Systematic Study of Effective Hydrothermal Synthesis to Fabricate Nb-Incorporated TiO2 for Oxygen Reduction Reaction
Abstract
:1. Introduction
2. Materials and Methods
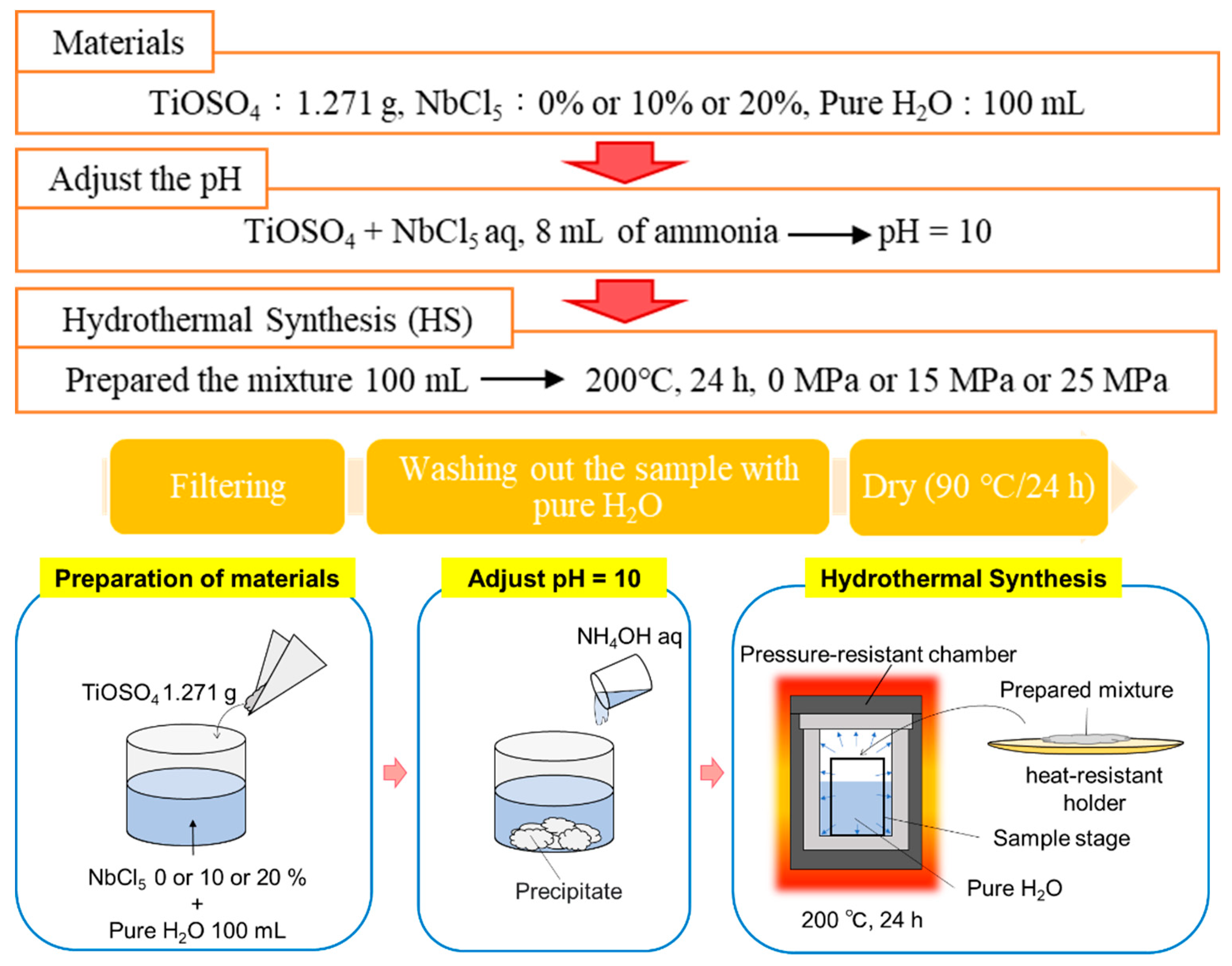
2.1. Preparation of Nb-Incorporated TiO2
2.2. Characterization
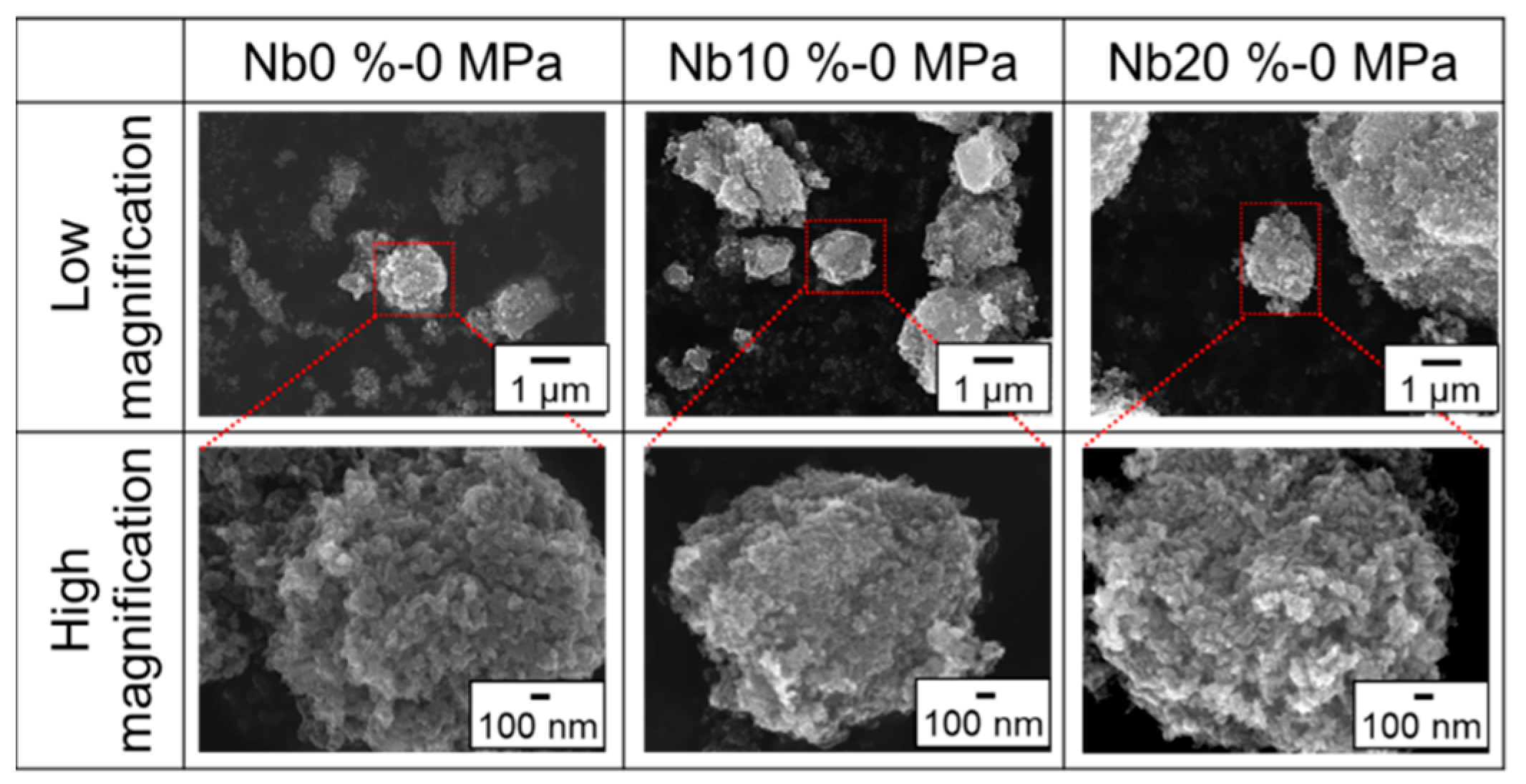
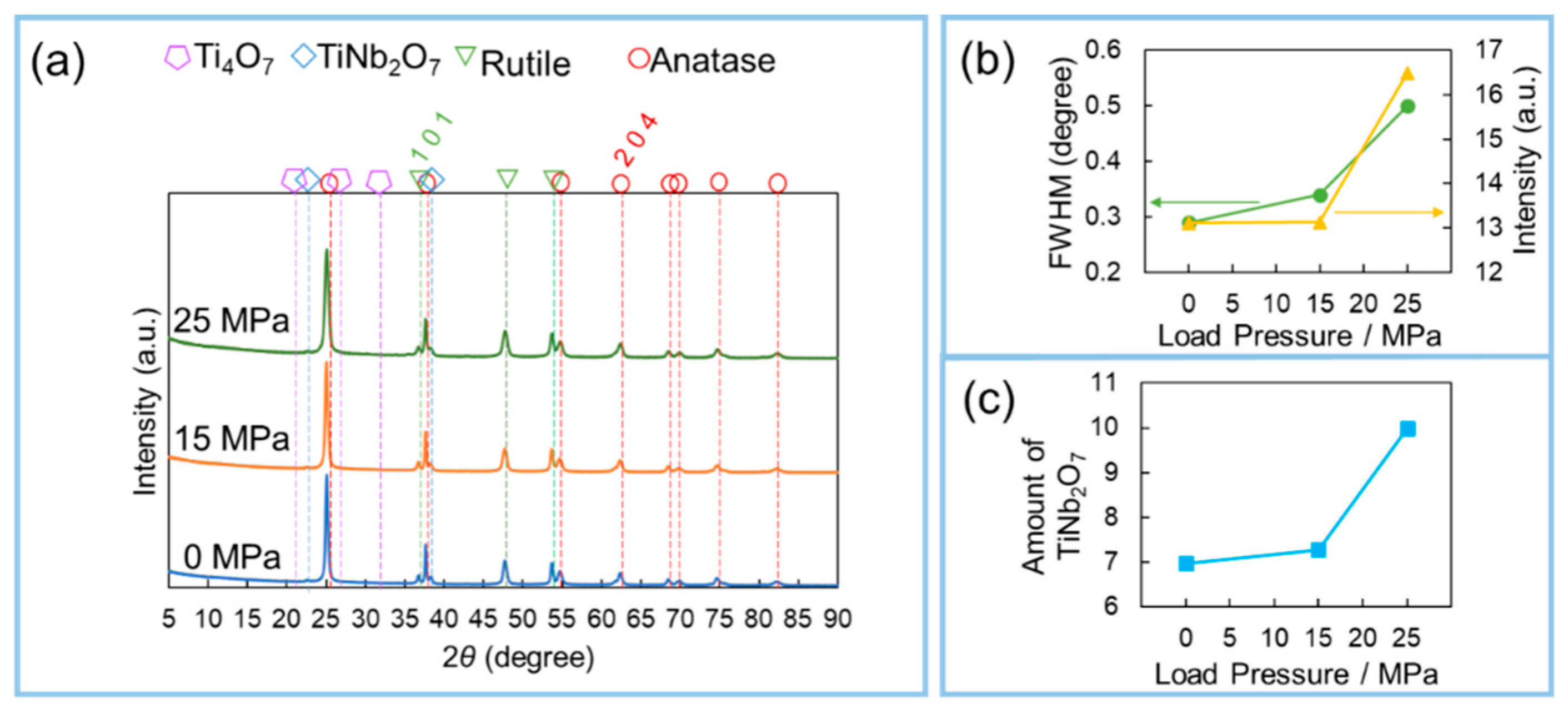
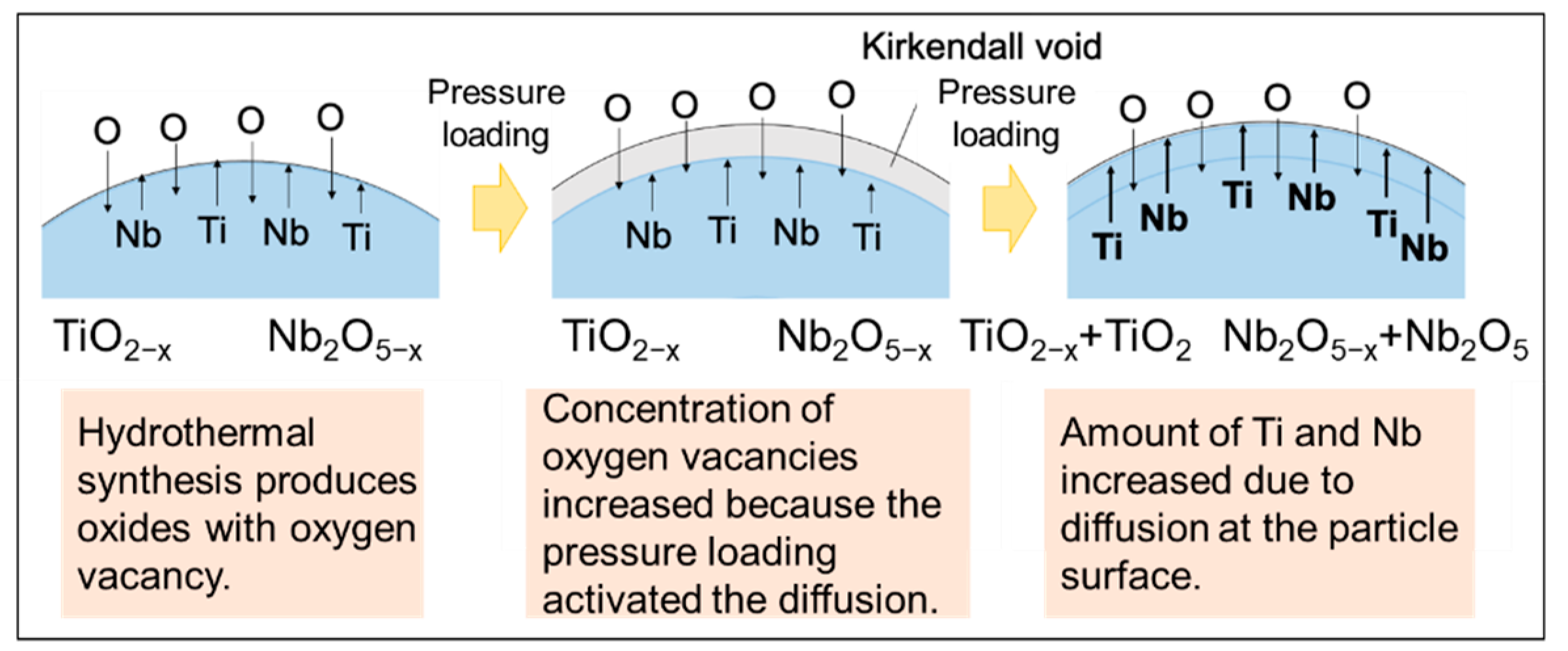
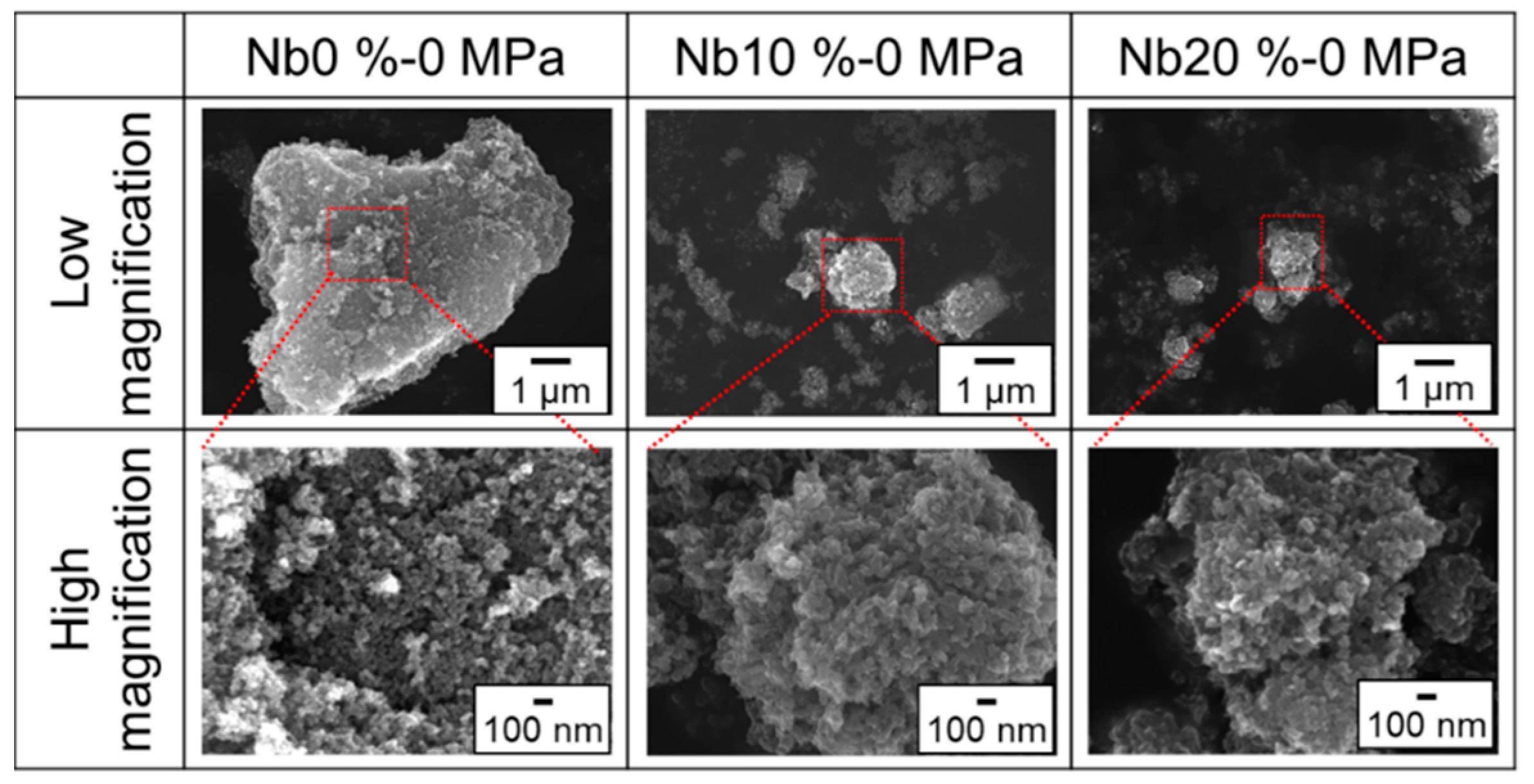
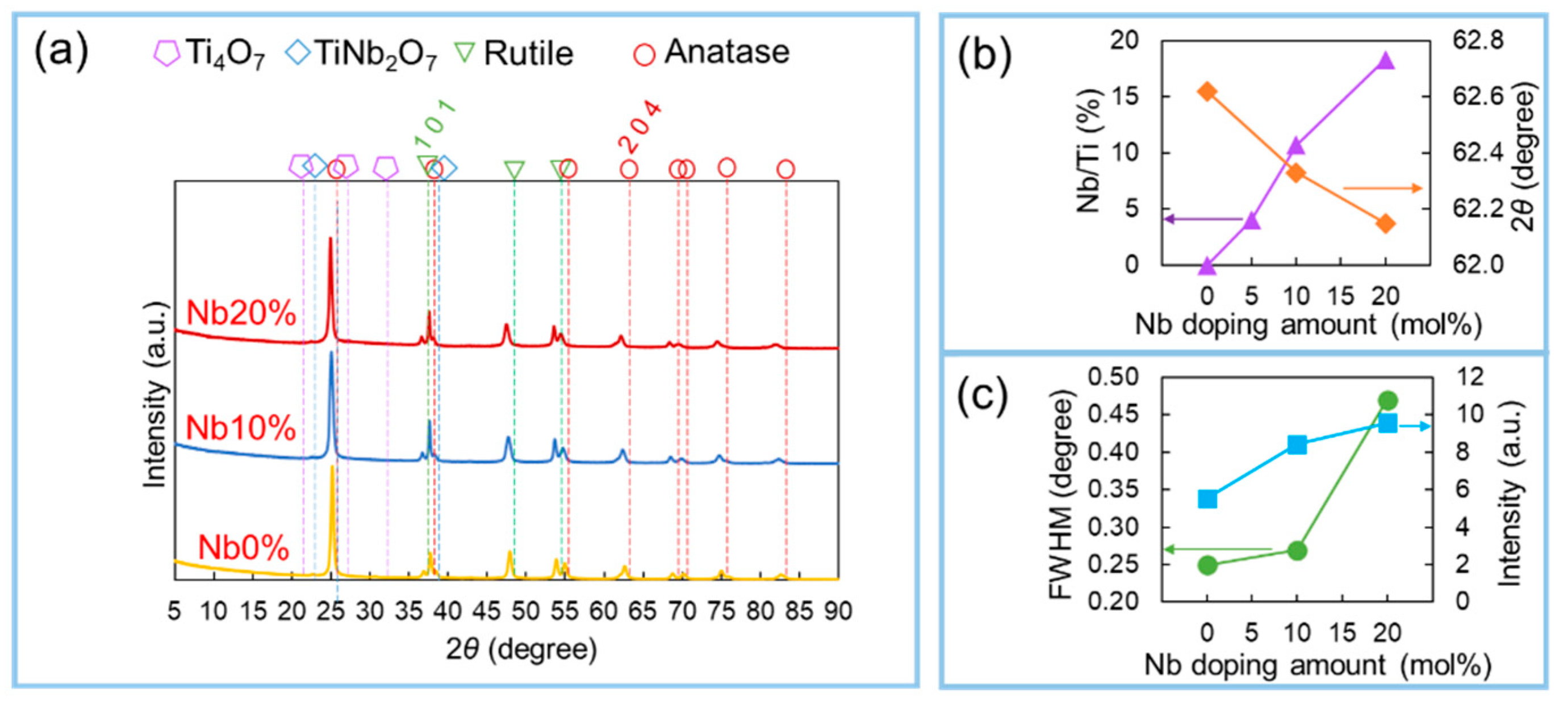
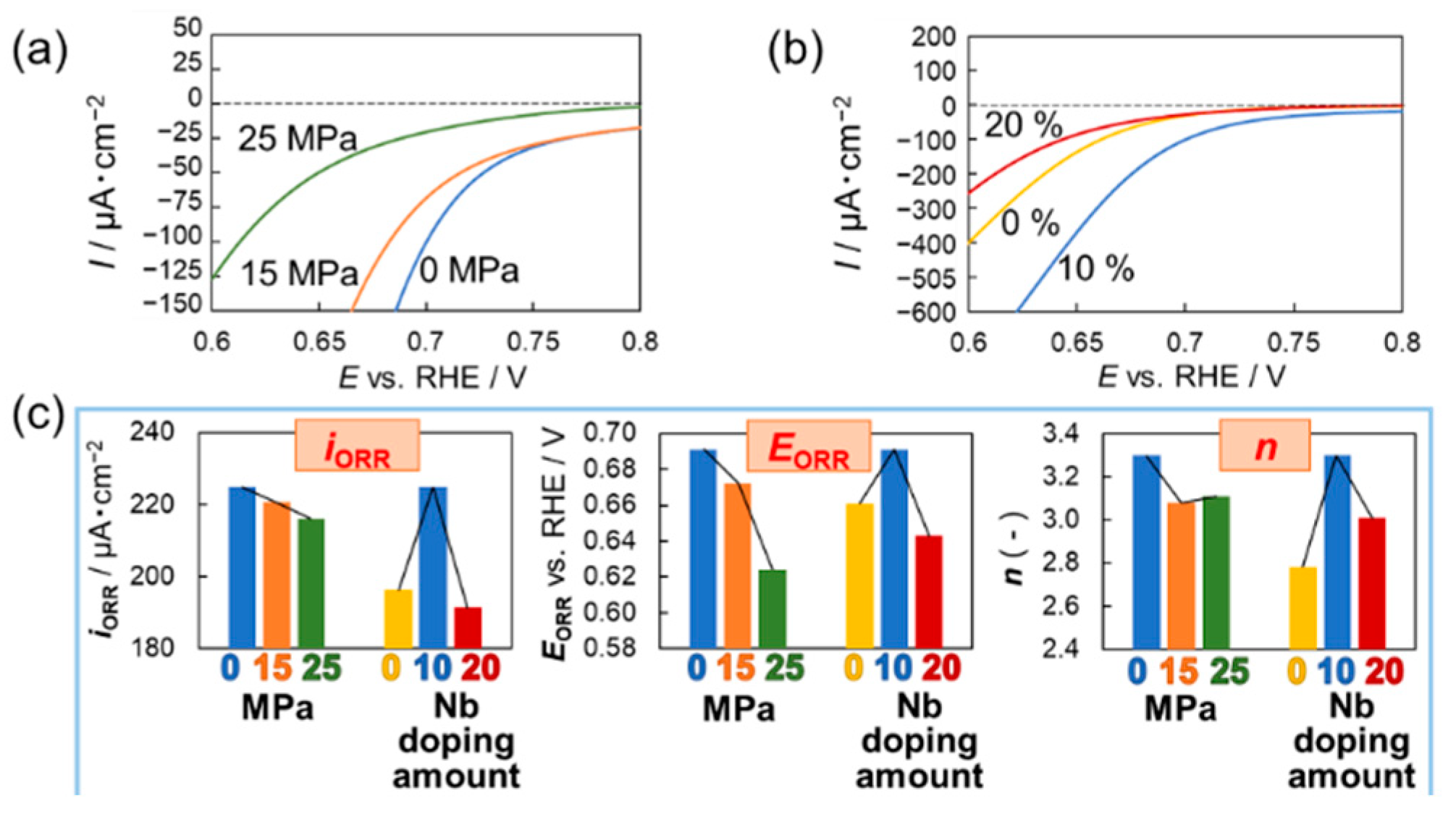
3. Results and Discussion
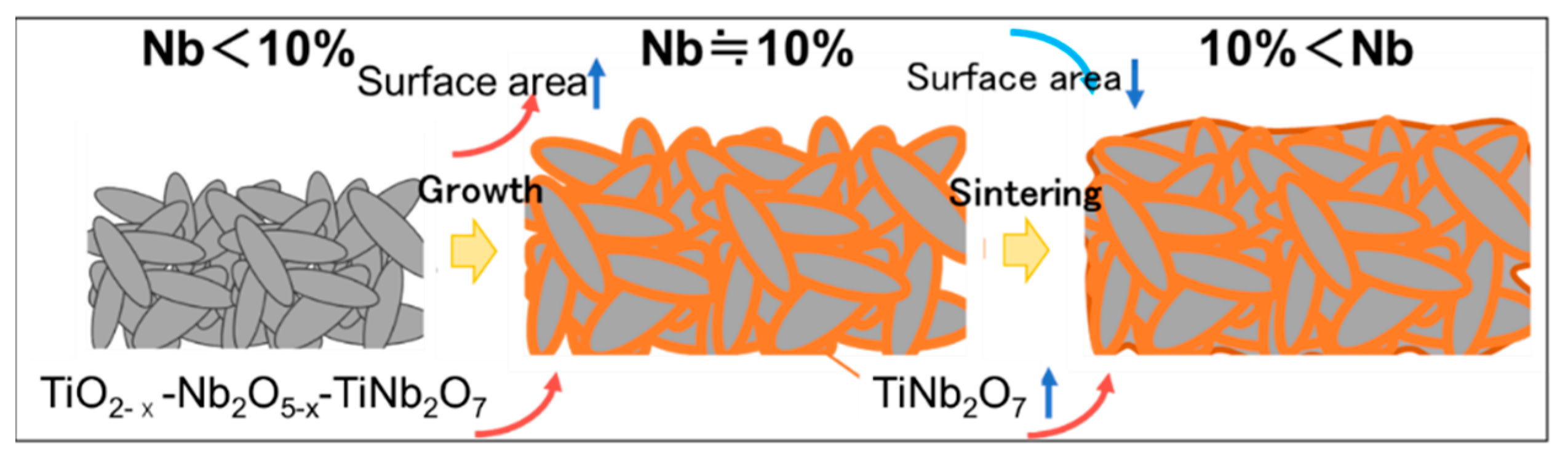
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Li, X.; Tai, L.; Shen, C.; Yang, J.; Sun, C.; Geng, H.; Zuo, X. Constructing electronic interconnected bimetallic selenide-filled porous carbon nanosheets for stable and highly efficient sodium-ion half/full batteries. Nanoscale 2021, 13, 18578–18585. [Google Scholar] [CrossRef]
- Ganyecz, Á.; Kállay, M. Oxygen reduction reaction on N-doped graphene: Effect of positions and scaling relations of adsorption energies. J. Phys. Chem. C Nanomater. Interfaces 2021, 125, 8551–8561. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chu, F.; Zeng, J.; Wang, Q.; Naren, T.; Li, Y.; Cheng, Y.; Lei, Y.; Wu, F. Single atom catalysts for fuel cells and rechargeable batteries: Principles, advances, and opportunities. ACS Nano 2021, 15, 210–239. [Google Scholar] [CrossRef] [PubMed]
- Kundu, A.; Mallick, S.; Ghora, S.; Raj, C.R. Advanced oxygen electrocatalyst for air-breathing electrode in Zn-air batteries. ACS Appl. Mater. Interfaces 2021, 13, 40172–40199. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, G.; Chen, X.; Zhang, Q.; Sui, J.; Li, C.; Zhang, Y.; Hu, J.; Yu, J.; Yu, L.; et al. Developing nitrogen and Co/Fe/Ni multi-doped carbon nanotubes as high-performance bifunctional catalyst for rechargeable zinc-air battery. J. Colloid Interface Sci. 2021, 593, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, W.; Ye, L.; Liu, J.; Wang, F. Hydrothermal-induced formation of well-defined hollow carbons with curvature-activated N-C Sites for Zn-air batteries. Chemistry 2021, 27, 6247–6253. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wang, C.; Wang, H.; Wang, J. A mass-producible integrative structure Pt alloy oxygen reduction catalyst synthesized with atomically dispersive metal–organic framework precursors. J. Colloid Interface Sci. 2021, 583, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zaman, S.; Tian, X.; Wang, Z.; Fang, W.; Xia, B.Y. Advanced platinum-based oxygen reduction electrocatalysts for fuel cells. Acc. Chem. Res. 2021, 54, 311–322. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, Y.; Tian, Z.; Jiang, K.; Li, Y.; Lin, Y.; Oloman, C.W.; Gyenge, E.L.; Su, J.; Chen, L. Enhanced catalytic performance of Pt by coupling with carbon defects. Innovation 2021, 2, 100161. [Google Scholar] [CrossRef]
- Wang, Z.; Mai, Y.; Yang, Y.; Shen, L.; Yan, C. Highly ordered Pt-based nanoparticles directed by the self-assembly of block copolymers for the oxygen reduction reaction. ACS Appl. Mater. Interfaces 2021, 13, 38138–38146. [Google Scholar] [CrossRef]
- Qiao, Z.; Wang, C.; Zeng, Y.; Spendelow, J.S.; Wu, G. Advanced nanocarbons for enhanced performance and durability of platinum catalysts in proton exchange membrane fuel cells. Small 2021, 17, e2006805. [Google Scholar] [CrossRef] [PubMed]
- Kothari, M.; Jeon, Y.; Miller, D.N.; Pascui, A.E.; Kilmartin, J.; Wails, D.; Ramos, S.; Chadwick, A.; Irvine, J.T.S. Platinum incorporation into titanate perovskites to deliver emergent active and stable platinum nanoparticles. Nat. Chem. 2021, 13, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Zhang, L.; Zhu, Y.; Shi, J.; Zhao, X.; Ren, X.; Li, S. Highly efficient catalytic properties of Sc and Fe single atoms stabilized on a honeycomb borophene/Al(111) heterostructure via a dual charge transfer effect. Nanoscale 2021, 13, 5875–5882. [Google Scholar] [CrossRef] [PubMed]
- Kostuch, A.; Rutkowska, I.A.; Dembinska, B.; Wadas, A.; Negro, E.; Vezzù, K.; Di Noto, V.; Kulesza, P.J. Enhancement of activity and development of low Pt content electrocatalysts for oxygen reduction reaction in acid media. Molecules 2021, 26, 5147. [Google Scholar] [CrossRef]
- Dogan, D.C.; Choi, J.; Seo, M.H.; Lee, E.; Jung, N.; Yim, S.D.; Yang, T.H.; Park, G.G. Enhancement of catalytic activity and durability of Pt nanoparticle through strong chemical interaction with electrically conductive support of Magneli phase titanium oxide. Nanomaterials 2021, 11, 829. [Google Scholar] [CrossRef]
- Sarkar, S.; Rawat, A.; Das, T.; Gaboardi, M.; Chakraborty, S.; Vinod, C.P.; Peter, S.C.; Non-Noble, S.-T. Metal-based ternary chalcogenide nanocrystals for Pt-like electrocatalytic hydrogen production. ChemSusChem 2021, 14, 3074–3083. [Google Scholar] [CrossRef]
- Atwa, M.; Li, X.; Wang, Z.; Dull, S.; Xu, S.; Tong, X.; Tang, R.; Nishihara, H.; Prinz, F.; Birss, V. Scalable nanoporous carbon films allow line-of-sight 3D atomic layer deposition of Pt: Towards a new generation catalyst layer for PEM fuel cells. Mater. Horiz. 2021, 8, 2451–2462. [Google Scholar] [CrossRef]
- Ott, S.; Orfanidi, A.; Schmies, H.; Anke, B.; Nong, H.N.; Hübner, J.; Gernert, U.; Gliech, M.; Lerch, M.; Strasser, P. Ionomer distribution control in porous carbon-supported catalyst layers for high-power and low Pt-loaded proton exchange membrane fuel cells. Nat. Mater. 2020, 19, 77–85. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef]
- Triantis, T.M.; Fotiou, T.; Kaloudis, T.; Kontos, A.G.; Falaras, P.; Dionysiou, D.D.; Pelaez, M.; Hiskia, A. Photocatalytic degradation and mineralization of microcystin-LR under UV-A, solar and visible light using nanostructured nitrogen doped TiO2. J. Hazard Mater. 2012, 211–212, 196–202. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Liu, N.; Han, Y.; Zhang, X.; Huang, H.; Lifshitz, Y.; Lee, S.T.; Zhong, J.; Kang, Z. Water splitting. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 2015, 347, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Eidsvåg, H.; Bentouba, S.; Vajeeston, P.; Yohi, S.; Velauthapillai, D. TiO2 as a photocatalyst for water splitting—An experimental and theoretical review. Molecules 2021, 26, 1687. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Liu, Y.; Ye, J.; Lin, Z.; Yang, X. Electrochemical oxidation of methyl orange by a Magneli phase Ti4O7 anode. Chemosphere 2020, 241, 125084. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, S.; Song, Y.; Xu, H.; Wang, Z.; Wang, W.; Zhao, Y. Performance evaluation of treating oil-containing restaurant wastewater in microbial fuel cell using in situ graphene/polyaniline modified titanium oxide anode. Environ. Technol. 2020, 41, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Takimoto, D.; Toda, Y.; Tominaka, S.; Mochizuki, D.; Sugimoto, W. Conductive nanosized Magneli-phase Ti4O7 with a Core@Shell structure. Inorg. Chem. 2019, 58, 7062–7068. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lettenmeier, P.; Golla-Schindler, U.; Gazdzicki, P.; Cañas, N.A.; Morawietz, T.; Hiesgen, R.; Hosseiny, S.S.; Gago, A.S.; Friedrich, K.A. Nanostructured Ir-supported on Ti4O7 as a cost-effective anode for proton exchange membrane (PEM) electrolyzers. Phys. Chem. Chem. Phys. 2016, 18, 4487–4495. [Google Scholar] [CrossRef]
- Galstyan, V.; Ponzoni, A.; Kholmanov, I.; Natile, M.M.; Comini, E.; Nematov, S.; Sberveglieri, G. Investigation of reduced graphene oxide and a Nb-doped TiO2 nanotube hybrid structure to improve the gas-sensing response and selectivity. ACS Sens. 2019, 4, 2094–2100. [Google Scholar] [CrossRef]
- Kong, L.; Wang, C.; Wan, F.; Li, L.; Zhang, X.; Liu, Y. Transparent Nb-doped TiO2 films with the [001] preferred orientation for efficient photocatalytic oxidation performance. Dalton Trans. 2017, 46, 15363–15372. [Google Scholar] [CrossRef]
- Nazir, S.; Behtash, M.; Cheng, J.; Luo, J.; Yang, K. Nb and Ta layer doping effects on the interfacial energetics and electronic properties of LaAlO3/SrTiO3 heterostructure: First-principles analysis. Phys. Chem. Chem. Phys. 2016, 18, 2379–2388. [Google Scholar] [CrossRef]
- Osorio-Guillén, J.; Lany, S.; Zunger, A. Atomic control of conductivity versus ferromagnetism in wide-gap oxides via selective doping: V, Nb, Ta in anatase TiO2. Phys. Rev. Lett. 2008, 100, 036601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davia, F.G.; Fernández, C.C.; Williams, F.J.; Calvo, E.J. Effect of porosity and active area on the assessment of catalytic activity of non-precious metal electrocatalyst for oxygen reduction. J. Phys. Condens Matter. 2021, 33. [Google Scholar] [CrossRef] [PubMed]
- Viera, M.; Riquelme, J.; Aliaga, C.; Marco, J.F.; Orellana, W.; Zagal, J.H.; Tasca, F. Oxygen reduction reaction at Penta-Coordinated Co phthalocyanines. Front. Chem. 2020, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Liu, Y.; Wang, C.; Liu, H. Assessing the electron transfer and oxygen mass transfer of the oxygen reduction reaction using a new electrode kinetic equation. Phys. Chem. Chem. Phys. 2018, 20, 16159–16166. [Google Scholar] [CrossRef]
- Peng, S.; Sun, S. Synthesis and characterization of monodisperse hollow Fe3O4 nanoparticles. Angew. Chem. Int. Ed. 2007, 46, 4155–4158. [Google Scholar] [CrossRef]
- Park, J.; Zheng, H.; Jun, Y.W.; Alivisatos, A.P. Hetero-epitaxial anion exchange yields single-crystalline hollow nanoparticles. J. Am. Chem. Soc. 2009, 131, 13943–13945. [Google Scholar] [CrossRef] [Green Version]
- Cabot, A.; Puntes, V.F.; Shevchenko, E.; Yin, Y.; Balcells, L.; Marcus, M.A.; Hughes, S.M.; Alivisatos, A.P. Vacancy coalescence during oxidation of iron nanoparticles. J. Am. Chem. Soc. 2007, 129, 10358–10360. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Shi, X.; Ji, S.; Wang, X.; Zhang, L.; Liang, H.; Brett, D.J.L.; Wang, X.; Wang, R. Tailoring hollow structure within NiCoP nanowire arrays via nanoscale Kirkendall diffusion to enhance hydrogen evolution reaction. Nanotechnology 2020, 31, 425404. [Google Scholar] [CrossRef]
- Lim, Y.; Lee, C.H.; Jun, C.H.; Kim, K.; Cheon, J.; Non-Kirkendall, M.-C. Morphology-conserving non-Kirkendall anion exchange of metal oxide nanocrystals. J. Am. Chem. Soc. 2020, 142, 9130–9134. [Google Scholar] [CrossRef]
- Ding, K.; Miao, Z.; Hu, B.; An, G.; Sun, Z.; Han, B.; Liu, Z. Study on the anatase to rutile phase transformation and controlled synthesis of rutile nanocrystals with the assistance of ionic liquid. Langmuir 2010, 26, 10294–10302. [Google Scholar] [CrossRef]
- Zhu, L.; Zhu, Z.; Zhou, J.; Qian, Y. Kirkendall effect modulated hollow red phosphorus nanospheres for high performance sodium-ion battery anodes. Chem. Commun. 2020, 56, 11795–11798. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Lisiecki, I.; Walls, M.; Pileni, M.P. Nanocrystallinity and the ordering of nanoparticles in two-dimensional superlattices: Controlled formation of either core/shell (Co/CoO) or hollow CoO nanocrystals. ACS Nano 2013, 7, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Anggara, K.; Lin, M.; Chan, Y. Formation of hollow iron oxide tetrapods via a shape-preserving nanoscale Kirkendall effect. Small 2014, 10, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.S.; Park, J.S.; Kang, Y.C. Preparation of hollow Fe2O3 nanorods and nanospheres by nanoscale Kirkendall diffusion, and their electrochemical properties for use in lithium-ion batteries. Sci. Rep. 2016, 6, 38933. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.D.; Tracy, J.B. Nanoparticle conversion chemistry: Kirkendall effect, galvanic exchange, and anion exchange. Nanoscale 2014, 6, 12195–12216. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wu, S.; Ma, J.; Mine, S.; Toyao, T.; Matsuoka, M.; Wang, L.; Zhang, J. Non-oxidative coupling of methane: N-type doping of niobium single atoms in TiO2-SiO2 induces electron localization. Angew. Chem. Int. Ed. 2021, 60, 11901–11909. [Google Scholar] [CrossRef]
- Duan, Y.; Zhao, G.; Liu, X.; Ma, J.; Chen, S.; Song, Y.; Pi, X.; Yu, X.; Yang, D.; Zhang, Y.; et al. Low-temperature processed tantalum/niobium co-doped TiO2 electron transport layer for high-performance planar perovskite solar cells. Nanotechnology 2021, 32, 245201. [Google Scholar] [CrossRef]
- Yan, W.; Liu, X. Niobium-doped TiO2: Effect of an interstitial oxygen atom on the charge state of niobium. Inorg. Chem. 2019, 58, 3090–3098. [Google Scholar] [CrossRef]
- Shi, R.; Huang, Y.; Li, M.; Zhu, Y.; He, X.; Jiang, R.; Lei, Z.; Liu, Z.; Sun, J. Synthesis of Ti4O7/Ti3O5 dual-phase nanofibers with coherent interface for oxygen reduction reaction electrocatalysts. Materials 2020, 13, 3142. [Google Scholar] [CrossRef]
- Becerril-Estrada, V.; Robles, I.; Martínez-Sánchez, C.; Godínez, L.A. Study of TiO2/Ti4O7 photo-anodes inserted in an activated carbon packed bed cathode: Towards the development of 3D-type photo-electro-Fenton reactors for water treatment. Electrochim. Acta 2020, 340, 135972. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.Y.; Numata, D.; Serizawa, A.; Sasaki, K.; Fukushima, K.; Hu, X.; Ishizaki, T. Systematic Study of Effective Hydrothermal Synthesis to Fabricate Nb-Incorporated TiO2 for Oxygen Reduction Reaction. Materials 2022, 15, 1633. https://doi.org/10.3390/ma15051633
Lee SY, Numata D, Serizawa A, Sasaki K, Fukushima K, Hu X, Ishizaki T. Systematic Study of Effective Hydrothermal Synthesis to Fabricate Nb-Incorporated TiO2 for Oxygen Reduction Reaction. Materials. 2022; 15(5):1633. https://doi.org/10.3390/ma15051633
Chicago/Turabian StyleLee, So Yoon, Daiki Numata, Ai Serizawa, Koudai Sasaki, Kaito Fukushima, Xiulan Hu, and Takahiro Ishizaki. 2022. "Systematic Study of Effective Hydrothermal Synthesis to Fabricate Nb-Incorporated TiO2 for Oxygen Reduction Reaction" Materials 15, no. 5: 1633. https://doi.org/10.3390/ma15051633
APA StyleLee, S. Y., Numata, D., Serizawa, A., Sasaki, K., Fukushima, K., Hu, X., & Ishizaki, T. (2022). Systematic Study of Effective Hydrothermal Synthesis to Fabricate Nb-Incorporated TiO2 for Oxygen Reduction Reaction. Materials, 15(5), 1633. https://doi.org/10.3390/ma15051633







