The Effect of ZnO, MgO, TiO2, and Na2O Modifiers on the Physical, Optical, and Radiation Shielding Properties of a TeTaNb Glass System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Optical Properties
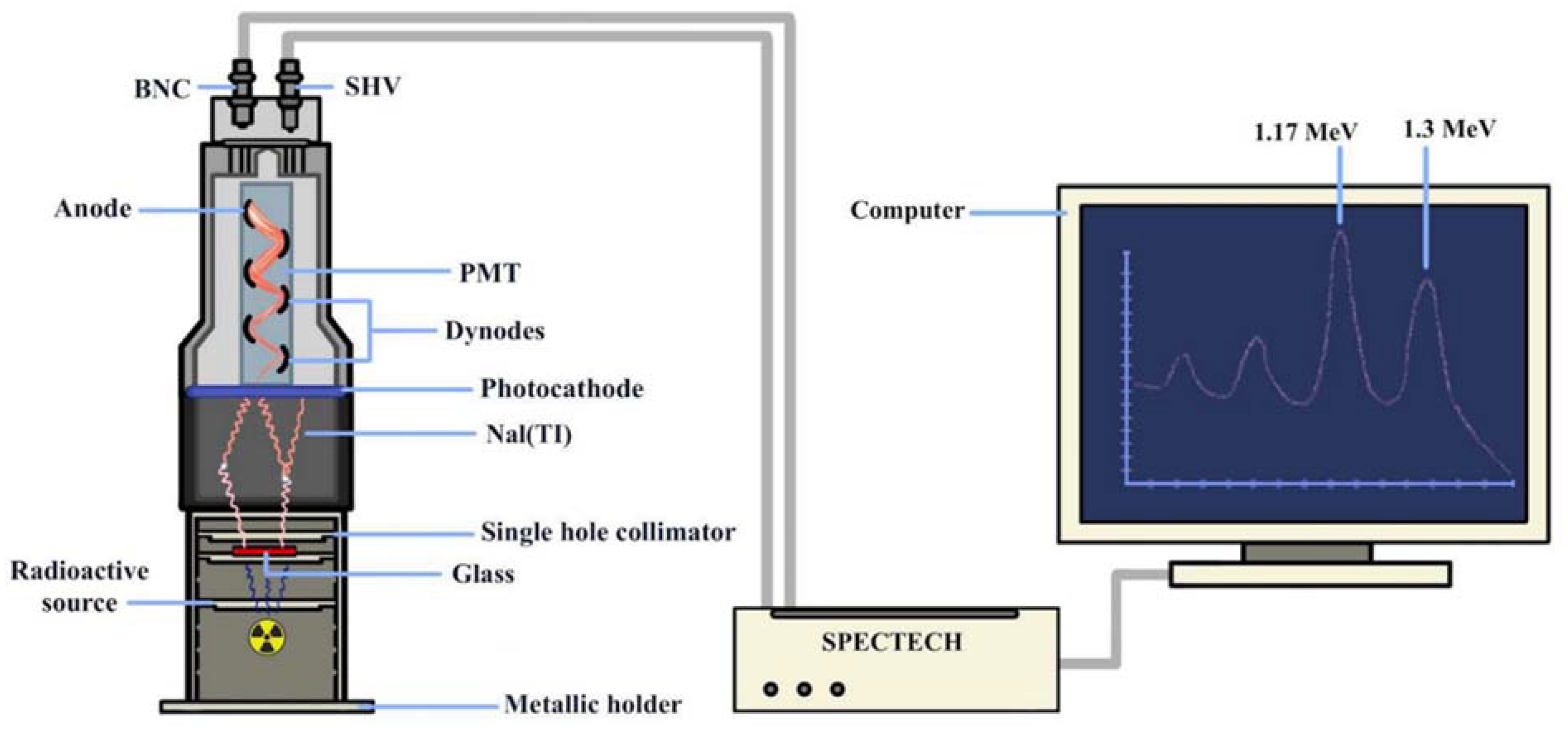
2.2. Measurement and Theoretical Evaluation of Glass Shielding Parameters
3. Results and Discussion
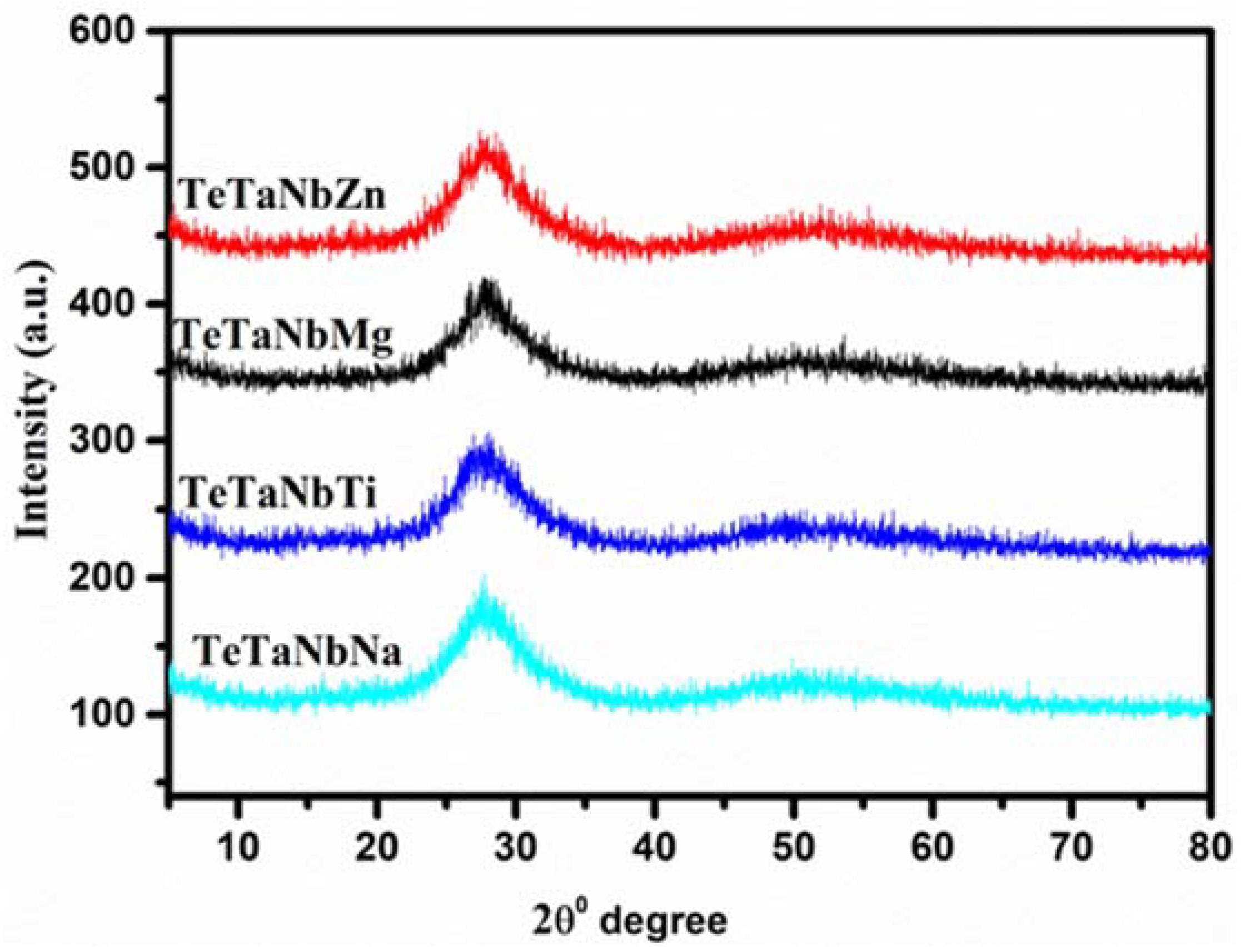
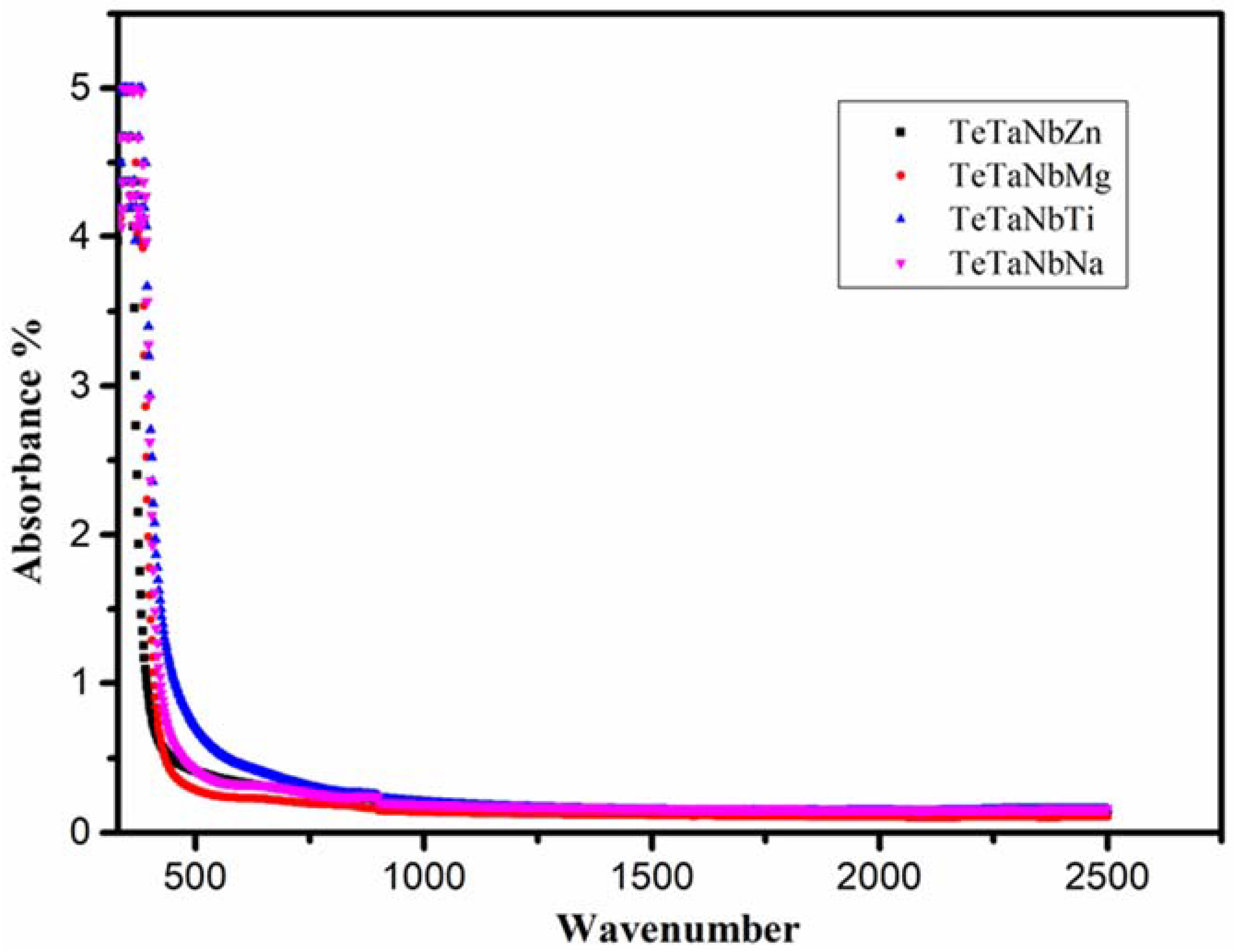
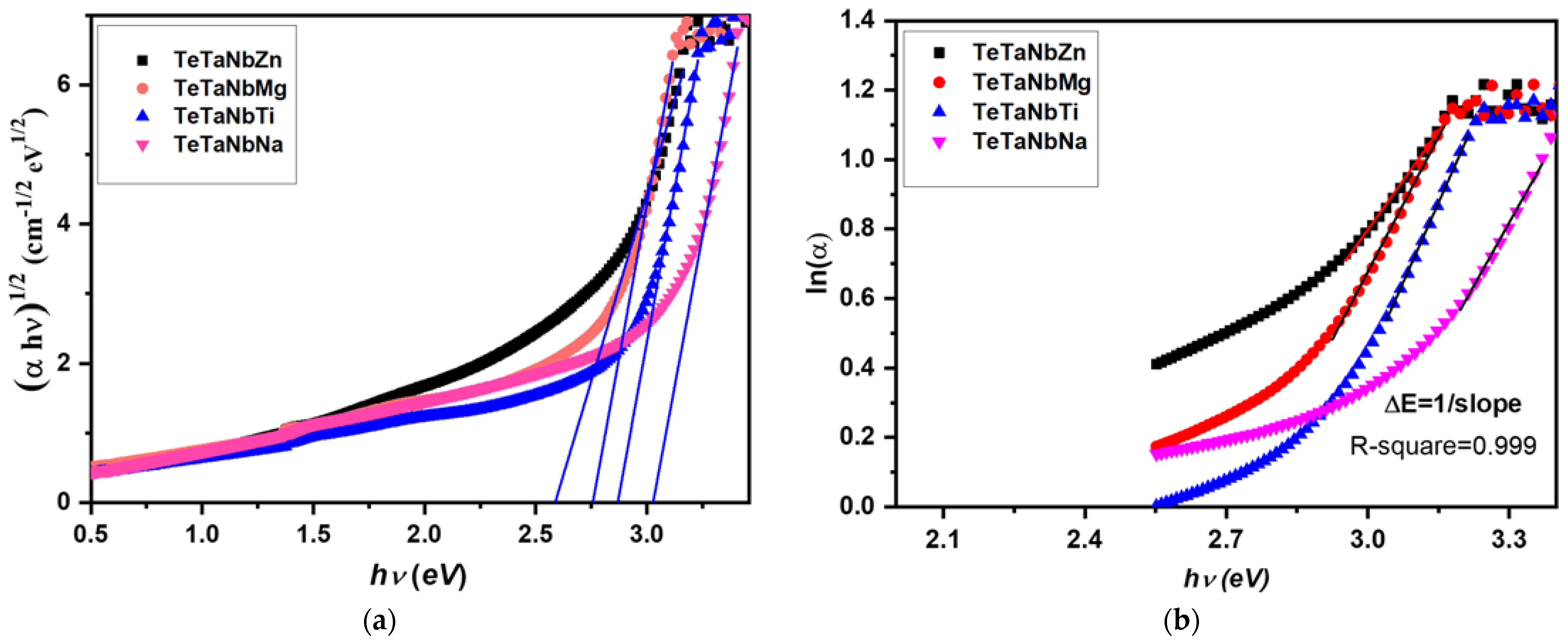
3.1. Optical and Physical Properties
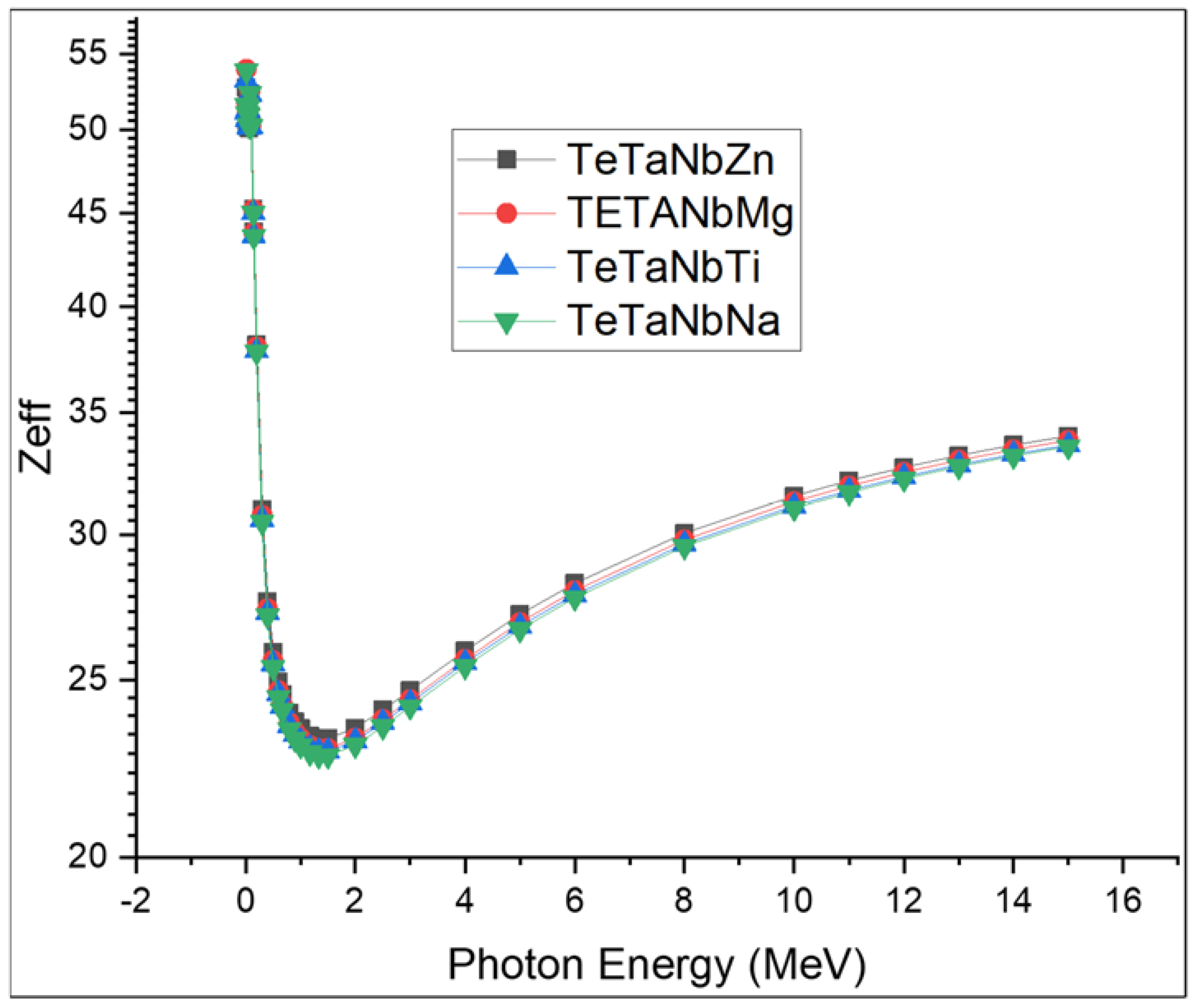
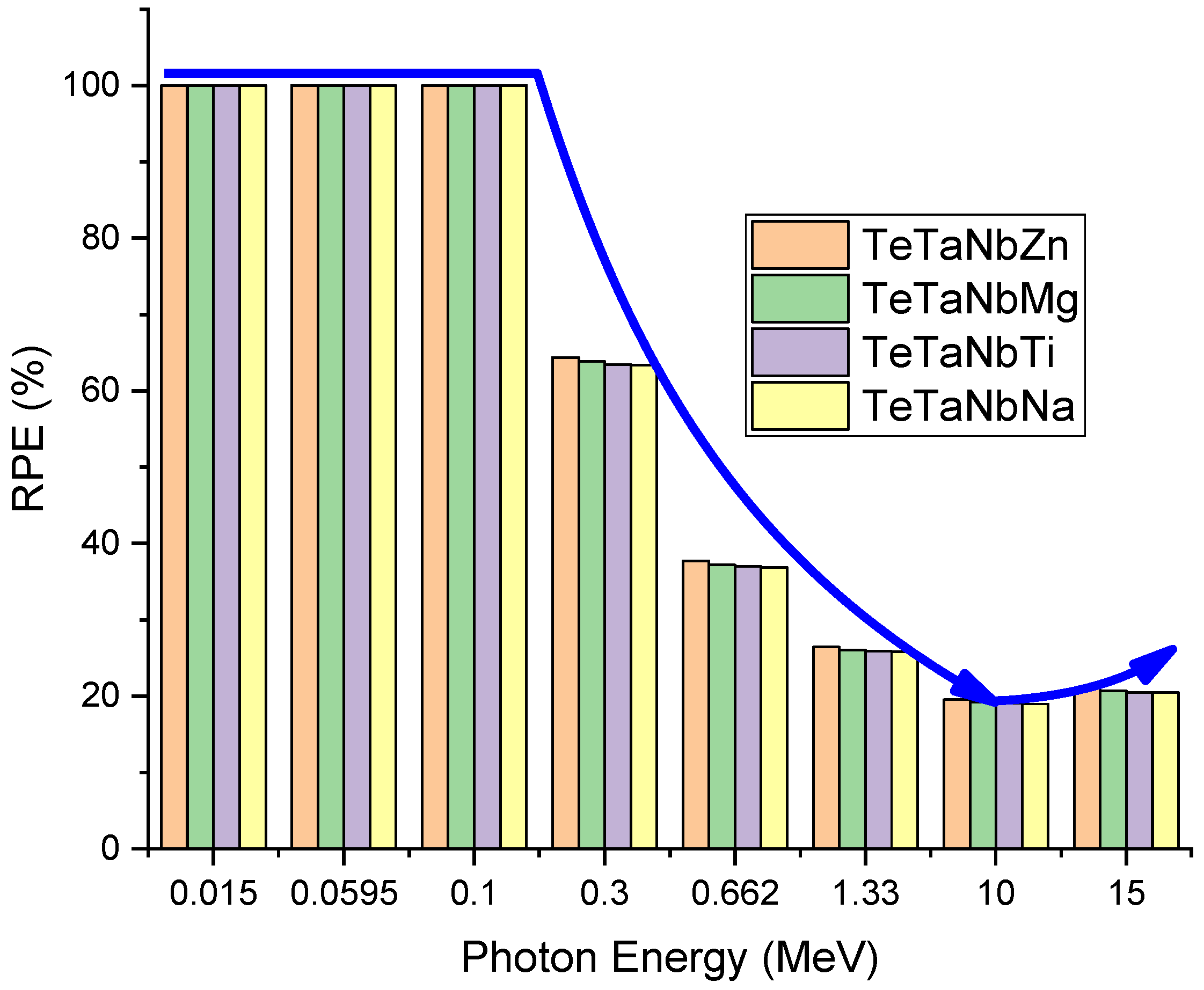
3.2. Radiation Shielding Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hussein, K.I.; Alqahtani, M.S.; Grelowska, I.; Reben, M.; Afifi, H.; Zahran, H.; Yaha, I.S.; Yousef, E.S. Optically transparent glass modified with metal oxides for X-rays and gamma rays shielding material. J. X-ray Sci. Technol. 2021, 29, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, M.S.; Almarhaby, A.M.; Hussein, K.I.; AbouDeif, Y.M.; Afifi, H.; Zahran, H.; Yaha, I.S.; Grelowska, I.; Yousef, E. Radiation attenuation and photoluminescence properties of host tellurite glasses doped with Er3+ ions. J. Instrum. 2021, 16, P01002. [Google Scholar] [CrossRef]
- Alqahtani, A.M.S.; Massoud, E.E.; Yahaa, I.S.; Yousef, E.S. An evaluation of the radiation protection characteristics of prototyped oxide glasses utilising Phy-X/PSD software. J. Instrum. 2020, 15, P08005. [Google Scholar]
- Rammah, Y.S.; Al-Buriahi, M.S.; El-Agawany, F.I.; AbouDeif, Y.M.; Yousef, E.S. Investigation of mechanical features and gamma-ray shielding efficiency of ternary TeO2-based glass systems containing Li2O, Na2O, K2O, or ZnO. Ceram. Int. 2020, 46, 27561. [Google Scholar] [CrossRef]
- Rammah, Y.S.; El-Agawany, F.I.; Abu El Soad, A.M.; Yousef, E.; El-Mesady, I.A. Ionizing radiation attenuation competences of gallium germanate-tellurite glasses utilizing MCNP5 simulation code and Phy-X/PSD program. Ceram. Int. 2020, 46, 22766. [Google Scholar] [CrossRef]
- Rammah, Y.S.; Olarinoye, I.O.; El-Agawany, F.I.; El-Adawy, A.; Yousef, E.S. The f-factor, neutron, gamma radiation and proton shielding competences of glasses with Pb or Pb/Bi heavy elements for nuclear protection applications. Ceram. Int. 2020, 46, 27163. [Google Scholar] [CrossRef]
- Gerward, L.; Guilbert, N.; Jensen, K.B.; Levring, H. WinXCom—A program for calculating X-ray attenuation coefficients. Radiat. Phys. Chem. 2004, 71, 653–654. [Google Scholar] [CrossRef]
- Hubbell, J.H.; Seltzer, S.M. Tables of X-ray Mass Attenuation Coefficients and Mass Energy-Absorption Coefficients 1 keV to 20 MeV for Elements Z = 1 to 92 and 48 Additional Substances of Dosimetric Interest; National Institute of Standards and Technology: Gaithersburg, MD, USA, 1995. [Google Scholar]
- Şakar, E.; Özpolat, Ö.F.; Alım, B.; Sayyed, M.I.; Kurudirek, M. Phy-X/PSD: Development of a user-friendly online software for calculation of parameters relevant to radiation shielding and dosimetry. Radiat. Phys. Chem. 2020, 166, 108496. [Google Scholar] [CrossRef]
- Hussein, K.I.; Alqahtani, M.S.; Algarni, H.; Zahran, I.S.; Yaha, I.; Grelowska, M.R.; Yousef, E.S. MIKE: A new computational tool for investigating radiation, optical and physical properties of prototyped shielding materials. J. Instrum. 2021, 16, T07004. [Google Scholar] [CrossRef]
- Souto, S.; Massot, M.; Balkanski, M.; Royer, D. Density and ultrasonic velocities in fast ionic conducting borate glasses. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol 1999, 64, 33–38. [Google Scholar] [CrossRef]
- Shamshad, L.; Rooh, G.; Limkitjaroenporn, P.; Srisittipokakun, N.; Chaiphaksa, W.; Kim, H.J.; Kaewkhao, J. A comparative study of gadolinium-based oxide and oxyfluoride glasses as low energy radiation shielding materials. Prog. Nucl. Energy 2017, 97, 53–59. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Qashou, S.I.; Khattari, Z.Y. Radiation shielding competence of newly developed TeO2–WO3 glasses. J. Alloys Compd. 2017, 96, 632–638. [Google Scholar] [CrossRef]
- Waly, E.S.A.; Fusco, M.A.; Bourham, M.A. Gamma-ray mass attenuation coefficient and half value layer factor of some oxide glass shielding materials. Ann. Nucl. Energy 2016, 96, 26–30. [Google Scholar] [CrossRef]
- Sayyed, M.I. Bismuth modified shielding properties of zinc boro-tellurite glasses. J. Alloys Comp. 2016, 688, 111–117. [Google Scholar] [CrossRef]
- Kaur, P.; Singh, D.; Singh, T. Heavy metal oxide glasses as gamma rays shielding material. Nucl. Eng. Des. 2016, 307, 364–376. [Google Scholar] [CrossRef]
- Bagheri, R.; Moghaddam, A.K.; Yousefnia, H. Gamma ray shielding study of barium–bismuth–borosilicate glasses as transparent shielding materials using MCNP-4C code, XCOM program, and available experimental data. Nucl. Eng. Technol. 2017, 49, 216–223. [Google Scholar] [CrossRef]
- Lakshminarayana, B.G.; Lira, S.O.; Sayyed, A.; Kityk, I.V.; Halimah, M.K.; Mahdi, M.A. X-ray photoelectron spectroscopy (XPS) and radiation shielding parameters investigations for zinc molybdenum boro-tellurite glasses containing different network modifiers. J. Mater. Sci. 2017, 52, 7394–7414. [Google Scholar] [CrossRef]
- Yongtao, Z.; Yunxia, Y.; Feihong, H.; Jing, R.; Shuanglong, Y.; Guorong, C.H. Characterization of new tellurite glasses and crystalline phases in the TeO2–PbO–Bi2O3–B2O3 system. J. Non-Cryst. Solids 2014, 386, 90–94. [Google Scholar]
- Guoying, Z.; Ying, T.; Huiyan, F.; Junjie, Z.; Hu, L. Properties and structures of Bi2O3–B2O3–TeO2 glass. J. Mater. Sci. Technol. 2013, 29, 209–214. [Google Scholar]
- Halimah, M.K.; Daud, W.M.; Sidek, H.A.A.; Zaidan, A.W.; Zainal, A.S. Optical properties of ternary tellurite glasses. Mater. Sci. 2010, 28, 173–180. [Google Scholar]
- Zaid, M.H.; Matori, K.A.; Aziz, S.H.; Zakaria, A.; Ghazali, M.S. Effect of ZnO on the physical properties and optical band gap of soda lime silicate glass. Int. J. Mol. Sci. 2012, 13, 7550–7558. [Google Scholar] [CrossRef] [Green Version]
- Sayyed, M.I.; Hamad, M.K.; Abu Mhareb, M.H.; Naseer, K.A.; Mahmoud, K.A.; Khandaker, M.U.; Osman, H.; Elesawy, B.H. Impact of Modifier Oxides on Mechanical and Radiation Shielding Properties of B2O3-SrO-TeO2-RO Glasses (Where RO = TiO2, ZnO, BaO, and PbO). Appl. Sci. 2021, 11, 10904. [Google Scholar] [CrossRef]
- Ma, X.; Peng, Z.; Li, J. Effect of Ta2O5 Substituting on Thermal and Optical Properties of High Refractive Index La2O3-Nb2O5 Glass System Prepared by Aerodynamic Levitation Method. J. Am. Ceram. Soc. 2015, 98, 770–773. [Google Scholar]
- Zhang, X.; Chen, Q.; Zhang, S. Ta2O5 Nanocrystals Strengthened Mechanical, Magnetic, and Radiation Shielding Properties of Heavy Metal Oxide Glass. Molecules 2021, 26, 4494. [Google Scholar] [CrossRef]
- Umair, M.M.; Yahya, A.K. Effect of Nb2O5 network stabilizer on elastic and optical properties of xNb2O5-(20-x)Bao-80TeO2 tellurite glass system. Chalcogenide Lett. 2015, 12, 287–300. [Google Scholar]
- Alalawi, A. Optical features and nuclear radiation shielding efficiency of ZnO-B2O3-Ta2O5 glasses. Phys. Scr. 2020, 95, 105302. [Google Scholar] [CrossRef]
- Agar, O.; Kavaz, E.; Altunsoy, E.E.; Kilicoglu, O.; Tekin, H.O.; Sayyed, M.I.; Erguzel, T.T.; Tarhan, N. Er2O3 effects on photon and neutron shielding properties of TeO2-Li2O-ZnO-Nb2O5 glass system. Results Phys. 2019, 13, 102277. [Google Scholar] [CrossRef]
- Sun, K.-H. Fundamental condition of glass formation. J. Am. Ceram. Soc. 1947, 30, 277–281. [Google Scholar] [CrossRef]
- Dimitrov, V.; Komatsu, T. An interpretation of optical properties of oxides and oxide glasses in terms of the electronic ion polarizability and average single bond strength (review). J. Univ. Chem. Technol. Metall. 2010, 45, 219–250. [Google Scholar] [CrossRef]
- Pawar, P.P.; Munishwar, S.R.; Gedam, R.S. Physical and optical properties of Dy3+/Pr3+ Co-doped lithium borate glasses for W-LED. J. Alloy. Compd. 2016, 660, 347–355. [Google Scholar] [CrossRef]
- Herzfeld, K.F. On Atomic Properties Which Make an Element a Metal. Phys. Rev. 1927, 29, 701–705. [Google Scholar] [CrossRef]
- Schott, C. Available online: https://www.schott.com/advanced_optics/english/products/optical-materials/special-materials/radiation-shielding-glasses/index.html (accessed on 18 January 2022).
- Alqahtani, M.S.; Hussein, K.I.; Afif, H.; Reben, M.; Grelowska, I.; Zahran, H.Y.; Yahia, I.S.; Yousef, E.S. Optical and radiation shielding characteristics of tellurite glass doped with different rare-earth oxides. J. X-ray Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Hubbell, J.H. Review of photon interaction cross section data in the medical and biological context. Phys. Med. Biol. 1999, 44, R1–R22. [Google Scholar] [CrossRef]
- Okunade, A. Parameters and computer software for the evaluation of mass attenuation and mass energy-absorption coefficients for body tissues and substitutes. J. Med. Phys. 2007, 32, 124–132. [Google Scholar] [CrossRef]
- Un, A.; Caner, T. The Direct-Z(eff) software for direct calculation of mass attenuation coefficient, effective atomic number and effective electron number. Ann. Nucl. Energy 2014, 65, 158–165. [Google Scholar] [CrossRef]
- Eyecioglu, O.; Karabul, Y.; El-Khayatt, A.M.; Iclli, O. ZXCOM: A software for computation of radiation sensing attributes. Radiat. Eff. Defects Solids 2016, 171, 965–977. [Google Scholar] [CrossRef]
- Elazoumi, S.H.; Sidek, H.A.A.; Rammah, Y.S.; El-Mallawany, R.; Halimah, M.K.; Matori, K.A.; Zaid, M.H.M. Effect of PbO on optical properties of tellurite glass. Results Phys. 2018, 8, 16–25. [Google Scholar] [CrossRef]
- Effendy, N.; Sidek, H.A.A.; Halimah, M.K.; Zaid, M.H.M. Enhancement on thermal, elastic and optical properties of new formulation tellurite glasses: Influence of ZnO as a glass modifier. Mater. Chem. Phys. 2021, 273, 125156. [Google Scholar] [CrossRef]
- Shaaban, K.S.; Zahran, H.Y.; Yahia, I.S.; Elsaeedy, H.I.; Shaaban, E.R.; Makhlouf, S.A.; Wahab, E.A. Mechanical and radiation-shielding properties of B2O3–P2O5–Li2O–MoO3 glasses. Appl. Phys. A 2020, 126, 804. [Google Scholar] [CrossRef]
- Ramak, K.; Yousef, E.; AlFaify, S.; Rüssel, C.; Maâlej, R. Raman, green and infrared emission cross-sections of Er3+ doped TZPPN tellurite glass. Opt. Mater. Express 2014, 4, 597–612. [Google Scholar]
- Weber, M.J. Probabilities for radiative and nonradiative decay of Er3+ in LaF3. Phys. Rev. 1967, 157, 262–272. [Google Scholar] [CrossRef]
- Elkhoshkhany, N.; El-Mallawany, R. Optical and kinetics parameters of lithium boro-tellurite glasses. Ceram. Int. 2015, 41, 3561–3567. [Google Scholar] [CrossRef]
- Upender, G.; Prasad, M. Raman, FTIR, thermal and optical properties of TeO2-Nb2O5-B2O3-V2O5 quaternary glass system. J. Taibah Univ. Sci. 2017, 11, 583–592. [Google Scholar]
- Sayyed, M.I.; Rammah, Y.S.; Laariedh, F.; Abouhaswa, A.S.; Badeche, T.B. Lead borate glasses doped by lanthanum: Synthesis, physical, optical, and gamma photon shielding properties. J. Non-Cryst. Solids 2020, 527, 119731. [Google Scholar] [CrossRef]
- Chinnamat, W.; Laopaiboon, R.; Laopaiboon, J.; Pencharee, S.; Bootjomchai, C. Influence of ionic radius modifying oxides on the elastic properties of glasses using ultrasonic techniques and FTIR spectroscopy. Phys. Chem. Glasses Eur. J. Glass Sci. Technol. B 2017, 58, 207–216. [Google Scholar] [CrossRef]
- Dutta, M.P.F.; Graca, M.A. Valente and S.K. Mendiratta, Structural characteristics and dielectric response of some zinc tellurite glasses and glass ceramics. Solid State Ion. 2013, 230, 66. [Google Scholar] [CrossRef]
- Stambouli, W.; Elhouichet, H.; Ferid, M. Study of thermal, structural and optical properties of tellurite glass with different TiO2 composition. Mol. Struct. 2012, 1028, 39–43. [Google Scholar] [CrossRef]
- Elkholy, H.; Othman, H.; Hager, I.; Ibrahim, M.; de Ligny, D. Thermal and optical properties of binary magnesium tellurite glasses and their link to the glass structure. J. Alloy. Compd. 2020, 823, 153781. [Google Scholar] [CrossRef]
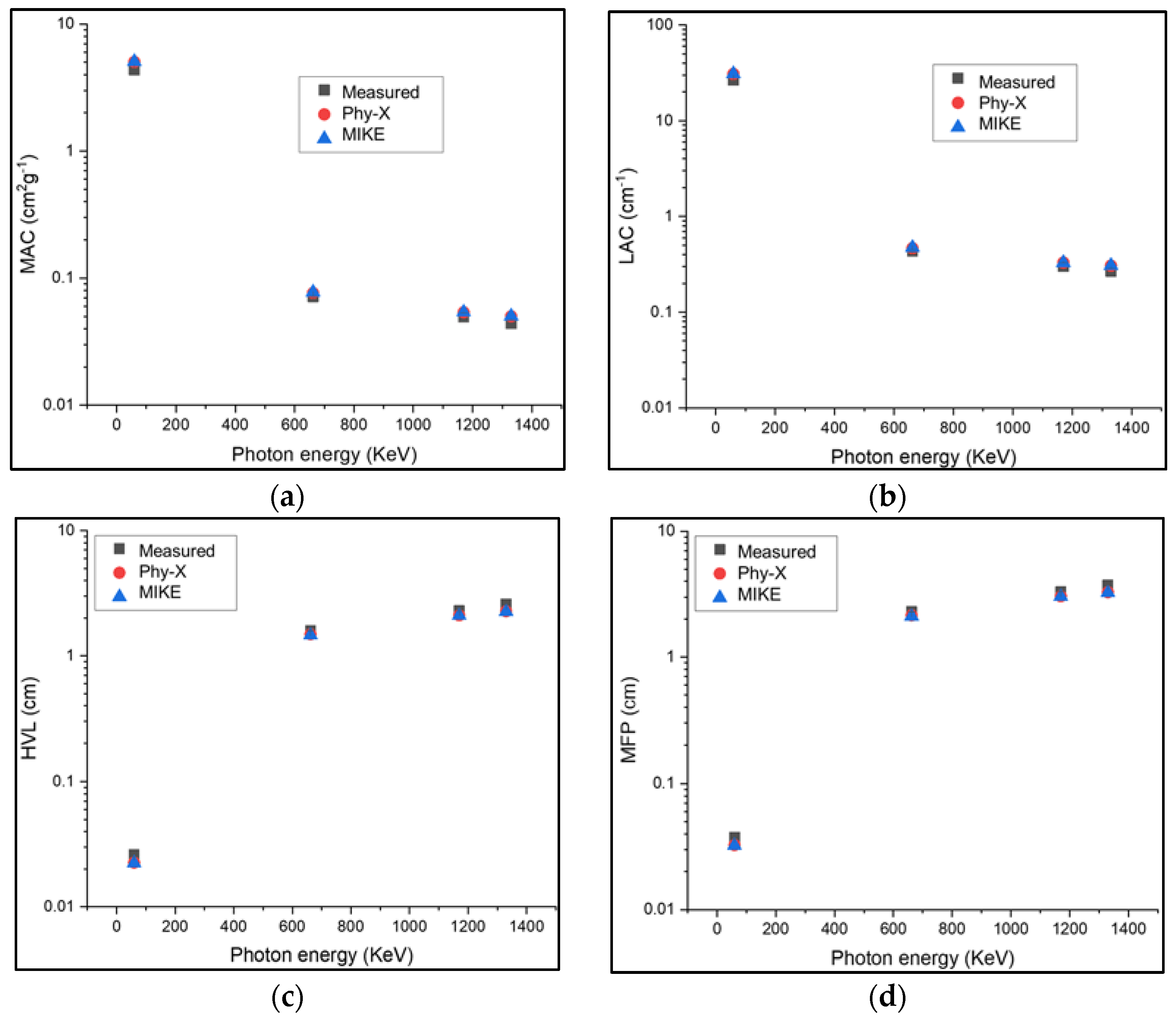

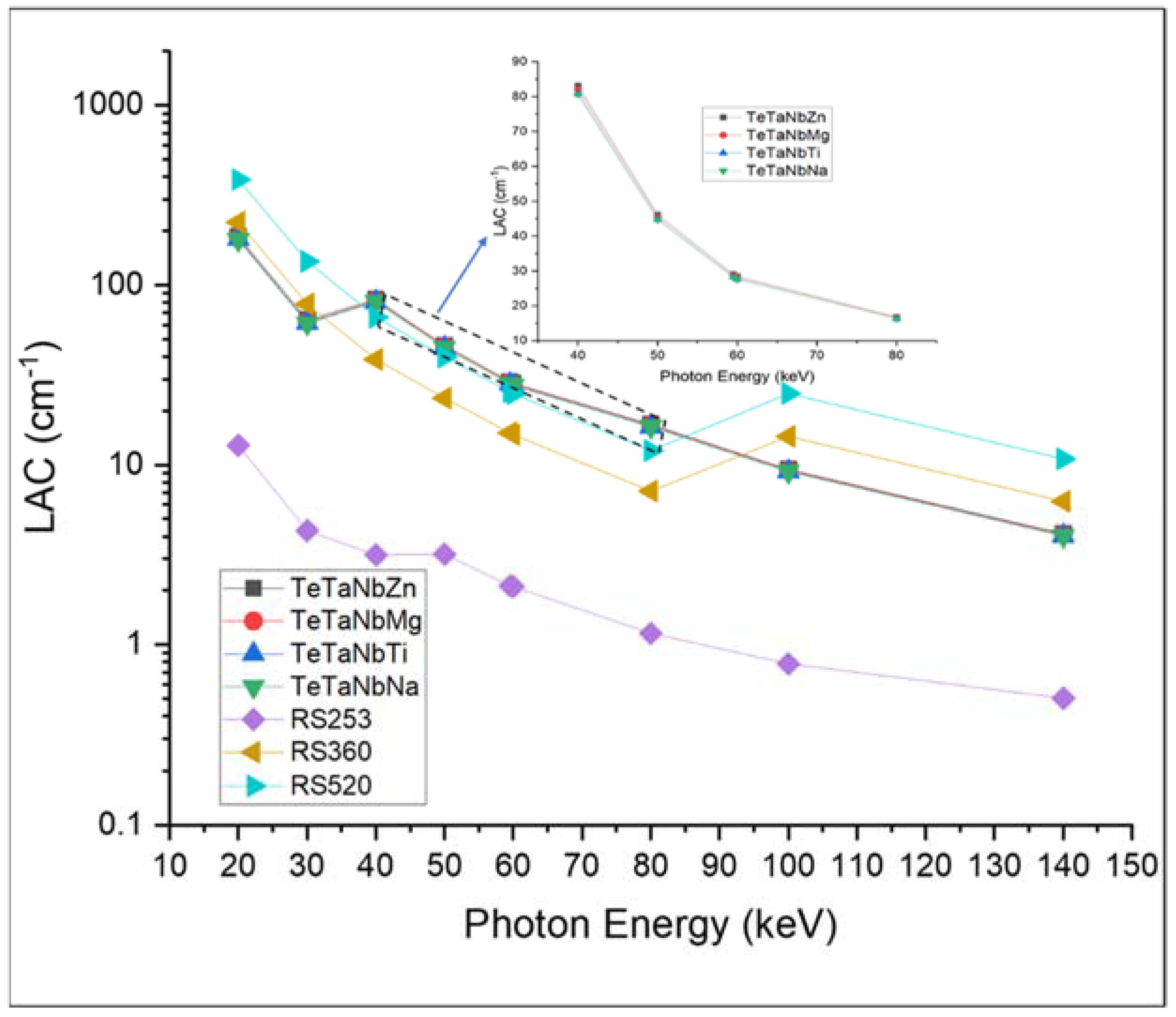
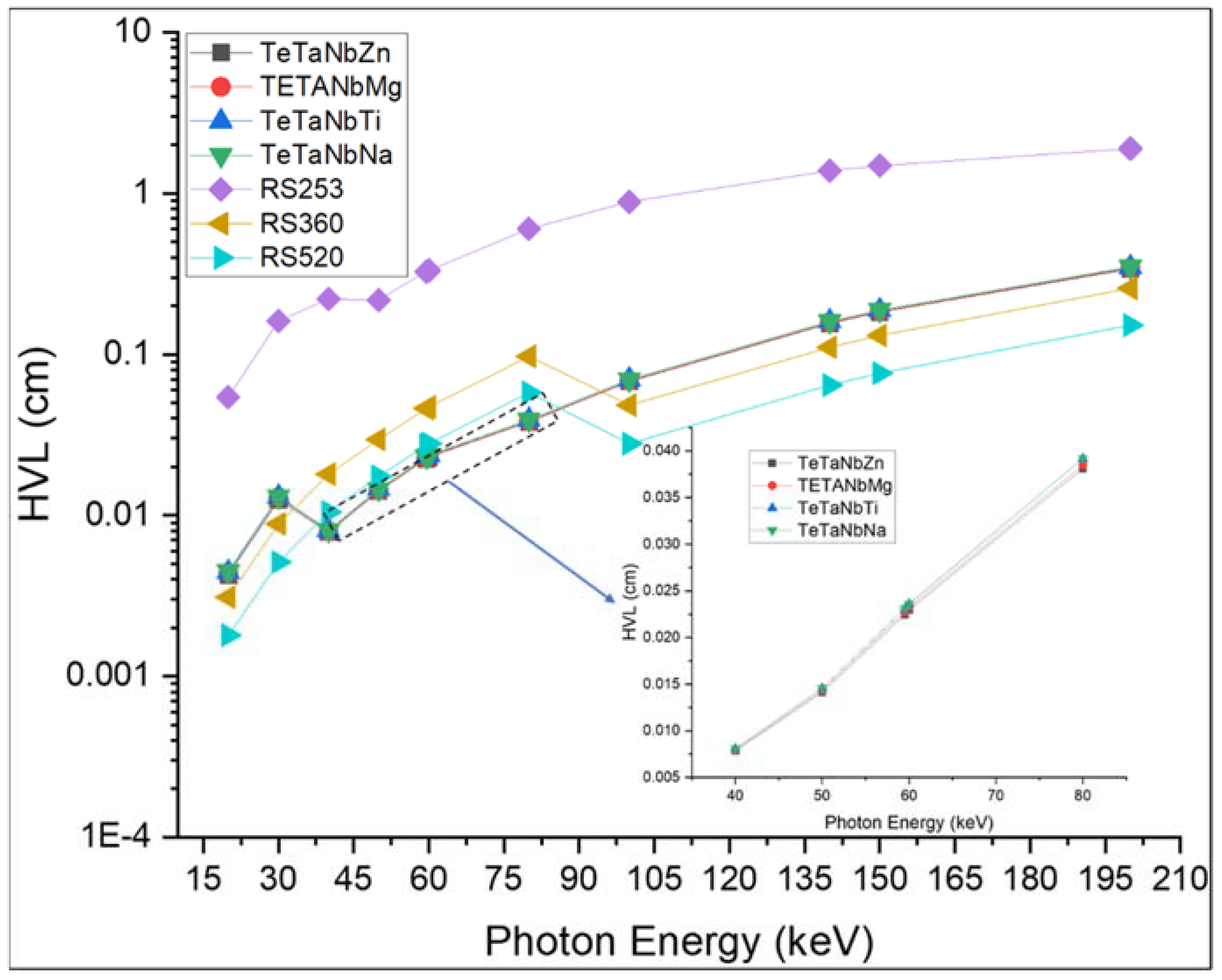
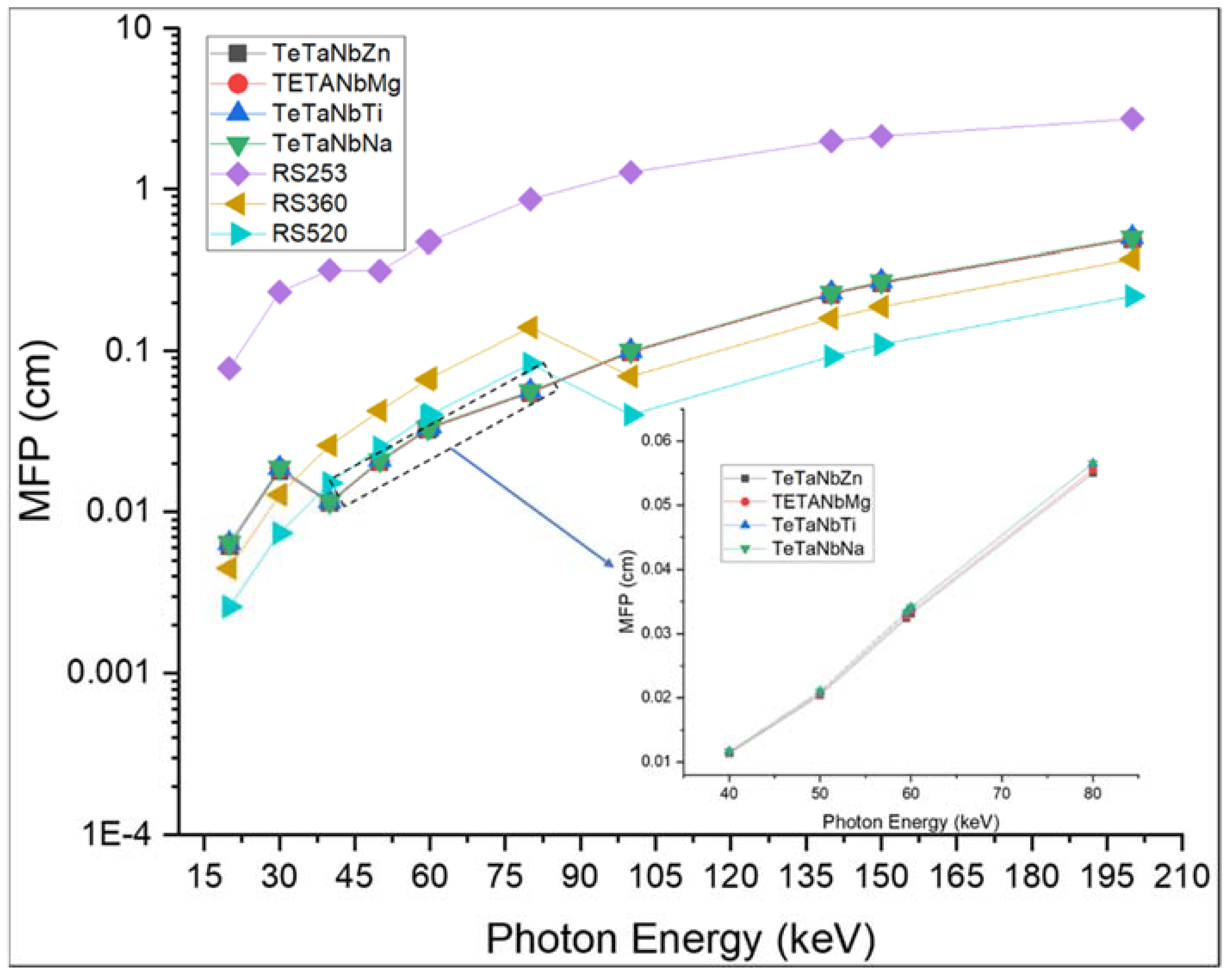

| Sample Code | Glass Composition | Density (g·cm−3) | Refractive Index |
|---|---|---|---|
| TeTaNbZn | 75TeO2–5Ta2O5–15Nb2O5–5ZnO | 6.1024 | 2.2005 |
| TeTaNbMg | 75TeO2–5Ta2O5–15Nb2O5–5MgO | 5.995 | 2.1367 |
| TeTaNbTi | 75TeO2–5Ta2O5–15Nb2O5–5TiO2 | 5.954 | 2.1186 |
| TeTaNbNa | 75TeO2–5Ta2O5–15Nb2O5–5Na2O | 5.9278 | 2.0967 |
| Sample Code | Vm (cm3·mol−1) | Vo (cm3 mol−1) | OPD (mol−1) | Energy Gap, Eopt (eV) | Urbach Energy, ΔE (eV) |
|---|---|---|---|---|---|
| TeTaNbZn | 26.0807 | 12.7223 | 78.6023 | 2.59 | 0.36 |
| TeTaNbMg | 26.2052 | 12.7830 | 78.2286 | 2.75 | 0.31 |
| TeTaNbTi | 26.7180 | 12.7229 | 78.5986 | 2.86 | 0.49 |
| TeTaNbNa | 26.6851 | 13.0171 | 76.8218 | 3.03 | 0.34 |
| Sample Code | Molar Polarizability, αm, Å3 | Molar Refraction, Rm (cm3 mol−1) | Metallization, M | χ3 × 10−12 Esu Third-Order Nonlinear |
|---|---|---|---|---|
| TeTaNbZn | 5.8117 | 14.6455 | 0.4385 | 1.49 |
| TeTaNbMg | 5.6473 | 14.2312 | 0.4569 | 1.11 |
| TeTaNbTi | 5.7003 | 14.3647 | 0.4624 | 1.02 |
| TeTaNbNa | 5.6226 | 14.1690 | 0.4690 | 1.02 |
| Photon Energy (keV) | Linear Attenuation Coefficients (LAC) cm2/g | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TeTaNbZn | TeTaNbMg | TeTaNbTi | TeTaNbNa | |||||||||
| Exp | Phy-X | MIKE | Exp | Phy-X | MIKE | Exp | Phy-X | MIKE | Exp | Phy-X | MIKE | |
| 59.5 | 26.571 ± 0.05 | 28.982 | 29.035 | 26.273 ± 0.039 | 28.609 | 28.661 | 25.613 ± 0.036 | 28.165 | 28.217 | 25.802 ± 0.041 | 28.129 | 28.181 |
| 622 | 0.435 ± 0.013 | 0.471 | 0.4709 | 0.411 ± 0.019 | 0.463 | 0.4630 | 0.404 ± 0.017 | 0.460 | 0.4595 | 0.3903 ± 0.024 | 0.458 | 0.4576 |
| 1170 | 0.302 ± 0.027 | 0.330 | 0.3308 | 0.297 ± 0.024 | 0.325 | 0.3251 | 0.287 ± 0.023 | 0.322 | 0.3229 | 0.2800 ± 0.017 | 0.321 | 0.3214 |
| 1330 | 0.267 ± 0.019 | 0.308 | 0.3082 | 0.264 ± 0.021 | 0.302 | 0.3028 | 0.255 ± 0.018 | 0.300 | 0.3008 | 0.2489± 0.020 | 0.321 | 0.2994 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussein, K.I.; Alqahtani, M.S.; Alzahrani, K.J.; Alqahtani, F.F.; Zahran, H.Y.; Alshehri, A.M.; Yahia, I.S.; Reben, M.; Yousef, E.S. The Effect of ZnO, MgO, TiO2, and Na2O Modifiers on the Physical, Optical, and Radiation Shielding Properties of a TeTaNb Glass System. Materials 2022, 15, 1844. https://doi.org/10.3390/ma15051844
Hussein KI, Alqahtani MS, Alzahrani KJ, Alqahtani FF, Zahran HY, Alshehri AM, Yahia IS, Reben M, Yousef ES. The Effect of ZnO, MgO, TiO2, and Na2O Modifiers on the Physical, Optical, and Radiation Shielding Properties of a TeTaNb Glass System. Materials. 2022; 15(5):1844. https://doi.org/10.3390/ma15051844
Chicago/Turabian StyleHussein, Khalid I., Mohammed S. Alqahtani, Khloud J. Alzahrani, Fawaz F. Alqahtani, Heba Y. Zahran, Ali M. Alshehri, Ibrahim. S. Yahia, Manuela. Reben, and El Sayed Yousef. 2022. "The Effect of ZnO, MgO, TiO2, and Na2O Modifiers on the Physical, Optical, and Radiation Shielding Properties of a TeTaNb Glass System" Materials 15, no. 5: 1844. https://doi.org/10.3390/ma15051844






