The Effect of Y3+ Addition on Morphology, Structure, and Electrical Properties of Yttria-Stabilized Tetragonal Zirconia Dental Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Method for Surface Investigation
2.3. Method for Structural Identification
2.4. Electrical Properties
3. Results and Discussion
3.1. Morphology Investigation
3.2. Structural Investigation
3.3. Electrical Investigation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manicone, P.F.; Rossi Iommetti, P.; Raffaelli, L. An Overview of Zirconia Ceramics: Basic Properties and Clinical Applications. J. Dent. 2007, 35, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Denry, I.; Kelly, J.R. State of the Art of Zirconia for Dental Applications. Dent. Mater. 2008, 24, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Ahamer, C.; Opitz, A.K.; Rupp, G.M.; Fleig, J. Revisiting the Temperature Dependent Ionic Conductivity of Yttria Stabilized Zirconia (YSZ). J. Electrochem. Soc. 2017, 164, F790–F803. [Google Scholar] [CrossRef]
- Al-baadani, A.; Sherief, R.; Kheireldean, A. Evaluation of internal adaptation of full contour zirconia crowns versus veneered zirconia crowns – in vitro study. Egypt. Dent. J. 2016, 62, 717–719. [Google Scholar] [CrossRef]
- Cotič, J.; Kocjan, A.; Panchevska, S.; Kosmač, T.; Jevnikar, P. In Vivo Ageing of Zirconia Dental Ceramics—Part II: Highly-Translucent and Rapid-Sintered 3Y-TZP. Dent. Mater. 2021, 37, 454–463. [Google Scholar] [CrossRef]
- Conrad, H.J.; Seong, W.-J.; Pesun, I.J. Current Ceramic Materials and Systems with Clinical Recommendations: A Systematic Review. J. Prosthet. Dent. 2007, 98, 389–404. [Google Scholar] [CrossRef]
- Mörmann, W.H. State of the Art of CAD/CAM Restorations: 20 Years of CEREC; Quintessence Publishing Company: New Malden, UK, 2006; ISBN 9781850971641. [Google Scholar]
- Jakovac, M.; Klaser, T.; Radatović, B.; Bafti, A.; Skoko, Ž.; Pavić, L.; Žic, M. Impact of Sandblasting on Morphology, Structure and Conductivity of Zirconia Dental Ceramics Material. Materials 2021, 14, 2834. [Google Scholar] [CrossRef]
- Jakovac, M.; Klaser, T.; Radatović, B.; Skoko, Ž.; Pavić, L.; Žic, M. Surface Characterization and Conductivity of Two Types of Lithium-Based Glass Ceramics after Accelerating Ageing. Materials 2020, 13, 5632. [Google Scholar] [CrossRef]
- Wokaun, C. XRD and Raman Identification of the Zirconia Modifications in Copper/zirconia and Palladium/zirconia Catalysts Prepared from Amorphous Precursors. J. Catal. 1991, 130, 657–661. [Google Scholar] [CrossRef]
- Kurpaska, L. Structural Properties of Zirconia – in-Situ High Temperature XRD Characterization. J. Mol. Struct. 2018, 1163, 287–293. [Google Scholar] [CrossRef]
- Jouili, M.; Andrieux, M.; Gallet, I.; Prud’Homme, N.; Ji, V. Texture and Residual Stress Analysis by XRD on Metastable Tetragonal Zirconia Films Obtained by MOCVD. ECS Trans. 2009, 25, 413–420. [Google Scholar] [CrossRef]
- Kim, H.-K.; Ahn, B. Effect of AlO Sandblasting Particle Size on the Surface Topography and Residual Compressive Stresses of Three Different Dental Zirconia Grades. Materials 2021, 14, 610. [Google Scholar] [CrossRef]
- Pereira, P.C.; Tango, R.N. Do the Different Sandblasting Protocols Influence on Flexural Strength of Zirconia Ceramic? Dent. Mater. 2013, 29, e22. [Google Scholar] [CrossRef]
- Tammam, R. Effect of sandblasting of zirconia abutment on surface roughness and bacterial adhesion. Egypt. Dent. J. 2017, 63, 1827–1831. [Google Scholar] [CrossRef]
- Žic, M. An Alternative Approach to Solve Complex Nonlinear Least-Squares Problems. J. Electroanal. Chem. 2016, 760, 85–96. [Google Scholar] [CrossRef]
- Žic, M. Solving CNLS Problems by Using Levenberg-Marquardt Algorithm: A New Approach to Avoid off-Limits Values during a Fit. J. Electroanal. Chem. 2017, 799, 242–248. [Google Scholar] [CrossRef]
- Žic, M.; Subotić, V.; Pereverzyev, S.; Fajfar, I. Solving CNLS Problems Using Levenberg-Marquardt Algorithm: A New Fitting Strategy Combining Limits and a Symbolic Jacobian Matrix. J. Electroanal. Chem. 2020, 866, 114171. [Google Scholar] [CrossRef]
- Boukamp, B. A Nonlinear Least Squares Fit Procedure for Analysis of Immittance Data of Electrochemical Systems. Solid State Ion. 1986, 20, 31–44. [Google Scholar] [CrossRef] [Green Version]
- Žic, M.; Pereverzyev, S., Jr.; Subotić, V.; Pereverzyev, S. Adaptive Multi-Parameter Regularization Approach to Construct the Distribution Function of Relaxation Times. GEM 2020, 11, 2. [Google Scholar] [CrossRef] [Green Version]
- Wan, T.H.; Saccoccio, M.; Chen, C.; Ciucci, F. Influence of the Discretization Methods on the Distribution of Relaxation Times Deconvolution: Implementing Radial Basis Functions with DRTtools. Electrochim. Acta 2015, 184, 483–499. [Google Scholar] [CrossRef]
- Žic, M.; Vlašić, L.; Subotic, V.; Pereverzyev, S.; Fajfar, I.; Kunaver, M. Extraction of Distribution Function of Relaxation Times by Using Levenberg-Marquardt Algorithm: A New Approach to Apply a Discretization Error Free Jacobian Matrix. J. Electrochem. Soc. 2022. [Google Scholar] [CrossRef]
- Badwal, S. Zirconia-Based Solid Electrolytes: Microstructure, Stability and Ionic Conductivity. Solid State Ionics 1992, 52, 23–32. [Google Scholar] [CrossRef]
- Chen, X.J.; Khor, K.A.; Chan, S.H.; Yu, L.G. Influence of Microstructure on the Ionic Conductivity of Yttria-Stabilized Zirconia Electrolyte. Mater. Sci. Eng. A Struct. Mater. 2002, 335, 246–252. [Google Scholar] [CrossRef]
- Strasser, T.; Preis, V.; Behr, M.; Rosentritt, M. Roughness, Surface Energy, and Superficial Damages of CAD/CAM Materials after Surface Treatment. Clin. Oral Investig. 2018, 22, 2787–2797. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.R.; Denry, I. Stabilized Zirconia as a Structural Ceramic: An Overview. Dent. Mater. 2008, 24, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Kosmač, T.; Oblak, C.; Jevnikar, P.; Funduk, N.; Marion, L. The Effect of Surface Grinding and Sandblasting on Flexural Strength and Reliability of Y-TZP Zirconia Ceramic. Dent. Mater. 1999, 15, 426–433. [Google Scholar] [CrossRef]
- Chevalier, J.; Gremillard, L.; Virkar, A.V.; Clarke, D.R. The Tetragonal-Monoclinic Transformation in Zirconia: Lessons Learned and Future Trends. J. Am. Ceram. Soc. 2009, 92, 1901–1920. [Google Scholar] [CrossRef]
- Koizumi, M.; Nishihara, M. Isostatic Pressing: Technology and Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1991; ISBN 9781851665969. [Google Scholar]
- Li, K.; Rao, J.; Ning, C. Optimized Sintering and Mechanical Properties of Y-TZP Ceramics for Dental Restorations by Adding Lithium Disilicate Glass Ceramics. J. Adv. Ceram. 2021, 10, 1326–1337. [Google Scholar] [CrossRef]
- Dahl, P.; Kaus, I.; Zhao, Z.; Johnsson, M.; Nygren, M.; Wiik, K.; Grande, T.; Einarsrud, M.-A. Densification and Properties of Zirconia Prepared by Three Different Sintering Techniques. Ceram. Int. 2007, 33, 1603–1610. [Google Scholar] [CrossRef]
- Manière, C.; Lee, G.; McKittrick, J.; Chan, S.; Olevsky, E.A. Modeling Zirconia Sintering Trajectory for Obtaining Translucent Submicronic Ceramics for Dental Implant Applications. Acta Mater. 2020, 188, 101–107. [Google Scholar] [CrossRef]
- da Silva, B.T.F.; Trevelin, L.T.; Schroeter, A.C.; Willers, A.E.; Cesar, P.F.; Matos, A.B. Effect of Silica Coating and Laser Treatment on the Flexural Strength, Surface Characteristics, and Bond Strength of a Dental Zirconia. Eur. J. Oral Sci. 2021, 129, e12754. [Google Scholar] [CrossRef]
- Kwon, S.-M.; Min, B.K.; Kim, Y.K.; Kwon, T.-Y. Influence of Sandblasting Particle Size and Pressure on Resin Bonding Durability to Zirconia: A Residual Stress Study. Materials 2020, 13, 5629. [Google Scholar] [CrossRef] [PubMed]
- Piconi, C.; Maccauro, G. Zirconia as a Ceramic Biomaterial. Biomaterials 1999, 20, 1–25. [Google Scholar] [CrossRef]
- Garvie, R.C.; Nicholson, P.S. Structure and Thermomechanical Properties of Partially Stabilized Zirconia in the CaO-ZrO2 System. J. Am. Ceram. Soc. 1972, 55, 152–157. [Google Scholar] [CrossRef]
- Belli, R.; Hurle, K.; Schürrlein, J.; Petschelt, A.; Werbach, K.; Peterlik, H.; Rabe, T.; Mieller, B.; Lohbauer, U. A Revised Relationship between Fracture Toughness and Y2O3 Content in Modern Dental Zirconias. ChemRxiv 2021. [Google Scholar] [CrossRef]
- Camposilvan, E.; Leone, R.; Gremillard, L.; Sorrentino, R.; Zarone, F.; Ferrari, M.; Chevalier, J. Aging Resistance, Mechanical Properties and Translucency of Different Yttria-Stabilized Zirconia Ceramics for Monolithic Dental Crown Applications. Dent. Mater. 2018, 34, 879–890. [Google Scholar] [CrossRef]
- Badwal, S.P.S.; Ciacchi, F.T. Oxygen-Ion Conducting Electrolyte Materials for Solid Oxide Fuel Cells. Ionics 2000, 6, 1–21. [Google Scholar] [CrossRef]
- Subbarao, E.; Maiti, H. Solid Electrolytes with Oxygen Ion Conduction. Solid State Ion. 1984, 11, 317–338. [Google Scholar] [CrossRef]
- Jiang, S. An Electrode Kinetics Study of H2 Oxidation on Ni/Y2O3âZrO2 Cermet Electrode of the Solid Oxide Fuel Cell. Solid State Ion. 1999, 123, 209–224. [Google Scholar] [CrossRef]
- Barsoukov, E.; Ross Macdonald, J. Impedance Spectroscopy: Theory, Experiment, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2005; ISBN 9780471716228. [Google Scholar]
- Yamamoto, O.; Takeda, Y.; Kanno, R.; Kohno, K. Electrical Conductivity of Tetragonal Stabilized Zirconia. J. Mater. Sci. 1990, 25, 2805–2808. [Google Scholar] [CrossRef]
- Levenberg, K. A Method for the Solution of Certain Non-Linear Problems in Least Squares. Quart. Appl. Math. 1944, 2, 164–168. [Google Scholar] [CrossRef] [Green Version]
- Marquardt, D.W. An Algorithm for Least-Squares Estimation of Nonlinear Parameters. J. Soc. Ind. Appl. Math. 1963, 11, 431–441. [Google Scholar] [CrossRef]
- Palmour, H., III; Davis, R.F.; Hare, T.M. (Eds.) Processing of Crystalline Ceramics; Springer: New York, NY, USA, 2012; ISBN 9781468433807. [Google Scholar]
- Moguš-Milanković, A.; Pavić, L.; Srilatha, K.; Srinivasa Rao, C.; Srikumar, T.; Gandhi, Y.; Veeraiah, N. Electrical, Dielectric and Spectroscopic Studies on MnO Doped LiI–AgI–B2O3 Glasses. J. Appl. Phys. 2012, 111, 013714. [Google Scholar] [CrossRef] [Green Version]
- Boora, M.; Malik, S.; Kumar, V.; Bala, M.; Arora, S.; Rohilla, S.; Kumar, A.; Dalal, J. Investigation of Structural and Impedance Spectroscopic Properties of Borate Glasses with High Li+ Concentration. Solid State Ion. 2021, 368, 115704. [Google Scholar] [CrossRef]
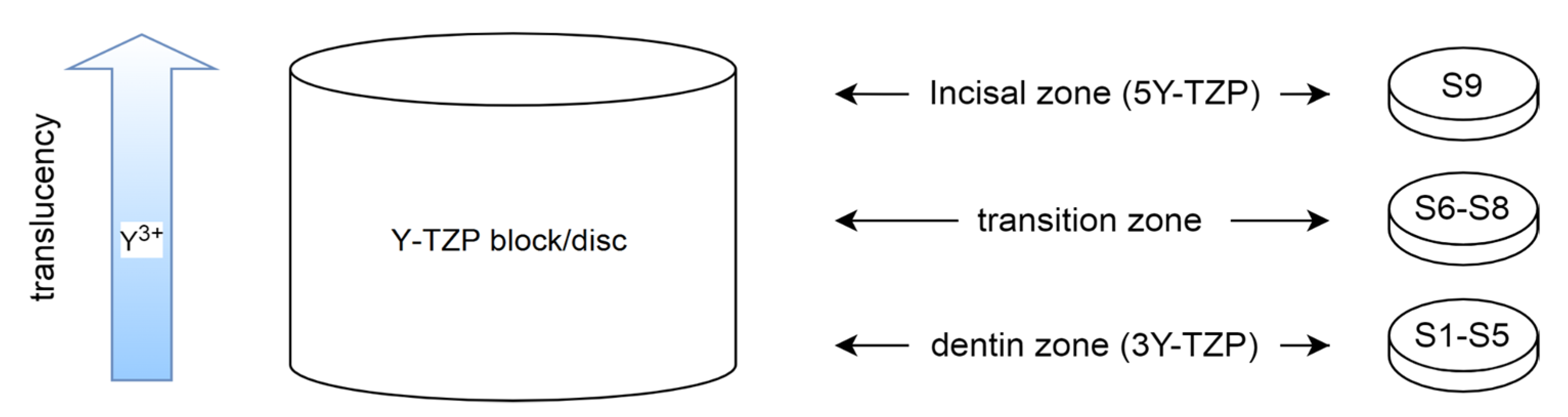
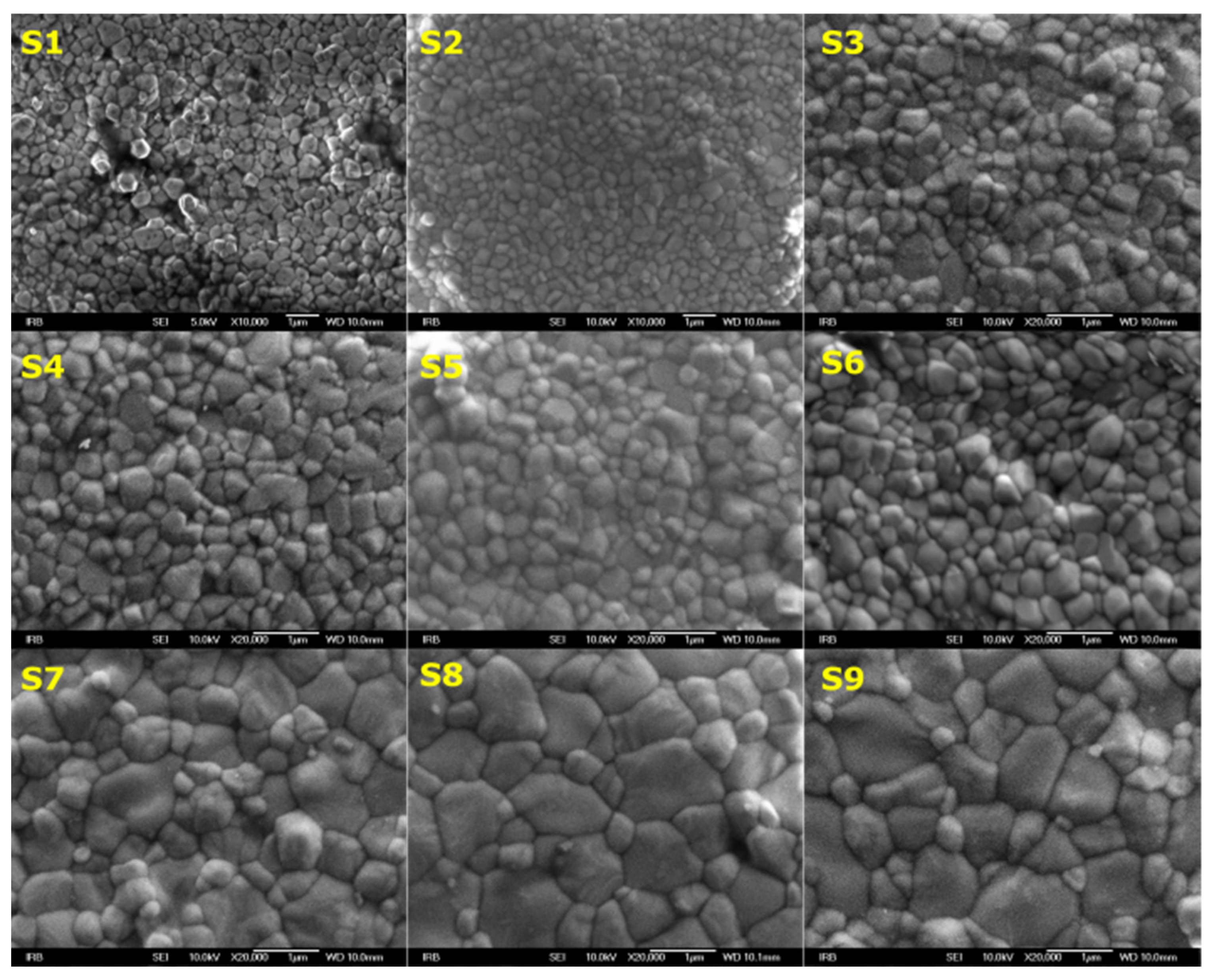


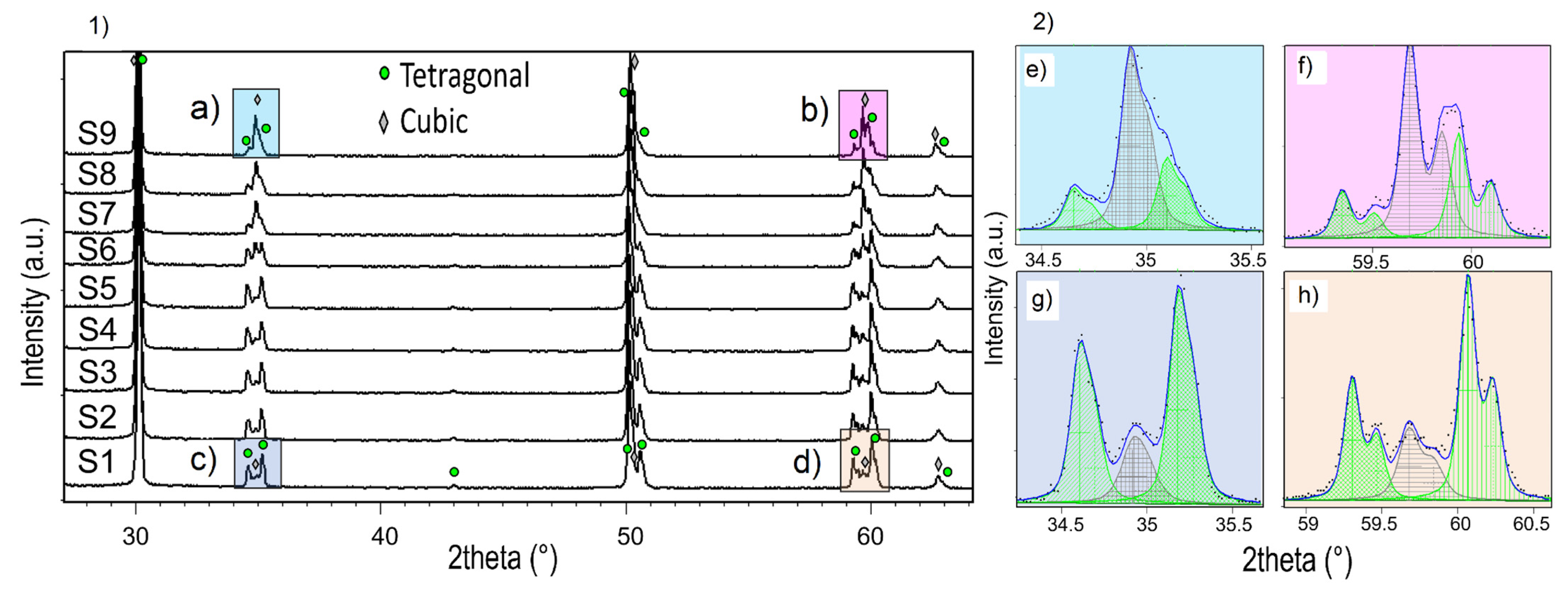
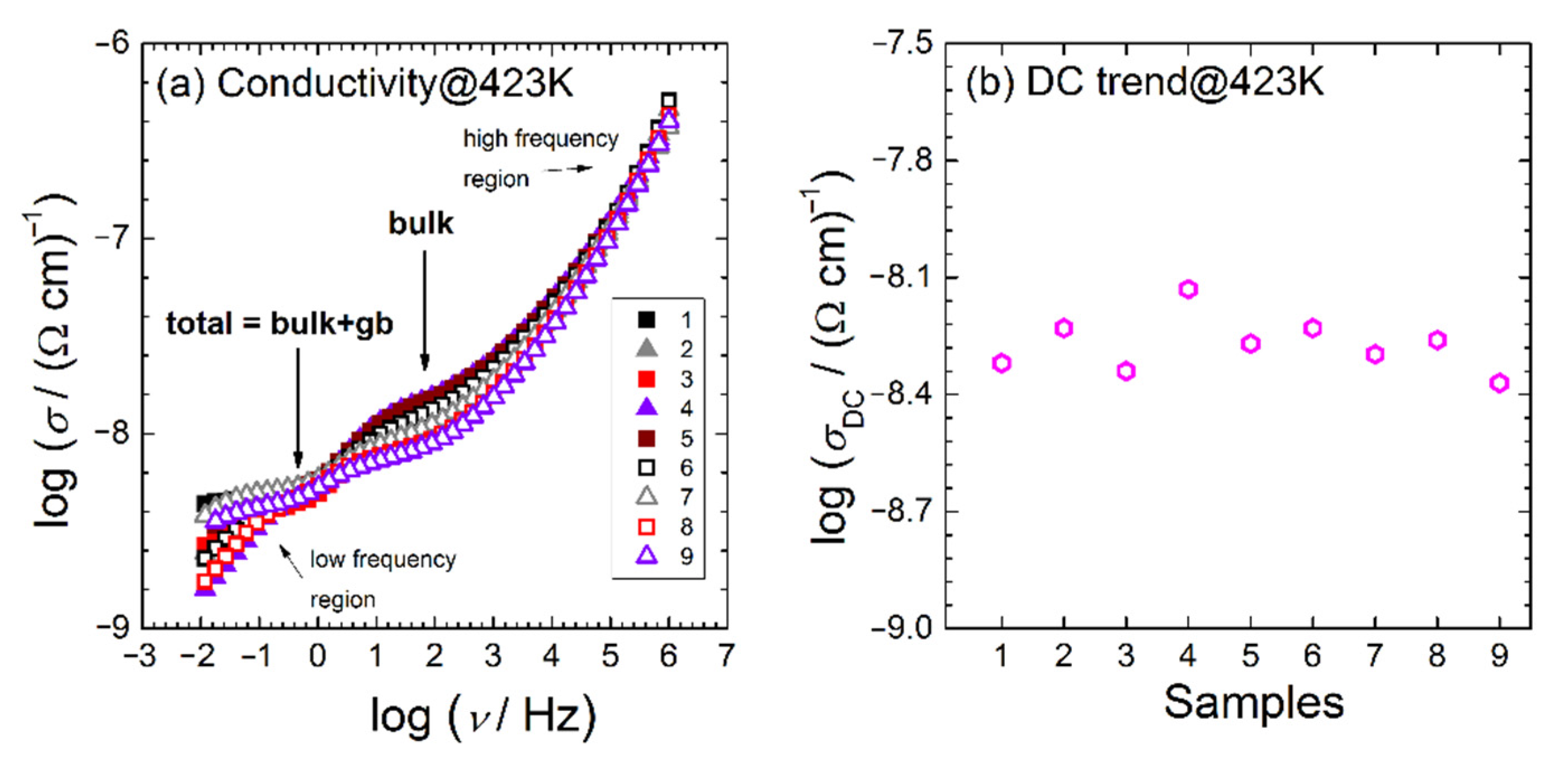

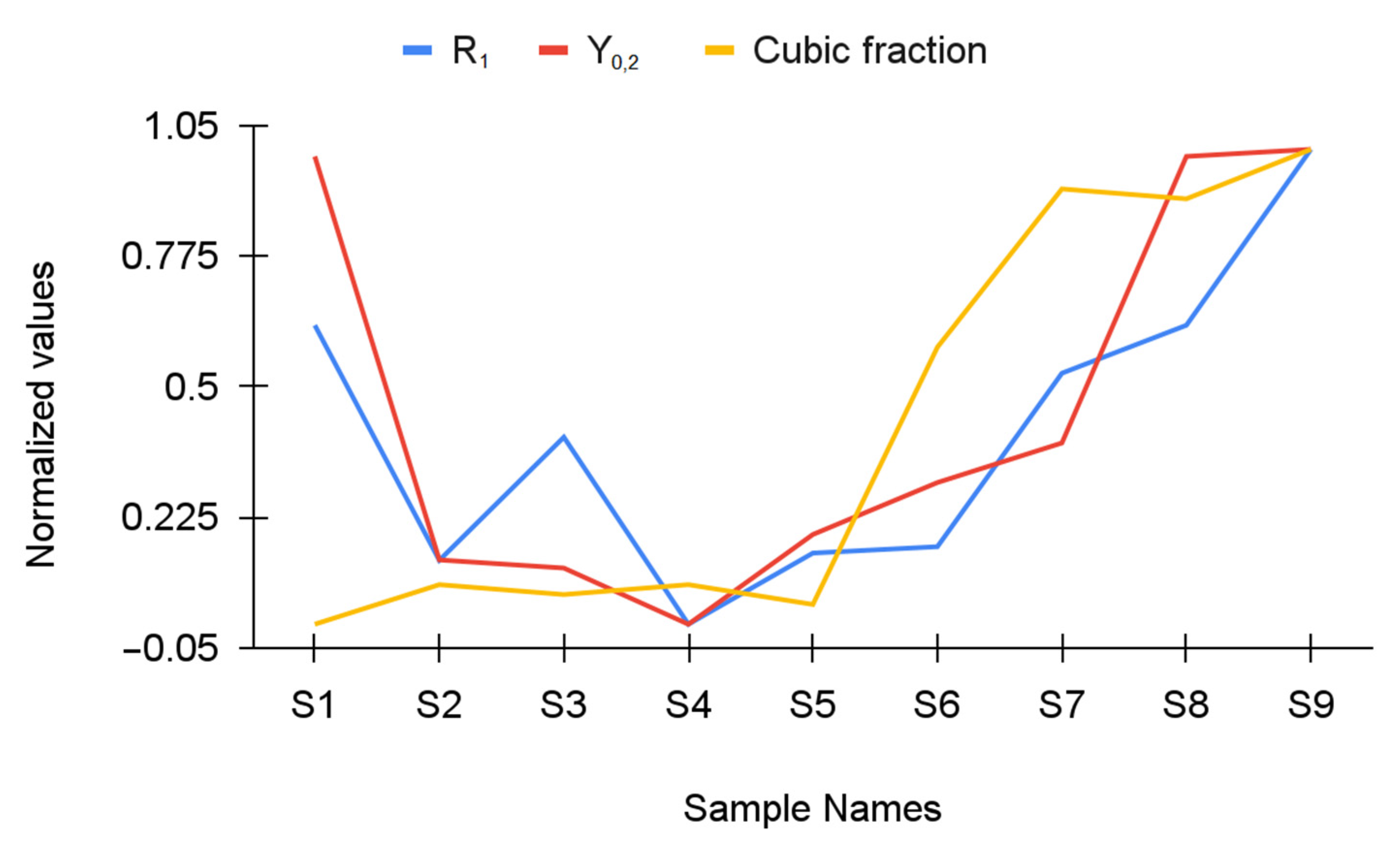
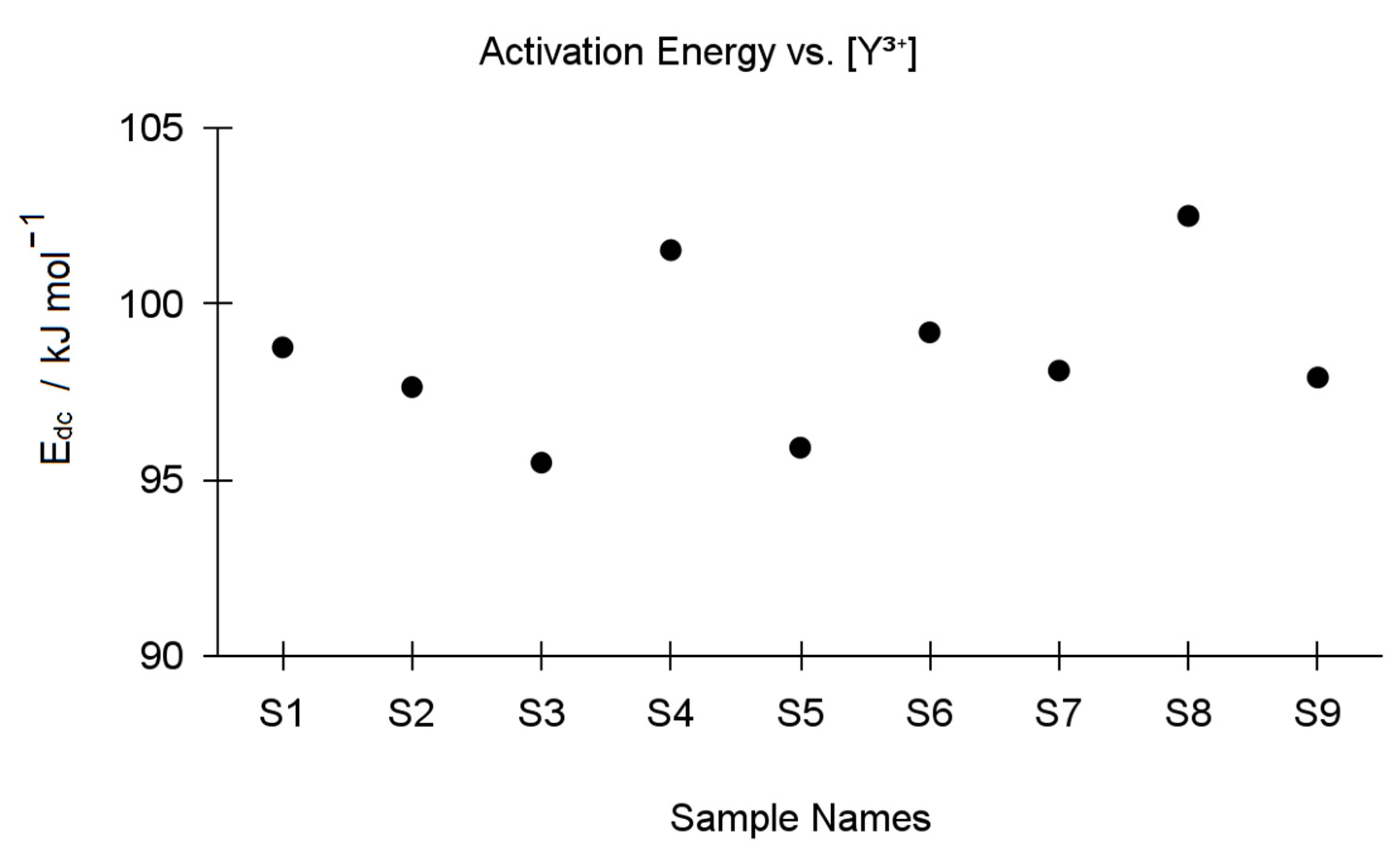
| Y-TZP Sample Names | ||||||||
|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 |
 | ||||||||
 | ||||||||
| Sample | Tetragonal Phase | Cubic Phase | |||
|---|---|---|---|---|---|
| a(Å) | c(Å) | % | a(Å) | % | |
| S1 | 3.6033(2) | 5.1778(3) | 78 | 5.1334(2) | 22 |
| S2 | 3.6036(2) | 5.1781(3) | 74 | 5.1338(3) | 26 |
| S3 | 3.6035(2) | 5.1783(3) | 75 | 5.1333(3) | 25 |
| S4 | 3.6032(2) | 5.1786(3) | 74 | 5.1329(3) | 26 |
| S5 | 3.6032(2) | 5.1781(3) | 76 | 5.1326(3) | 24 |
| S6 | 3.6034(2) | 5.1768(3) | 50 | 5.1305(3) | 50 |
| S7 | 3.6036(2) | 5.1742(3) | 34 | 5.1294(3) | 66 |
| S8 | 3.6063(2) | 5.1730(3) | 35 | 5.1292(3) | 65 |
| S9 | 3.6120(2) | 5.1713(3) | 30 | 5.1301(3) | 70 |
| Sample | R1 (Ω) | Y0,1 (S secn cm−1) | n1 | R2 (Ω) | Y0,2 (S secn cm−1) | n2 | Y0,el (S secn cm−1) | n3 |
|---|---|---|---|---|---|---|---|---|
| S1 | 1.21 × 106 | 1.15 × 10−10 | 0.87 | 3.00 × 105 | 1.88 × 10−8 | 0.95 | 3.11 × 10−7 | 0.38 |
| S2 | 9.16 × 105 | 1.50 × 10−10 | 0.85 | 8.52 × 105 | 7.38 × 10−9 | 0.88 | 4.74 × 10−7 | 0.40 |
| S3 | 1.07 × 106 | 2.06 × 10−10 | 0.82 | 1.25 × 106 | 7.15 × 10−9 | 0.83 | 6.97 × 10−7 | 0.46 |
| S4 | 8.36 × 105 | 1.35 × 10−10 | 0.87 | 4.14 × 105 | 5.56 × 10−9 | 1.03 | 1.78 × 10−7 | 0.43 |
| S5 | 9.25 × 105 | 1.66 × 10−10 | 0.84 | 9.52 × 105 | 8.10 × 10−9 | 0.85 | 7.27 × 10−7 | 0.44 |
| S6 | 9.33 × 105 | 1.42 × 10−10 | 0.86 | 6.64 × 105 | 9.57 × 10−9 | 0.87 | 4.33 × 10−7 | 0.35 |
| S7 | 1.15 × 106 | 1.80 × 10−10 | 0.82 | 5.95 × 105 | 1.07 × 10−8 | 0.86 | 1.12 × 10−6 | 0.44 |
| S8 | 1.21 × 106 | 1.15 × 10−10 | 0.87 | 3.00 × 105 | 1.88 × 10−8 | 0.95 | 3.11 × 10−7 | 0.38 |
| S9 | 1.43 × 106 | 1.66 × 10−10 | 0.83 | 7.46 × 105 | 1.90 × 10−8 | 0.83 | 9.70 × 10−7 | 0.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakovac, M.; Klaser, T.; Bafti, A.; Skoko, Ž.; Pavić, L.; Žic, M. The Effect of Y3+ Addition on Morphology, Structure, and Electrical Properties of Yttria-Stabilized Tetragonal Zirconia Dental Materials. Materials 2022, 15, 1800. https://doi.org/10.3390/ma15051800
Jakovac M, Klaser T, Bafti A, Skoko Ž, Pavić L, Žic M. The Effect of Y3+ Addition on Morphology, Structure, and Electrical Properties of Yttria-Stabilized Tetragonal Zirconia Dental Materials. Materials. 2022; 15(5):1800. https://doi.org/10.3390/ma15051800
Chicago/Turabian StyleJakovac, Marko, Teodoro Klaser, Arijeta Bafti, Željko Skoko, Luka Pavić, and Mark Žic. 2022. "The Effect of Y3+ Addition on Morphology, Structure, and Electrical Properties of Yttria-Stabilized Tetragonal Zirconia Dental Materials" Materials 15, no. 5: 1800. https://doi.org/10.3390/ma15051800
APA StyleJakovac, M., Klaser, T., Bafti, A., Skoko, Ž., Pavić, L., & Žic, M. (2022). The Effect of Y3+ Addition on Morphology, Structure, and Electrical Properties of Yttria-Stabilized Tetragonal Zirconia Dental Materials. Materials, 15(5), 1800. https://doi.org/10.3390/ma15051800








