Solid-State-Activated Sintering of ZnAl2O4 Ceramics Containing Cu3Nb2O8 with Superior Dielectric and Thermal Properties
Abstract
:1. Introduction
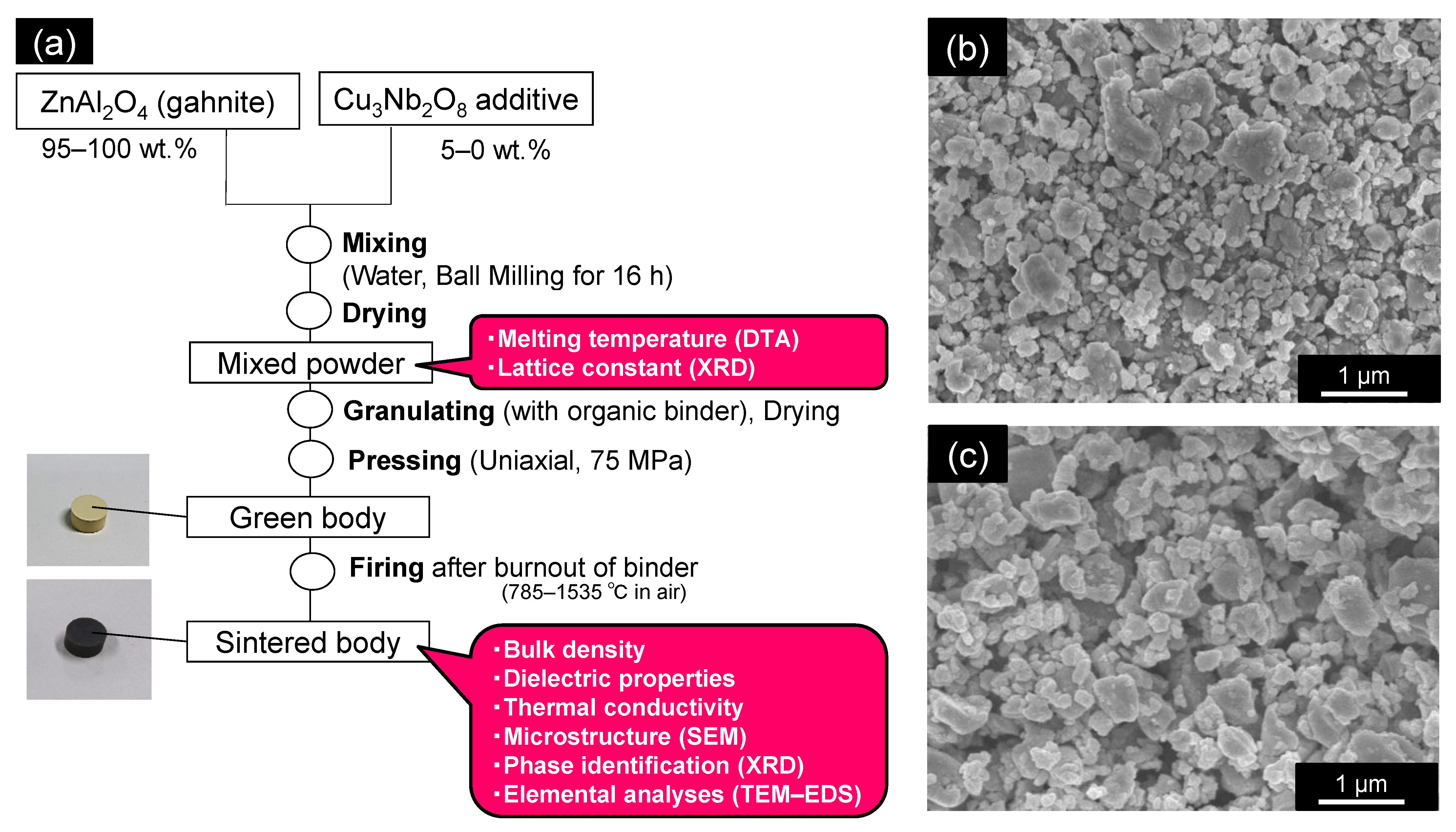
2. Materials and Methods
2.1. Fabricating Sintered Samples
2.2. Characterization
3. Results and Discussion
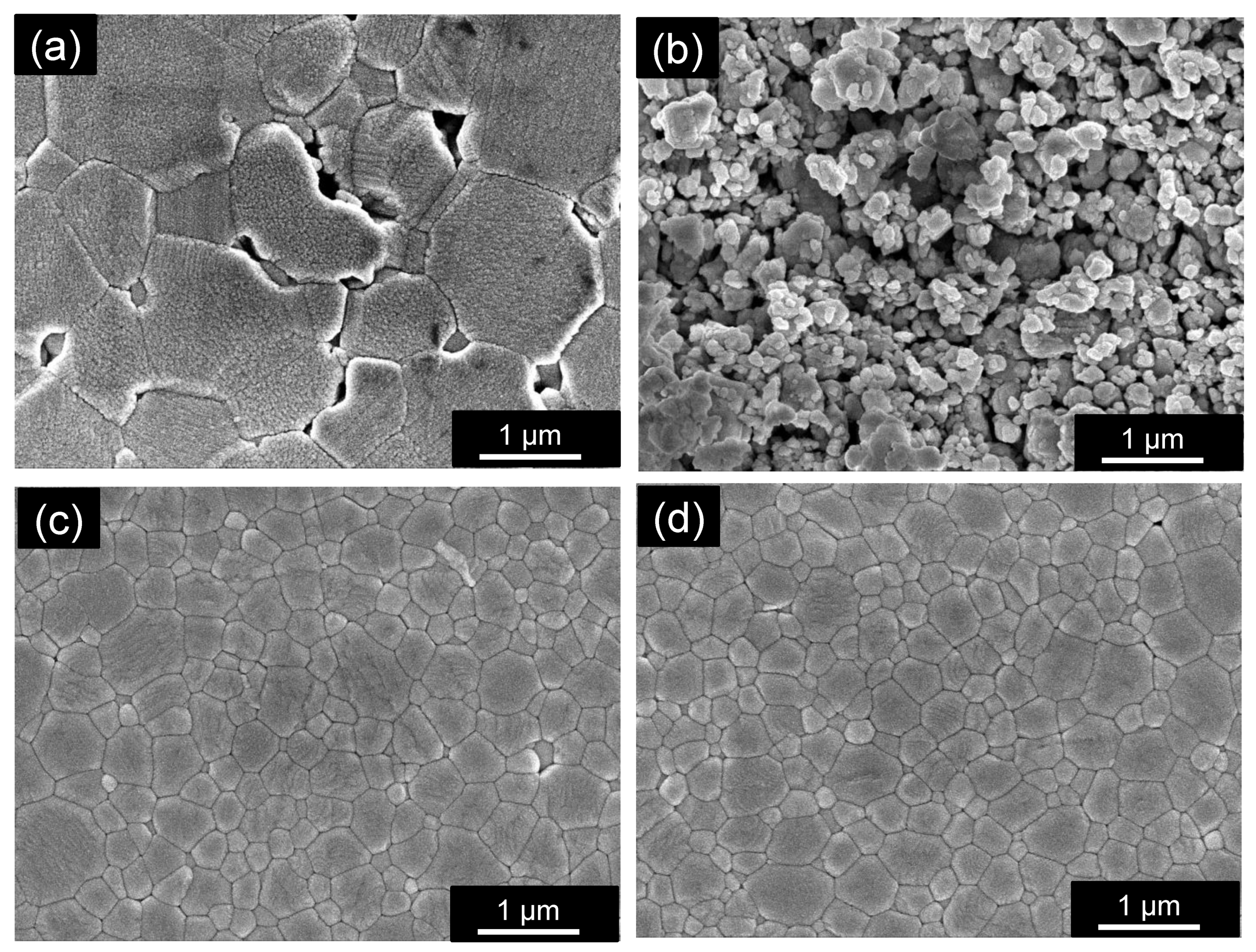
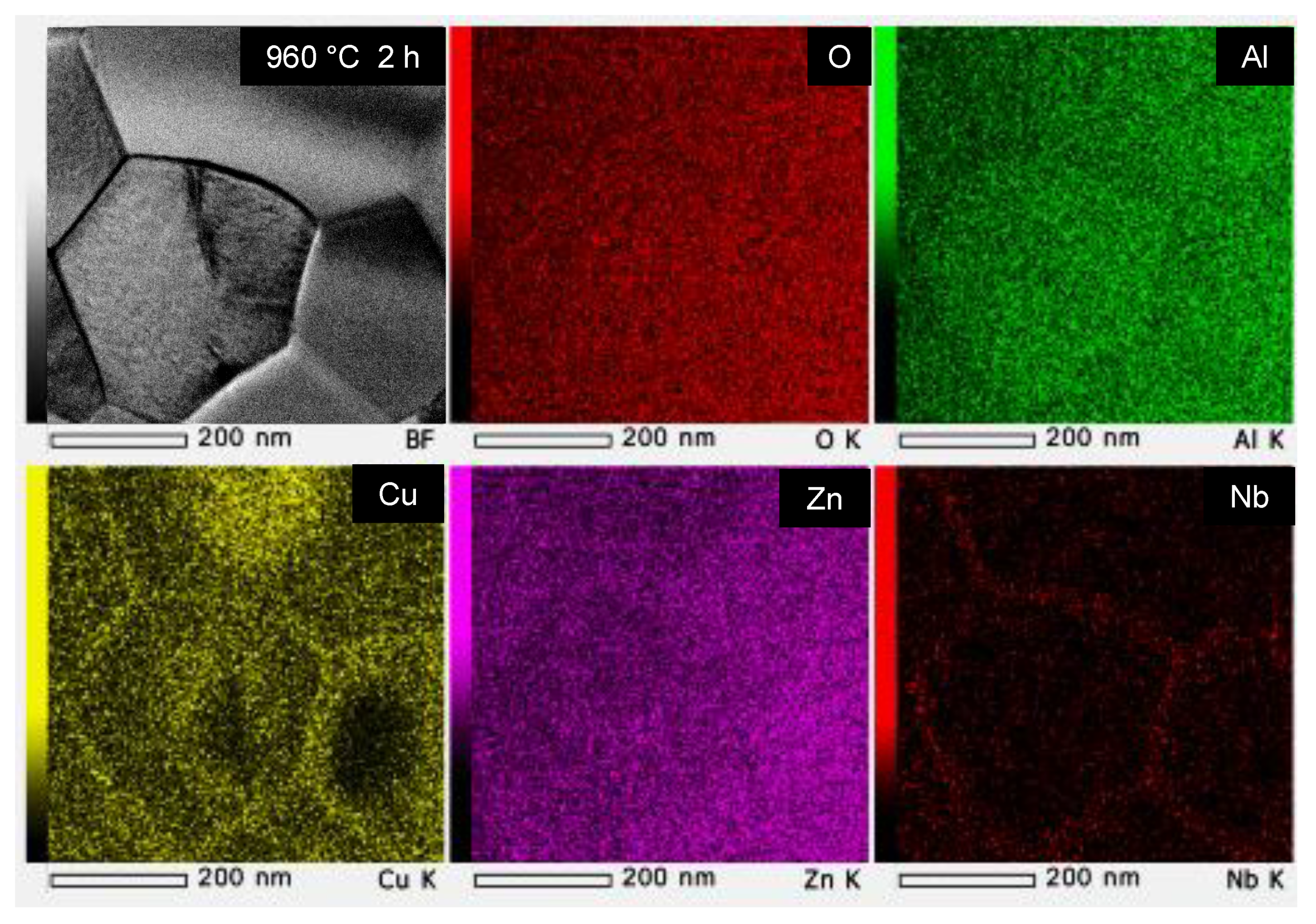
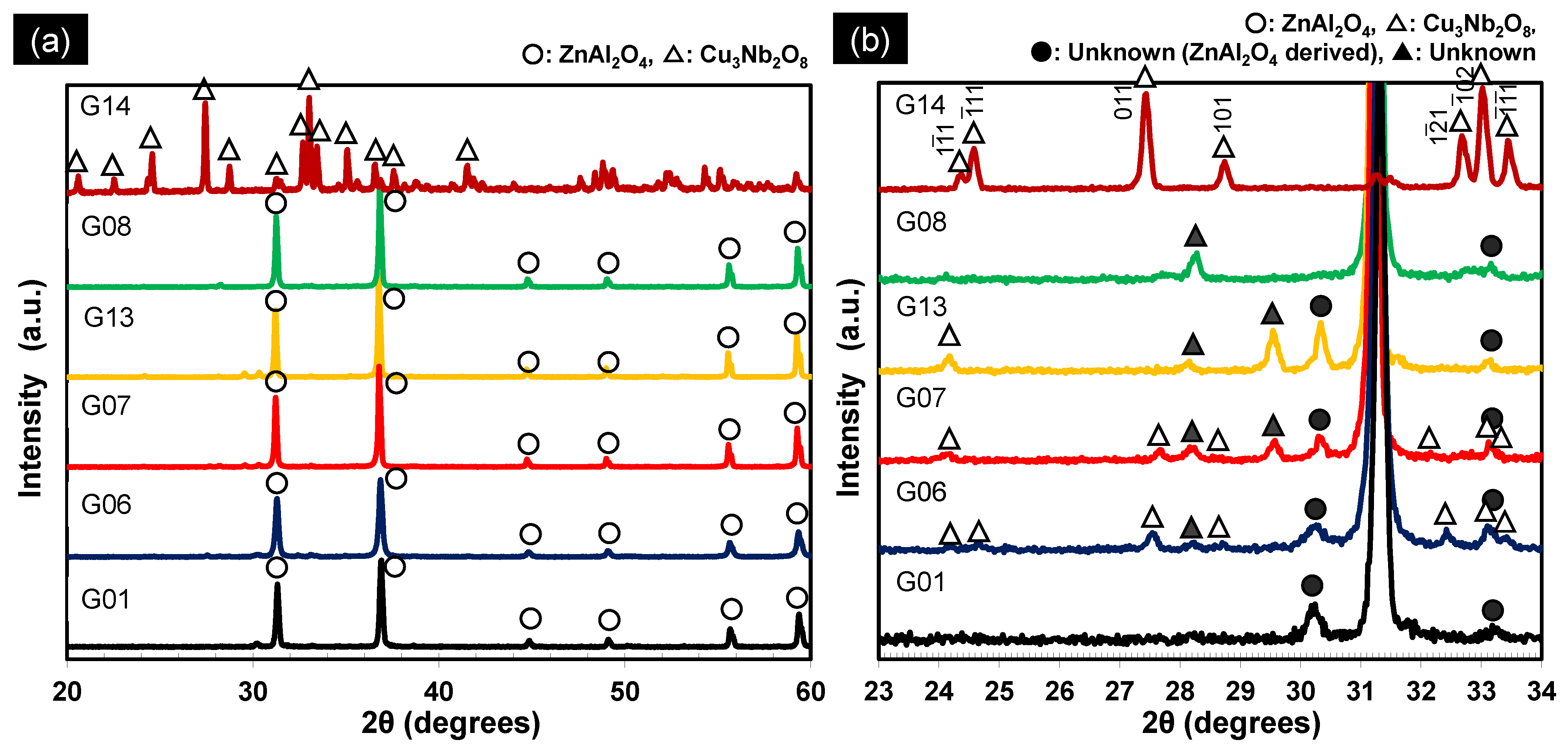
3.1. Effect of the Cu3Nb2O8 Additive and Its Calcination on the Properties of ZnAl2O4
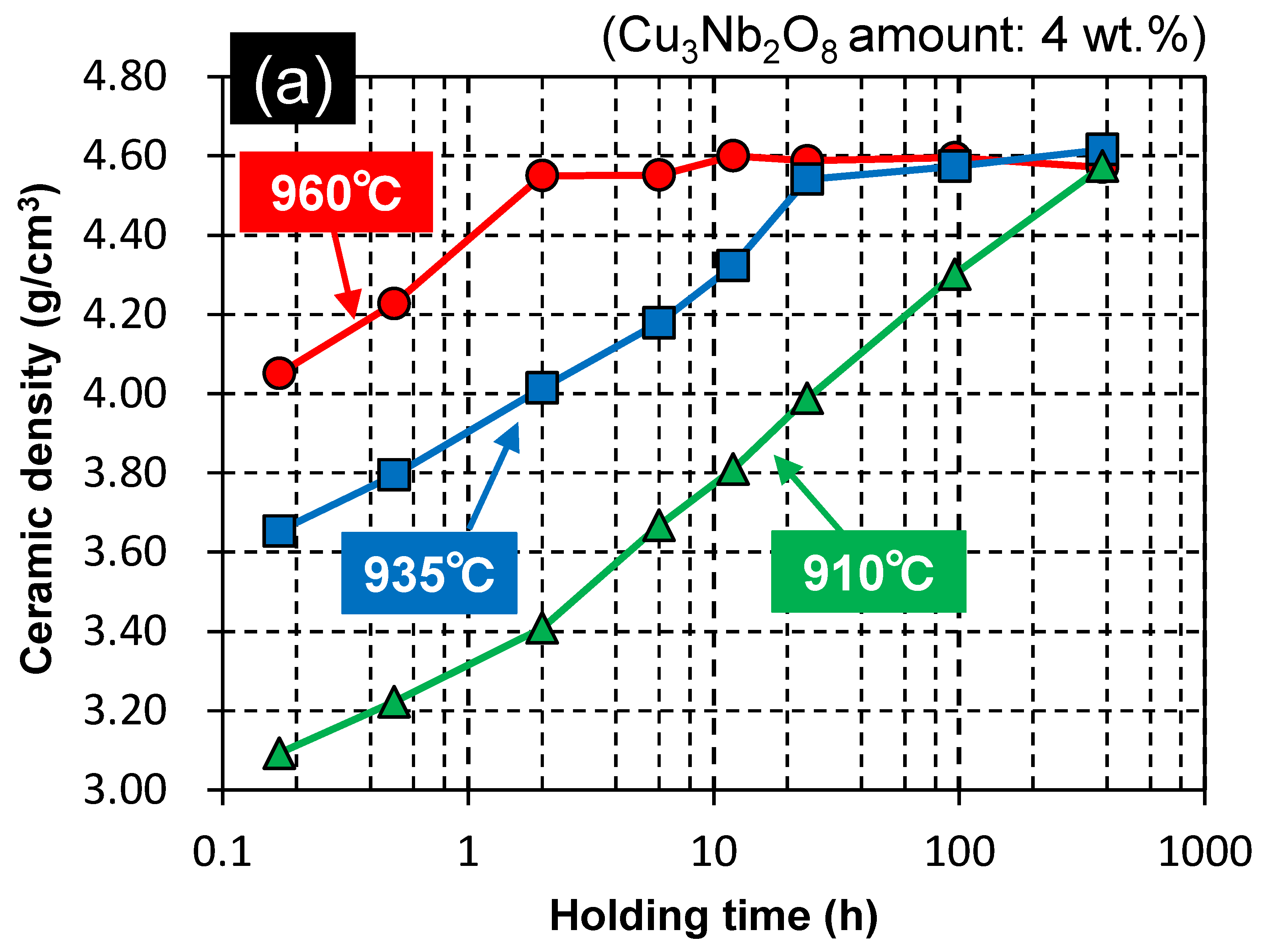
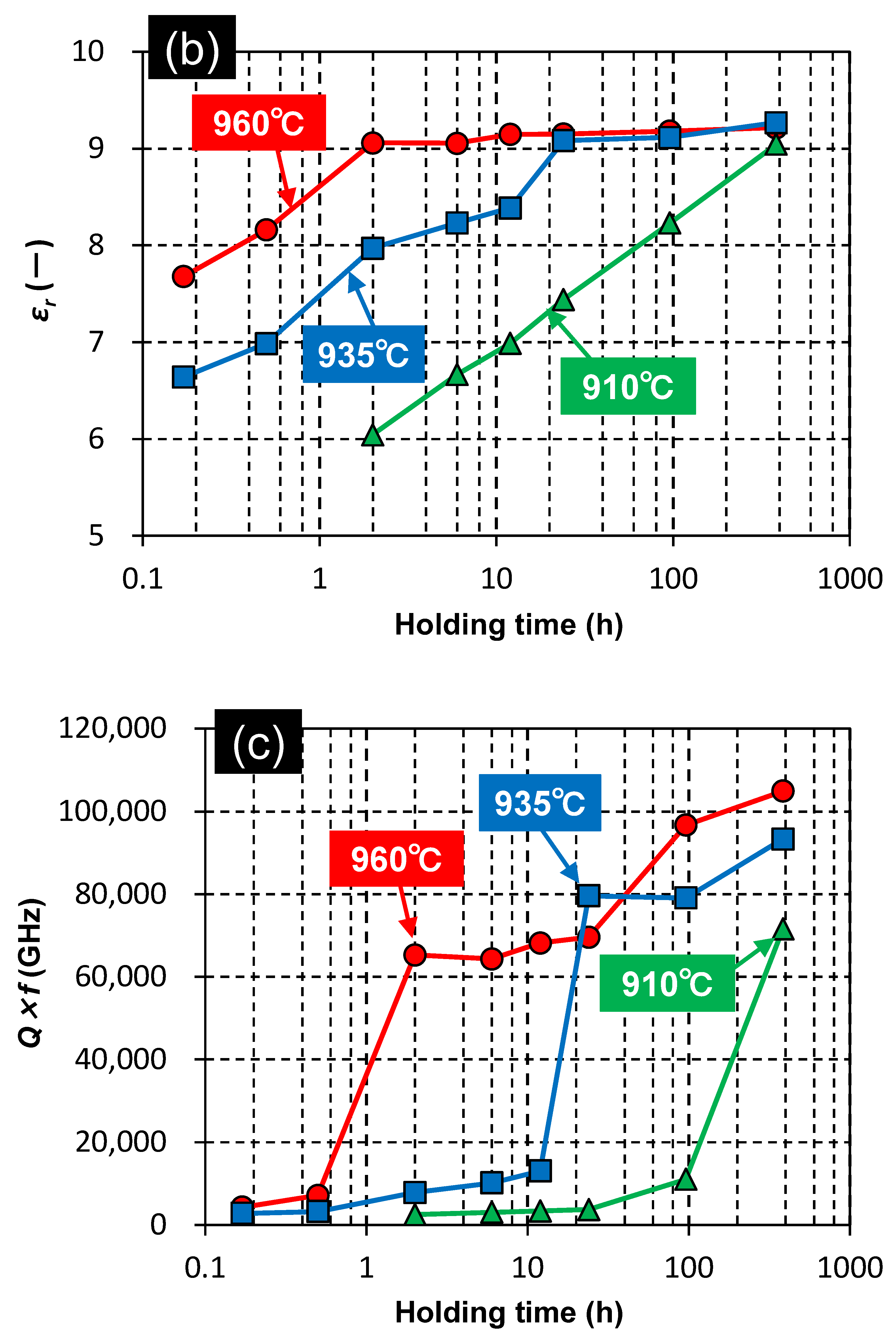
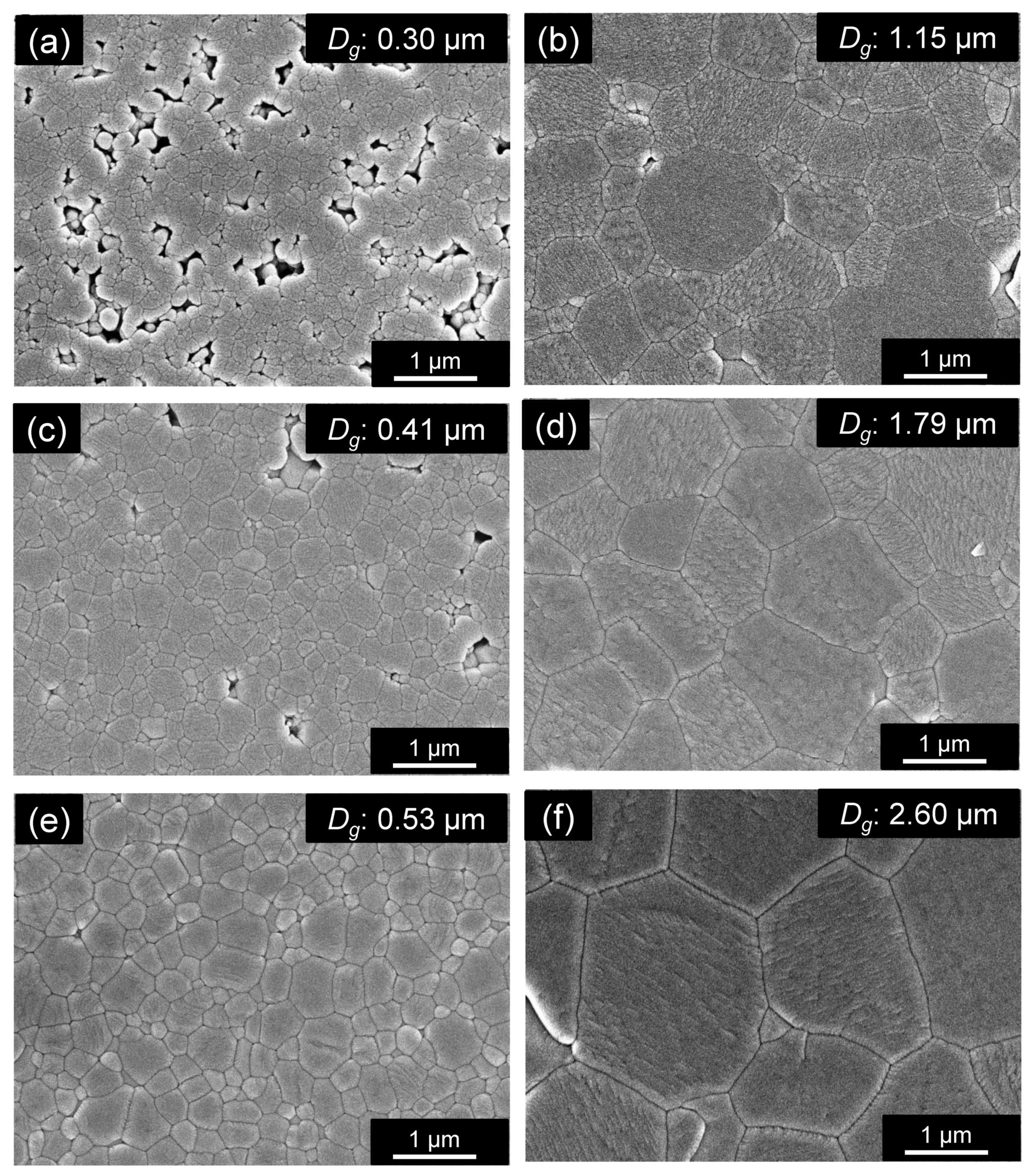
3.2. Effect of Holding Time on the Properties of ZnAl2O4
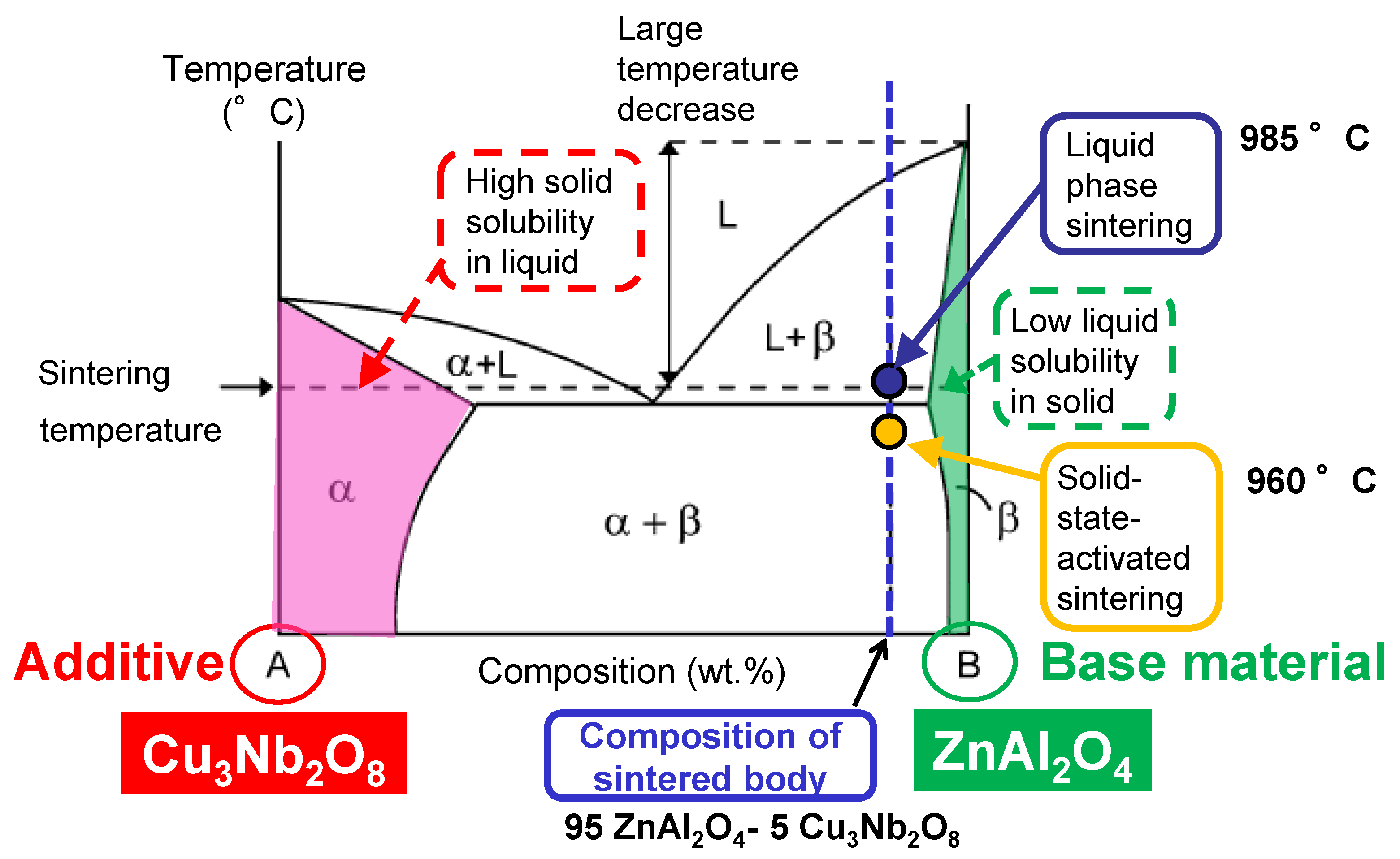
3.3. Proposed Sintering Mechanism
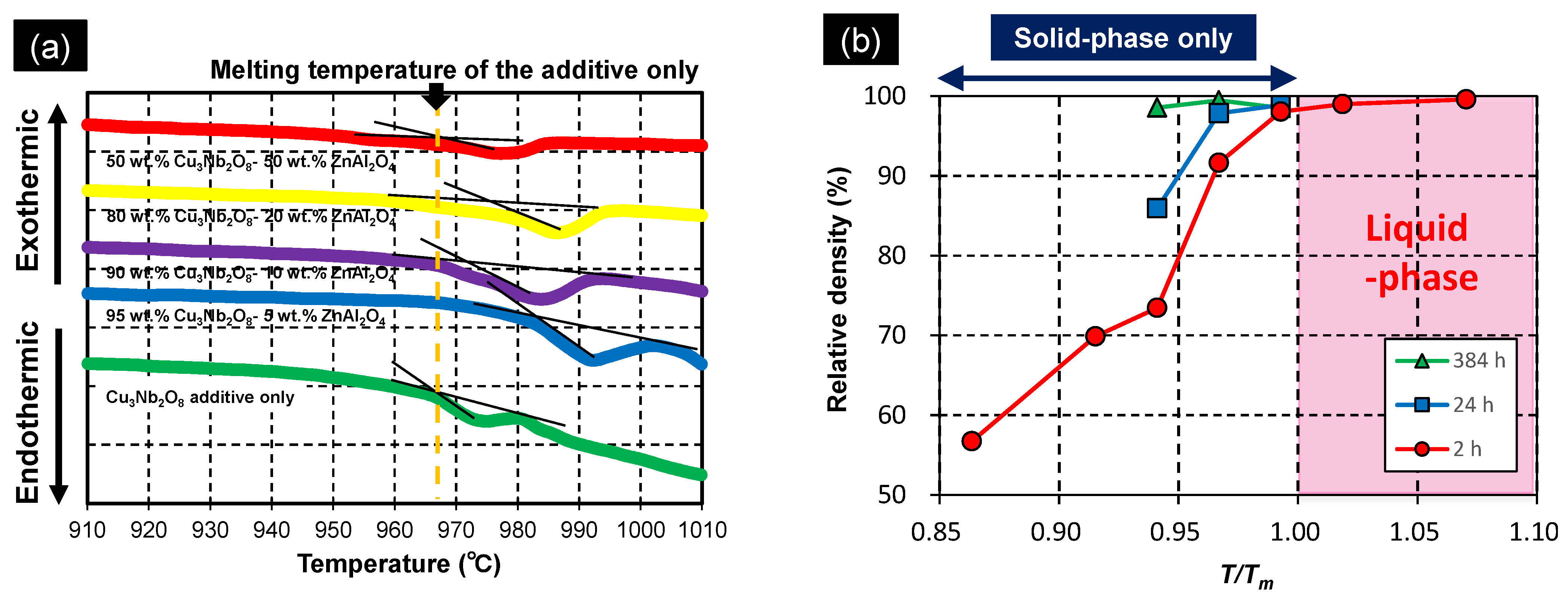
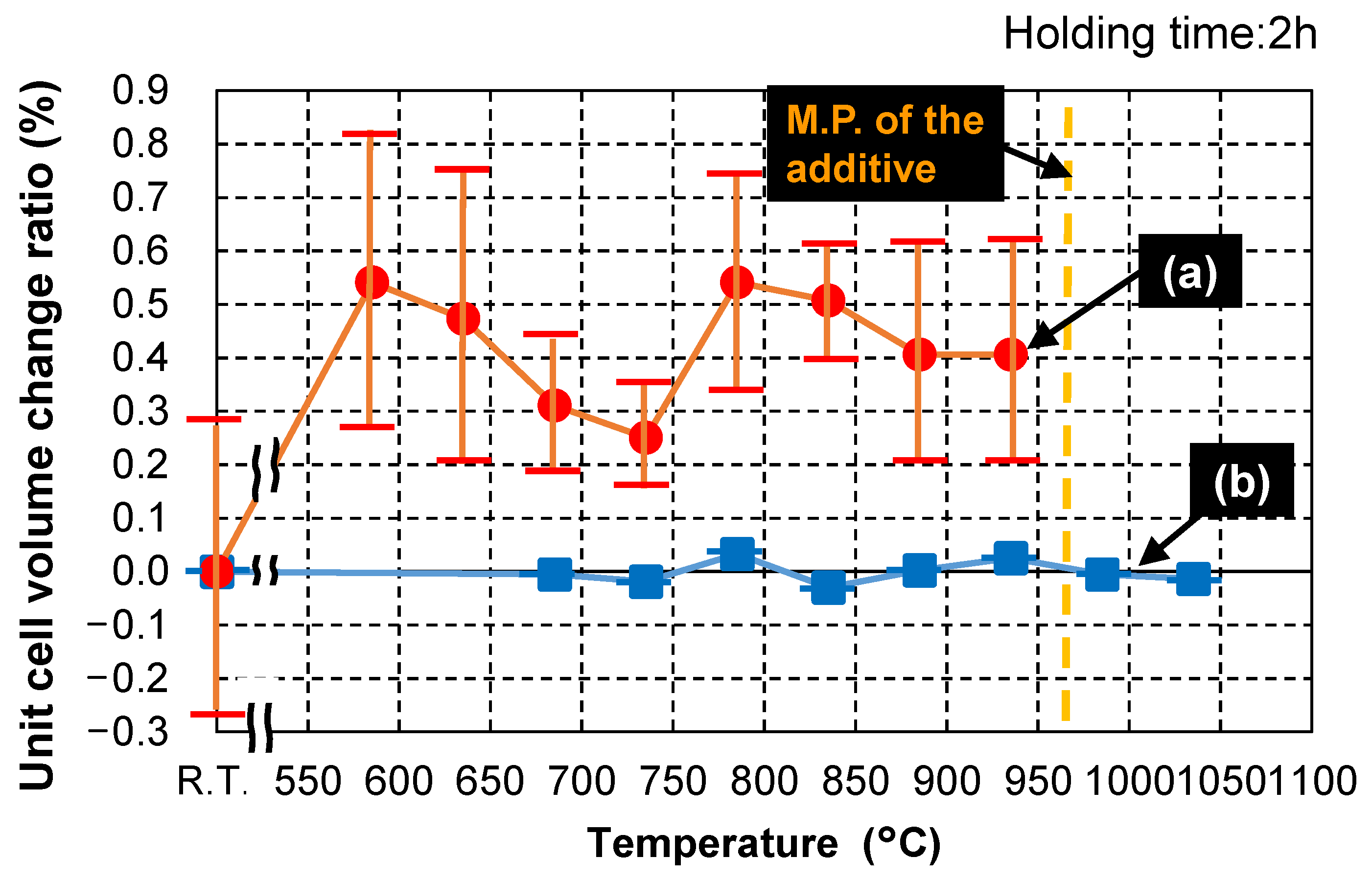
3.3.1. Melting Temperature
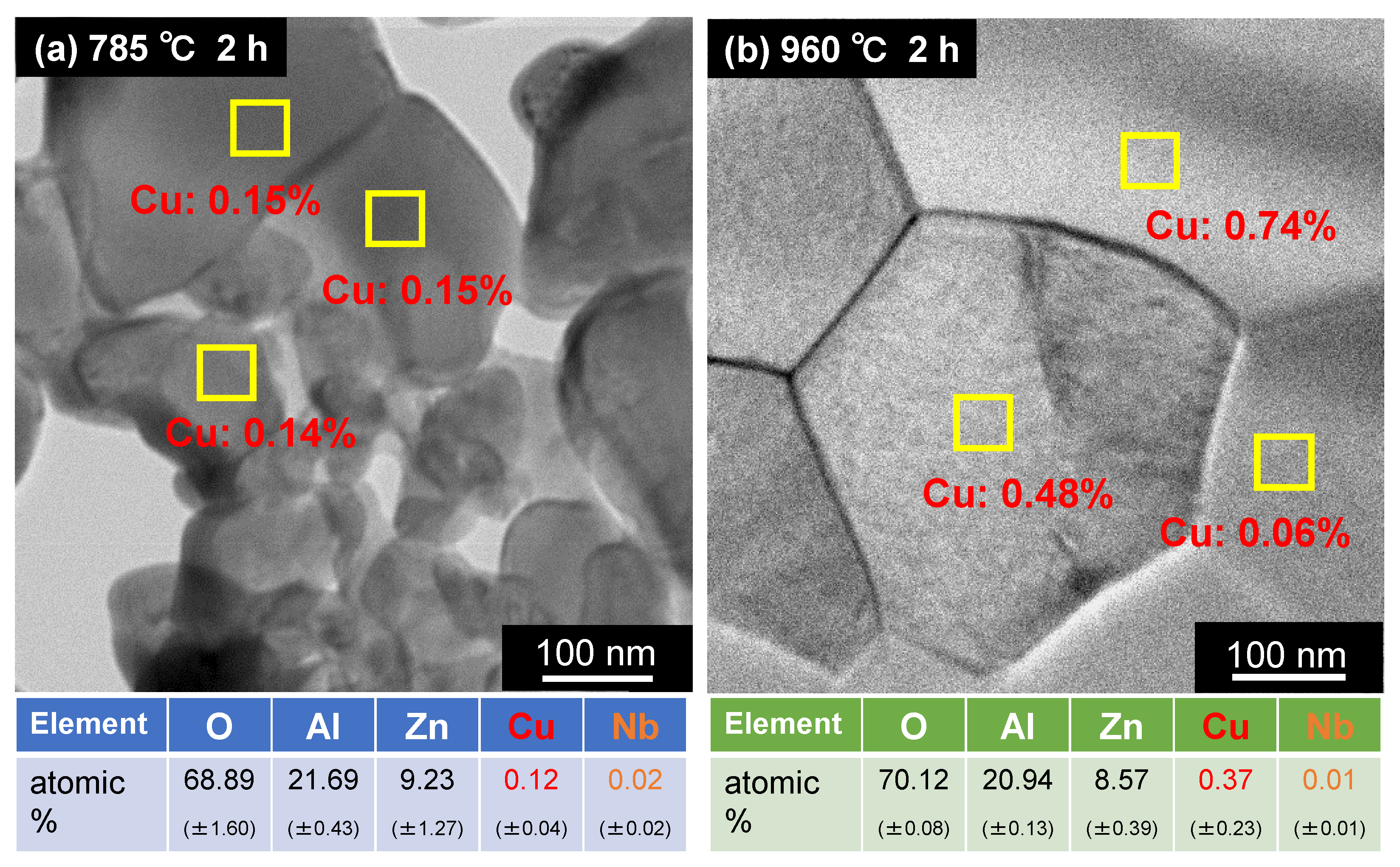
3.3.2. Degree of Solid Solution
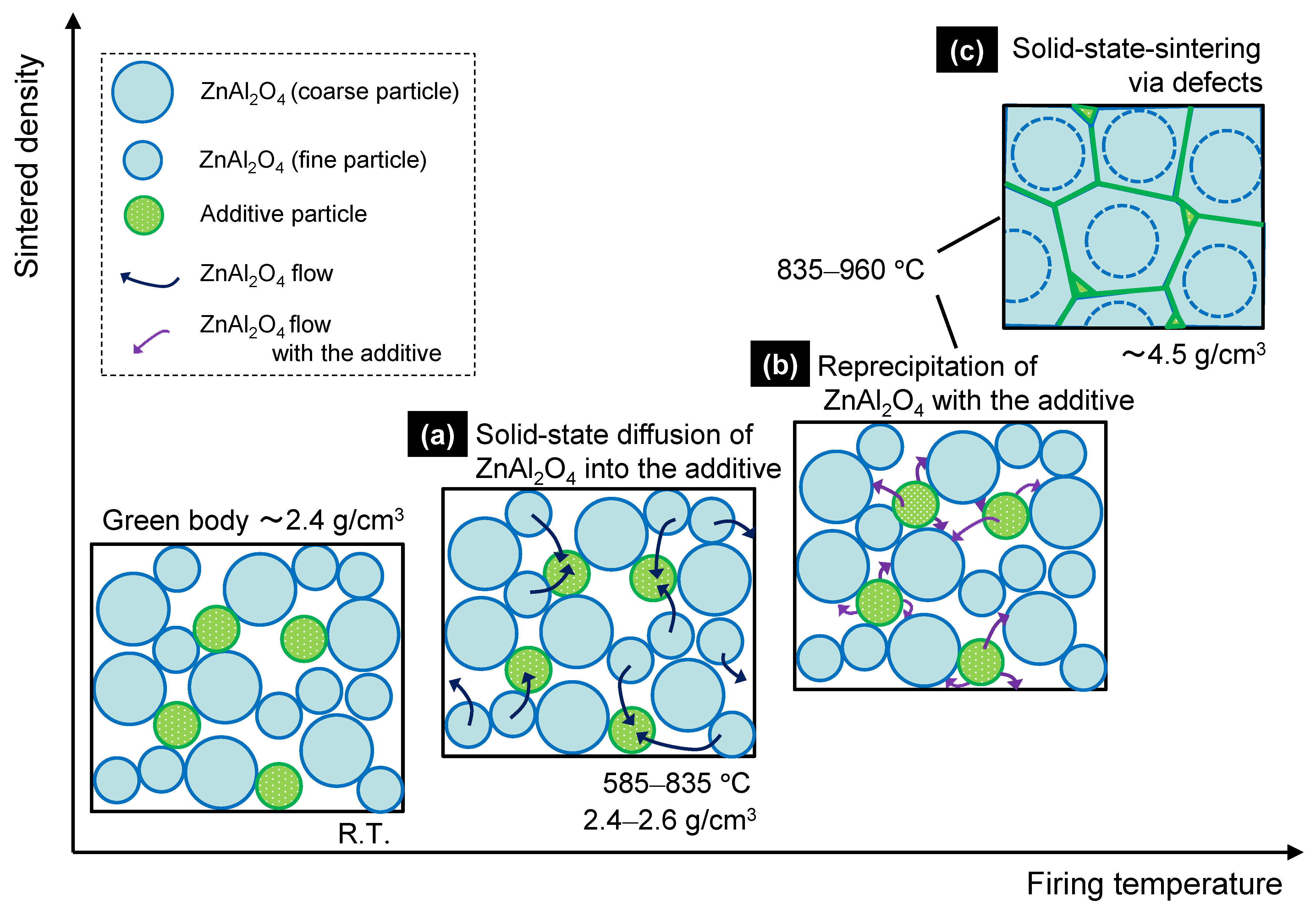
3.3.3. Solid-State-Activated Sintering Model in This Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Imanaka, Y. Multilayered Low Temperature Cofired Ceramics (LTCC) Technology; Springer: New York, NY, USA, 2005; pp. 1–18. [Google Scholar]
- Sebastian, M.T. Dielectric Materials for Wireless Communication; Elsevier: New York, NY, USA, 2008; pp. 445–512. [Google Scholar]
- Sebastian, M.T.; Wang, H.; Jantunen, H. Low temperature co-fired ceramics with ultra-low sintering temperature: A review. Curr. Opin. Solid State Mater. Sci. 2016, 20, 151–170. [Google Scholar] [CrossRef]
- Sebastian, M.T.; Jantunen, H. Low loss dielectric materials for LTCC applications. Int. Mater. Rev. 2008, 53, 58–90. [Google Scholar] [CrossRef]
- Induja, I.J.; Abhilash, P.; Arun, S.; Surendran, K.P.; Sebastian, M.T. LTCC tapes based on Al2O3–BBSZ glass with improved thermal conductivity. Ceram. Int. 2015, 41, 13572–13581. [Google Scholar] [CrossRef]
- Yin, L.; Yang, L.; Yang, W.; Guo, Y.; Ma, K.; Li, S.; Zhang, J. Thermal design and analysis of multi-chip LED module with ceramic substrate. Solid-State Electron. 2010, 54, 1520–1524. [Google Scholar] [CrossRef]
- Shimizu, M.; Tomioka, S.; Yoshida, N.; Kameyama, I.; Okuda, K. Varistor and Its Manufacturing Method. Japanese Patent Publication No. 2015-156406, 2015. [Google Scholar]
- Shigeno, K.; Katsumura, H.; Kagata, H.; Asano, H.; Inoue, O. Preparation and characterization of low temperature sintered alumina by CuO-TiO2-Nb2O5-Ag2O additives. Ferroelectrics 2007, 356, 189–196. [Google Scholar] [CrossRef]
- Shigeno, K.; Kuraoka, Y.; Asakawa, T.; Fujimori, H. Sintering mechanism of low-temperature co-fired alumina featuring superior thermal conductivity. J. Am. Ceram. Soc. 2021, 104, 2017–2029. [Google Scholar] [CrossRef]
- Surendran, K.P.; Santha, N.; Mohanan, P.; Sebastian, M.T. Temperature stable low loss ceramic dielectrics in (1−x) ZnA12O4–xTiO2 system for microwave substrate applications. Eur. Phys. J. 2004, B41, 301–306. [Google Scholar] [CrossRef]
- Surendran, K.P.; Sebastian, M.T.; Manjusha, M.V.; Philip, J. A low loss, dielectric substrate in ZnA12O4–TiO2 system for microelectronic applications. J. Appl. Phys. 2005, 98, 044101. [Google Scholar] [CrossRef]
- Roshni, S.; Sebastian, M.T.; Surendran, K.P. Can zinc aluminate-titania composite be an alternative for alumina as microelectronic substrate? Sci. Rep. 2017, 7, 40839. [Google Scholar] [CrossRef] [Green Version]
- Fu, P.; Wang, Z.Y.; Lin, Z.D.; Liu, Y.Q.; Roy, V.A.L. The microwave dielectric properties of transparent ZnAl2O4 ceramics fabricated by spark plasma sintering. J. Mater. Sci. Mater. Electron. 2017, 28, 9589–9595. [Google Scholar] [CrossRef]
- Thomas, S.; Sebastian, M.T. Effect of B2O3-Bi2O3-SiO2-ZnO glass on the sintering and microwave dielectric properties of 0.83ZnAl2O4-0.17TiO2. Mater. Res. Bull. 2008, 43, 843–851. [Google Scholar] [CrossRef]
- Takahashi, S.; Kan, A.; Ogawa, H. Microwave dielectric properties and cation distributions of Zn1-3xAl2+2xO4 ceramics with defect structures. J. Eur. Ceram. Soc. 2017, 37, 3059–3064. [Google Scholar] [CrossRef]
- Lan, X.-K.; Li, J.; Zou, Z.-Y.; Xie, M.-Q.; Fan, G.-F.; Lu, W.-Z.; Lei, W. Improved sinterability and microwave dielectric properties of [Zn0.5Ti0.5]3+-doped ZnAl2O4 spinel solid solution. J. Am. Ceram. Soc. 2019, 102, 5952–5957. [Google Scholar] [CrossRef]
- Shigeno, K.; Kaneko, S.; Nakashima, H.; Suenaga, K.; Fujimori, H. Low-temperature sintering of gahnite ceramic using Cu-Nb-O additive and evaluation of dielectric and thermal properties. J. Electron. Mater. 2020, 49, 6046–6054. [Google Scholar] [CrossRef]
- Nunes, S.I.; Bradt, R.C. Grain growth of ZnO in ZnO-Bi2O3 ceramics with Al2O3 additions. J. Am. Ceram. Soc. 1995, 78, 2469–2475. [Google Scholar] [CrossRef]
- Kim, D.-W.; Kim, I.-T.; Park, B.; Hong, K.-S.; Kim, J.-H. Microwave dielectric properties of (1−x)Cu3Nb2O8−xZn3Nb2O8 ceramics. J. Mater. Res. 2001, 16, 1465–1470. [Google Scholar] [CrossRef]
- Matsui, K.; Matsumoto, A.; Uehara, M.; Enomoto, N.; Hojo, J. Sintering kinetics at isothermal shrinkage: Effect of specific surface area on the initial sintering stage of fine zirconia powder. J. Am. Ceram. Soc. 2007, 90, 44–49. [Google Scholar] [CrossRef]
- Takada, T.; Wang, S.F.; Yoshikawa, S.; Jang, S.-J.; Newnham, R.E. Effect of glass addition on BaO-TiO2-WO3 microwave ceramics. J. Am. Ceram. Soc. 1994, 77, 1909–1916. [Google Scholar] [CrossRef]
- Hakki, B.W.; Coleman, P.D. A dielectric resonator method of measuring inductive capacities in the millimeter range. IRE Trans. Microw. Theory Tech. 1960, 8, 402–410. [Google Scholar] [CrossRef]
- Yamaguchi, T. Characterization techniques of ceramic: Properties of sintered bodies. Ceram. Jpn. 1984, 19, 520–529. [Google Scholar]
- Tamura, H. Microwave dielectric losses caused by lattice defects. J. Eur. Ceram. Soc. 2006, 26, 1775–1780. [Google Scholar] [CrossRef]
- Zheng, C.W.; Fan, X.C.; Chen, X.M. Analysis of infrared reflection spectra of (Mg1−xZnx)Al2O4 microwave dielectric ceramics. J. Am. Ceram. Soc. 2008, 91, 490–493. [Google Scholar] [CrossRef]
- Penn, S.J.; Alford, N.M.; Templeton, A.; Wang, X.; Xu, M.; Reece, M.; Schrapel, K. Effect of porosity and grain size on the microwave dielectric properties of sintered alumina. J. Am. Ceram. Soc. 1997, 80, 1885–1888. [Google Scholar] [CrossRef]
- German, R.M.; Farooq, S.; Kipphut, C.M. Kinetics of liquid sintering. Mater. Sci. Eng. A 1988, 105/106, 215–224. [Google Scholar] [CrossRef]
- German, R.M.; Suri, P.; Park, S.J. Liquid phase sintering. J. Mater. Sci. 2009, 44, 1–39. [Google Scholar] [CrossRef] [Green Version]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Phillips, D.S.; Mitchell, T.E.; Heuer, A.H. Precipitation in star sapphire III. Chemical effects accompanying precipitation. Philos. Mag. 1980, A42, 417–432. [Google Scholar] [CrossRef]
- Kingery, W.D.; Bowen, H.K.; Uhlmann, D.R. Introduction to Ceramics, 2nd ed.; Wiley: New York, NY, USA, 1976; pp. 413–429. [Google Scholar]
- Liu, H.-H.; Jean, J.-H. Processing and properties of a low-fire, high-thermal-conductivity alumina with CuTiNb2O8. Int. J. Ceram. Eng. Sci. 2020, 2, 38–45. [Google Scholar] [CrossRef] [Green Version]


| Sample | ZnAl2O4 Quantity | Additive Quantity | Additive Conditions | Firing Temperature | Holding Time | Comparison Purpose |
|---|---|---|---|---|---|---|
| wt.% | °C | h | ||||
| G01 | 100 | 0 | Calcined | 1485 | 2 | *1) |
| G02 | 99 | 1 | Calcined | 1185 | 2 | *1) |
| G03 | 98 | 2 | Calcined | 1085 | 2 | *1) |
| G04 | 97 | 3 | Calcined | 1035 | 2 | *1) |
| G05 | 96 | 4 | Calcined | 960 | 2 | *1) *4) |
| G06 | 95 | 5 | Calcined | 785 | 2 | *2) |
| G07 | 95 | 5 | Calcined | 960 | 2 | *1) *2) *3) |
| G08 | 95 | 5 | Non-calcined | 960 | 2 | *3) |
| G09 | 96 | 4 | Calcined | 910 | 2 | *4) |
| G10 | 96 | 4 | Calcined | 910 | 384 | *4) |
| G11 | 96 | 4 | Calcined | 935 | 2 | *4) |
| G12 | 96 | 4 | Calcined | 935 | 384 | *4) |
| G13 | 96 | 4 | Calcined | 960 | 384 | *4) |
| G14 | 0 | 100 | Calcined | 885 | 2 | *1) |
| Sample | Additive Quantity | Additive Conditions | Firing Temp. | Holding Time | Ceramic Density | Relative Density | Dg | εr | Q × f | τf | κ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| wt.% | °C | h | g/cm3 | % | μm | — | GHz | ppm·K−1 | W·m−1·K−1 | ||
| G01 | 0 | Calcined | 1485 | 2 | 4.47 | 97.0 | 2.10 | 9.0 | 16,100 | −73 | 27.3 |
| G02 | 1 | Calcined | 1185 | 2 | 4.50 | 97.5 | 3.10 | 9.0 | 98,200 | −63 | 12.2 |
| G03 | 2 | Calcined | 1085 | 2 | 4.57 | 98.9 | 1.36 | 9.1 | 81,700 | −60 | 10.2 |
| G04 | 3 | Calcined | 1035 | 2 | 4.60 | 99.4 | 0.89 | 9.3 | 77,000 | −60 | 8.8 |
| G05 | 4 | Calcined | 960 | 2 | 4.55 | 98.1 | 0.53 | 9.1 | 65,300 | −54 | 8.9 |
| G06 | 5 | Calcined | 785 | 2 | 2.56 | 55.0 | 0.20 | ✕ | ✕ | ✕✕ | ✕✕ |
| G07 | 5 | Calcined | 960 | 2 | 4.55 | 97.8 | 0.55 | 9.2 | 60,800 | −56 | 7.9 |
| G08 | 5 | Non-calcined | 960 | 2 | 4.52 | 97.2 | 0.57 | 9.0 | 51,300 | −63 | 8.5 |
| G09 | 4 | Calcined | 910 | 2 | 3.41 | 73.5 | 0.30 | 6.0 | 2480 | ×× | ×× |
| G10 | 4 | Calcined | 910 | 384 | 4.57 | 98.6 | 1.15 | 9.0 | 71,500 | −54 | 11.3 |
| G11 | 4 | Calcined | 935 | 2 | 4.01 | 86.4 | 0.41 | 8.0 | 7840 | ×× | ×× |
| G12 | 4 | Calcined | 935 | 384 | 4.62 | 99.5 | 1.79 | 9.3 | 93,300 | −61 | 10.2 |
| G13 | 4 | Calcined | 960 | 384 | 4.57 | 98.5 | 2.60 | 9.2 | 105,000 | −56 | 10.1 |
| G14 | 100 | Calcined | 885 | 2 | 5.37 | 95.0 | 10.1 | 15.1 | 18,700 | −70 | 6.1 |
| R01* | — | — | 900–1100 [4] | — | — | — | — | <15 [4,5] | >1000 [4] | <±100 [4] | 2–7 [4,5] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shigeno, K.; Yano, T.; Fujimori, H. Solid-State-Activated Sintering of ZnAl2O4 Ceramics Containing Cu3Nb2O8 with Superior Dielectric and Thermal Properties. Materials 2022, 15, 1770. https://doi.org/10.3390/ma15051770
Shigeno K, Yano T, Fujimori H. Solid-State-Activated Sintering of ZnAl2O4 Ceramics Containing Cu3Nb2O8 with Superior Dielectric and Thermal Properties. Materials. 2022; 15(5):1770. https://doi.org/10.3390/ma15051770
Chicago/Turabian StyleShigeno, Koichi, Takuma Yano, and Hirotaka Fujimori. 2022. "Solid-State-Activated Sintering of ZnAl2O4 Ceramics Containing Cu3Nb2O8 with Superior Dielectric and Thermal Properties" Materials 15, no. 5: 1770. https://doi.org/10.3390/ma15051770






