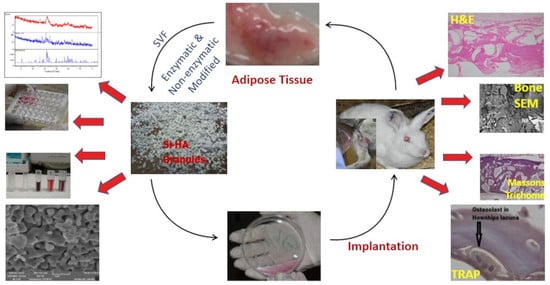
Osteogenic Induction with Silicon Hydroxyapatite Using Modified Autologous Adipose Tissue-Derived Stromal Vascular Fraction: In Vitro and Qualitative Histomorphometric Analysis
Abstract
:1. Introduction
2. Material and Methods
2.1. Synthesis of Silocon-Substituted Hydroxyapatite
2.2. Material Characterization
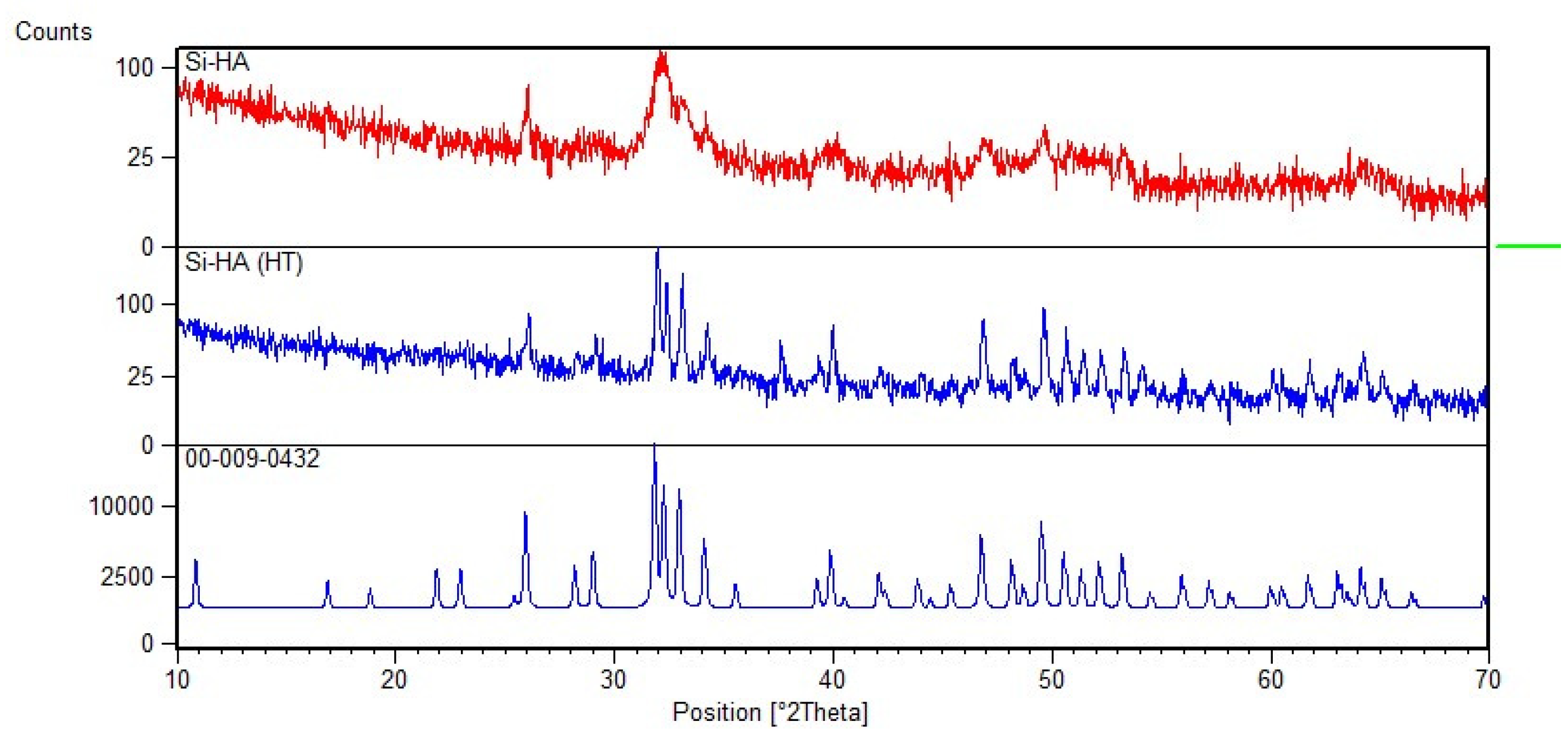
2.2.1. XRD
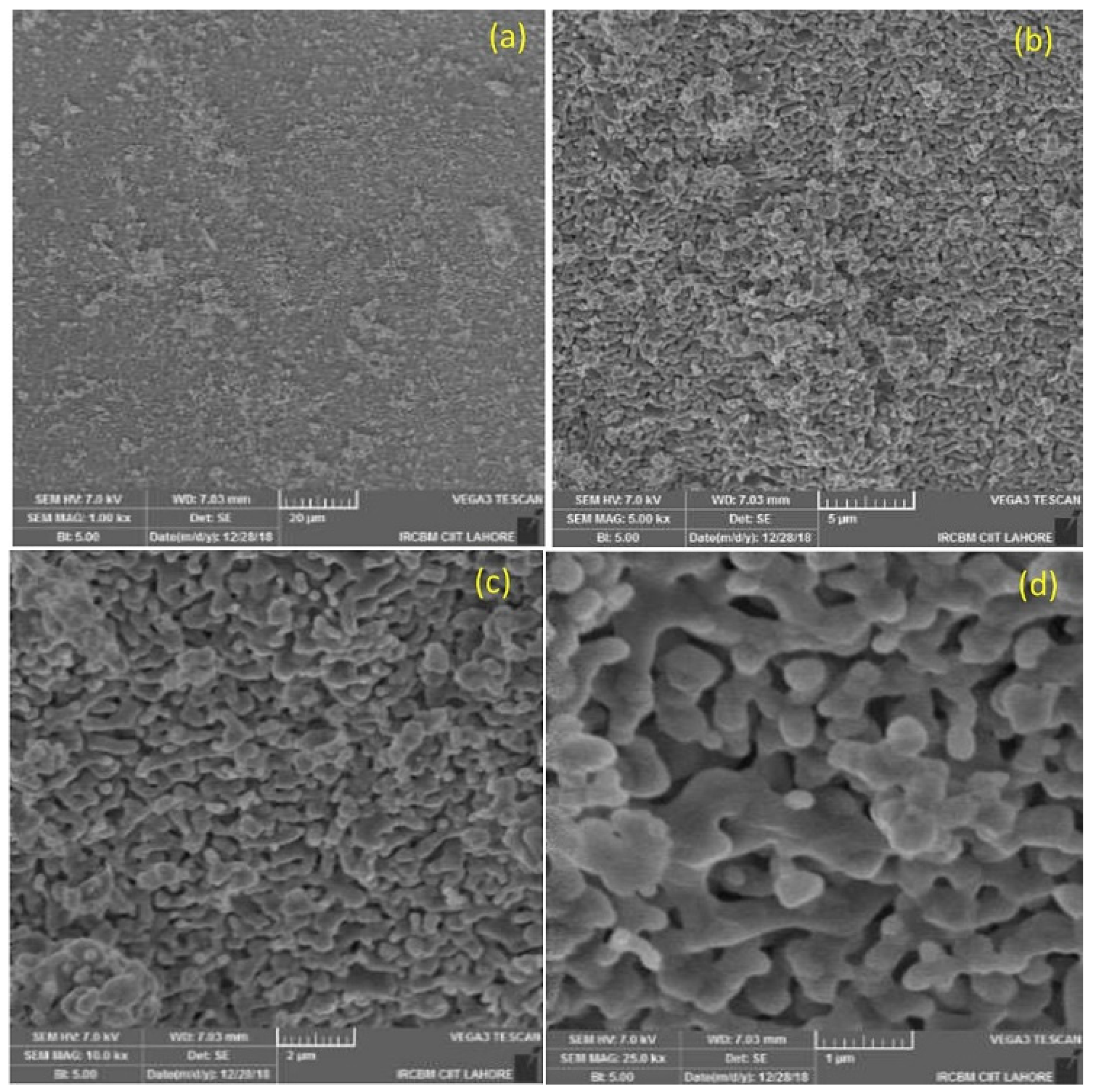
2.2.2. SEM
2.2.3. FTIR
2.3. In Vitro Studies
2.3.1. Hemolysis Essay
2.3.2. Blood Complete Picture
2.4. Cytotoxicity Analysis
2.4.1. Cell Culture
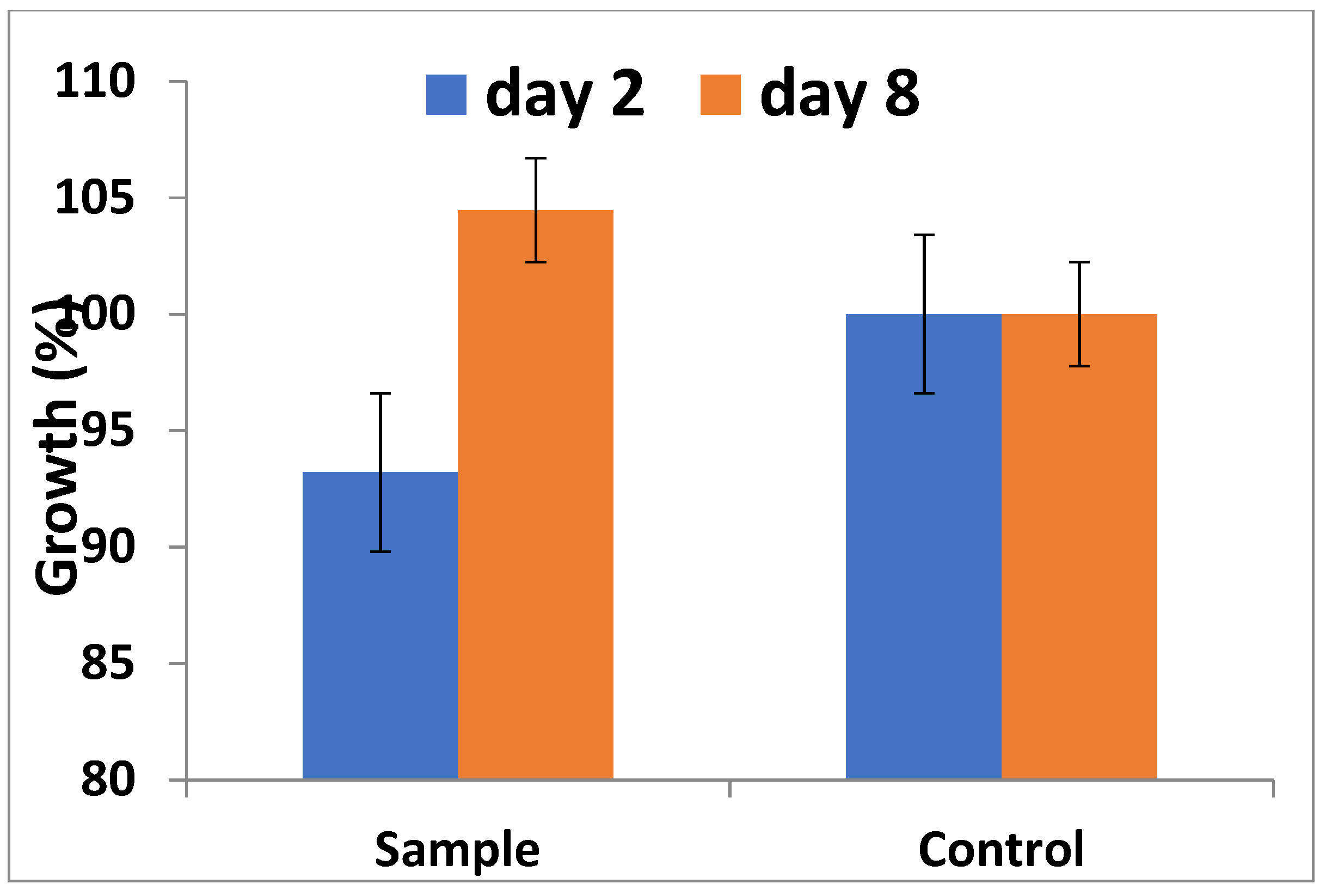
2.4.2. Alamar Blue Assay
2.5. In Vivo Studies
2.5.1. Sample Size Calculation
2.5.2. Surgical Procedure: Fat Harvesting
2.6. SVF Isolation: Enzymatic Digestion
2.7. SVF Isolation: Non Enzymatic (Modified)
2.8. Surgical Procedure for Bone Defects
2.9. Animal Sacrifice and Sample Manipulation
2.10. Histomorphometric Analysis
2.11. SEM of Bone
2.12. Statistical Analysis
3. Results and Discussion
3.1. XRD
3.2. FTIR
3.3. Scanning Electron Microscopy
3.4. Cytotoxicity Analysis: Alamar Blue Assay
3.5. Hemolysis Essay
3.6. Histomorphometry
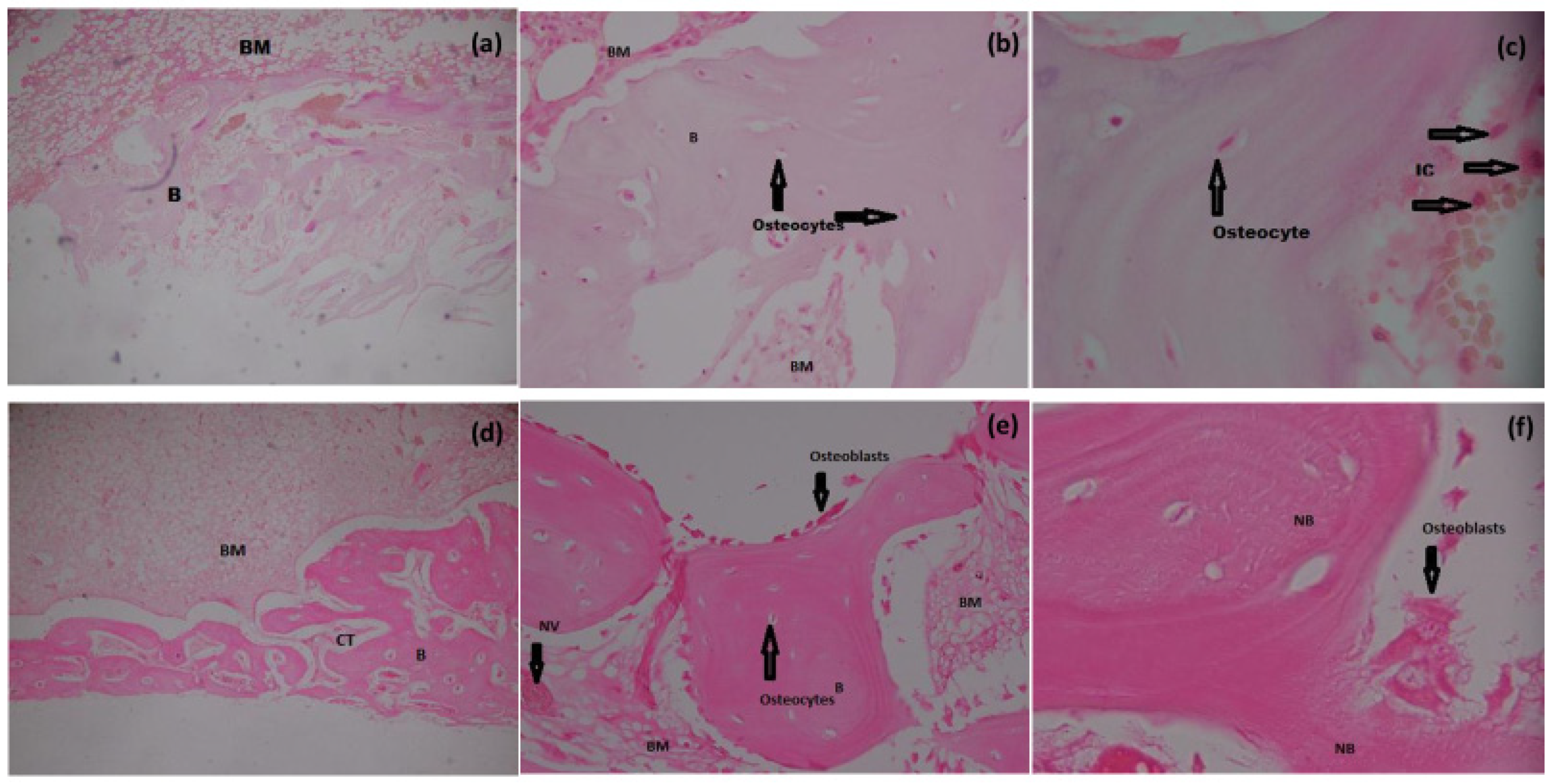
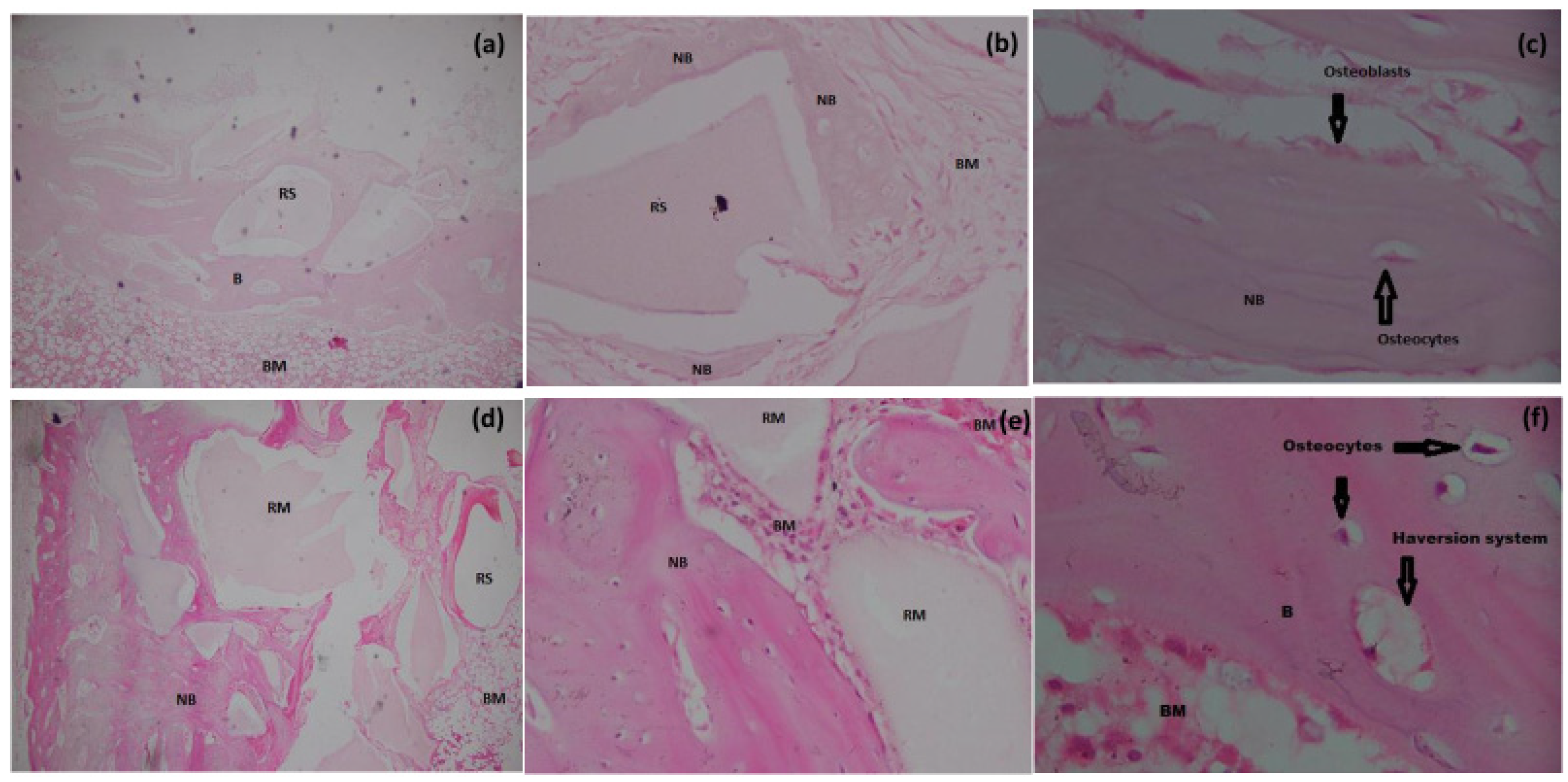
3.6.1. H&E Staining
3.6.2. Masson’s Trichome Staining
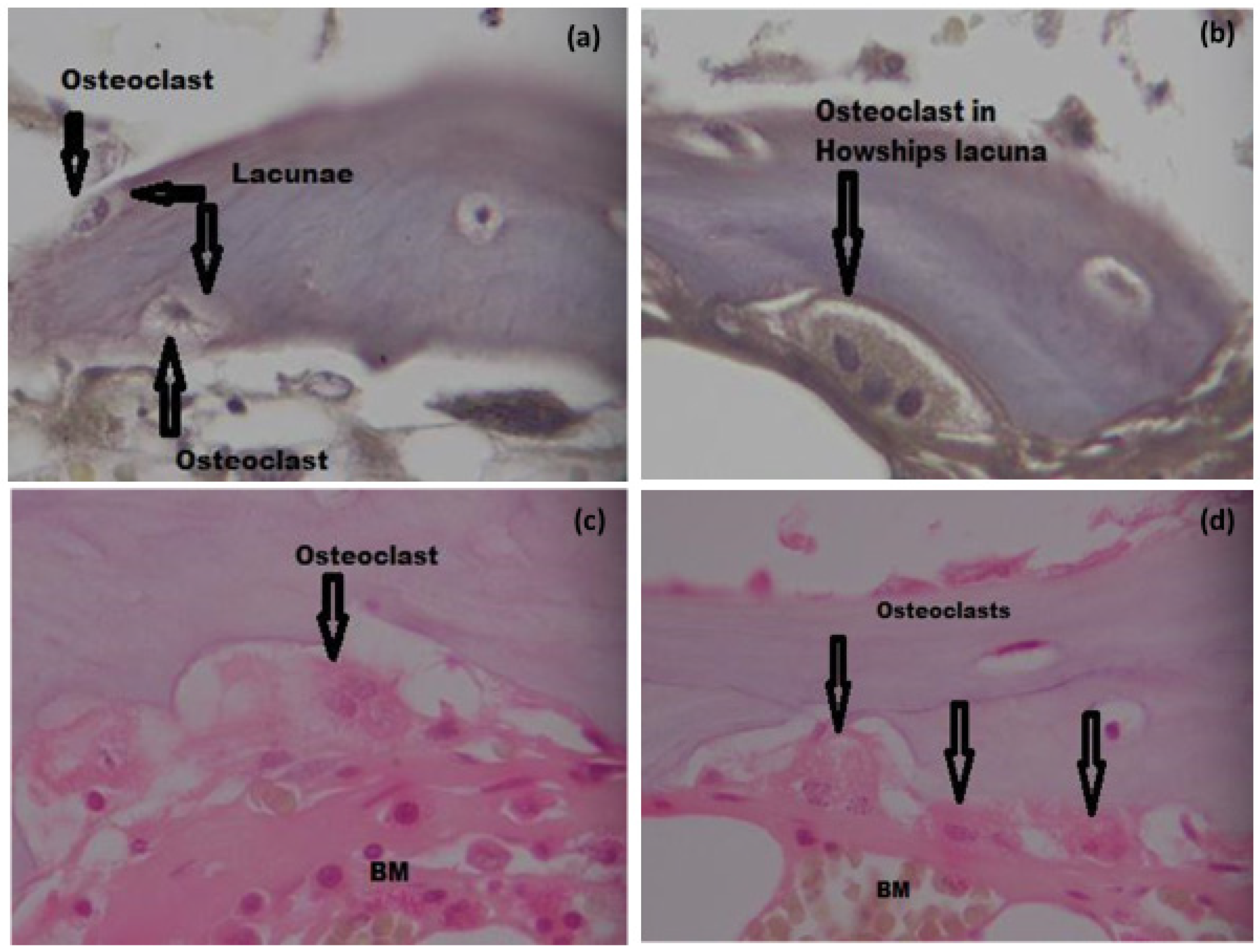
3.6.3. TRAP Staining and Osteoclast Count
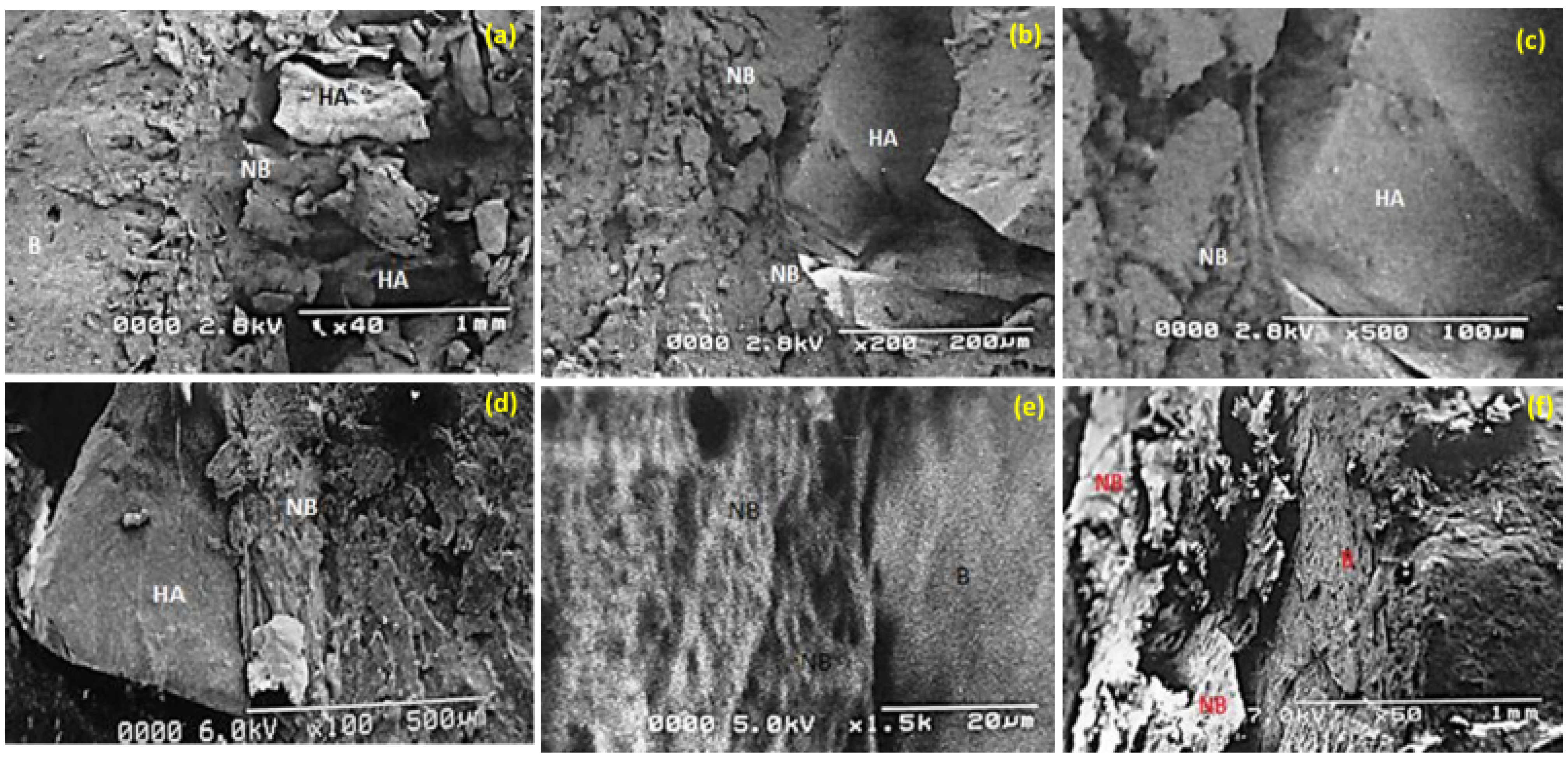
3.7. SEM: Biomaterial Interface
3.8. Conclusions
3.9. Study Limitations
3.10. Future Recommendations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, G.; Liu, Z.; Liu, Y.; Yu, J.; Wang, X.; Tan, Z.; Ye, X. Recent trends in the development of bone regenerative biomaterials. Front. Cell. Dev. Biol. 2021, 9, 665813. [Google Scholar] [CrossRef]
- Wang, C.; Wang, S.; Li, K.; Ju, Y.; Li, J.; Zhang, Y.; Li, J.; Liu, X.; Shi, X.; Zhao, Q. Preparation of laponite bioceramics for potential bone tissue engineering applications. PLoS ONE 2014, 9, e99585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polo-Corrales, L.; Latorre-Esteves, M.; Ramirez-Vick, J.E. Scaffold design for bone regeneration. J. Nanosc. Nanotechnol. 2014, 14, 15–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanner, M.C.; Hagelskamp, S.; Vlachopoulos, W.; Miska, M.; Findeisen, S.; Grimm, A.; Schmidmaier, G.; Haubruck, P. Non-union treatment based on the “diamond concept” is a clinically effective and safe treatment option in older adults. Clin. Interv. Aging 2020, 15, 1221. [Google Scholar] [CrossRef]
- Kono, T.; Sakae, T.; Nakada, H.; Kaneda, T.; Okada, H. Confusion between Carbonate Apatite and Biological Apatite (Carbonated Hydroxyapatite) in Bone and Teeth. Minerals 2022, 12, 170. [Google Scholar] [CrossRef]
- Khan, A.S.; Chaudhry, A.A. Handbook of Ionic Substituted Hydroxyapatites; Woodhead Publishing: Sawston, UK, 2019. [Google Scholar]
- Götz, W.; Tobiasch, E.; Witzleben, S.; Schulze, M. Effects of silicon compounds on biomineralization, osteogenesis, and hard tissue formation. Pharmaceutics 2019, 11, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ku, J.-K.; Hong, I.; Lee, B.-K.; Yun, P.-Y.; Lee, J.K. Dental alloplastic bone substitutes currently available in Korea. J. Korean Assoc. Oral. Maxillofac. Surg. 2019, 45, 51–67. [Google Scholar] [CrossRef] [Green Version]
- Fiume, E.; Magnaterra, G.; Rahdar, A.; Verné, E.; Baino, F. Hydroxyapatite for Biomedical Applications: A Short Overview. Ceramics 2021, 4, 542–563. [Google Scholar] [CrossRef]
- Chaudhry, A.A.; Khalid, H.; Zahid, M.; Ijaz, K.; Akhtar, H.; Younas, B.; Manzoor, F.; Iqbal, F.; Rehman, I.U. Zinc containing calcium phosphates obtained via microwave irradiation of suspensions. J. Mat. Chem. Phys. 2022, 276, 124921. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Navarrete, D.A. Biological Apatites in Bone and Teeth. In Nanoceramics in Clinical Use: From Materials to Applications, 2nd ed.; Vallet-Regi, M., Arcos Navarrete, D., Eds.; Royal Society of Chemistry: London, UK, 2016; pp. 1–29. [Google Scholar]
- Vasconcellos, L.M.R.D.; Nascimento, R.D.; Cairo, C.A.A.; Leite, D.D.O.; Santos, E.L.D.S.; Campos, G.E.; Prado, R.F.D.; Jardini, M.A.N.; Vasconcellos, L.G.O.D.; Carvalho, Y.R. Porous titanium associated with CaP coating: In vivo and in vitro osteogenic performance. Mat. Res. 2017, 21. [Google Scholar] [CrossRef] [Green Version]
- Casarrubios, L.; Gómez-Cerezo, N.; Sánchez-Salcedo, S.; Feito, M.; Serrano, M.; Saiz-Pardo, M.; Ortega, L.; De Pablo, D.; Díaz-Güemes, I.; Fernández-Tomé, B. Silicon substituted hydroxyapatite/VEGF scaffolds stimulate bone regeneration in osteoporotic sheep. Acta Biomat. 2020, 101, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Kralick, A.E.; Zemel, B.S. Evolutionary perspectives on the developing skeleton and implications for lifelong health. Front. Endocrinol. 2020, 11, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazini, L.; Ezzoubi, M.; Malka, G. Overview of current adipose-derived stem cell (ADSCs) processing involved in therapeutic advancements: Flow chart and regulation updates before and after COVID-19. Stem Cell Res. Ther. 2021, 12, 1. [Google Scholar] [CrossRef]
- Tsuji, W.; Rubin, J.P.; Marra, K.G. Adipose-derived stem cells: Implications in tissue regeneration. World J. Stem. Cells 2014, 6, 312. [Google Scholar] [CrossRef]
- Da Silva, H.M.; Mateescu, M.; Damia, C.; Champion, E.; Soares, G.; Anselme, K. Importance of dynamic culture for evaluating osteoblast activity on dense silicon-substituted hydroxyapatite. Colloids Surf. B Biointerfaces 2010, 80, 138–144. [Google Scholar] [CrossRef]
- Labjar, H.; Chaair, H. Synthesis and Characterization of Apatite Silicated Powders with Wet Precipitation Method. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2021; p. 00106. [Google Scholar] [CrossRef]
- Bandgar, S.S.; Yadav, H.M.; Shirguppikar, S.S.; Shinde, M.A.; Shejawal, R.V.; Kolekar, T.V.; Bamane, S.R. Enhanced hemolytic biocompatibility of hydroxyapatite by chromium (Cr3+) doping in hydroxyapatite nanoparticles synthesized by solution combustion method. J. Korean Ceram. Soc. 2017, 54, 158–166. [Google Scholar] [CrossRef] [Green Version]
- Koç, A.; Elçin, A.E.; Elçin, Y.M. Ectopic osteogenic tissue formation by MC3T3-E1 cell-laden chitosan/hydroxyapatite composite scaffold. Nanomed. Biotechnol. 2016, 44, 1440–1447. [Google Scholar] [CrossRef]
- Jahan, K.; Manickam, G.; Tabrizian, M.; Murshed, M. In vitro and in vivo investigation of osteogenic properties of self-contained phosphate-releasing injectable purine-crosslinked chitosan-hydroxyapatite constructs. Sci. Rep. 2020, 10, 11603. [Google Scholar] [CrossRef]
- Barbosa, M.A.L.; Iwaki Filho, L.; Iwaki, L.C.V.; Natali, M.R.M.; Takeshita, W.M.; Sabio, S. Histologic and histomorphometric study of bone repair around short dental implants inserted in rabbit tibia, associated with tricalcium phosphate graft bone. Acta Sci. Health Sci. 2014, 36, 257–263. [Google Scholar] [CrossRef] [Green Version]
- Blanco, J.F.; García-Briñon, J.; Benito-Garzón, L.; Pescador, D.; Muntión, S.; Sánchez-Guijo, F. Human bone marrow mesenchymal stromal cells promote bone regeneration in a xenogeneic rabbit model: A preclinical study. Stem Cells Int. 2018, 2018, 7089484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seman, C.N.Z.C.; Zakaria, Z.; Sharifudin, M.A.; Ahmad, A.C.; Awang, M.S.; Yusof, N.M.; Buyong, Z. Model of A Critical Size Defect in the New Zealand White Rabbit’s Tibia. IIUM Med. J. Malays. 2018, 17, 13–18. [Google Scholar] [CrossRef]
- Kim, A.; Kim, D.-H.; Song, H.-R.; Kang, W.-H.; Kim, H.-J.; Lim, H.-C.; Cho, D.-W.; Bae, J.-H. Repair of rabbit ulna segmental bone defect using freshly isolated adipose-derived stromal vascular fraction. Cytotherapy 2012, 14, 296–305. [Google Scholar] [CrossRef]
- Saxer, F.; Scherberich, A.; Todorov, A.; Studer, P.; Miot, S.; Schreiner, S.; Güven, S.; Tchang, L.A.; Haug, M.; Heberer, M. Implantation of stromal vascular fraction progenitors at bone fracture sites: From a rat model to a first-in-man study. Stem Cells 2016, 34, 2956–2966. [Google Scholar] [CrossRef]
- Rhee, S.C.; Ji, Y.-h.; Gharibjanian, N.A.; Dhong, E.S.; Park, S.H.; Yoon, E.-S. In vivo evaluation of mixtures of uncultured freshly isolated adipose-derived stem cells and demineralized bone matrix for bone regeneration in a rat critically sized calvarial defect model. Stem Cells Dev. 2011, 20, 233–242. [Google Scholar] [CrossRef]
- Toplu, G.; Ozcelik, D.; Serin, M.; Erdem, H.; Topacoglu, A.T. Adipose tissue-derived stromal vascular fraction increases osteogenesis in an experimental design zygomatic bone defect model. J. Craniofac. Surg. 2017, 28, 2179–2182. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadoya, Y.; Al-Saffar, N.; Kobayashi, A.; Revell, P. The expression of osteoclast markers on foreign body giant cells. Bone Miner. 1994, 27, 85–96. [Google Scholar] [CrossRef]
- Camassa, J.A.; Diogo, C.C.; Bordelo, J.; Bonelli, M.d.A.; Viegas, C.A.; Azevedo, J.T.; Dourado, N.; Dias, I.R. Tartrate-resistant acid phosphate as biomarker of bone turnover over the lifespan and different physiologic stages in sheep. BMC Vet. Res. 2017, 13, 239. [Google Scholar] [CrossRef]
- Abid, W.K.; Mukhtar, Y.H.A. Repair of surgical bone defects grafted with hydroxylapatite+ β-TCP combined with hyaluronic acid and collagen membrane in rabbits: A histological study. J. Taibah Univ. Med. Sci. 2019, 14, 14–24. [Google Scholar] [CrossRef]
- Camargo, A.F.d.F.; Baptista, A.M.; Natalino, R.; Camargo, O.P.d. Bioactive glass in cavitary bone defects: A comparative experimental study in rabbits. Acta Ortopédica Brasileira 2015, 23, 202–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucaciu, O.; Soriţău, O.; Gheban, D.; Ciuca, D.R.; Virtic, O.; Vulpoi, A.; Dirzu, N.; Câmpian, R.; Băciuţ, G.; Popa, C. Dental follicle stem cells in bone regeneration on titanium implants. BMC Biotechnol. 2015, 15, 114. [Google Scholar] [CrossRef] [Green Version]
- Vogely, H.C.; Oosterbos, C.; Puts, E.; Nijhof, M.; Nikkels, P.; Fleer, A.; Tonino, A.; Dhert, W.; Verbout, A. Effects of hydroxyapatite coating on Ti-6 Al-4V implant-site infection in a rabbit tibial model. J. Orthop. Res. 2000, 18, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Marchat, D.; Zymelka, M.; Coelho, C.; Gremillard, L.; Joly-Pottuz, L.; Babonneau, F.; Esnouf, C.; Chevalier, J.; Bernache-Assollant, D. Accurate characterization of pure silicon-substituted hydroxyapatite powders synthesized by a new precipitation route. Acta Biomater. 2013, 9, 6992–7004. [Google Scholar] [CrossRef] [Green Version]
- Matamoros-Veloza, Z.; Rendon-Angeles, J.C.; Yanagisawa, K.; Ueda, T.; Zhu, K.; Moreno-Perez, B. Preparation of silicon hydroxyapatite nanopowders under microwave-assisted hydrothermal method. Nanomaterials 2021, 11, 1548. [Google Scholar] [CrossRef]
- Moreno-Perez, B.; Matamoros-Veloza, Z.; Rendon-Angeles, J.C.; Yanagisawa, K.; Onda, A.; Pérez-Terrazas, J.E.; Mejia-Martínez, E.E.; Díaz, O.B.; Rodríguez-Reyes, M. Synthesis of silicon-substituted hydroxyapatite using hydrothermal process. Boletín de la Sociedad Española de Cerámica y Vidrio 2020, 59, 50–64. [Google Scholar] [CrossRef]
- Habibovic, P.; de Groot, K. Osteoinductive biomaterials—Properties and relevance in bone repair. J. Tissue Eng. Regen. Med. 2007, 1, 25–32. [Google Scholar] [CrossRef]
- Bundela, H.; Bajpai, A. Designing of hydroxyapatite-gelatin based porous matrix as bone substitute: Correlation with biocompatibility aspects. Express Polym. Lett 2008, 2, 201–213. [Google Scholar] [CrossRef]
- Suresh Kumar, C.; Dhanaraj, K.; Vimalathithan, R.; Ilaiyaraja, P.; Suresh, G. Hydroxyapatite for bone related applications derived from sea shell waste by simpleprecipitation method. J. Asian Ceram. Soc. 2020, 8, 416–429. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.-T. Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Gao, C.; Peng, S.; Feng, P.; Shuai, C. Bone biomaterials and interactions with stem cells. Bone Res. 2017, 5, 17059. [Google Scholar] [CrossRef] [PubMed]
- Montoya, C.; Du, Y.; Gianforcaro, A.L.; Orrego, S.; Yang, M.; Lelkes, P.I. On the road to smart biomaterials for bone research: Definitions, concepts, advances, and outlook. Bone Res. 2021, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Al-Nasiry, S.; Geusens, N.; Hanssens, M.; Luyten, C.; Pijnenborg, R. The use of Alamar Blue assay for quantitative analysis of viability, migration and invasion of choriocarcinoma cells. Hum. Reprod. 2007, 22, 1304–1309. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Zhang, K.; Shi, C.; Liu, L.; Yan, G.; Liu, C.; Zhou, Y.; Hu, Y.; Sun, H.; Yang, B. Small molecules modified biomimetic gelatin/hydroxyapatite nanofibers constructing an ideal osteogenic microenvironment with significantly enhanced cranial bone formation. Int. J. Nanomed. 2018, 13, 7167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Hadad, A.A.; Peón, E.; García-Galván, F.R.; Barranco, V.; Parra, J.; Jiménez-Morales, A.; Galván, J.C. Biocompatibility and corrosion protection behaviour of hydroxyapatite sol-gel-derived coatings on Ti6Al4V alloy. Materials 2017, 10, 94. [Google Scholar] [CrossRef] [Green Version]
- Herrera-Alonso, A.E.; Ibarra-Alonso, M.C.; Esparza-González, S.C.; Estrada-Flores, S.; García-Cerda, L.A.; Martínez-Luévanos, A. Biomimetic Growth of Hydroxyapatite on SiO2 Microspheres to Improve Its Biocompatibility and Gentamicin Loading Capacity. Materials 2021, 14, 6941. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, X.; Zhou, Y.; Cai, M.; Lin, K.; Fang, B.; Xia, L. The synergistic promotion of osseointegration by nanostructure design and silicon substitution of hydroxyapatite coatings in a diabetic model. J. Mater. Chem. B 2020, 8, 2754–2767. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, B.; Sarfaraz, Z.; Muhammad, N.; Ahmad, P.; Iqbal, J.; Khan, Z.U.H.; Gonfa, G.; Iqbal, F.; Jamal, A.; Rahim, A. Ionic liquid as a potential solvent for preparation of collagen-alginate-hydroxyapatite beads as bone filler. J. Biomater. Sci. Polym. Ed. Polymer. Ed. 2018, 29, 1168–1184. [Google Scholar] [CrossRef]
- Zhang, C.; Yan, B.; Cui, Z.; Cui, S.; Zhang, T.; Wang, X.; Liu, D.; Yang, R.; Jiang, N.; Zhou, Y. Bone regeneration in minipigs by intrafibrillarly-mineralized collagen loaded with autologous periodontal ligament stem cells. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Florencio-Silva, R.; Sasso, G.R.d.S.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of bone tissue: Structure, function, and factors that influence bone cells. Biomed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [Green Version]
- Rahimnia, A.; Hesarikia, H.; Rahimi, A.; Karami, S.; Kaviani, K. Evaluation and comparison of synthesised hydroxyapatite in bone regeneration: As an in vivo study. J. Taibah Univ. Med. Sci. 2021, 16, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Veremeev, A.; Bolgarin, R.; Nesterenko, V.; Andreev-Andrievskiy, A.; Kutikhin, A. Native bovine hydroxyapatite powder, demineralised bone matrix powder, and purified bone collagen membranes are efficient in repair of critical-sized rat calvarial defects. Materials 2020, 13, 3393. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Suarez, A.; Rizo-Gorrita, M.; Suárez-Vega, D.; Velazco, G.; Rodriguez Gelfenstein, I.; Vázquez-Pachón, C.; Serrera-Figallo, M.-Á.; Torres-Lagares, D. Effectiveness of silicon platelet-rich fibrin and autologous bone on bone regeneration in rabbit calvarian defects: A radiological and histological study. Appl. Sci. 2021, 11, 4074. [Google Scholar] [CrossRef]
- Lendeckel, S.; Jödicke, A.; Christophis, P.; Heidinger, K.; Wolff, J.; Fraser, J.K.; Hedrick, M.H.; Berthold, L.; Howaldt, H.-P. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: Case report. Craniomaxillofac. Surg. 2004, 32, 370–373. [Google Scholar] [CrossRef]
- Anitha, A.; Menon, D.; Sivanarayanan, T.; Koyakutty, M.; Mohan, C.C.; Nair, S.V.; Nair, M.B. Bioinspired composite matrix containing hydroxyapatite-silica core-shell nanorods for bone tissue engineering. ACS Appl. Mater. Interfaces 2017, 9, 26707–26718. [Google Scholar] [CrossRef]
- Farré-Guasch, E.; Bravenboer, N.; Helder, M.N.; Schulten, E.A.; Ten Bruggenkate, C.M.; Klein-Nulend, J. Blood vessel formation and bone regeneration potential of the stromal vascular fraction seeded on a calcium phosphate scaffold in the human maxillary sinus floor elevation model. Materials 2018, 11, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyberg, E.; Farris, A.; O’Sullivan, A.; Rodriguez, R.; Grayson, W. Comparison of stromal vascular fraction and passaged adipose-derived stromal/stem cells as point-of-care agents for bone regeneration. Tissue Eng. Part A 2019, 25, 1459–1469. [Google Scholar] [CrossRef]
- Kuroshima, S.; Sasaki, M.; Nakajima, K.; Tamaki, S.; Hayano, H.; Sawase, T. Transplantation of Noncultured Stromal Vascular Fraction Cells of Adipose Tissue Ameliorates Osteonecrosis of the Jaw–Like Lesions in Mice. J. Bone Min. Res. 2018, 33, 154–166. [Google Scholar] [CrossRef] [Green Version]
- Kamenaga, T.; Kuroda, Y.; Nagai, K.; Tsubosaka, M.; Takashima, Y.; Kikuchi, K.; Fujita, M.; Ikuta, K.; Anjiki, K.; Maeda, T. Cryopreserved human adipose-derived stromal vascular fraction maintains fracture healing potential via angiogenesis and osteogenesis in an immunodeficient rat model. Stem Cell Res. Ther. 2021, 12, 110. [Google Scholar] [CrossRef]
- Roato, I.; Belisario, D.C.; Compagno, M.; Verderio, L.; Sighinolfi, A.; Mussano, F.; Genova, T.; Veneziano, F.; Pertici, G.; Perale, G. Adipose-derived stromal vascular fraction/xenohybrid bone scaffold: An alternative source for bone regeneration. Stem Cells Int. 2018, 2018, 4126379. [Google Scholar] [CrossRef]
- Justesen, J.; Pedersen, S.B.; Stenderup, K.; Kassem, M. Subcutaneous adipocytes can differentiate into bone-forming cells in vitro and in vivo. Tissue Eng. 2004, 10, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Dongen, J.A.v.; Harmsen, M.C.; Stevens, H.P. Isolation of stromal vascular fraction by fractionation of adipose tissue. Methods Mol. Biol. 2019, 91–103. [Google Scholar] [CrossRef]
- Gimble, J.M.; Katz, A.J.; Bunnell, B.A. Adipose-derived stem cells for regenerative medicine. Circ. Res. 2007, 100, 1249–1260. [Google Scholar] [CrossRef]
- Grandfield, K.; Palmquist, A.; Engqvist, H.; Thomsen, P. Resolving the CaP-bone interface: A review of discoveries with light and electron microscopy. Biomatter 2012, 2, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Shah, F.A.; Ruscsák, K.; Palmquist, A. 50 years of scanning electron microscopy of bone—A comprehensive overview of the important discoveries made and insights gained into bone material properties in health, disease, and taphonomy. Bone Res. 2019, 7, 15. [Google Scholar] [CrossRef] [Green Version]
- Kunjalukkal Padmanabhan, S.; Nitti, P.; Stanca, E.; Rochira, A.; Siculella, L.; Raucci, M.G.; Madaghiele, M.; Licciulli, A.; Demitri, C. Mechanical and Biological Properties of Magnesium-and Silicon-Substituted Hydroxyapatite Scaffolds. Materials 2021, 14, 6942. [Google Scholar] [CrossRef] [PubMed]
- Storti, G.; Scioli, M.G.; Kim, B.-S.; Orlandi, A.; Cervelli, V. Adipose-derived stem cells in bone tissue engineering: Useful tools with new applications. Stem Cells Int. 2019, 2019, 3673857. [Google Scholar] [CrossRef] [Green Version]


| S. No | Groups | Sample Size | Bone Reconstruction Procedure | |
|---|---|---|---|---|
| 1. | A | Control Group | 5 | Defect was kept as such |
| 2. | B | Experimental group 1 | 5 | Defect was closed by placing bioceramic alone |
| 3. | C | Experimental group 2 | 5 | Defect was closed by placing bioceramic along with SVF * (enzymatic dissociation) |
| 4. | D | Experimental group 3 | 5 | Defect was closed by placing bioceramic along with SVF * (non-enzymatic, modified) |
| S. No | Perimeter | Grading | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| 1 | Bone formation | Absent | Present at the periphery | Centrally | Present centrally & at the periphery | - |
| 2 | Haversion canals | |||||
| 3 | Osteoblasts | |||||
| 4 | Osteocytes | |||||
| 5 | Osteoclasts | |||||
| 6 | Mature bone | |||||
| 7 | Immature bone | |||||
| 8 | Bone formation closing the defect | Absent | Present between 1% to 25% | Present between 26% to 50% | Present between 51% to 75% | Present between 76% to 100% |
| 9 | Vascularization | Absent | Present at the surface | Present in depth | - | - |
| 10 | Inflammation | Present | Absent | - | - | - |
| 11 | Granulation tissue | Present | Absent | - | - | - |
| S. No | Categories | Scores | ||||
|---|---|---|---|---|---|---|
| None | Mild | Moderate | Severe | |||
| 1. | Medullary canal | Leukocytes | 0 | 1 | 2 | 3 |
| Micro abscesses | 0 | 1 | 2 | 3 | ||
| Fibrosis | 0 | 1 | 2 | 3 | ||
| Granulation tissue | 0 | 1 | 2 | 3 | ||
| 2. | Cortex | Destruction of cortex | 0 | 1 | 2 | 3 |
| Enlarged Haversian canals | 0 | 1 | 2 | 3 | ||
| Leukocytes | 0 | 1 | 2 | 3 | ||
| Micro abscesses | 0 | 1 | 2 | 3 | ||
| Granulation tissue | 0 | 1 | 2 | 3 | ||
| 3. | New bone formation | (>50%) 0 | (25–50%) 1 | (1–25%) 2 | (0%) 3 | |
| 4. | Maximum (worst) score | 30 | ||||
| S. No | Sample No | OD Sample | OD −ve Control | OD +ve Control | % Hemolysis |
|---|---|---|---|---|---|
| 1 | Sample 1 | 0.079 | 0.04 | 1.82 | 2.19 |
| 2 | Sample 2 | 0.018 | 0.04 | 1.82 | 1.23 |
| 3 | Sample 3 | 0.102 | 0.04 | 1.82 | 3.66 |
| Parameters | Gp-1 (n = 5) | Gp-2 (n = 5) | Gp-3 (n = 5) | Gp-4 (n = 5) | p-Value |
|---|---|---|---|---|---|
| Histological Evaluation Grading Score | 10.20 ± 0.44 | 26.40 ± 1.34 | 27.20 ± 2.16 | 25.00 ± 1.41 | <0.001 |
| Group Comparison | Gp-1 vs. Gp-2 | Gp-2 vs. Gp-3 | Gp-1 vs. Gp-3 | Gp-2 vs. Gp-4 | Gp-1 vs. Gp-4 | Gp-3 vs. Gp-4 |
|---|---|---|---|---|---|---|
| Histological Evaluation Grading Score | <0.001 (p value) | 0.826 (p value) | <0.001 (p value) | 0.460 (p value) | <0.001 (p value) | 0.126 (p value) |
| S. No | Animal No | Group I | Group II | Group III | Group IV | Group V |
|---|---|---|---|---|---|---|
| 1 | 1 | 2 | 2 | 2 | 2 | 1 |
| 2 | 2 | 2 | 2 | 2 | 1 | 2 |
| 3 | 3 | 2 | 3 | 2 | 1 | 1 |
| 4 | 4 | 2 | 3 | 2 | 2 | 1 |
| 5 | 5 | 2 | 2 | 3 | 2 | 1 |
| Sum | 10 | 12 | 11 | 8 | 6 | |
| Mean | 2 | 2.4 | 2.2 | 1.6 | 1.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.M.; Butt, S.A.; Chaudhry, A.A.; Rashid, A.; Ijaz, K.; Majeed, A.; Gul, H. Osteogenic Induction with Silicon Hydroxyapatite Using Modified Autologous Adipose Tissue-Derived Stromal Vascular Fraction: In Vitro and Qualitative Histomorphometric Analysis. Materials 2022, 15, 1826. https://doi.org/10.3390/ma15051826
Khan MM, Butt SA, Chaudhry AA, Rashid A, Ijaz K, Majeed A, Gul H. Osteogenic Induction with Silicon Hydroxyapatite Using Modified Autologous Adipose Tissue-Derived Stromal Vascular Fraction: In Vitro and Qualitative Histomorphometric Analysis. Materials. 2022; 15(5):1826. https://doi.org/10.3390/ma15051826
Chicago/Turabian StyleKhan, Muhammad Marghoob, Shadab Ahmed Butt, Aqif Anwar Chaudhry, Amir Rashid, Kashif Ijaz, Asifa Majeed, and Hashmat Gul. 2022. "Osteogenic Induction with Silicon Hydroxyapatite Using Modified Autologous Adipose Tissue-Derived Stromal Vascular Fraction: In Vitro and Qualitative Histomorphometric Analysis" Materials 15, no. 5: 1826. https://doi.org/10.3390/ma15051826
APA StyleKhan, M. M., Butt, S. A., Chaudhry, A. A., Rashid, A., Ijaz, K., Majeed, A., & Gul, H. (2022). Osteogenic Induction with Silicon Hydroxyapatite Using Modified Autologous Adipose Tissue-Derived Stromal Vascular Fraction: In Vitro and Qualitative Histomorphometric Analysis. Materials, 15(5), 1826. https://doi.org/10.3390/ma15051826