Some Aspects of Oxidation and Reduction Processes in Ti–Al and Ti–Al–Nb Systems
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
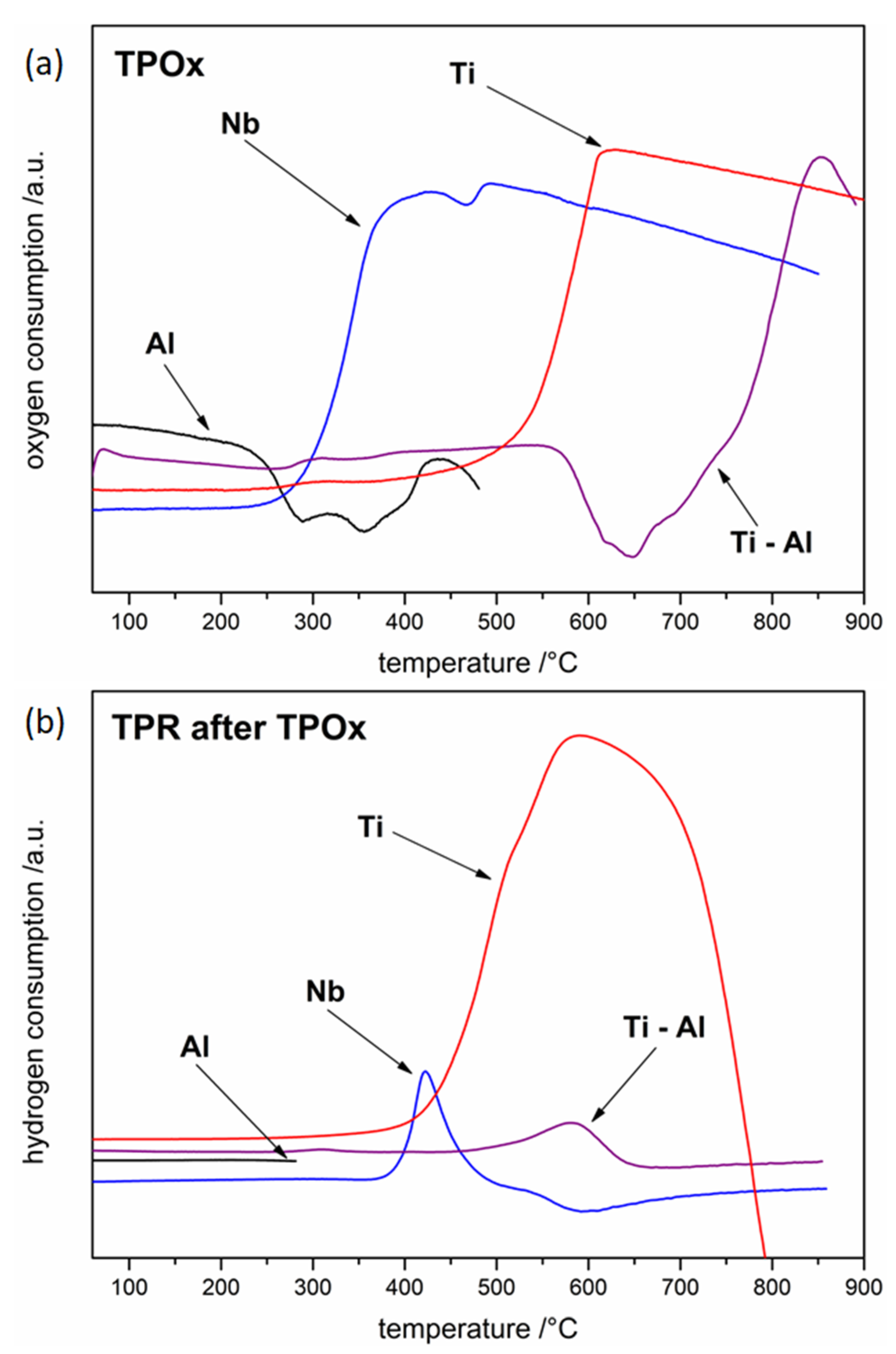
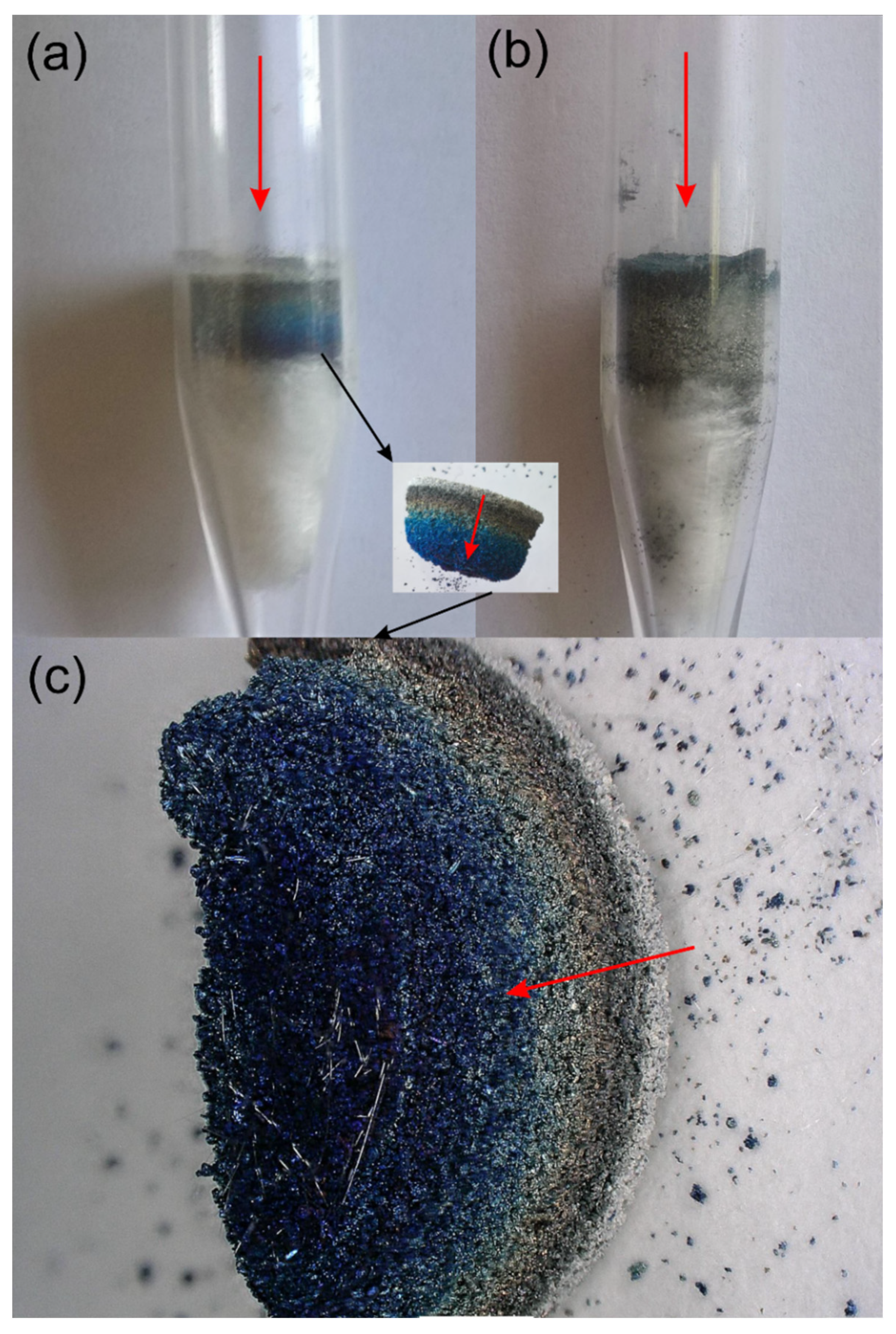
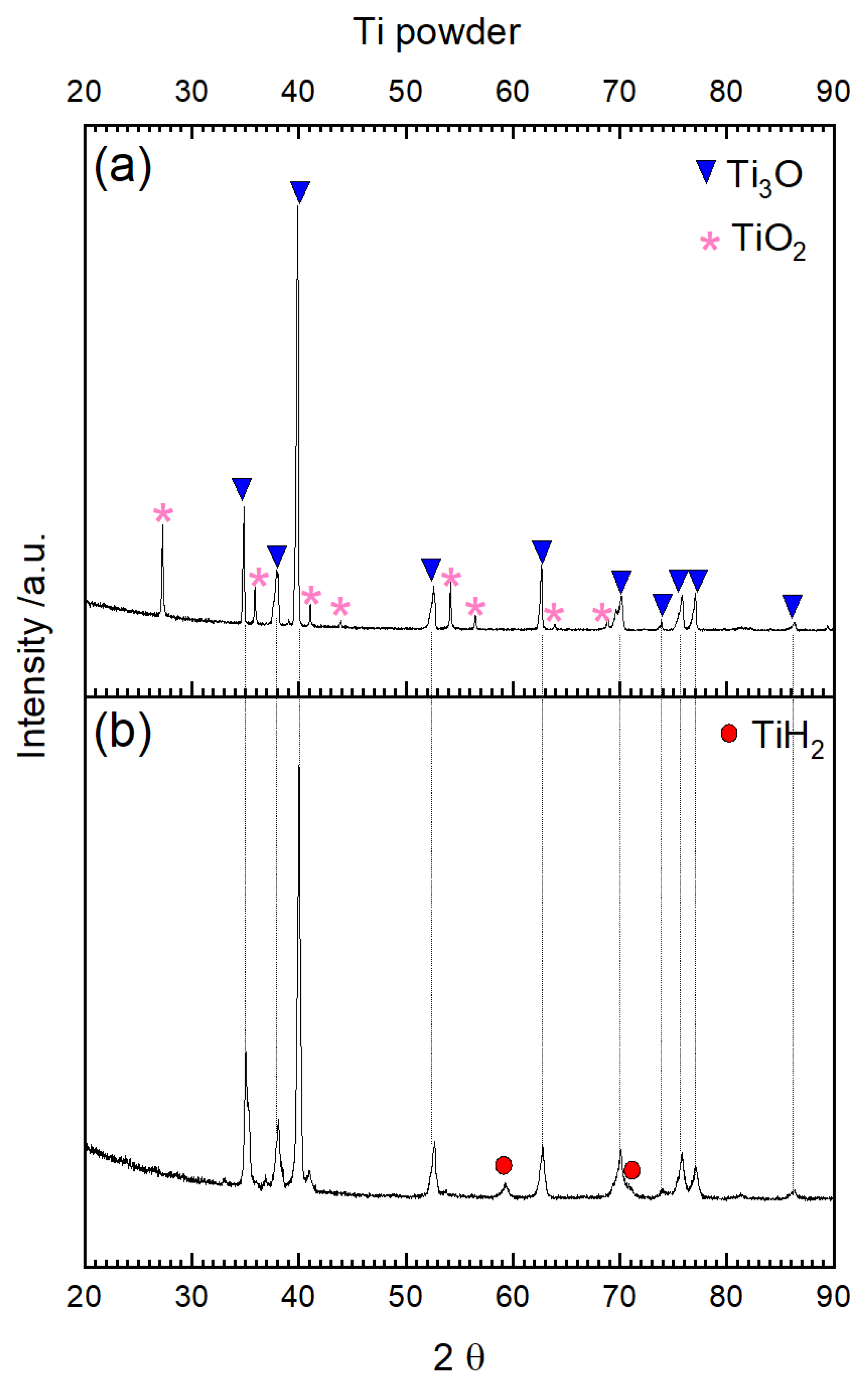
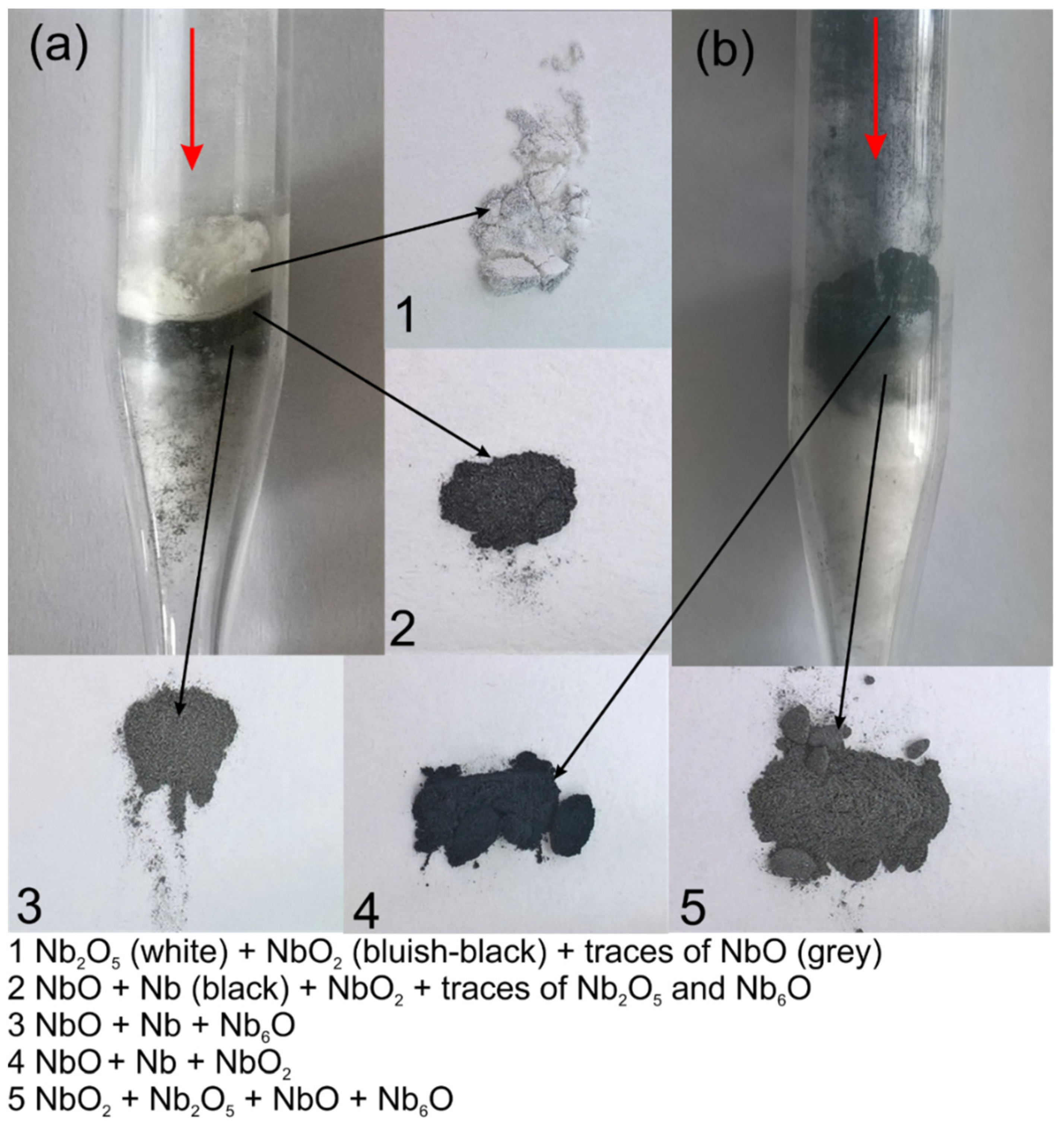
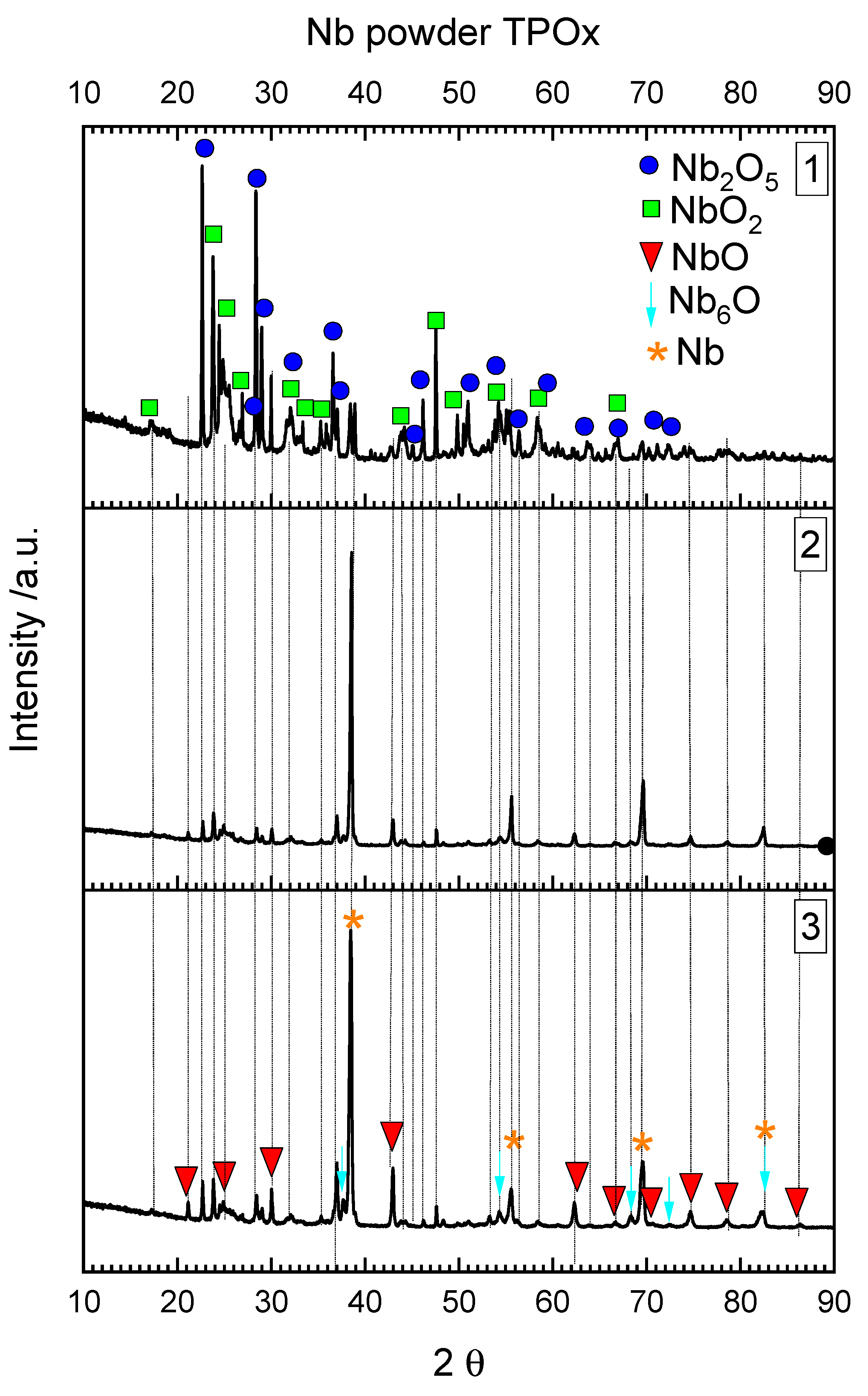
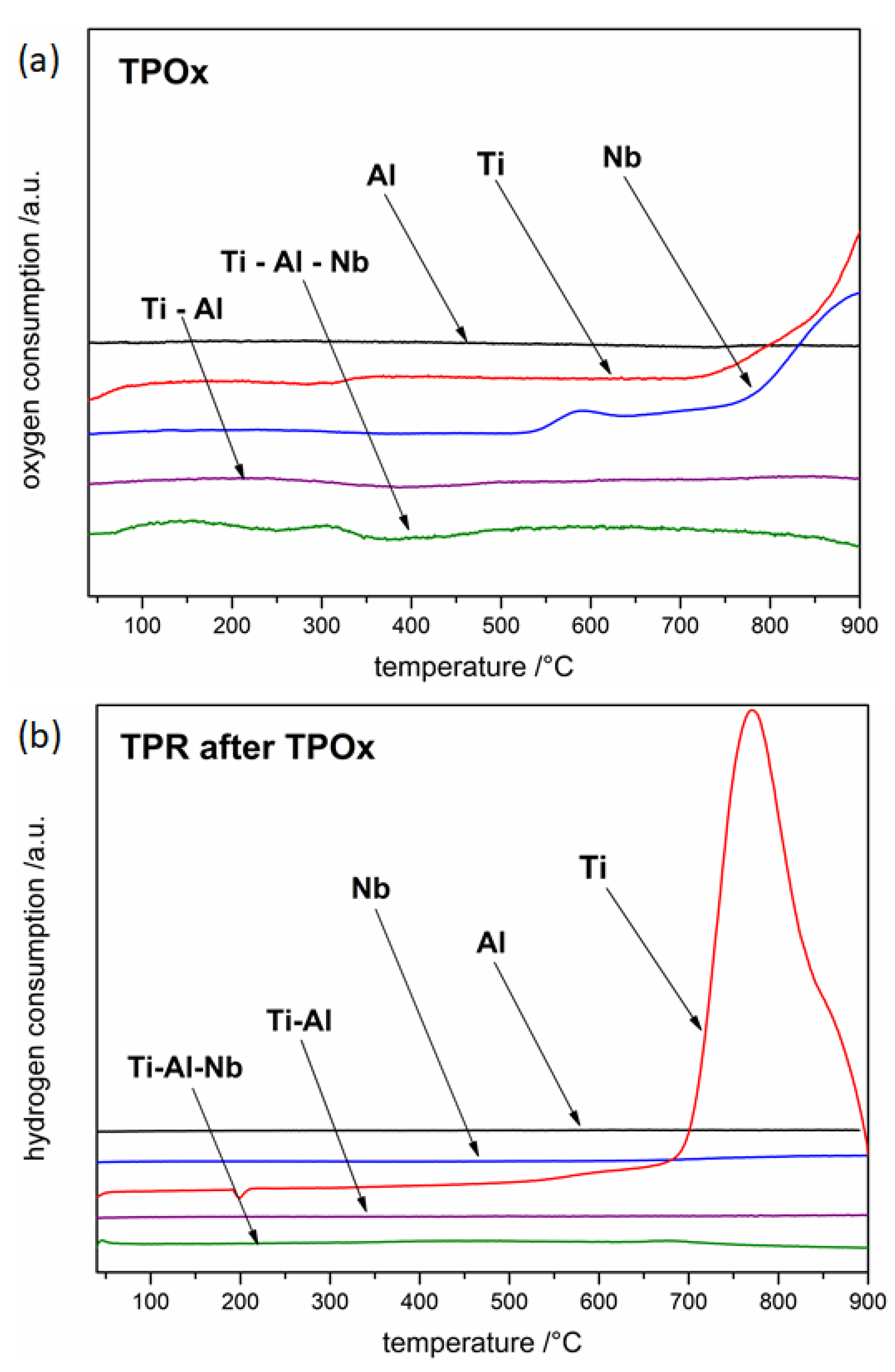
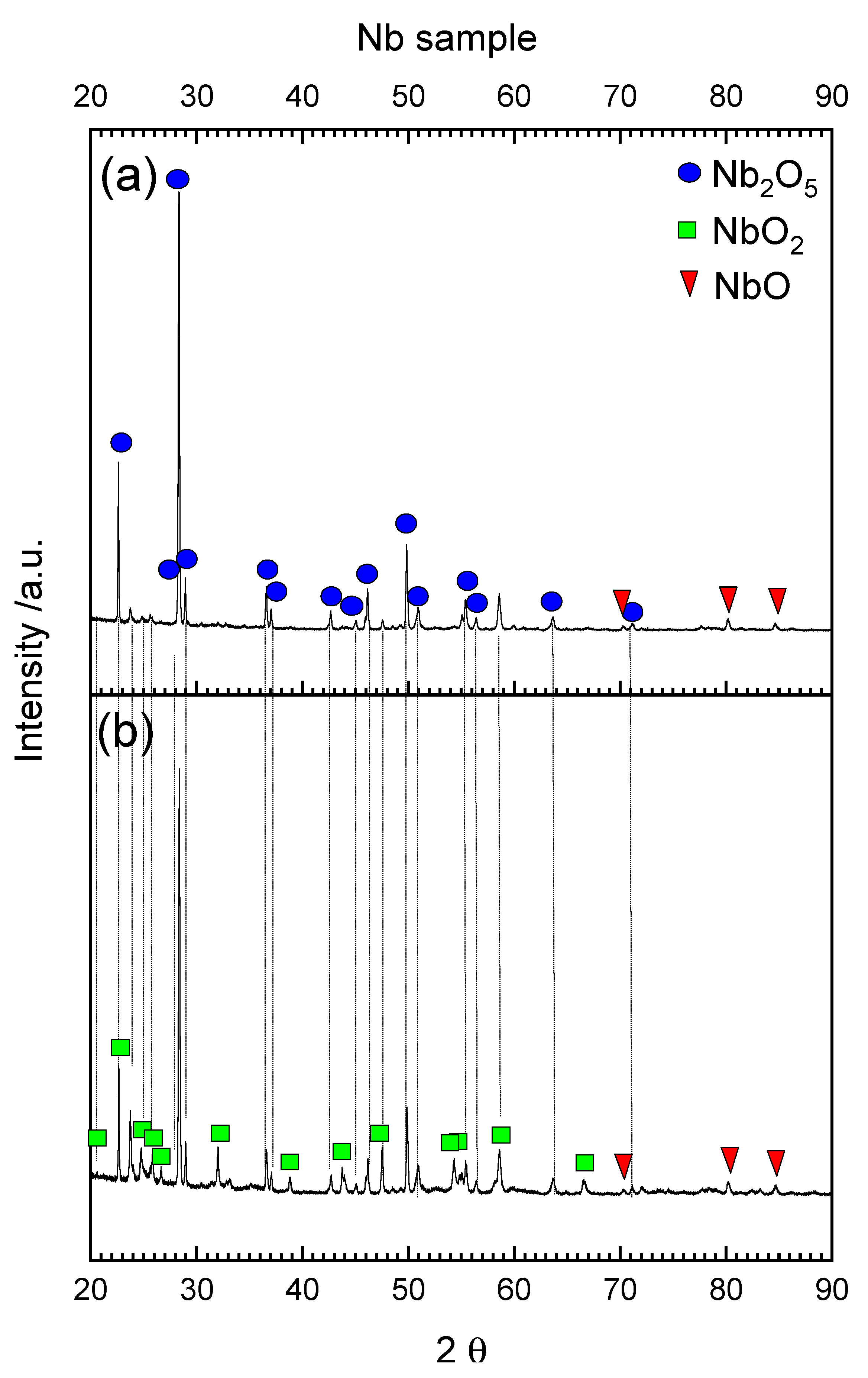
3.1. Oxidation and Reduction of Powders
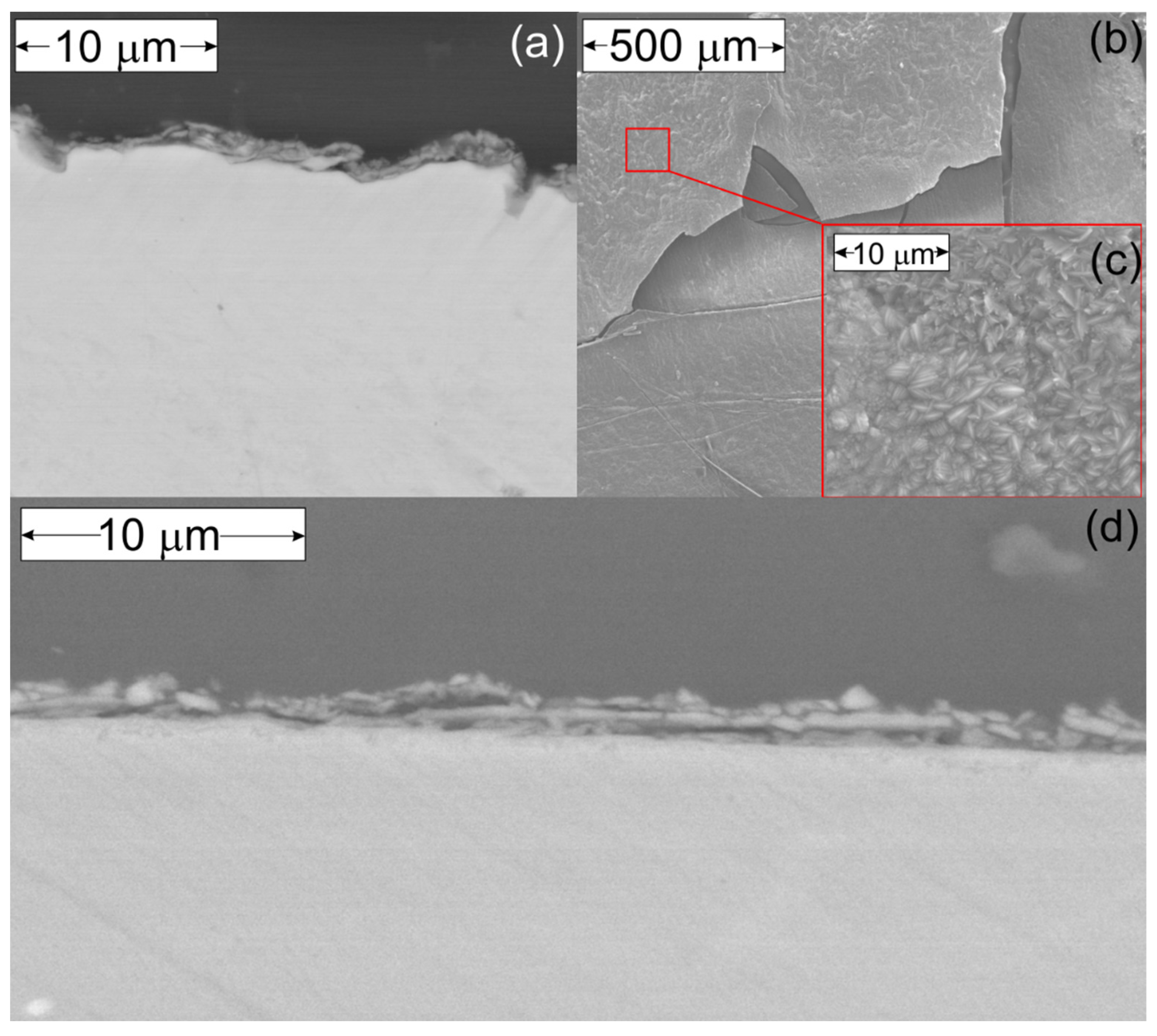
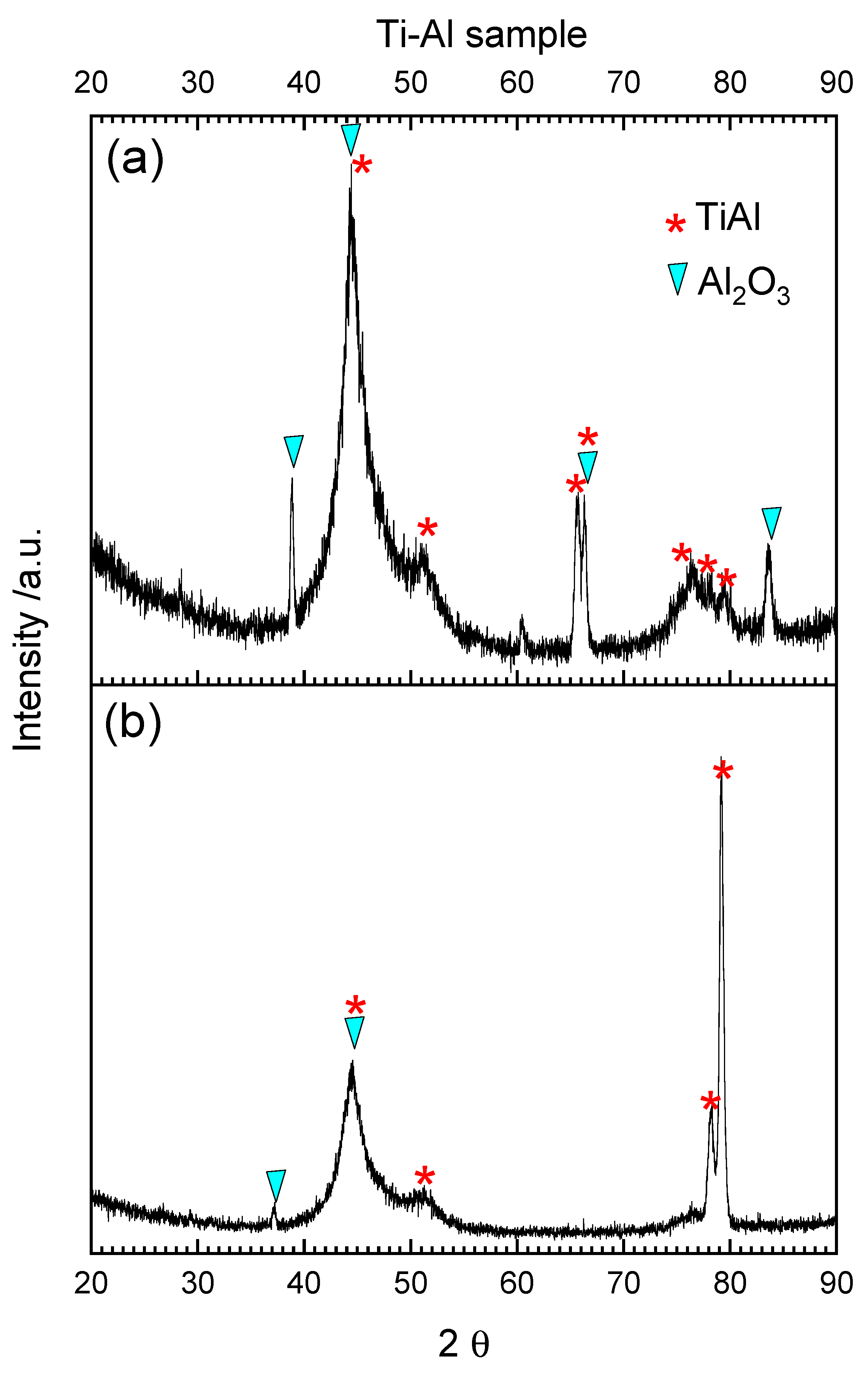
3.2. Oxidation and Reduction of Solid Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, G.; Bernasek, S.L.; Schwartz, J.V. Oxidation of a polycrystalline titanium surface by oxygen and water. Surf. Sci. 2000, 458, 80–90. [Google Scholar] [CrossRef]
- Vaquila, I.; Vergara, L.I.; Passeggi, M.C.G., Jr.; Vidal, R.A.; Ferrón, J. Chemical reactions at surfaces: Titanium oxidation. Surf. Coat. Tech. 1999, 122, 67–71. [Google Scholar] [CrossRef]
- Okamoto, H. O-Ti (Oxygen-Titanium). J. Phase Equilib. Diffus. 2011, 32, 473–474. [Google Scholar] [CrossRef]
- Unnam, J.; Shenoy, R.N.; Clark, R.K. Oxidation of Commercial Purity Titanium. Oxid. Met. 1986, 26, 231–252. [Google Scholar] [CrossRef]
- Kofstad, P.; Hauffe, K. Oxydation von Titan. Werkst. Korros. 1956, 11, 642–649. [Google Scholar] [CrossRef]
- Kofstad, P. High-temperature oxidation of titanium. J. Less Common Met. 1967, 12, 449–464. [Google Scholar] [CrossRef]
- NIST Chemistry WebBook. Available online: https://webbook.nist.gov/chemistry/ (accessed on 9 November 2021).
- Marshall, P.; O’Connor, P.B.; Chan, W.-T.; Kristof, P.V.; Goddard, J.D. Reactions of boron and aluminum atoms with small molecules. In Gas Phase Metal Reactions; Fontijn, A., Ed.; Elsevier Science Publishers B.V.: North Holland, Netherlands, 1992; pp. 147–177. [Google Scholar]
- Garland, N.L. Kinetic Studies of Boron and Aluminum Species. In Gas Phase Metal Reactions; Fontijn, A., Ed.; Elsevier Science Publishers B.V.: North Holland, The Netherlands, 1992; pp. 73–91. [Google Scholar]
- Vorozhtsov, A.B.; Lerner, M.; Rodkevich, N.; Nie, H.; Abraham, A.; Schoenitz, M.; Dreizin, E.L. Oxidation of nano-sized aluminum powders. Thermochim. Acta 2016, 636, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Nico, C.; Monteiro, T.; Graça, M.P.F. Niobium oxides and niobates physical properties: Review and prospects. Prog. Mater. Sci. 2016, 80, 1–37. [Google Scholar] [CrossRef]
- Delheusy, M.; Stierle, A.; Kasper, N.; Kurta, R.P.; Vlad, A.; Dosch, H.; Antoine, C.; Resta, A.; Lundgren, E.; Andersen, J. X-ray investigation of subsurface interstitial oxygen at Nb/oxide interfaces. Appl. Phys. Lett. 2008, 92, 1–3. [Google Scholar] [CrossRef]
- Nico, C.; Rino, L.; Mtos, M.; Monteiro, M.; Costa, F.M.; Monteiro, T.; Graca, M.P.F. NbO/Nb2O5 core-shells by thermal oxidations. J. Eur. Ceram. Soc. 2013, 33, 15–16. [Google Scholar] [CrossRef]
- Newbery, E.; Pring, J.N. The reduction of metallic oxides with hydrogen at high pressures. Proc. R. Soc. Lond. A 1916, 639, 276–285. [Google Scholar] [CrossRef]
- Rojas, E.; Delgado, J.J.; Guerrero-Pérez, M.O.; Bañares, M.A. Performance of NiO and Ni–Nb–O active phases during the ethane ammoxidation into acetonitrile. Catal. Sci. Technol. 2013, 3, 3173–3182. [Google Scholar] [CrossRef]
- Appel, F.; Wagner, R. Microstructure and deformation of two-phase γ-Titanium Aluminides. Mater. Sci. Eng. R. 1998, 5, 187–268. [Google Scholar] [CrossRef]
- Voice, W.E.; Henderson, M.; Shelton, E.F.J.; Wu, X. Gamma titanium aluminide, TNB. Intermetallics 2005, 9, 959–964. [Google Scholar] [CrossRef]
- Mitoraj, M.; Godlewska, E.; Heintz, O.; Geoffroy, N.; Fontana, S.; Chevalier, S. Scale composition and oxidation mechanism of the Ti-46Al-8Nb alloy in air at 700 and 800 °C. Intermetallics 2011, 1, 39–47. [Google Scholar] [CrossRef]
- Yue, X.; Shen, J.; Xiong, Y.; Li, Q.; Zheng, S. Microstructure, fracture toughness and high-temperature tensile propertyof large size Ti–46Al–5Nb-0.18C-0.3Si alloy with oriented lamella by electromagnetic confinement directional solidification. Mat. Sci. Eng. A 2021, 812, 141139. [Google Scholar] [CrossRef]
- Ibáñez-Péreza, J.; Nób, M.L.; Oehringc, M.; Clemensd, H.; San Juan, J.M. Influence of Nb on Ti diffusion in γ-TiAl intermetallics studied by mechanical spectroscopy. J. Alloys Compd. 2021, 867, 158880. [Google Scholar] [CrossRef]
- Lin, J.P.; Zhao, L.L.; Li, G.Y.; Zhang, L.Q.; Song, X.P.; Ye, F.; Chen, G.L. Effect of Nb on oxidation behavior of high Nb containing TiAl alloys. Intermetallics 2011, 2, 131–136. [Google Scholar] [CrossRef]
- Cui, Y.; Aoyagi, K.; Koizumi, Y.; Yang, C.; Bian, H.; Hayasaka, Y.; Fujieda, T.; Chiba, A. Effect of niobium addition on tensile properties and oxidation resistance of a titanium-based alloy. Corros. Sci. 2021, 180, 109198. [Google Scholar] [CrossRef]
- Pan, D.; Ru, Y.; Liu, T.; Wang, Y.; Yu, F.; Chen, S.; Yan, X.; Fan, B.; Li, R. Highly efficient and stable ordered mesoporous Ti-Al composite oxide catalyst for oxidative dehydrogenation of ethylbenzene to styrene with CO2. Chem. Eng. Sci. 2022, 15, 117388. [Google Scholar] [CrossRef]
- Jostsons, A.; Malin, A.S. The ordered structure of Ti3O. Acta Cryst. 1968, 2, 211–213. [Google Scholar] [CrossRef]
- Peng, W.; Zeng, W.; Zhang, Y.; Shi, C.; Quan, B.; Wu, J. The Effect of Colored Titanium Oxides on the Color Change on the Surface of Ti-5Al-5Mo-5V-1Cr-1Fe Alloy. J. Mater. Eng. Perform. 2013, 22, 2588–2593. [Google Scholar] [CrossRef]
- Zhu, Q.; Peng, Y.; Lin, L.; Fan, C.-M.; Gao, G.-Q.; Wang, R.-X.; Xu, A.-W. Stable blue TiO2-x nanoparticles for efficient visible light photocatalysts. J. Mater. Chem. A 2014, 2, 4429–4437. [Google Scholar] [CrossRef]
- Qiu, J.; Li, S.; Gray, E.; Liu, H.; Gu, Q.-F.; Sun, C.; Lai, C.; Zhao, H.; Zhang, S. Hydrogenation Synthesis of Blue TiO2 for High-Performance Lithium-Ion Batteries. J. Phys. Chem. C 2014, 118, 8824–8830. [Google Scholar] [CrossRef]
- Kong, L.; Wang, C.; Zheng, H.; Zhang, X.; Liu, Y. Defect-Induced Yellow Color in Nb-Doped TiO2 and Its Impact on Visible-Light Photocatalysis. J. Phys. Chem. C 2015, 119, 16623–16632. [Google Scholar] [CrossRef]
- Wu, Q.; Huang, F.; Zhao, M.; Xua, J.; Zhou, J.; Wang, Y. Ultra-small yellow defective TiO2 nanoparticles for co-catalyst free photocatalytic hydrogen production. Nano Energy 2016, 24, 63–71. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Shu, X.; Wan, D.; Wei, N.; Yu, X.; Breese, M.B.H.; Venkatesan, T.; Xue, J.M.; Liu, Y.; et al. From Titanium Sesquioxide to Titanium Dioxide: Oxidation-Induced Structural, Phase, and Property Evolution. Chem. Mater. 2018, 13, 4383–4392. [Google Scholar] [CrossRef]
- Khader, M.M.; Kheiri, F.M.-N.; El-Anadouli, B.E.; Ateya, B.G. Mechanism of Reduction of Rutile with Hydrogen. J. Phys. Chem. 1993, 97, 6074–6077. [Google Scholar] [CrossRef]
- Bratan, V.; Munteanu, C.; Hornoiu, C.; Vasile, A.; Papa, F.; State, R.; Preda, S.; Culita, D.; Ionescu, N.I. CO oxidation over Pd supported catalysts —In situ study of the electric and catalytic properties. Appl. Catal. B-Environ. 2017, 207, 166–173. [Google Scholar] [CrossRef]
- Zhang, W.; Sadedin, D.R.; Reuter, M.A.; McCallum, J.C. The de-oxidation of partially oxidized titanium by hydrogen plasma. Mater. Forum 2007, 31, 76–83. [Google Scholar]
- Lindemann, I.; Herrich, M.; Gebel, B.; Schmidt, R.; Stoeck, U.; Uhlemann, M.; Gebert, A. Synthesis of spherical nanocrystalline titanium hydride powder via calciothermic low temperature reduction. Scripta Mater. 2017, 130, 256–259. [Google Scholar] [CrossRef]
- Murray, J.L.; Wriedt, H.A. The O-Ti (Oxygen-Titanium) System. J. Phase Equilib. 1987, 8, 148–165. [Google Scholar] [CrossRef]
- Williams, D.N.; Koehl, B.G.; Bartlett, E.S. The reaction of titanium with hydrogen gas at ambient temperatures. J. Less-Common Met. 1969, 4, 385–398. [Google Scholar] [CrossRef]
- Jeurgens, L.P.H.; Sloof, W.G.; Tichelaar, F.D.; Mittemeijer, E.J. Thermodynamic stability of amorphous oxide films on metals: Application to aluminum oxide films on aluminum substrates. Phys. Rev. B 2000, 62, 4707–4719. [Google Scholar] [CrossRef] [Green Version]
- Jeurgens, L.P.H.; Sloof, W.G.; Tichelaar, F.D.; Mittemeijer, J. Structure and morphology of aluminium-oxide films formed by thermal oxidation of aluminium. Thin Solid Film 2002, 418, 89–101. [Google Scholar] [CrossRef]
- Hoivik, N.D.; Elam, J.W.; Linderman, R.J.; Bright, V.M.; George, S.M.; Lee, Y.C. Atomic layer deposited protective coatings for micro-electromechanical systems. Sens. Actuators A 2003, 1–2, 100–108. [Google Scholar] [CrossRef]
- Sanchez-Lopez, J.C.; Gonzalez-Elipe, A.R.; Fernandez, A. Passivation of Nano Crystalline Al Prepared by the Gas. J. Mater. Res. 1998, 3, 703–707. [Google Scholar] [CrossRef]
- Reichel, F.; Jeurgens, L.P.H.; Richter, G.; Mittemeije, E.J. Amorphous versus crystalline state for ultrathin Al2O3 overgrowths on Al Substrates. J. Appl. Phys. 2008, 103, 1–10. [Google Scholar] [CrossRef]
- Trunov, M.A.; Schoenitz, M.; Zhu, X.; Dreizin, E.L. Effect of polymorphic phase transformations in Al2O3 film on oxidation kinetics of aluminum powders. Combust. Flame 2005, 4, 310–318. [Google Scholar] [CrossRef]
- Hasani, S.; Panjepour, M.; Shamanian, M. The Oxidation Mechanism of Pure Aluminum Powder Particles. Oxid. Met. 2012, 3, 179–195. [Google Scholar] [CrossRef]
- Barin, I.; Knacke, O. Thermochemical Properties of Inorganic Substances; Springer: Berlin, Germany, 1973; pp. 11, 29, 30–32, 316, 323. [Google Scholar]
- Braaten, O.; Kjekshus, A.; Kvande, H. The Possible Reduction of Alumina to Aluminum Using Hydrogen. JOM 2000, 52, 47–53. [Google Scholar] [CrossRef]
- Talbot, D.E.J.; Anyalebechi, P.N. Solubility of hydrogen in liquid aluminium. Mater. Sci. Tech. 1988, 1, 1–4. [Google Scholar] [CrossRef]
- Weller, S.W.; Montagna, A.A. Studies of alumina I. Reaction with hydrogen at elevated temperatures. J. Catalysis 1971, 3, 303–311. [Google Scholar] [CrossRef]
- Niebuhr, J. Die niederen Oxide des Niobs. J. Less Common Met. 1966, 11, 191–203. [Google Scholar] [CrossRef]
- Ishikawa, K.; Habaguchi, H.; Obata, N.; Kobori, Y.; Ohtsu, N.; Aoki, K. Formation of surface oxides and its effects on the hydrogen permeability of Nb40Ti30Ni30 alloy. Int. J. Hydrogen Energ. 2016, 10, 5269–5275. [Google Scholar] [CrossRef]
- Shamrai, V.F.; Blagoveshchenski, Y.V.; Gordeev, A.S.; Mitin, A.V.; Drobinova, I.A. Structural States and Electrical Conductivity of Oxidized Niobium Nanopowders. Russ. Metall. Met. 2007, 4, 322–326. [Google Scholar] [CrossRef]
- Honada, N.; Ichikawa, T.; Isobe, S.; Nakagawa, T.; Tokoyoda, K.; Honma, T.; Fuji, H.; Kojima, Y. X-ray Absorption Spectroscopic Study on Valence State and Local Atomic Structure of Transition Metal Oxides Doped in MgH2. J. Phys. Chem. C 2009, 30, 13450–13455. [Google Scholar] [CrossRef]
- Takahashi, K.; Isobe, S.; Ohnuki, S. The catalytic effect of Nb, NbO and Nb2O5 with different surface planes on dehydrogenation in MgH2: Density functional theory study. J. Alloy. Comp. 2013, 1, S25–S28. [Google Scholar] [CrossRef]
- Batalu, D.; Coşmeleaţǎ, G.; Aloman, A. Critical analysis of the Ti-Al phase diagrams. UPB Sci. Bull. B Chem. Mater. Sci. 2006, 68, 77–90. [Google Scholar]
- Pan, L.; Ai, M.; Huang, C.; Yin, L.; Liu, X.; Zhang, R.; Wang, S.; Jiang, Z.; Zhang, X.; Zou, J.-J.; et al. Manipulating spin polarization of titanium dioxide for efficient photocatalysis. Nat. Commun. 2020, 418, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Neacsu, E.I.; Constantin, V.; Yanushkevich, K.; Donath, C.; Anastasescu, M.; Popescu, A.M. Modification on Ti-6Al-4V Alloy During Corrosion in a High Temperature Ionic Liquid. Rev. Chim. 2020, 4, 201–219. [Google Scholar] [CrossRef]
- Hidnert, P. Thermal expansion of titanium. J. Res. Nat. Bur. Stand. 1943, 30, 101–105. [Google Scholar] [CrossRef]
- Hummer, D.R.; Heaney, P.J.; Post, J.E. Thermal expansion of anatase and rutile between 300 and 575 K using synchrotron powder X-ray diffraction. Powder Diffraction 2007, 4, 352–357. [Google Scholar] [CrossRef]
- McKee, D.W.; Huang, S.-C. High Temperature Ordered Intermetallic Alloys IV. In Proceedings of the 4th MRS Symposium, Boston, MA, USA, 27–30 November 1990; Johnson, L.A., Pope, D.P., Stiegler, J.O., Eds.; Materials Research Society: Pittsburgh, PA, USA, 1991; p. 939. [Google Scholar]
- Meier, G.H. Fundamentals of the oxidation of high-temperature intermetallics. In Oxidation of High-Temperature Intermetallics; Grobstein, T., Doychak, J., Eds.; The Minerals, Metals & Materials Society: Pittsburgh, PA, USA, 1988; pp. 1–17. [Google Scholar]
- Shida, Y.; Anada, H. Oxidation Behavior of Binary TiAl Alloys in High Temperature Air Environment. Mater. Trans. JIM 1993, 3, 236–242. [Google Scholar] [CrossRef] [Green Version]
- Rahmel, A.; Quadakkers, W.J.; Schütze, M. Foundamentals of TiAl Oxidation—A Critical Review. Werkst. Korros. 1995, 5, 271–285. [Google Scholar] [CrossRef]
- Oliveira, R.M.; Hoshida, L.; Oliveira, A.C.; Silva, M.M.N.F.; Pichon, L.; Santos, N.M. Evaluation of the resistance to oxidation of niobium treated by high temperature nitrogen Plasma Based Ion Implantation. Surf. Coat. Tech. 2017, 312, 110–116. [Google Scholar] [CrossRef]
- Maurice, V.; Despert, G.; Zanna, S.; Josso, P.; Bacos, M.-P.; Marcus, P. XPS study of the initial stages of oxidation of α2-Ti3Al and γ-TiAl intermetallic alloys. Acta Mater. 2007, 10, 3315–3325. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitoraj-Królikowska, M.; Drożdż, E. Some Aspects of Oxidation and Reduction Processes in Ti–Al and Ti–Al–Nb Systems. Materials 2022, 15, 1640. https://doi.org/10.3390/ma15051640
Mitoraj-Królikowska M, Drożdż E. Some Aspects of Oxidation and Reduction Processes in Ti–Al and Ti–Al–Nb Systems. Materials. 2022; 15(5):1640. https://doi.org/10.3390/ma15051640
Chicago/Turabian StyleMitoraj-Królikowska, Marzena, and Ewa Drożdż. 2022. "Some Aspects of Oxidation and Reduction Processes in Ti–Al and Ti–Al–Nb Systems" Materials 15, no. 5: 1640. https://doi.org/10.3390/ma15051640
APA StyleMitoraj-Królikowska, M., & Drożdż, E. (2022). Some Aspects of Oxidation and Reduction Processes in Ti–Al and Ti–Al–Nb Systems. Materials, 15(5), 1640. https://doi.org/10.3390/ma15051640





