Tuning the Defects of Two-Dimensional Layered Carbon/TiO2 Superlattice Composite for a Fast Lithium-Ion Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Electrochemical Characterization
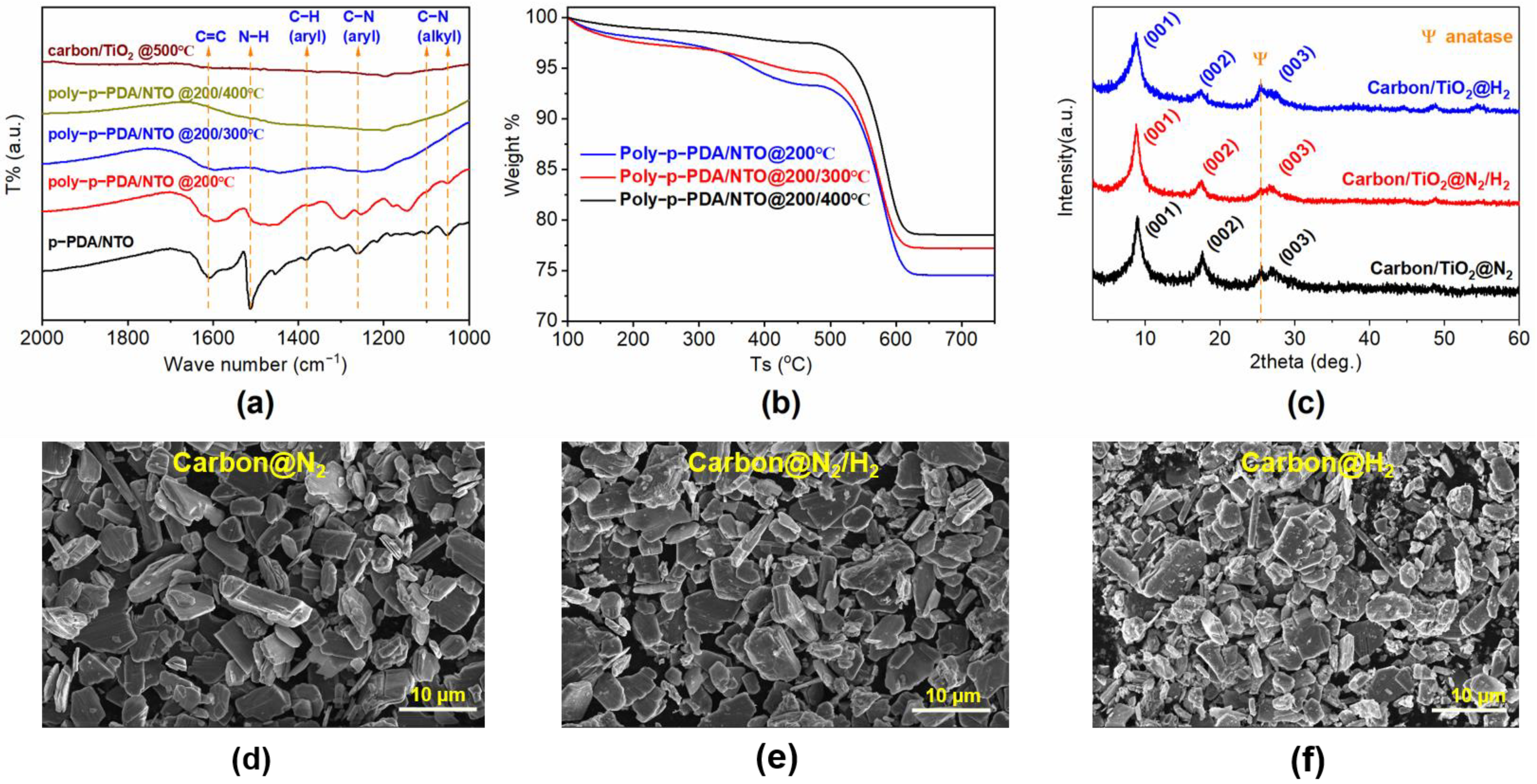
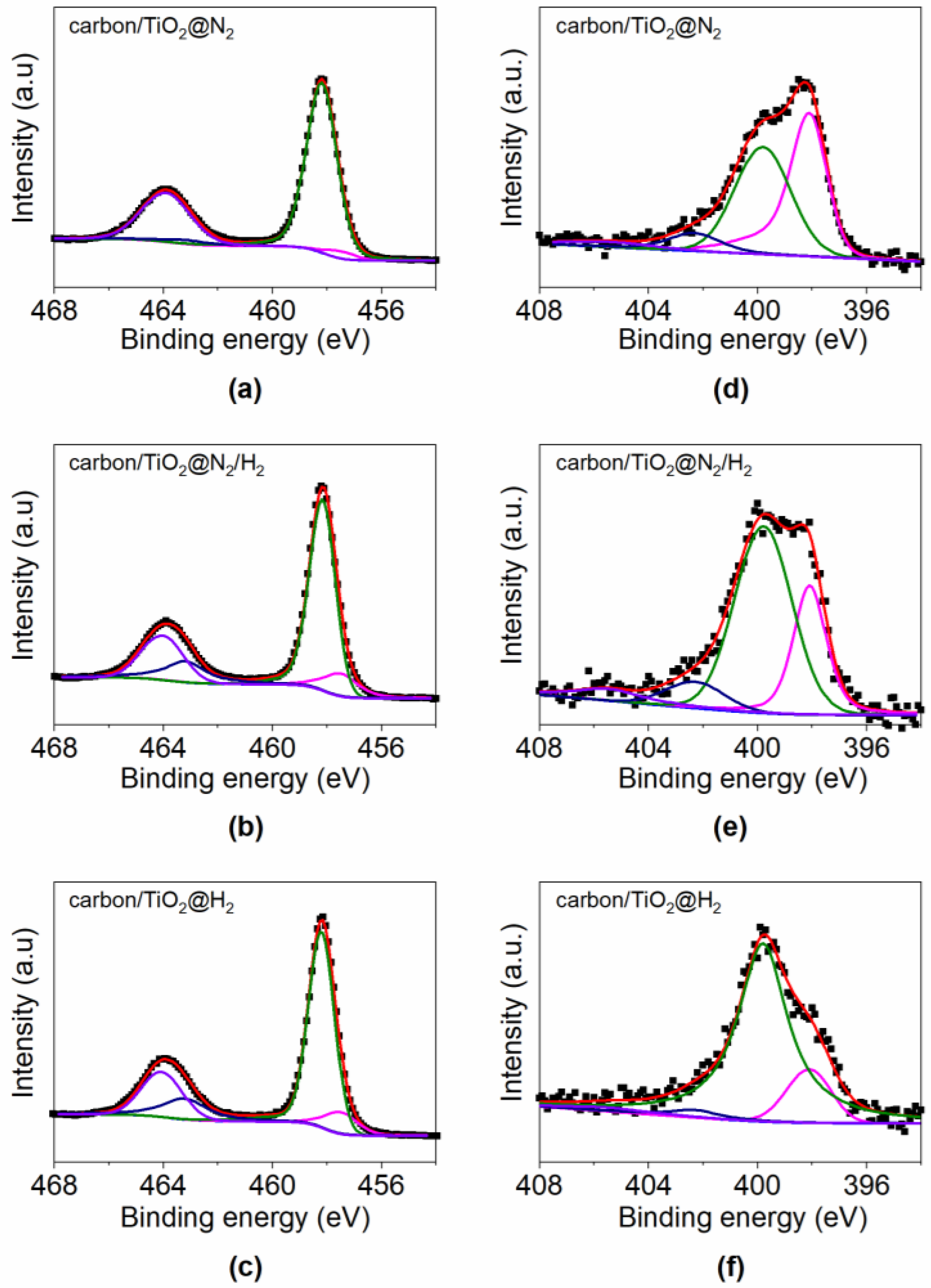
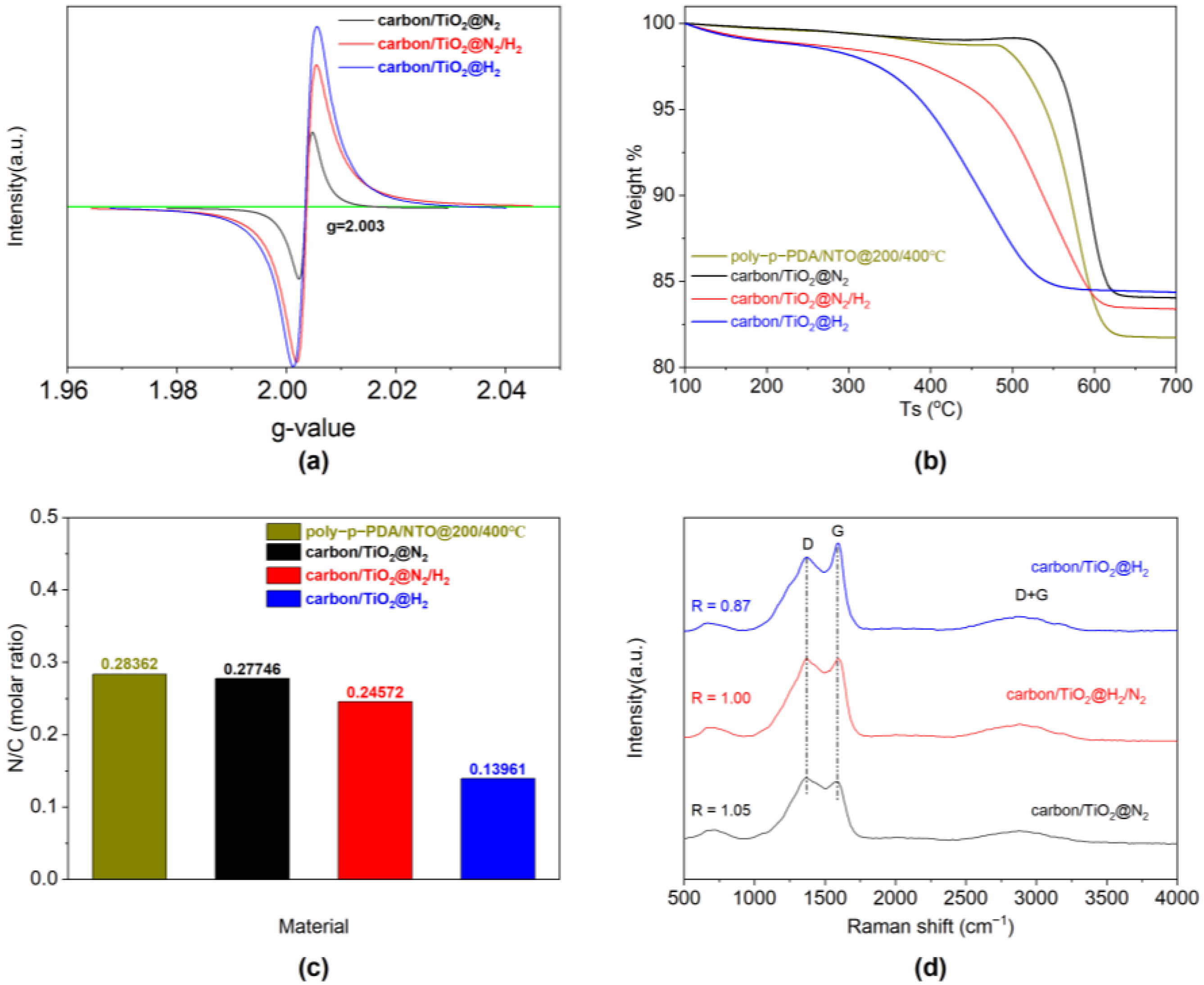
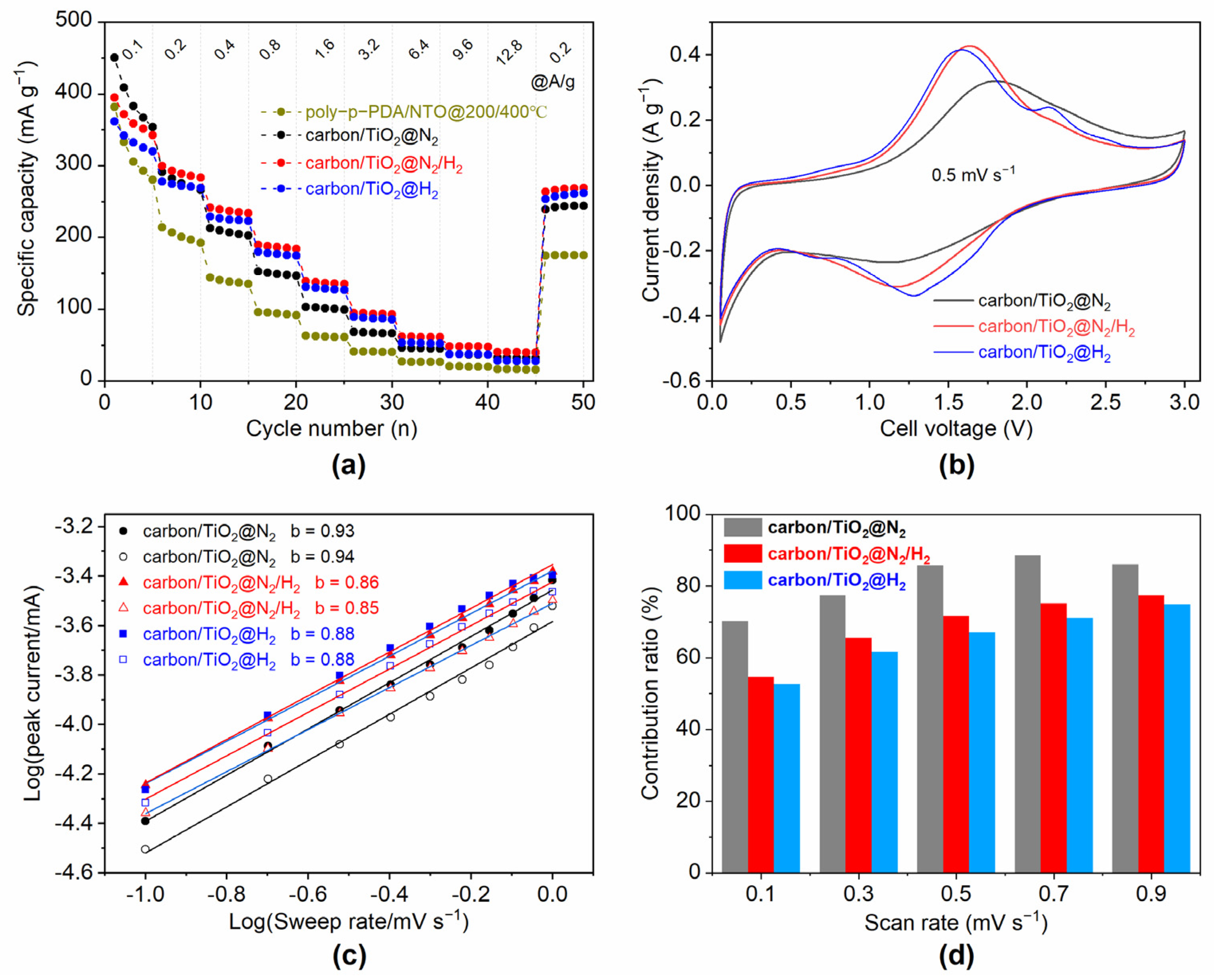
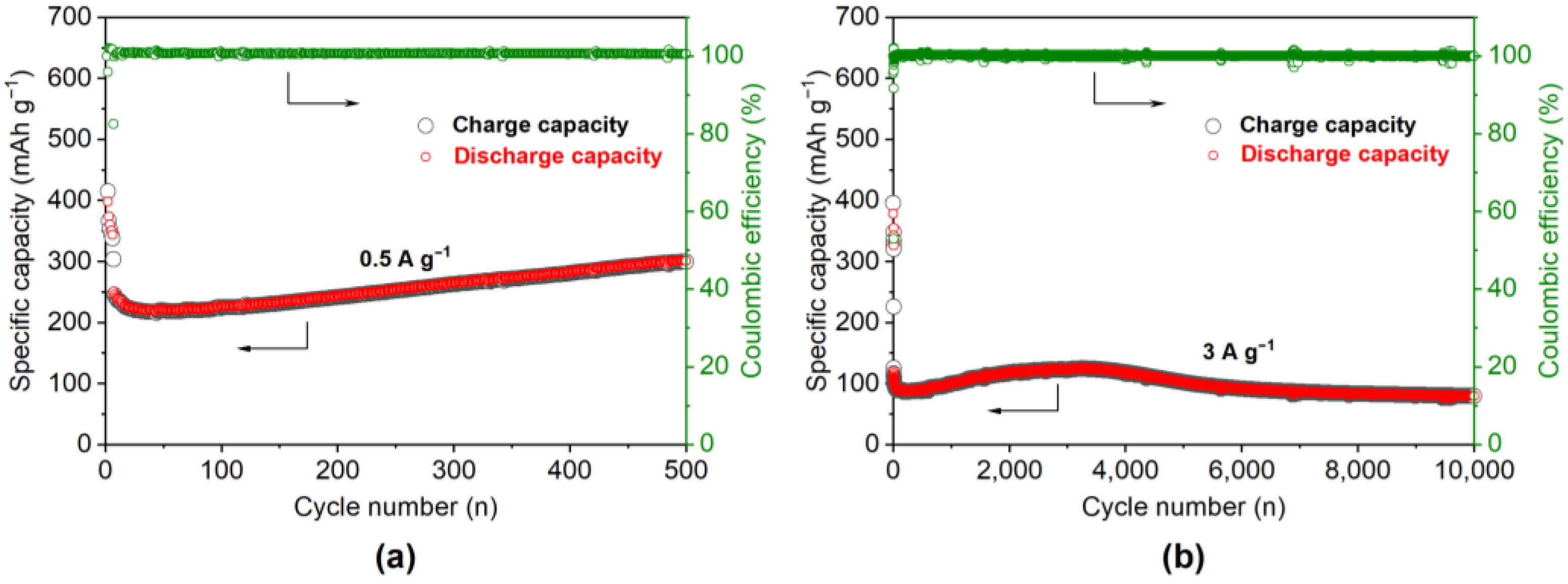
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dylla, A.G.; Henkelman, G.; Stevenson, K.J. Lithium insertion in nanostructured TiO2 (B) architectures. Acc. Chem. Res. 2013, 46, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Xu, J.; Yang, X.; Lu, F.; He, S.; Yang, J.; Fan, H.; Wu, M. Ultrathin anatase TiO2 nanosheets embedded with TiO2-B nanodomains for lithium-ion storage: Capacity enhancement by phase boundaries. Adv. Energy. Mater. 2014, 5, 1401756. [Google Scholar] [CrossRef]
- Cheng, X.; Hu, M.; Huang, R.; Jiang, J. Hf-free synthesis of anatase TiO2 nanosheets with largely exposed and clean {001} facets and their enhanced rate performance as anodes of lithium-ion battery. ACS Appl. Mater. Int. 2014, 6, 19176–19183. [Google Scholar] [CrossRef]
- Wang, D.; Kou, R.; Choi, D.; Yang, Z.; Nie, Z.; Li, J.; Saraf, L.V.; Hu, D.; Zhang, J.; Graff, G.L.; et al. Ternary self-assembly of ordered metal oxide-graphene nanocomposites for electrochemical energy storage. ACS Nano 2010, 4, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Qu, S.; Yang, M.; Zhang, J. Materials and electrode engineering of high capacity anodes in lithium ion batteries. J. Power Sources 2020, 450, 227697. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.; Sougrati, M.T.; Feng, Z.; Leconte, Y.; Fisher, A.; Srinivasan, M.; Xu, Z. A review on design strategies for carbon based metal oxides and sulfides nanocomposites for high performance Li and Na ion battery anodes. Adv. Energy Mater. 2017, 7, 1601424. [Google Scholar] [CrossRef]
- Mo, R.; Lei, Z.; Sun, K.; Rooney, D. Facile synthesis of anatase TiO2 quantum-dot/graphene-nanosheet composites with enhanced electrochemical performance for lithium-ion batteries. Adv. Mater. 2014, 26, 2084–2088. [Google Scholar] [CrossRef]
- Fu, W.; Li, Y.; Chen, M.; Hu, Y.; Liu, B.; Zhang, K.; Zhan, C.; Zhang, M.; Shen, Z. An orderly arrangement of layered carbon nanosheet/TiO2 nanosheet stack with superior artificially interfacial lithium pseudocapacity. J. Power Sources 2020, 468, 228363. [Google Scholar] [CrossRef]
- Hu, Y.; Li, Y.; Cheng, J.; Chen, M.; Fu, W.; Liu, B.; Zhang, M.; Shen, Z. Intercalation of carbon nanosheet into layered TiO2 grain for highly interfacial lithium storage. ACS Appl. Mater. Int. 2020, 12, 21709–21719. [Google Scholar] [CrossRef]
- Yao, Z.; Yin, H.; Zhou, L.; Pan, G.; Wang, Y.; Xia, X.; Wu, J.; Wang, X.; Tu, J. Ti3+ self-doped li4Ti5O12 anchored on N-doped carbon nanofiber arrays for ultrafast lithium-ion storage. Small 2019, 15, 1905296. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, L.; Lu, X.; Liu, L.; Liu, L.; Sun, X.; Yin, Y.; Oswald, S.; Zou, Z.; Ding, F.; et al. Tunable pseudocapacitance in 3d TiO2-δ nanomembranes enabling superior lithium storage performance. ACS Nano 2017, 11, 821–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Song, W.; Hou, H.; Zhang, Y.; Jing, M.; Jia, X.; Ji, X. Ti3+ self-doped dark rutile TiO2 ultrafine nanorods with durable high-rate capability for lithium-ion batteries. Adv. Funct. Mater. 2015, 25, 6793–6801. [Google Scholar] [CrossRef]
- Wang, X.; Sun, G.; Routh, P.; Kim, D.H.; Huang, W.; Chen, P. Heteroatom-doped graphene materials: Syntheses, properties and applications. Chem. Soc. Rev. 2014, 43, 7067–7098. [Google Scholar] [CrossRef] [Green Version]
- Czerw, R.; Terrones, M.; Charlier, J.C.; Blase, X.; Foley, B.; Kamalakaran, R.; Grobert, N.; Terrones, H.; Tekleab, D.; Ajayan, P.M.; et al. Identification of electron donor states in N-doped carbon nanotubes. Nano Lett. 2001, 1, 457–460. [Google Scholar] [CrossRef] [Green Version]
- Nevidomskyy, A.H.; Csanyi, G.; Payne, M.C. Chemically active substitutional nitrogen impurity in carbon nanotubes. Phys. Rev. Lett. 2003, 91, 105502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latil, S.; Roche, S.; Mayou, D.; Charlier, J.C. Mesoscopic transport in chemically doped carbon nanotubes. Phys. Rev. Lett. 2004, 92, 256805. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, M.; Wickramaratne, N.P.; Jaroniec, M.; Dou, S.; Dai, L. High-performance sodium ion batteries based on three-dimensional anode from nitrogen-doped graphene foams. Adv. Mater. 2015, 27, 2042–2048. [Google Scholar] [CrossRef]
- Xiao, K.; Liu, Y.; Hu, P.; Yu, G.; Sun, Y.; Zhu, D. N-type field-effect transistors made of an individual nitrogen-doped multiwalled carbon nanotube. J. Am. Chem. Soc. 2005, 127, 8614–8617. [Google Scholar] [CrossRef]
- Qiao, L.; Zheng, W.; Xu, H.; Zhang, L.; Jiang, Q. Field emission properties of N-doped capped single-walled carbon nanotubes: A first-principles density-functional study. J. Chem. Phys. 2007, 126, 164702. [Google Scholar] [CrossRef]
- Zhong, Z.; Lee, G.; Mo, C.; Hong, S.; Kang, J. Tailored field-emission property of patterned carbon nitride nanotubes by a selective doping of substitutional N (sN) and pyridine-like N (pN) atoms. Chem. Mater. 2007, 19, 2918–2920. [Google Scholar] [CrossRef]
- Yang, S.; Shin, W.; Kang, J. The nature of graphite- and pyridinelike nitrogen conFigureurations in carbon nitride nanotubes: Dependence on diameter and helicity. Small 2008, 4, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Tan, Z.; Zhang, M.; Li, L.; Wang, Y.; Guan, L.; Tan, X.; Pan, L.; Fang, H.; Wu, M. Facile and cost-effective manipulation of hierarchical carbon nanosheets for pseudocapacitive lithium/potassium storage. Carbon 2020, 165, 296–305. [Google Scholar] [CrossRef]
- Zheng, F.; Yang, Y.; Chen, Q. High lithium anodic performance of highly nitrogen-doped porous carbon prepared from a metal-organic framework. Nat. Commun. 2014, 5, 5261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zhou, Z.; Wang, L. CN(x) nanotubes with pyridinelike structures: P-type semiconductors and Li storage materials. J. Chem. Phys. 2008, 129, 104703. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xue, X.; Liu, P.; Wang, C.; Yi, X.; Hu, Y.; Ma, L.; Zhu, G.; Chen, R.; Chen, T.; et al. Atomic substitution enabled synthesis of vacancy-rich two-dimensional black TiO2-x nanoflakes for high-performance rechargeable magnesium batteries. ACS Nano 2018, 12, 12492–12502. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Jo, Y.; Prasanna, K.; Santhoshkumar, P.; Joe, Y.; Vediappan, K.; Gnanamuthu, R.; Lee, C. Bandgap tuned and oxygen vacant TiO2−x anode materials with enhanced electrochemical properties for lithium ion batteries. J. Ind. Eng. Chem. 2019, 71, 177–183. [Google Scholar] [CrossRef]
- Ye, K.; Li, K.; Lu, Y.; Guo, Z.; Ni, N.; Liu, H.; Huang, Y.; Ji, H.; Wang, P. An overview of advanced methods for the characterization of oxygen vacancies in materials. TrAC-Trend. Anal. Chem. 2019, 116, 102–108. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, G.; Wang, M.; Li, J.; Lu, T.; Pan, L. Covalent-organic-frameworks derived N-doped porous carbon materials as anode for superior long-life cycling lithium and sodium ion batteries. Carbon 2017, 116, 686–694. [Google Scholar] [CrossRef]
- Reddy, A.L.M.; Srivastava, A.; Gowda, S.R.; Gullapalli, H.; Dubey, M.; Ajayan, P.M. Synthesis of nitrogen-doped graphene films for lithium battery application. ACS Nano 2010, 4, 6337–6342. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Shao, X.; Cao, D. Nitrogen-doped graphene nanosheets as anode materials for lithium ion batteries: A first-principles study. J. Mater. Chem. 2012, 22, 8911–8915. [Google Scholar] [CrossRef]
- Shah, S.A.; Shen, X.; Xie, M.; Zhu, G.; Ji, Z.; Zhou, H.; Xu, K.; Yue, X.; Yuan, A.; Zhu, J.; et al. Nickel@nitrogen-doped carbon@MoS2 nanosheets: An efficient electrocatalyst for hydrogen evolution reaction. Small 2019, 15, 1804545. [Google Scholar] [CrossRef]
- Wang, B.; Xu, B.; Liu, T.; Liu, P.; Guo, C.; Wang, S.; Wang, Q.; Xiong, Z.; Wang, D.; Zhao, X. Mesoporous carbon-coated LiFePO4 nanocrystals co-modified with graphene and Mg2+ doping as superior cathode materials for lithium ion batteries. Nanoscale 2014, 6, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, C.; Ji, G.; Lee, J. Nitrogen-doped carbon-encapsulation of Fe3O4 for increased reversibility in Li+ storage by the conversion reaction. J. Mater. Chem. 2012, 22, 7845–7850. [Google Scholar] [CrossRef]
- Wood, K.N.; O’Hayre, R.; Pylypenko, S. Recent progress on nitrogen/carbon structures designed for use in energy and sustainability applications. Energy Environ. Sci. 2014, 7, 1212–1249. [Google Scholar] [CrossRef]
- Ding, Z.; Zhao, L.; Suo, L.; Jiao, Y.; Meng, S.; Hu, Y.; Wang, Z.; Chen, L. Towards understanding the effects of carbon and nitrogen-doped carbon coating on the electrochemical performance of Li4Ti5O12 in lithium ion batteries: A combined experimental and theoretical study. Phys. Chem. Chem. Phys. 2011, 13, 15127–15133. [Google Scholar] [CrossRef]
- Das, D.; Kim, S.; Lee, K.R.; Singh, A.K. Li diffusion through doped and defected graphene. Phys. Chem. Chem. Phys. 2013, 15, 15128–15134. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.; Chen, Q. Improved performance of graphene doped with pyridinic n for Li-ion battery: A density functional theory model. Phys. Chem. Chem. Phys. 2013, 15, 12982–12987. [Google Scholar] [CrossRef]
- Lee, J.; Li, Z.; Zhu, L.; Xie, S.; Cui, X. Ti3+ self-doped TiO2 via facile catalytic reduction over Al(acac)3 with enhanced photoelectrochemical and photocatalytic activities. Appl. Catal. B-Environ. 2018, 224, 715–724. [Google Scholar] [CrossRef]
- Duan, J.; Hou, H.; Liu, X.; Yan, C.; Liu, S.; Meng, R.; Hao, Z.; Yao, Y.; Liao, Q. In situ Ti3+-doped TiO2 nanotubes anode for lithium ion battery. J. Porous Mater. 2016, 23, 837–843. [Google Scholar] [CrossRef]
- Wang, J.; Polleux, J.; Lim, J.; Dunn, B. Pseudocapacitive contributions to electrochemical energy storage in TiO2 (anatase) nanoparticles. J. Phys. Chem. C 2007, 111, 14925–14931. [Google Scholar] [CrossRef]
- Brezesinski, T.; Wang, J.; Polleux, J.; Dunn, B.; Tolbert, S.H. Templated nanocrystal-based porous TiO2 films for next-generation electrochemical capacitors. J. Am. Chem. Soc. 2009, 131, 1802–1809. [Google Scholar] [CrossRef] [PubMed]
- Augustyn, V.; Come, J.; Lowe, M.A.; Kim, J.W.; Taberna, P.L.; Tolbert, S.H.; Abruna, H.D.; Simon, P.; Dunn, B. High-rate electrochemical energy storage through Li+ intercalation pseudocapacitance. Nat. Mater. 2013, 12, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Duan, H.; Xu, B.; Zhang, L.; Hu, Y.; Zhao, C.; Wang, Z.; Chen, L.; Yang, Y. Lithium storage in nitrogen-rich mesoporous carbon materials. Energy Environ. Sci. 2012, 5, 7950–7955. [Google Scholar] [CrossRef]
- Yan, Y.; Yin, Y.; Xin, S.; Guo, Y.; Wan, L. Ionothermal synthesis of sulfur-doped porous carbons hybridized with graphene as superior anode materials for lithium-ion batteries. Chem. Commun. (Camb.) 2012, 48, 10663–10665. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Gu, B.; Wang, J.; Li, A.; Zhang, M.; Shen, Z. Tuning the Defects of Two-Dimensional Layered Carbon/TiO2 Superlattice Composite for a Fast Lithium-Ion Storage. Materials 2022, 15, 1625. https://doi.org/10.3390/ma15051625
Liu B, Gu B, Wang J, Li A, Zhang M, Shen Z. Tuning the Defects of Two-Dimensional Layered Carbon/TiO2 Superlattice Composite for a Fast Lithium-Ion Storage. Materials. 2022; 15(5):1625. https://doi.org/10.3390/ma15051625
Chicago/Turabian StyleLiu, Bingheng, Bo Gu, Jingxian Wang, Anchang Li, Ming Zhang, and Zhongrong Shen. 2022. "Tuning the Defects of Two-Dimensional Layered Carbon/TiO2 Superlattice Composite for a Fast Lithium-Ion Storage" Materials 15, no. 5: 1625. https://doi.org/10.3390/ma15051625






