Version]
- Błaszkiewicz, P.; Kotkowiak, M. Gold-Based Nanoparticles Systems in Phototherapy-Current Strategies. Curr. Med. Chem. 2019, 25, 5914–5929. [Google Scholar] [CrossRef]
- Khlebtsov, B.; Panfilova, E.; Khanadeev, V.; Bibikova, O.; Terentyuk, G.; Ivanov, A.; Rumyantseva, V.; Shilov, I.; Ryabova, A.; Loshchenov, V.; et al. Nanocomposites Containing Silica-Coated Gold–Silver Nanocages and Yb–2,4-Dimethoxyhematoporphyrin: Multifunctional Capability of IR-Luminescence Detection, Photosensitization, and Photothermolysis. ACS Nano 2011, 5, 7077–7089. [Google Scholar] [CrossRef]
- Chen, B.; Cao, J.; Zhang, K.; Zhang, Y.-N.; Lu, J.; Iqbal, M.Z.; Zhang, Q.; Kong, X. Synergistic Photodynamic and Photothermal Therapy of BODIPY-Conjugated Hyaluronic Acid Nanoparticles. J. Biomater. Sci. Polym. Ed. 2021, 32, 2028–2045. [Google Scholar] [CrossRef]
- Pinto, A.; Pocard, M. Photodynamic Therapy and Photothermal Therapy for the Treatment of Peritoneal Metastasis: A Systematic Review. Pleura Peritoneum 2018, 3, 20180124. [Google Scholar] [CrossRef]
- Deng, X.; Shao, Z.; Zhao, Y. Solutions to the Drawbacks of Photothermal and Photodynamic Cancer Therapy. Adv. Sci. 2021, 8, 2002504. [Google Scholar] [CrossRef]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical Development and Potential of Photothermal and Photodynamic Therapies for Cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef]
- Guo, M.; Mao, H.; Li, Y.; Zhu, A.; He, H.; Yang, H.; Wang, Y.; Tian, X.; Ge, C.; Peng, Q.; et al. Dual Imaging-Guided Photothermal/Photodynamic Therapy Using Micelles. Biomaterials 2014, 35, 4656–4666. [Google Scholar] [CrossRef] [Green Version]
- Yan, F.; Wu, H.; Liu, H.; Deng, Z.; Liu, H.; Duan, W.; Liu, X.; Zheng, H. Molecular Imaging-Guided Photothermal/Photodynamic Therapy against Tumor by iRGD-Modified Indocyanine Green Nanoparticles. J. Control. Release 2015, 224, 217–228. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, W.; Cui, Y.; Zhou, N.; Shen, J. Near-Infrared Light-Mediated Photodynamic/Photothermal Therapy Nanoplatform by the Assembly of Fe3O4 Carbon Dots with Graphitic Black Phosphorus Quantum Dots. Int. J. Nanomed. 2018, 13, 2803–2819. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zheng, B.-D.; Peng, X.-H.; Li, S.-Z.; Ying, J.-W.; Zhao, Y.; Huang, J.-D.; Yoon, J. Phthalocyanines as Medicinal Photosensitizers: Developments in the Last Five Years. Co-ord. Chem. Rev. 2019, 379, 147–160. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, Z.; Ren, Y.; Chen, X.; Zhang, W.; Zhu, X.; Mao, Z.; Shen, J.; Nie, S. Advances in Nanomaterials for Use in Photothermal and Photodynamic Therapeutics (Review). Mol. Med. Rep. 2019, 20, 5–15. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Yang, J.; Luo, L.; Jiang, M.; Qin, B.; Yin, H.; Zhu, C.; Yuan, X.; Zhang, J.; Luo, Z.; et al. Targeting Photodynamic and Photothermal Therapy to the Endoplasmic Reticulum Enhances Immunogenic Cancer Cell Death. Nat. Commun. 2019, 10, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Dykman, L.A.; Khlebtsov, N.G. Multifunctional Gold-Based Nanocomposites for Theranostics. Biomaterials 2016, 108, 13–34. [Google Scholar] [CrossRef]
- Choi, J.; Lee, S.-E.; Park, J.-S.; Kim, S.Y. Gold Nanorod-Photosensitizer Conjugates with Glutathione-Sensitive Linkages for Synergistic Cancer Photodynamic/Photothermal Therapy. Biotechnol. Bioeng. 2018, 115, 1340–1354. [Google Scholar] [CrossRef]
- Jang, B.; Park, J.-Y.; Tung, C.-H.; Kim, I.-H.; Choi, Y. Gold Nanorod−Photosensitizer Complex for Near-Infrared Fluorescence Imaging and Photodynamic/Photothermal Therapy In Vivo. ACS Nano 2011, 5, 1086–1094. [Google Scholar] [CrossRef]
- Wang, S.; Huang, P.; Nie, L.; Xing, R.; Liu, D.; Wang, Z.; Lin, J.; Chen, S.; Niu, G.; Lu, G.; et al. Single Continuous Wave Laser Induced Photodynamic/Plasmonic Photothermal Therapy Using Photosensitizer-Functionalized Gold Nanostars. Adv. Mater. 2013, 25, 3055–3061. [Google Scholar] [CrossRef] [Green Version]
- Terentyuk, G.; Panfilova, E.; Khanadeev, V.; Chumakov, D.; Genina, E.; Bashkatov, A.; Tuchin, V.; Bucharskaya, A.; Maslyakova, G.; Khlebtsov, N.; et al. Gold Nanorods with a Hematoporphyrin-Loaded Silica Shell for Dual-Modality Photodynamic and Photothermal Treatment of Tumors in Vivo. Nano Res. 2014, 7, 325–337. [Google Scholar] [CrossRef]
- Zhang, S.; Lv, H.; Zhao, J.; Cheng, M.; Sun, S. Synthesis of Porphyrin-Conjugated Silica-Coated Au Nanorods for Synergistic Photothermal Therapy and Photodynamic Therapy of Tumor. Nanotechnology 2019, 30, 265102. [Google Scholar] [CrossRef]
- Liu, Z.; Xie, F.; Xie, J.; Chen, J.; Li, Y.; Lin, Q.; Luo, F.; Yan, J. New-Generation Photosensitizer-Anchored Gold Nanorods for a Single Near-Infrared Light-Triggered Targeted Photodynamic–Photothermal Therapy. Drug Deliv. 2021, 28, 1769–1784. [Google Scholar] [CrossRef]
- Wang, L.; Jacques, S.L.; Zheng, L. MCML—Monte Carlo Modeling of Light Transport in Multi-Layered Tissues. Comput. Methods Programs Biomed. 1995, 47, 131–146. [Google Scholar] [CrossRef]
- Wilson, B.C.; Adam, G. A Monte Carlo Model for the Absorption and Flux Distributions of Light in Tissue. Med. Phys. 1983, 10, 824–830. [Google Scholar] [CrossRef]
- Avetisyan, A.Y.; Yakunin, A.N.; Tuchin, V.V. On the Problem of Local Tissue Hyperthermia Control: Multiscale Modelling of Pulsed Laser Radiation Action on a Medium with Embedded Nanoparticles. Quantum Electron. 2011, 40, 1081–1088. [Google Scholar] [CrossRef]
- Shao, J.; Griffin, R.J.; Galanzha, E.I.; Kim, J.-W.; Koonce, N.; Webber, J.; Mustafa, T.; Biris, A.S.; Nedosekin, D.; Zharov, V.P. Photothermal Nanodrugs: Potential of TNF-Gold Nanospheres for Cancer Theranostics. Sci. Rep. 2013, 3, 1293. [Google Scholar] [CrossRef]
- Oraevsky, A.; Lapotko, D. Laser Activated Nanothermolysis of Cells. U.S. Patent 13/136,939, 23 February 2012. [Google Scholar]
- Yakunin, A.N.; Avetisyan, Y.A.; Tuchin, V.V. Quantification of Laser Local Hyperthermia Induced by Gold Plasmonic Nanoparticles. J. Biomed. Opt. 2015, 20, 51030. [Google Scholar] [CrossRef]
- Maksimova, I.L.; Akchurin, G.G.; Terentyuk, G.S.; Khlebtsov, B.; Ermolaev, I.A.; Skaptsov, A.A.; Revzina, E.M.; Tuchin, V.; Khlebtsov, N.G. Laser Photothermolysis of Biological Tissues by Using Plasmon-Resonance Particles. Quantum Electron. 2008, 38, 536–542. [Google Scholar] [CrossRef]
- Welch, A.J.; Wissler, E.H.; Priebe, L.A. Significance of Blood Flow in Calculations of Temperature in Laser Irradiated Tissue. IEEE Trans. Biomed. Eng. 1980, BME-27, 164–166. [Google Scholar] [CrossRef]
- Ramasamy, M.; Lee, S.S.; Yi, D.K.; Kim, K. Magnetic, Optical Gold Nanorods for Recyclable Photothermal Ablation of Bacteria. J. Mater. Chem. B 2013, 2, 981–988. [Google Scholar] [CrossRef]
- Millenbaugh, N.J.; Baskin, J.B.; DeSilva, M.N.; Elliott, W.R.; Glickman, R.D. Photothermal Killing of Staphylococcus Aureus Using Antibody-Targeted Gold Nanoparticles. Int. J. Nanomed. 2015, 10, 1953–1960. [Google Scholar] [CrossRef] [Green Version]
- Mocan, L.; Tabaran, F.A.; Mocan, T.; Pop, T.; Moşteanu, O.; Agoşton-Coldea, L.; Matea, C.T.; Gonciar, D.; Zdrehus, C.; Iancu, C. Laser Thermal Ablation of Multidrug-Resistant Bacteria Using Functionalized Gold Nanoparticles. Int. J. Nanomed. 2017, 12, 2255–2263. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, N.N.; Alkilany, A.M.; Khalil, E.A.; Al-Bakri, A.G. Nano-Photothermal Ablation Effect of Hydrophilic and Hydrophobic Functionalized Gold Nanorods on Staphylococcus Aureus and Propionibacterium Acnes. Sci. Rep. 2018, 8, 6881. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Deng, W.; Yang, F.; Wu, Z.; Huang, M.; Gu, M. Gold Nanoparticles Decorated Graphene Oxide/Nanocellulose Paper for NIR Laser-Induced Photothermal Ablation of Pathogenic Bacteria. Carbohydr. Polym. 2018, 198, 206–214. [Google Scholar] [CrossRef]
- Penders, J.; Stolzoff, M.; Hickey, D.J.; Andersson, M.; Webster, T.J. Shape-Dependent Antibacterial Effects of Non-Cytotoxic Gold Nanoparticles. Int. J. Nanomed. 2017, 12, 2457–2468. [Google Scholar] [CrossRef] [Green Version]
- Yakunin, A.; Zarkov, S.V.; Avetisyan, Y.A.; Akchurin, G.G., Jr.; Tuchina, E.S.; Tuchin, V. Modeling of Hyperthermia Induced by Functionalized Gold Nanorods Bound to Staphylococcus Aureus under NIR Laser Radiation. In Proceedings of the Saratov Fall Meeting 2018: Optical and Nano-Technologies for Biology and Medicine, Saratov, Russia, 24–28 September 2018; Volume 11065, pp. 279–288. [Google Scholar] [CrossRef]
- Tuchin, V.V.; Popp, J.; Zakharov, V. Multimodal Optical Diagnostics of Cancer; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- El-Sayed, I.H.; Huang, X.; El-Sayed, M.A. Selective Laser Photo-Thermal Therapy of Epithelial Carcinoma Using Anti-EGFR Antibody Conjugated Gold Nanoparticles. Cancer Lett. 2006, 239, 129–135. [Google Scholar] [CrossRef]
- Manuchehrabadi, N.; Chen, Y.; Lebrun, A.; Ma, R.; Zhu, L. Computational Simulation of Temperature Elevations in Tumors Using Monte Carlo Method and Comparison to Experimental Measurements in Laser Photothermal Therapy. J. Biomech. Eng. 2013, 135, 121007. [Google Scholar] [CrossRef] [Green Version]
- Youn, J.-I. The Effect of an Optical Clearing Agent on Tissue Prior to 1064-nm Laser Therapy. Med. Lasers 2021, 10, 146–152. [Google Scholar] [CrossRef]
- Chu, Y.; Liao, S.; Liao, H.; Lu, Y.; Geng, X.; Wu, D.; Pei, J.; Wang, Y. Second Near-Infrared Photothermal Therapy with Superior Penetrability through Skin Tissues. CCS Chem. 2021, 3, 3289–3300. [Google Scholar] [CrossRef]
- Jia, H.; Chen, B.; Li, D. Dynamic Optical Absorption Characteristics of Blood after Slow and Fast Heating. Lasers Med. Sci. 2017, 32, 513–525. [Google Scholar] [CrossRef]
- Yaroslavsky, A.N.; Schulze, P.C.; Yaroslavsky, I.V.; Schober, R.; Ulrich, F.; Schwarzmaier, H.-J. Optical Properties of Selected Native and Coagulated Human Brain Tissues in Vitro in the Visible and Near Infrared Spectral Range. Phys. Med. Biol. 2002, 47, 2059–2073. [Google Scholar] [CrossRef]
- Yu, T.; Qi, Y.; Zhu, J.; Xu, J.; Gong, H.; Luo, Q.; Zhu, D. Elevated-Temperature-Induced Acceleration of PACT Clearing Process of Mouse Brain Tissue. Sci. Rep. 2017, 7, 38848. [Google Scholar] [CrossRef] [Green Version]
- Laufer, J.; Simpson, R.; Kohl, M.; Essenpreis, M.; Cope, M. Effect of Temperature on the Optical Properties of ex Vivo Human Dermis and Subdermis. Phys. Med. Biol. 1998, 43, 2479–2489. [Google Scholar] [CrossRef] [Green Version]
- Iorizzo, T.W.; Jermain, P.R.; Salomatina, E.; Muzikansky, A.; Yaroslavsky, A.N. Temperature Induced Changes in the Optical Properties of Skin in Vivo. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Skinner, M.G.; Everts, S.; Reid, A.D.; Vitkin, A.; Lilge, L.; Sherar, M.D. Changes in Optical Properties Ofex Vivorat Prostate due to Heating. Phys. Med. Biol. 2000, 45, 1375–1386. [Google Scholar] [CrossRef]
- Nagarajan, V.K.; Gogineni, V.R.; White, S.B.; Yu, B. Real Time Evaluation of Tissue Optical Properties during Thermal Ablation of ex Vivo Liver Tissues. Int. J. Hyperth. 2018, 35, 176–182. [Google Scholar] [CrossRef] [Green Version]
- Nagarajan, V.K.; Ward, J.M.; Yu, B. Association of Liver Tissue Optical Properties and Thermal Damage. Lasers Surg. Med. 2020, 52, 779–787. [Google Scholar] [CrossRef]
- Genin, V.D.; Bucharskaya, A.B.; Genina, E.A.; Terentyuk, G.S.; Khlebtsov, N.G.; Tuchin, V.V.; Bashkatov, A.N. Optical Properties of Model Cholangiocarcinoma Tissues in the Spectral Range of 350–2250 nm in Laser Photothermolysis Treatment. In Saratov Fall Meeting 2020: Optical and Nanotechnologies for Biology and Medicine; International Society for Optics and Photonics: Bellingham, WA, USA, 2021; Volume 11845, p. 118450Z. [Google Scholar] [CrossRef]
- Genin, V.D.; Bucharskaya, A.B.; Navolokin, N.A.; Terentyuk, G.S.; Khlebtsov, N.G.; Tuchin, V.V.; Genina, E.A. Impact of Immersion Agents on Optical Parameters of Tissues in the Process of Laser Photothermal Tumor Therapy: A Pilot Study. Opt. Spectrosc. 2022. in print. [Google Scholar]
- Bian, W.; Wang, Y.; Pan, Z.; Chen, N.; Li, X.; Wong, W.-L.; Liu, X.; He, Y.; Zhang, K.; Lu, Y.-J. Review of Functionalized Nanomaterials for Photothermal Therapy of Cancers. ACS Appl. Nano Mater. 2021, 4, 11353–11385. [Google Scholar] [CrossRef]
- Yuan, M.; Xu, S.; Zhang, Q.; Zhao, B.; Feng, B.; Ji, K.; Yu, L.; Chen, W.; Hou, M.; Xu, Y.; et al. Bicompatible Porous Co3O4 Nanoplates with Intrinsic Tumor Metastasis Inhibition for Multimodal Imaging and DNA Damage–Mediated Tumor Synergetic Photothermal/Photodynamic Therapy. Chem. Eng. J. 2020, 394, 124874. [Google Scholar] [CrossRef]
- Zhao, T.; Shen, X.; Li, L.; Guan, Z.; Gao, N.; Yuan, P.; Yao, S.Q.; Xu, Q.-H.; Xu, G.Q. Gold Nanorods as Dual Photo-Sensitizing and Imaging Agents for Two-Photon Photodynamic Therapy. Nanoscale 2012, 4, 7712–7719. [Google Scholar] [CrossRef]
- Jiang, C.; Zhao, T.; Yuan, P.; Gao, N.; Pan, Y.; Guan, Z.; Zhou, N.; Xu, Q.-H. Two-Photon Induced Photoluminescence and Singlet Oxygen Generation from Aggregated Gold Nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 4972–4977. [Google Scholar] [CrossRef]
- Vankayala, R.; Huang, Y.-K.; Kalluru, P.; Chiang, C.-S.; Hwang, K.C. First Demonstration of Gold Nanorods-Mediated Photodynamic Therapeutic Destruction of Tumors via Near Infra-Red Light Activation. Small 2013, 10, 1612–1622. [Google Scholar] [CrossRef]
- Vankayala, R.; Kuo, C.-L.; Sagadevan, A.; Chen, P.-H.; Chiang, C.-S.; Hwang, K.C. Morphology Dependent Photosensitization and Formation of Singlet Oxygen (1Δg) by Gold and Silver Nanoparticles and Its Application in Cancer Treatment. J. Mater. Chem. B 2013, 1, 4379–4387. [Google Scholar] [CrossRef]
- Vankayala, R.; Lin, C.-C.; Kalluru, P.; Chiang, C.-S.; Hwang, K.C. Gold Nanoshells-Mediated Bimodal Photodynamic and Photothermal Cancer Treatment Using Ultra-Low Doses of Near Infra-Red Light. Biomaterials 2014, 35, 5527–5538. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, X.; Li, N.; Wang, B.; He, S. Absorption-Dependent Generation of Singlet Oxygen from Gold Bipyramids Excited under Low Power Density. RSC Adv. 2015, 5, 81897–81904. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef]
- Dahl, O. Interaction of Hyperthermia and Chemotherapy. In Recent Results in Cancer Research; Springer: Berlin/Heidelberg, Germany, 1988; Volume 107, pp. 157–169. [Google Scholar] [CrossRef]
- Urano, M. Invited Review: For the Clinical Application of Thermochemotherapy Given at Mild Temperatures. Int. J. Hyperth. 1999, 15, 79–107. [Google Scholar] [CrossRef]
- Gajewski, T.F.; Schreiber, H.; Fu, Y.-X. Innate and Adaptive Immune Cells in the Tumor Microenvironment. Nat. Immunol. 2013, 14, 1014–1022. [Google Scholar] [CrossRef] [Green Version]
- Turubanova, V.D.; Mishchenko, T.A.; Balalaeva, I.V.; Efimova, I.; Peskova, N.N.; Klapshina, L.G.; Lermontova, S.A.; Bachert, C.; Krysko, O.; Vedunova, M.V.; et al. Novel Porphyrazine-Based Photodynamic Anti-Cancer Therapy Induces Immunogenic Cell Death. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Liu, Y.; Chongsathidkiet, P.; Crawford, B.M.; Odion, R.; Dechant, C.A.; Kemeny, H.R.; Cui, X.; Maccarini, P.F.; Lascola, C.D.; Fecci, P.E.; et al. Plasmonic Gold Nanostar-Mediated Photothermal Immunotherapy for Brain Tumor Ablation and Immunologic Memory. Immunotherapy 2019, 11, 1293–1302. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, T.; Feng, T.; Wan, Y.; Blum, N.T.; Liu, C.; Zheng, C.; Zhao, Z.; Jiang, T.; Wang, J.; et al. Plasmonic Modulation of Gold Nanotheranostics for Targeted NIR-II Photothermal-Augmented Immunotherapy. Nano Today 2020, 35, 100987. [Google Scholar] [CrossRef]
- Liu, Y.; Chorniak, E.; Odion, R.; Etienne, W.; Nair, S.K.; Maccarini, P.; Palmer, G.M.; Inman, B.A.; Vo-Dinh, T. Plasmonic Gold Nanostars for Synergistic Photoimmunotherapy to Treat Cancer. Nanophotonics 2021, 10, 3295–3302. [Google Scholar] [CrossRef]
- Jung, B.-K.; Lee, Y.K.; Hong, J.; Ghandehari, H.; Yun, C.-O. Mild Hyperthermia Induced by Gold Nanorod-Mediated Plasmonic Photothermal Therapy Enhances Transduction and Replication of Oncolytic Adenoviral Gene Delivery. ACS Nano 2016, 10, 10533–10543. [Google Scholar] [CrossRef]
- Chen, G.; Ding, L.; Wu, P.; Zhou, Y.; Sun, M.; Wang, K.; Oupický, D. Polymeric Micelleplexes for Improved Photothermal Endosomal Escape and Delivery of siRNA. Polym. Adv. Technol. 2018, 29, 2593–2600. [Google Scholar] [CrossRef]
- Chong, L.M.; Tng, D.J.H.; Tan, L.L.Y.; Chua, M.L.K.; Zhang, Y. Recent Advances in Radiation Therapy and Photodynamic Therapy. Appl. Phys. Rev. 2021, 8, 041322. [Google Scholar] [CrossRef]
- Cai, R.; Xiang, H.; Yang, D.; Lin, K.-T.; Wu, Y.; Zhou, R.; Gu, Z.; Yan, L.; Zhao, Y.; Tan, W. Plasmonic AuPt@CuS Heterostructure with Enhanced Synergistic Efficacy for Radiophotothermal Therapy. J. Am. Chem. Soc. 2021, 143, 16113–16127. [Google Scholar] [CrossRef]
- Kayani, Z.; Islami, N.; Behzadpour, N.; Zahraie, N.; Imanlou, S.; Tamaddon, P.; Salehi, F.; Daneshvar, F.; Perota, G.; Sorati, E.; et al. Combating Cancer by Utilizing Noble Metallic Nanostructures in Combination with Laser Photothermal and X-Ray Radiotherapy. J. Drug Deliv. Sci. Technol. 2021, 65, 102689. [Google Scholar] [CrossRef]
- Nichols, J.W.; Bae, Y.H. EPR: Evidence and Fallacy. J. Control. Release 2014, 190, 451–464. [Google Scholar] [CrossRef]
- Hare, J.I.; Lammers, T.; Ashford, M.B.; Puri, S.; Storm, G.; Barry, S.T. Challenges and Strategies in Anti-Cancer Nanomedicine Development: An Industry Perspective. Adv. Drug Deliv. Rev. 2017, 108, 25–38. [Google Scholar] [CrossRef] [Green Version]
- Pires, L.; Demidov, V.; Wilson, B.C.; Salvio, A.G.; Moriyama, L.; Bagnato, V.S.; Vitkin, I.A.; Kurachi, C. Dual-Agent Photodynamic Therapy with Optical Clearing Eradicates Pigmented Melanoma in Preclinical Tumor Models. Cancers 2020, 12, 1956. [Google Scholar] [CrossRef]
- Tuchin, V.V.; Genina, E.A.; Tuchina, E.S.; Svetlakova, A.V.; Svenskaya, Y.I. Optical Clearing of Tissues: Issues of Antimicrobial Phototherapy and Drug Delivery. Adv. Drug Deliv. Rev. 2021, 180, 114037. [Google Scholar] [CrossRef]
- Khlebtsov, B.; Burov, A.; Pylaev, T.; Savkina, A.; Prikhozhdenko, E.; Bratashov, D.; Khlebtsov, N. Improving SERS Bioimaging of Subcutaneous Phantom in Vivo with Optical Clearing. J. Biophotonics 2021, e202100281. [Google Scholar] [CrossRef]
- Tuchin, V.V.; Zhu, D.; Genina, E.A. Handbook of Tissue Optical Clearing: New Prospects in Optical Imaging; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar] [CrossRef]
- Sokolovskaya, O.I.; Sergeeva, E.A.; Golovan, L.A.; Kashkarov, P.K.; Khilov, A.V.; Kurakina, D.A.; Orlinskaya, N.Y.; Zabotnov, S.V.; Kirillin, M.Y. Numerical Simulation of Enhancement of Superficial Tumor Laser Hyperthermia with Silicon Nanoparticles. Photonics 2021, 8, 580. [Google Scholar] [CrossRef]
- Bucharskaya, A.; Maslyakova, G.; Terentyuk, G.; Yakunin, A.; Avetisyan, Y.; Bibikova, O.; Tuchina, E.; Khlebtsov, B.; Khlebtsov, N.; Tuchin, V. Towards Effective Photothermal/Photodynamic Treatment Using Plasmonic Gold Nanoparticles. Int. J. Mol. Sci. 2016, 17, 1295. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bucharskaya, A.B.; Khlebtsov, N.G.; Khlebtsov, B.N.; Maslyakova, G.N.; Navolokin, N.A.; Genin, V.D.; Genina, E.A.; Tuchin, V.V. Photothermal and Photodynamic Therapy of Tumors with Plasmonic Nanoparticles: Challenges and Prospects. Materials 2022, 15, 1606. https://doi.org/10.3390/ma15041606
Bucharskaya AB, Khlebtsov NG, Khlebtsov BN, Maslyakova GN, Navolokin NA, Genin VD, Genina EA, Tuchin VV. Photothermal and Photodynamic Therapy of Tumors with Plasmonic Nanoparticles: Challenges and Prospects. Materials. 2022; 15(4):1606. https://doi.org/10.3390/ma15041606
Chicago/Turabian StyleBucharskaya, Alla B., Nikolai G. Khlebtsov, Boris N. Khlebtsov, Galina N. Maslyakova, Nikita A. Navolokin, Vadim D. Genin, Elina A. Genina, and Valery V. Tuchin. 2022. "Photothermal and Photodynamic Therapy of Tumors with Plasmonic Nanoparticles: Challenges and Prospects" Materials 15, no. 4: 1606. https://doi.org/10.3390/ma15041606
APA StyleBucharskaya, A. B., Khlebtsov, N. G., Khlebtsov, B. N., Maslyakova, G. N., Navolokin, N. A., Genin, V. D., Genina, E. A., & Tuchin, V. V. (2022). Photothermal and Photodynamic Therapy of Tumors with Plasmonic Nanoparticles: Challenges and Prospects. Materials, 15(4), 1606. https://doi.org/10.3390/ma15041606









