Towards Replacing Titanium with Copper in the Bipolar Plates for Proton Exchange Membrane Water Electrolysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Deposition of Coatings by VPS
2.2. Physico-Chemical Characterization of Coatings
2.3. Half-Cell Corrosion Testing
2.4. Post-Test Analytic
2.5. PEMWE Test
3. Results and Discussion
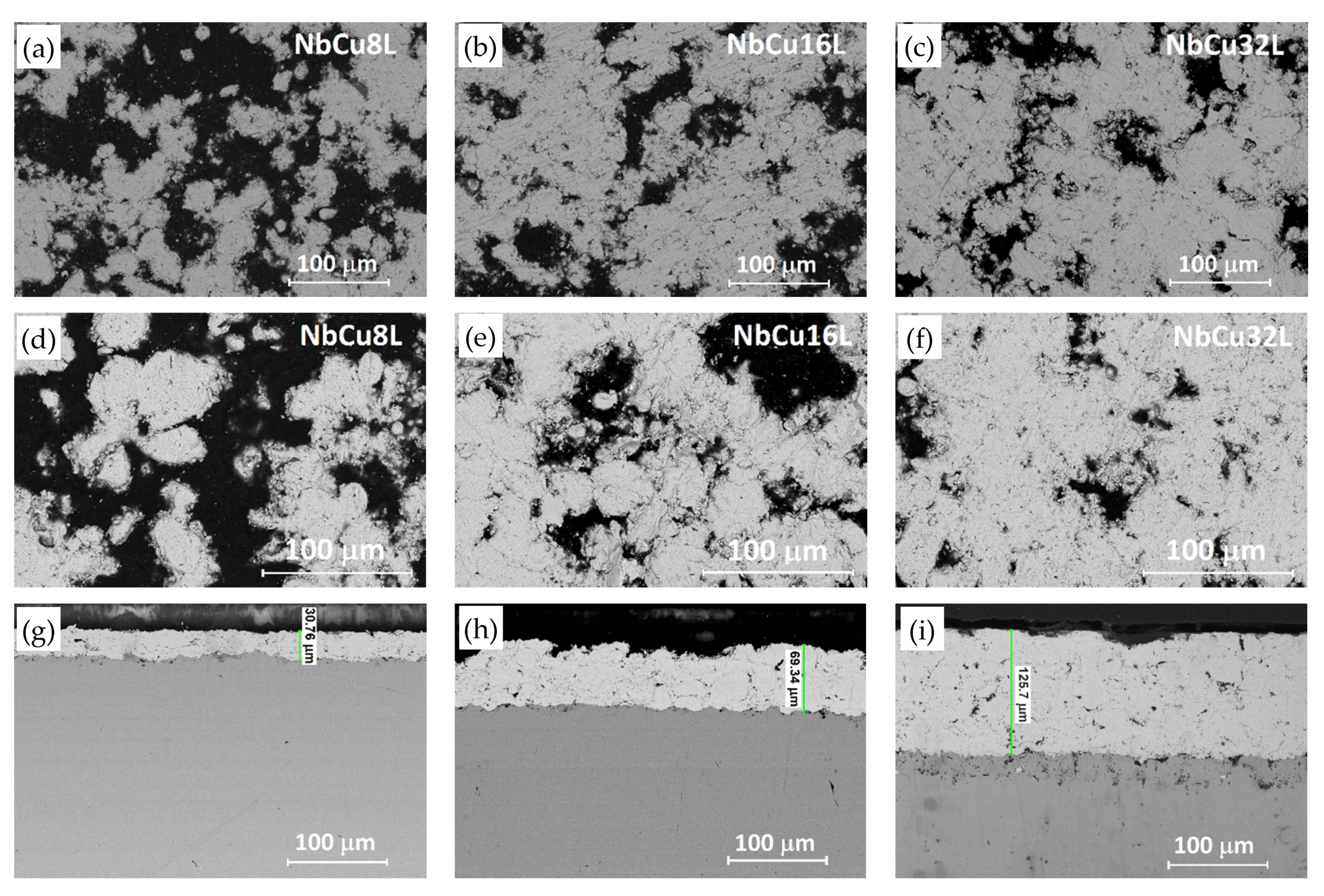
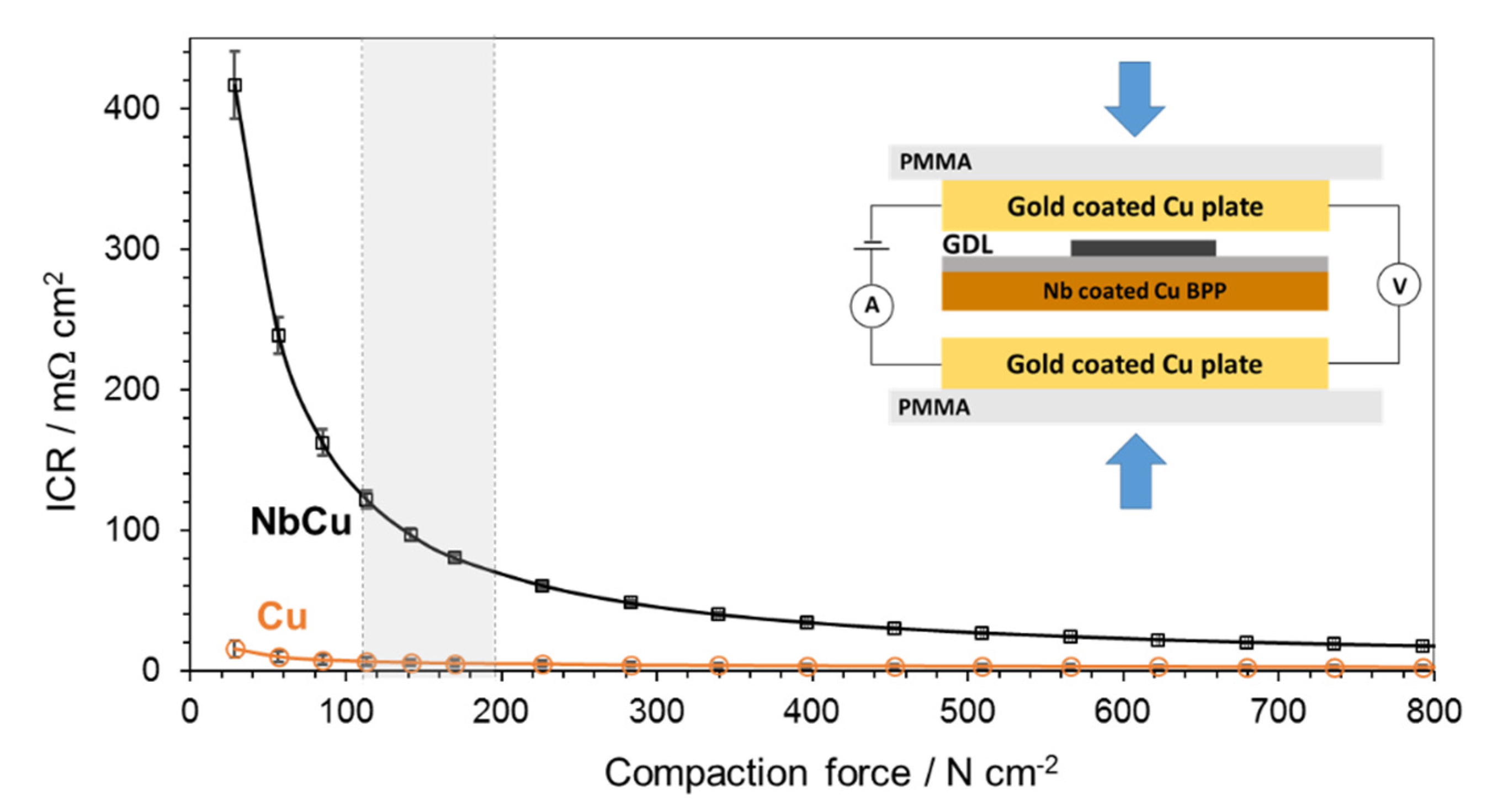
3.1. Physico-Chemical Characterization of Coatings
3.2. Half-Cell Corrosion Testing
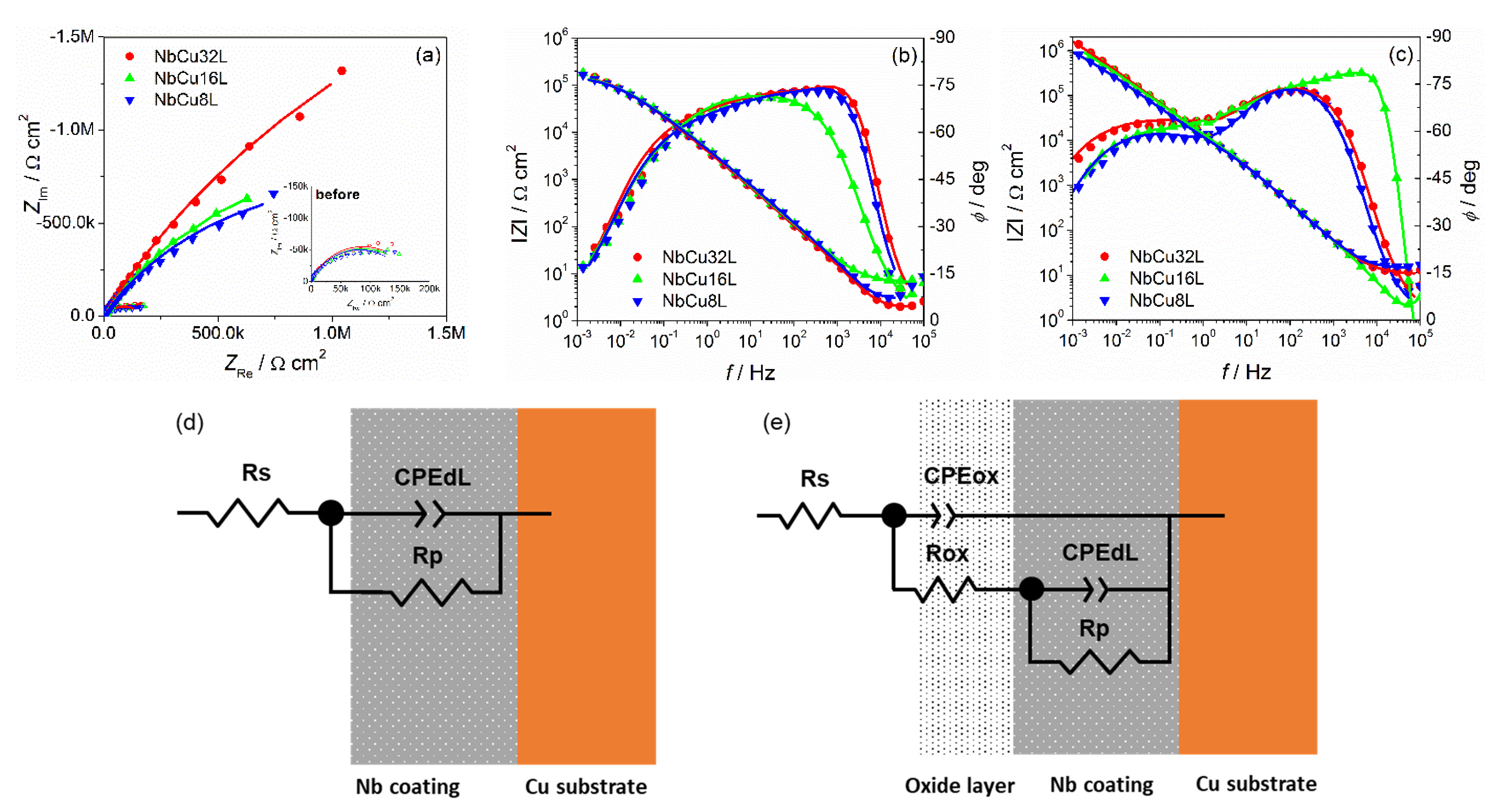
3.3. Electrochemical Impedance Spectroscopy
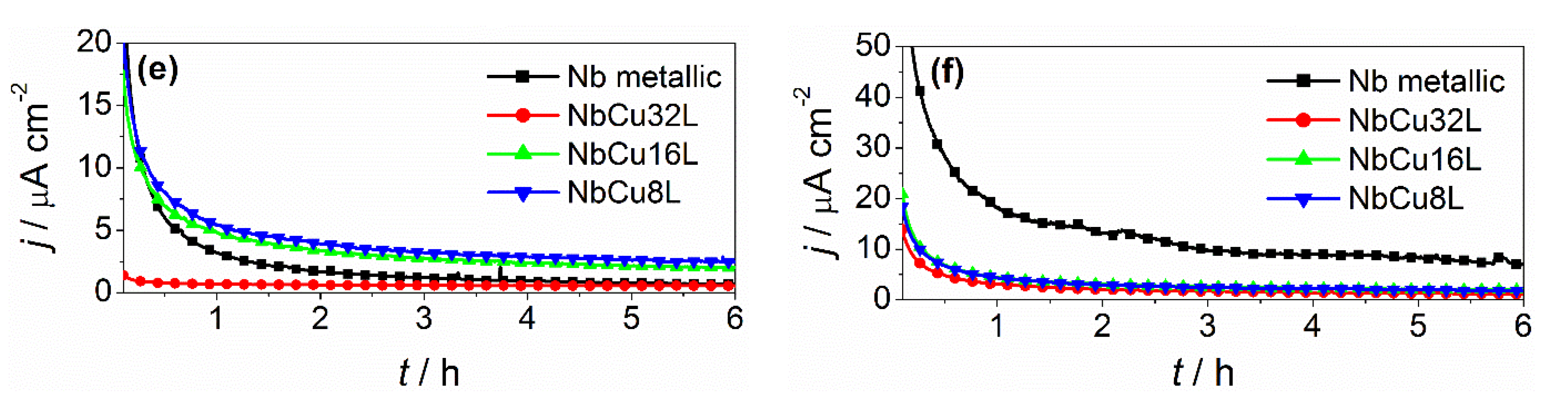
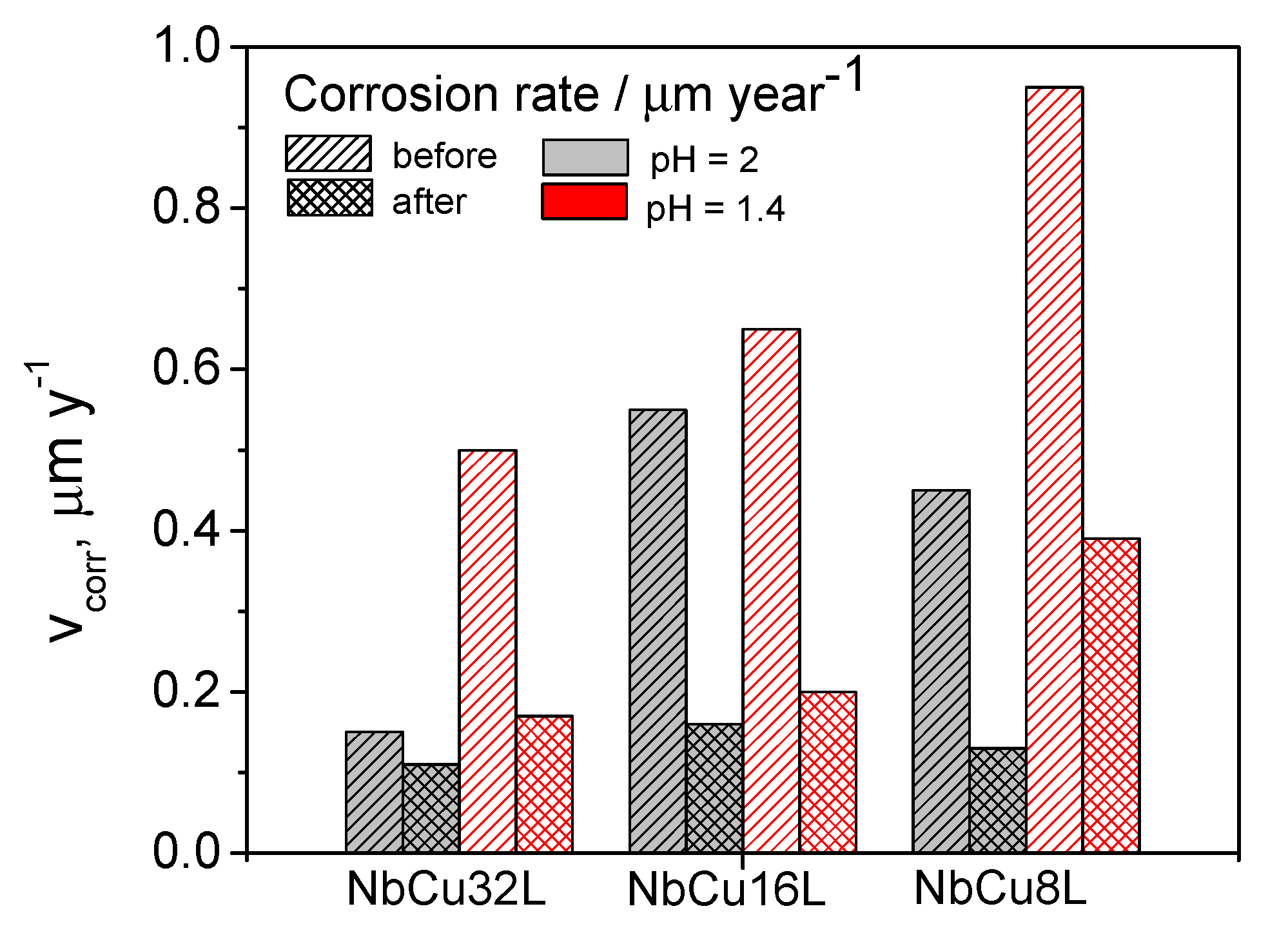
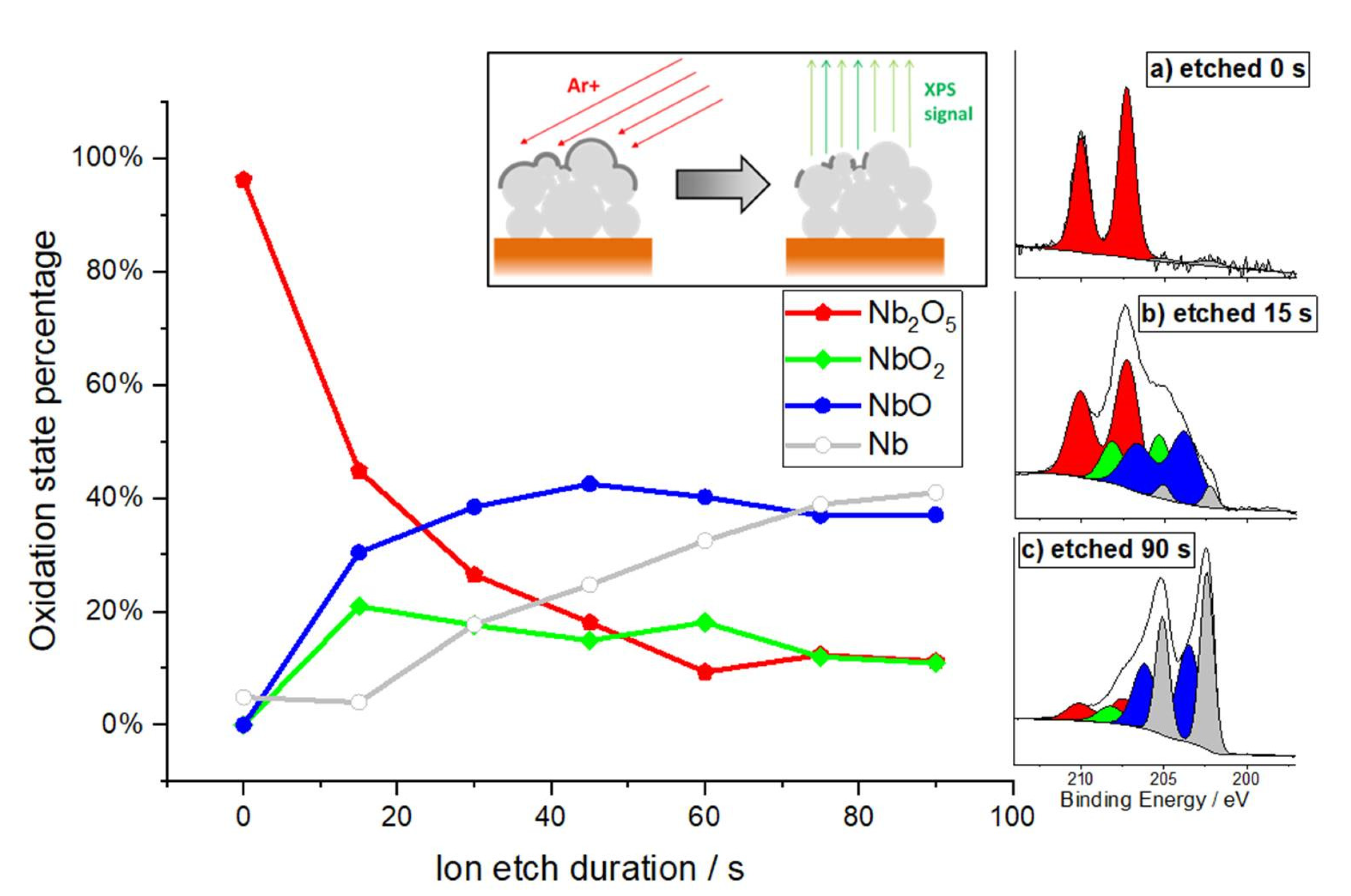
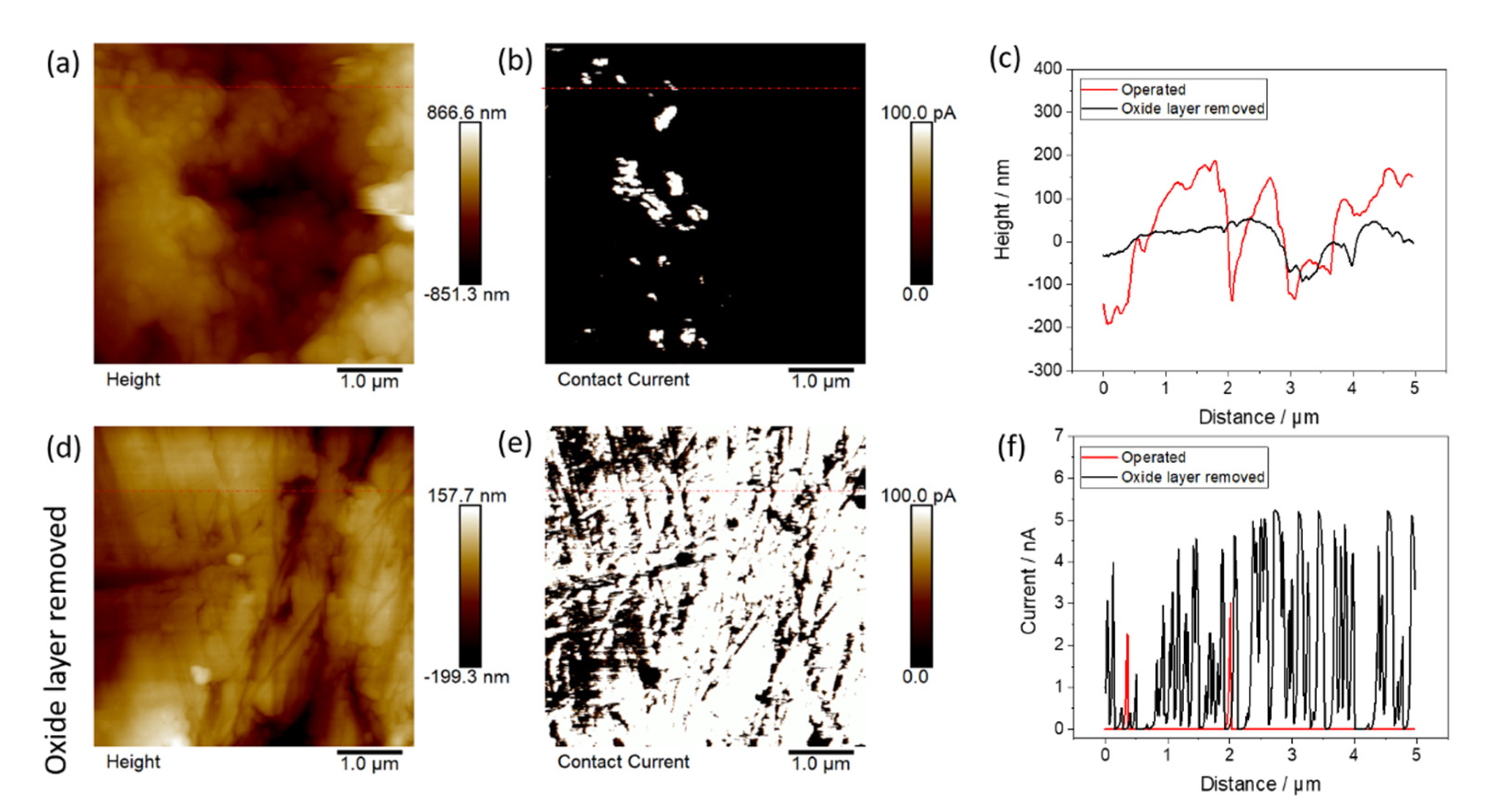
3.4. Post-Test Analytic
3.5. PEMWE Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Press, R.J.; Santhanam, K.S.V.; Miri, M.J.; Bailey, A.V.; Takacs, G.A. Introduction to Hydrogen Technology; John Wiley & Sons: Hoboken, NJ, USA, 2009; p. 211. [Google Scholar]
- Dincer, I.; Acar, C. Review and evaluation of hydrogen production methods for better sustainability. Int. J. Hydrogen Energy 2015, 40, 11094–11111. [Google Scholar] [CrossRef]
- Wittstadt, U. Electrolysis: Hydrogen production using electricity. In Hydrogen as a Future Energy Carrier; Züttel, A., Borgschulte, A., Schlapbach, L., Eds.; Wiley-VCH: Weinheim, Germany, 2008; p. 155. [Google Scholar]
- Millet, P.; Ngameni, R.; Grigoriev, S.A.; Mbemba, N.; Brisset, F.; Ranjbari, A.; Etiévant, C. PEM water electrolyzers: From electrocatalysis to stack development. Int. J. Hydrogen Energy 2010, 35, 5043–5052. [Google Scholar] [CrossRef]
- Langemann, M.; Fritz, D.L.; Müller, M.; Stolten, D. Validation and characterization of suitable materials for bipolar plates in PEM water electrolysis. Int. J. Hydrogen Energy 2015, 40, 11385–11391. [Google Scholar] [CrossRef]
- Lædre, S.; Kongstein, O.E.; Oedegaard, A.; Karoliussen, H.; Seland, F. Materials for Proton Exchange Membrane water electrolyzer bipolar plates. Int. J. Hydrogen Energy 2017, 42, 2713–2723. [Google Scholar] [CrossRef]
- Pantò, F.; Siracusano, S.; Briguglio, N.; Aricò, A.S. Durability of a recombination catalyst-based membrane-electrode assembly for electrolysis operation at high current density. Appl. Energy 2020, 279, 115809. [Google Scholar] [CrossRef]
- Siracusano, S.; Van Dijk, N.; Backhouse, R.; Merlo, L.; Baglio, V.; Aricò, A.S. Degradation issues of PEM electrolysis MEAs. Renew. Energy 2018, 123, 52–57. [Google Scholar] [CrossRef]
- Siracusano, S.; Trocino, S.; Briguglio, N.; Pantò, F.; Aricò, A.S. Analysis of performance degradation during steady-state and load-thermal cycles of proton exchange membrane water electrolysis cells. J. Power Sources 2020, 468, 228390. [Google Scholar] [CrossRef]
- Dodwell, J.; Maier, M.; Majasan, J.; Jervis, R.; Castanheira, L.; Shearing, P.; Hinds, G.; Brett, D.J.L. Open-circuit dissolution of platinum from the cathode in polymer electrolyte membrane water electrolysers. J. Power Sources 2021, 498, 229937. [Google Scholar] [CrossRef]
- Burch, M.J.; Lewinski, K.A.; Buckett, M.I.; Luopa, S.; Sun, F.; Olson, E.J.; Steinbach, A.J. A novel work-flow to study Ir electrode thinning and dissolution in proton exchange membrane water electrolyzers. J. Power Sources 2021, 500, 229978. [Google Scholar] [CrossRef]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Chen, Z.; Duan, X.; Wei, W.; Wang, S.; Ni, B.-J. Electrocatalysts for acidic oxygen evolution reaction: Achievements and perspectives. Nano Energy 2020, 78, 105392. [Google Scholar] [CrossRef]
- Siracusano, S.; Baglio, V.; Grigoriev, S.A.; Merlo, L.; Fateev, V.N.; Aricò, A.S. The influence of iridium chemical oxidation state on the performance and durability of oxygen evolution catalysts in PEM electrolysis. J. Power Sources 2017, 366, 105–114. [Google Scholar] [CrossRef]
- Rozain, C.; Mayousse, E.; Guillet, N.; Millet, P. Influence of iridium oxide loadings on the performance of PEM water electrolysis cells: Part I–Pure IrO2-based anodes. Appl. Catal. B 2016, 182, 153–160. [Google Scholar] [CrossRef]
- Solà-Hernández, L.; Claudel, F.; Maillard, F.; Beauger, C. Doped tin oxide aerogels as oxygen evolution reaction catalyst supports. Int. J. Hydrogen Energy 2019, 44, 24331–24341. [Google Scholar] [CrossRef]
- Saveleva, V.A.; Wang, L.; Kasian, O.; Batuk, M.; Hadermann, J.; Gallet, J.-J.; Bournel, F.; Alonso-Vante, N.; Ozouf, G.; Beauger, C.; et al. Insight into the Mechanisms of High Activity and Stability of Iridium Supported on Antimony-Doped Tin Oxide Aerogel for Anodes of Proton Exchange Membrane Water Electrolyzers. ACS Catal. 2020, 10, 2508–2516. [Google Scholar] [CrossRef]
- Genova-Koleva, R.V.; Alcaide, F.; Álvarez, G.; Cabot, P.L.; Grande, H.-J.; Martínez-Huerta, M.V.; Miguel, O. Supporting IrO2 and IrRuOx nanoparticles on TiO2 and Nb-doped TiO2 nanotubes as electrocatalysts for the oxygen evolution reaction. J. Energy Chem. 2019, 34, 227–239. [Google Scholar] [CrossRef] [Green Version]
- Böhm, L.; Näther, J.; Underberg, M.; Kazamer, N.; Holtkotte, L.; Rost, U.; Marginean, G.; Wirkert, F.; Brodmann, M.; Hülser, T.; et al. Pulsed electrodeposition of iridium catalyst nanoparticles on titanium suboxide supports for application in PEM electrolysis. Mat. Today Proc. 2021, 45, 4254–4259. [Google Scholar] [CrossRef]
- Kim, I.G.; Lim, A.; Jang, J.H.; Lee, K.-Y.; Nah, I.W.; Park, S. Leveraging metal alloy-hybrid support interaction to enhance oxygen evolution kinetics and stability in proton exchange membrane water electrolyzers. J. Power Sources 2021, 501, 230002. [Google Scholar] [CrossRef]
- Jiang, G.; Yu, H.; Hao, J.; Chi, J.; Fan, Z.; Yao, D.; Qin, B.; Shao, Z. An effective oxygen electrode based on Ir0.6Sn0.4O2 for PEM water electrolyzers. J. Energy Chem. 2019, 39, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Buttler, A.; Spliethoff, H. Current status of water electrolysis for energy storage, grid balancing and sector coupling via power-to-gas and power-to-liquids: A review. Renew. Sust. Energ. Rev. 2018, 82, 2440–2454. [Google Scholar] [CrossRef]
- Grigoriev, S.A.; Fateev, V.N.; Bessarabov, D.G.; Millet, P. Current status, research trends, and challenges in water electrolysis science and technology. Int. J. Hydrogen Energy 2020, 45, 26036–26058. [Google Scholar] [CrossRef]
- Bertuccioli, L.; Chan, A.; Hart, D.; Lehner, F.; Madden, B.; Standen, E. Study on Development of Water Electrolysis in the EU by E4tech Sàrl with Element Energy Ltd. for the Fuel Cells and Hydrogen Joint Undertaking. 2014. Available online: https://www.fch.europa.eu/sites/default/files/study%20electrolyser_0-Logos_0_0.pdf (accessed on 10 January 2022).
- Ayers, K.E.; Anderson, E.B.; Capuano, C.; Carter, B.; Dalton, L.; Hanlon, G.; Manco, J.; Niedzwiecki, M. Research Advances towards Low Cost, High Efficiency PEM Electrolysis. ECS Transactions 2010, 33, 3–15. [Google Scholar] [CrossRef]
- Gago, A.S.; Ansar, S.A.; Saruhan, B.; Schultz, U.; Lettenmeier, P.; Cañas, N.A.; Gazdzicki, P.; Morawietz, T.; Hiesgen, R.; Arnold, J.; et al. Protective coatings on stainless steel bipolar plates for proton exchange membrane (PEM) electrolysers. J. Power Sources 2016, 307, 815–825. [Google Scholar] [CrossRef] [Green Version]
- Lettenmeier, P.; Wang, R.; Abouatallah, R.; Burggraf, F.; Gago, A.S.; Friedrich, K.A. Coated Stainless Steel Bipolar Plates for Proton Exchange Membrane Electrolyzers. J. Electrochem. Soc. 2016, 163, F3119. [Google Scholar] [CrossRef]
- Lettenmeier, P.; Wang, R.; Abouatallah, R.; Saruhan, B.; Freitag, O.; Gazdzicki, P.; Morawietz, T.; Hiesgen, R.; Gago, A.S.; Friedrich, K.A. Low-Cost and Durable Bipolar Plates for Proton Exchange Membrane Electrolyzers. Sci. Rep. 2017, 7, 44035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.-S.; Lee, I.-S.; Choi, J.-Y.; Jun, S.; Kim, D.; Cha, B.-C.; Kim, D.-W. Corrosion Behavior of Niobium-Coated 316L Stainless Steels as Metal Bipolar Plates for Polymer Electrolyte Membrane Fuel Cells. Materials 2021, 14, 4972. [Google Scholar] [CrossRef] [PubMed]
- Atapour, M.; Rajaei, V.; Trasatti, S.; Casaletto, M.P.; Chiarello, G.L. Thin Niobium and Niobium Nitride PVD Coatings on AISI 304 Stainless Steel as Bipolar Plates for PEMFCs. Coatings 2020, 10, 889. [Google Scholar] [CrossRef]
- Shi, K.; Li, X.; Zhao, Y.; Li, W.-W.; Wang, S.-B.; Xie, X.-F.; Yao, L.; Jensen, J.O.; Li, Q.-F. Corrosion Behavior and Conductivity of TiNb and TiNbN Coated Steel for Metallic Bipolar Plates. Appl. Sci. 2019, 9, 2568. [Google Scholar] [CrossRef] [Green Version]
- Arsova, I.L.; Prusi, A.R.; Arsov, L.D. Ellipsometric study of anodic oxide films formed on niobium surfaces. J. Solid State Electrochem. 2003, 7, 217–222. [Google Scholar] [CrossRef]
- Arsova, I.; Prusi, A.; Grčev, T.; Arsov, L. Electrochemical characterization of the passive films formed on niobium surfaces in H2SO4 solutions. J. Serb. Chem. Soc. 2006, 71, 177–187. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J. Is niobium more corrosion-resistant than commercially pure titanium in fluoride-containing artificial saliva? Electrochim. Acta 2017, 233, 151–166. [Google Scholar] [CrossRef]
- Li, N.; Araya, S.S.; Cui, X.; Kær, S.K. The effects of cationic impurities on the performance of proton exchange membrane water electrolyzer. J. Power Sources 2020, 473, 228617. [Google Scholar] [CrossRef]
- Rasband, W.S. (1997–2005) ImageJ US. National Institutes of Health, Bethesda, Maryland, USA. Available online: http://rsb.info.nih.gov/ij/ (accessed on 10 January 2022).
- Hesse, R.; Chassé, T.; Szargan, R. Peak shape analysis of core level photoelectron spectra using UNIFIT for WINDOWS. Fresenius J. Anal. Chem. 1999, 365, 48–54. [Google Scholar] [CrossRef]
- Brodmann, M.; Greda, M.; Mutascu, C.; Roth, J. Energy Conversion Apparatus, in Particular Fuel Cell Stack or Electrolyzer. International Patent WO 002011069625 A1, 2011. [Google Scholar]
- Rost, U.; Roth, J.; Brodmann, M. Modular Polymer Electrolyte Membrane Fuel Cell and Electrolyser Stack Design with Hydraulic Compression in Power and Energy Student Summit, Dortmund, Germany. 2015. Available online: https://eldorado.tu-dortmund.de/handle/2003/33986 (accessed on 10 January 2022).
- Wirkert, F.J.; Roth, J.; Jagalski, S.; Neuhaus, P.; Rost, U.; Brodmann, M. A modular design approach for PEM electrolyser systems with homogeneous operation conditions and highly efficient heat management. Int. J. Hydrogen Energy 2020, 45, 1226–1235. [Google Scholar] [CrossRef]
- JRC, EU Harmonised Polarisation Curve Test Method for Low Temperature Water Electrolysis, European Commission. 2018. Available online: https://www.fch.europa.eu/sites/default/files/Polarisation%20curve%20JRC.PDF (accessed on 10 January 2022).
- Chen, D.; Pegler, A.; Dwivedi, G.; De Wet, D.; Dorfman, M. Thermal Cycling Behavior of Air Plasma-Sprayed and Low-Pressure Plasma-Sprayed Environmental Barrier Coatings. Coatings 2021, 11, 868. [Google Scholar] [CrossRef]
- Mrdak, M.; Lačnjevac, Č.; Rakin, M. Mechanical and structural features of Nb coating layers deposited on steel substrates in a vacuum chamber. ZASTITA MATERIJALA 2018, 59, 167–172. [Google Scholar] [CrossRef]
- Kellenberger, A.; Duca, D.; Vaszilcsin, N.; Craciunescu, C.M. Electrochemical Evaluation of Niobium Corrosion Resistance in Simulated Anodic PEM Electrolyzer Environment. Int. J. Electrochem. Sci. 2020, 15, 10664–10673. [Google Scholar] [CrossRef]
- Liu, Y.; Min, L.; Zhang, W.; Wang, Y. High-Performance Graphene Coating on Titanium Bipolar Plates in Fuel Cells via Cathodic Electrophoretic Deposition. Coatings 2021, 11, 437. [Google Scholar] [CrossRef]
- U.S. Department of Energy. Hydrogen and Fuel cell Technologies Office Multi-Year Research, Development, and Demonstration Plan. 2014. Available online: https://www.energy.gov/sites/default/files/2017/05/f34/fcto_myrdd_fuel_cells.pdf (accessed on 10 January 2022).
- Young, L. Anodic oxide films on niobium: Thickness, dielectric constant, dispersion, reflection minima, formation field strength, and surface area. Can. J. Chem. 1960, 38, 1141–1147. [Google Scholar] [CrossRef]
- Seah, M.P.; Dench, W.A. Quantitative electron spectroscopy of surfaces: A standard data base for electron inelastic mean free path is solids. Surf. Interf. Anal. 1979, 1, 2–11. [Google Scholar] [CrossRef]
- Matsunami, N.; Yamamura, Y.; Itikawa, Y.; Itoh, N.; Kazumata, Y.; Miyagawa, S.; Morita, K.; Shimizu, R.; Tawara, H. Energy Dependence of the Yields of Ion-Induced Sputtering of Monatomic Solids, IPPJ-AM-32; Institute of Plasma Physics, Nagoya University: Nagoya, Japan, 1983. [Google Scholar]
- Hackemüller, F.J.; Borgardt, E.; Panchenko, O.; Müller, M.; Bram, M. Manufacturing of Large-Scale Titanium-Based Porous Transport Layers for Polymer Electrolyte membrane Electrolysis by Tape Casting. Adv. Eng. Mater. 2019, 21, 1801201. [Google Scholar] [CrossRef]
- Lettenmeier, P.; Kolb, S.; Sata, N.; Fallisch, A.; Zielke, L.; Thiele, S.; Gago, A.S.; Friedrich, K.A. Comprehensive investigation of novel pore-graded gas diffusion layers for high-performance and cost-effective proton exchange membrane electrolyzers. Energy Environ. Sci. 2017, 10, 2521–2533. [Google Scholar] [CrossRef] [Green Version]


| Chemical Composition | Weight |
|---|---|
| Resin | |
| Phenol, polymer with formaldehyde, glycidyl ether | 60–90% |
| Alkyl (C12–14) glycidyl ether | 5–15% |
| Oxirane, [((2-ethylhexyl)oxy)methyl] | 0–10% |
| Bisphenol A–Epichlorohydrin polymer | 0–5% |
| Hardener | |
| 1,2-Cyclohexanediamine | 40–70% |
| Poly[oxy(methyl-1,2-ethanediyl)], alpha-hydro-omega-(2-aminomethylethoxy)- ether with 2-ethyl-2-(hydroxymethyl)-1,3-propanediol (3:1) | 20–50% |
| 2,2′,2″-nitrilotriethanol | 0–5% |
| Piperazine | 0–2% |
| Corrosion Parameters | Nb Metallic | NbCu8L | NbCu16L | NbCu32L | ||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | |
| Ecorr (V) | 0.146 | 0.220 | 0.196 | 0.362 | 0.217 | 0.413 | 0.126 | 0.243 |
| jcorr (µA cm−2) | 1.20 | 0.15 | 0.064 | 0.018 | 0.078 | 0.023 | 0.021 | 0.015 |
| ba (mV decade−1) | 194 | 362 | 151 | 227 | 162 | 189 | 164 | 199 |
| bc (mV decade−1) | −269 | −121 | −162 | −112 | −163 | −126 | −111 | −115 |
| RP (kΩ cm2) | 40.8 | 262.5 | 530.2 | 1809.2 | 452.3 | 1427.2 | 1473.3 | 1956.8 |
| Corrosion Parameters | Nb Metallic | NbCu8L | NbCu16L | NbCu32L | ||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | |
| Ecorr (V) | 0.278 | 0.383 | 0.335 | 0.415 | 0.303 | 0.460 | 0.240 | 0.407 |
| jcorr (µA cm−2) | 1.23 | 0.45 | 0.133 | 0.054 | 0.092 | 0.029 | 0.070 | 0.024 |
| ba (mV decade−1) | 176 | 326 | 184 | 342 | 140 | 296 | 163 | 259 |
| bc (mV decade−1) | −289 | −270 | −173 | −121 | −165 | −99 | −141 | −98 |
| RP (kΩ cm2) | 38.6 | 142.5 | 291.1 | 718.7 | 357.5 | 1110.8 | 469.0 | 1286.3 |
| Parameter | Nb Metallic | NbCu8L | NbCu16L | NbCu32L | ||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | |
| RS (Ω) | 11.4 (0.4%) | 10.5 (1.0%) | 2.9 (2.2%) | 14.0 (0.6%) | 6.5 (1.5%) | 10.1 (11.7%) | 2.0 (1.9%) | 10.4 (0.9%) |
| CPE-Tox (F cm−2 sn−1) | 1.08 × 10−4 (0.4%) | 1.74 × 10−5 (0.8%) | 8.70 × 10−6 (2.0%) | 9.65 × 10−6 (1.5%) | 4.12 × 10−5 (5.8%) | 1.08 × 10−5 (1.5%) | 9.73 × 10−6 (1.8%) | 1.01 × 10−5 (1.7%) |
| nox | 0.86 (0.1%) | 0.88 (0.2%) | 1 (fixed) | 0.86 (0.2%) | 0.85 (0.8%) | 0.85 (0.5%) | 1 (fixed) | 0.85 (0.3%) |
| Cox (F cm−2) | 3.63 × 10−5 | 5.37 × 10−6 | 8.70 × 10−6 | 2.26 × 10−6 | 9.44 × 10−6 | 2.18 × 10−6 | 9.73 × 10−6 | 2.01 × 10−6 |
| dox (nm) | 1.0 | 6.7 | 2.5 | 9.5 | 2.5 | 10.9 | 2.7 | 13.0 |
| Rox (kΩ cm2) | 31.6 (0.6%) | 92.8 (0.9%) | 0.07 (19.1%) | 9.6 (4.6%) | 0.05 (fixed) | 17.3 (21.0%) | 0.06 (16.7%) | 12.9 (7.5%) |
| CPE-Tdl (F cm−2 sn−1) | - | - | 5.14 × 10−5 (1.2%) | 1.81 × 10−5 (0.9%) | 1.71 × 10−5 (13.7%) | 1.21 × 10−5 (5.7%) | 5.39 × 10−5 (1.2%) | 1.13 × 10−5 (1.5%) |
| ndl | - | - | 0.67 (0.6%) | 0.63 (0.7%) | 0.47 (8.5%) | 0.63 (4.1%) | 0.69 (0.6%) | 0.65 (0.7%) |
| RP (kΩ cm2) | - | - | 175.8 (2.6%) | 2110.0 (5.8%) | 223.4 (7.7%) | 2744.2 (25.2%) | 195.4 (2.9%) | 6948.4 (9.1%) |
| χ2 | 5.4 × 10−4 | 2.5 × 10−3 | 5.2 × 10−3 | 5.8 × 10−4 | 2.1 × 10−3 | 1.7 × 10−2 | 5.1 × 10−3 | 8.4 × 10−4 |
| Sample | RP-Tafel (kΩ cm2) | RP-EIS (kΩ cm2) | ||||||
|---|---|---|---|---|---|---|---|---|
| Before pH = 2 | After pH = 2 | Before pH = 1.4 | After pH = 1.4 | Before pH = 2 | After pH = 2 | Before pH = 1.4 | After pH = 1.4 | |
| Nb metallic | 40.8 | 262.5 | 38.6 | 142.5 | 30.8 | 259.4 | 31.6 | 92.8 |
| NbCu8L | 530.2 | 1809.2 | 291.1 | 718.7 | 366.4 | 1913.6 | 175.8 | 2110.0 |
| NbCu16L | 452.3 | 1427.2 | 357.5 | 1110.8 | 262.5 | 4537.6 | 223.4 | 2744.2 |
| NbCu32L | 1473.3 | 1956.8 | 469.0 | 1286.3 | 1459.4 | 9008.4 | 195.4 | 6948.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kellenberger, A.; Vaszilcsin, N.; Duca, D.; Dan, M.L.; Duteanu, N.; Stiber, S.; Morawietz, T.; Biswas, I.; Ansar, S.A.; Gazdzicki, P.; et al. Towards Replacing Titanium with Copper in the Bipolar Plates for Proton Exchange Membrane Water Electrolysis. Materials 2022, 15, 1628. https://doi.org/10.3390/ma15051628
Kellenberger A, Vaszilcsin N, Duca D, Dan ML, Duteanu N, Stiber S, Morawietz T, Biswas I, Ansar SA, Gazdzicki P, et al. Towards Replacing Titanium with Copper in the Bipolar Plates for Proton Exchange Membrane Water Electrolysis. Materials. 2022; 15(5):1628. https://doi.org/10.3390/ma15051628
Chicago/Turabian StyleKellenberger, Andrea, Nicolae Vaszilcsin, Delia Duca, Mircea Laurentiu Dan, Narcis Duteanu, Svenja Stiber, Tobias Morawietz, Indro Biswas, Syed Asif Ansar, Pawel Gazdzicki, and et al. 2022. "Towards Replacing Titanium with Copper in the Bipolar Plates for Proton Exchange Membrane Water Electrolysis" Materials 15, no. 5: 1628. https://doi.org/10.3390/ma15051628
APA StyleKellenberger, A., Vaszilcsin, N., Duca, D., Dan, M. L., Duteanu, N., Stiber, S., Morawietz, T., Biswas, I., Ansar, S. A., Gazdzicki, P., Wirkert, F. J., Roth, J., Rost, U., Brodmann, M., Gago, A. S., & Friedrich, K. A. (2022). Towards Replacing Titanium with Copper in the Bipolar Plates for Proton Exchange Membrane Water Electrolysis. Materials, 15(5), 1628. https://doi.org/10.3390/ma15051628







