Effect of Electroless Cu Plating Ti3AlC2 Particles on Microstructure and Properties of Gd2O3/Cu Composites
Abstract
:1. Introduction
2. Materials and Methods
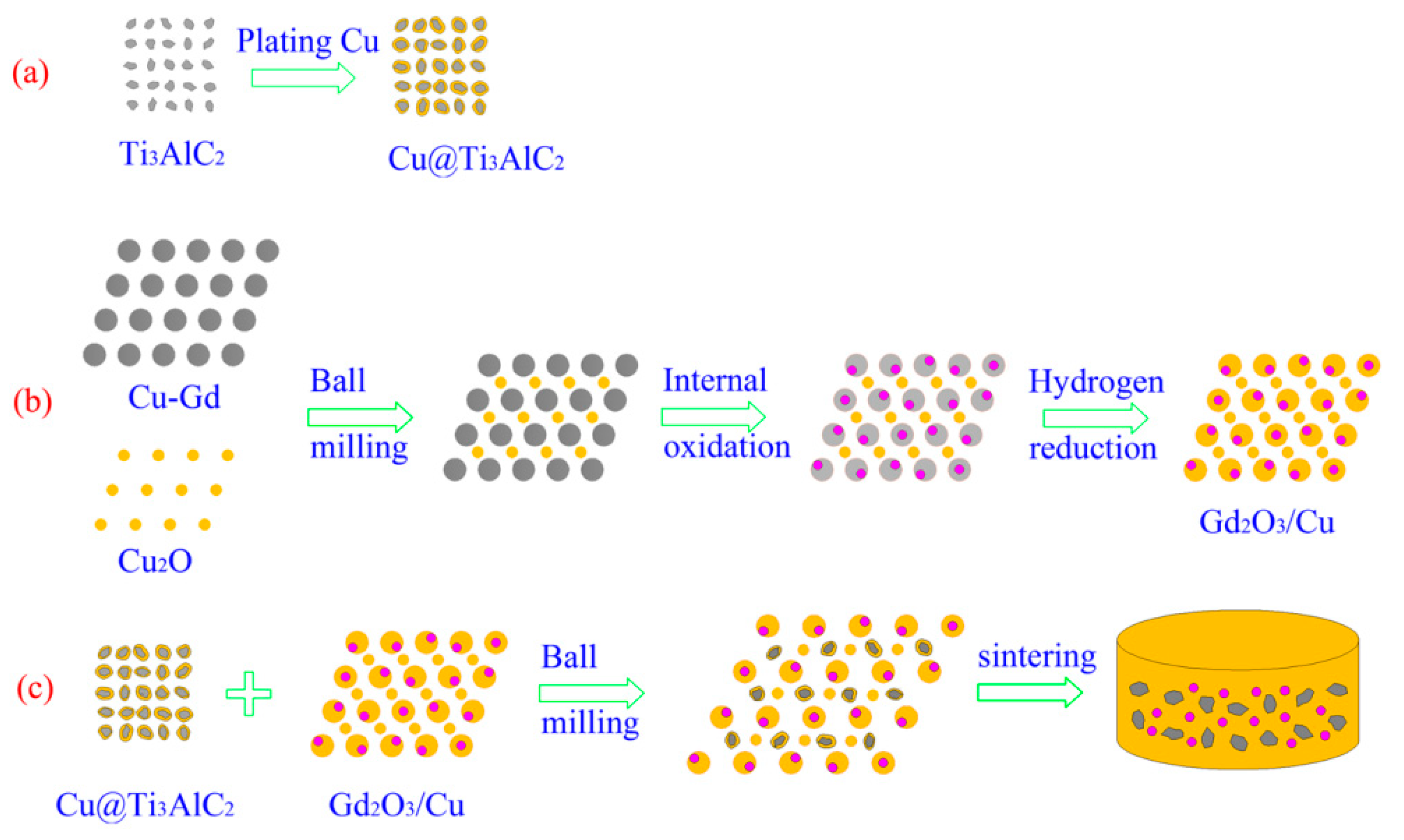
2.1. Raw Materials and Preparation Procedure
2.1.1. Electroless Cu Plating on the Surface of the Ti3AlC2 Particles
2.1.2. In Situ Synthesis of Gd2O3 Reinforced Phase
2.1.3. The Ball Milling and Sintering of the Cu@Ti3AlC2 and Gd2O3/Cu Powders
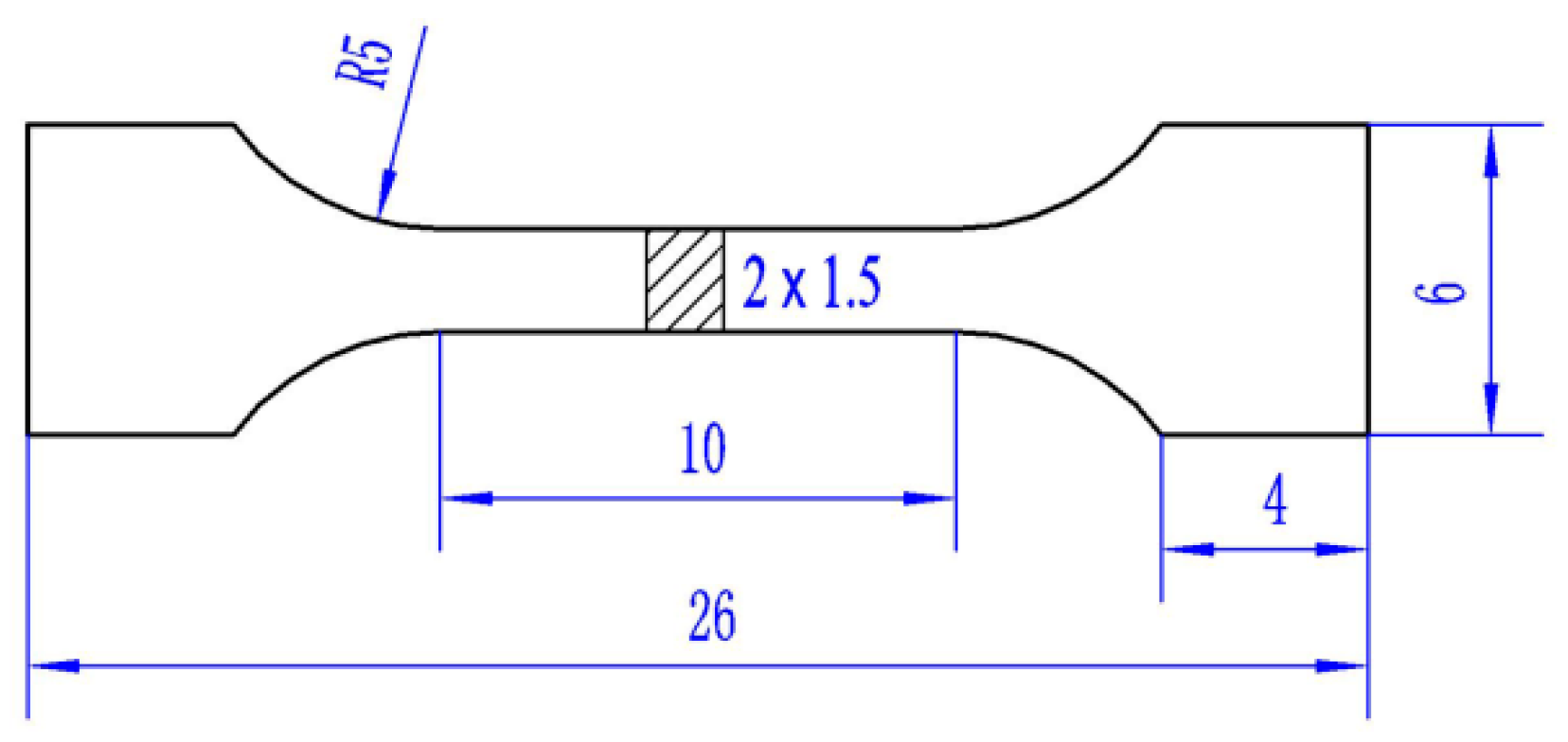
2.2. Test Methods
3. Results and Discussion
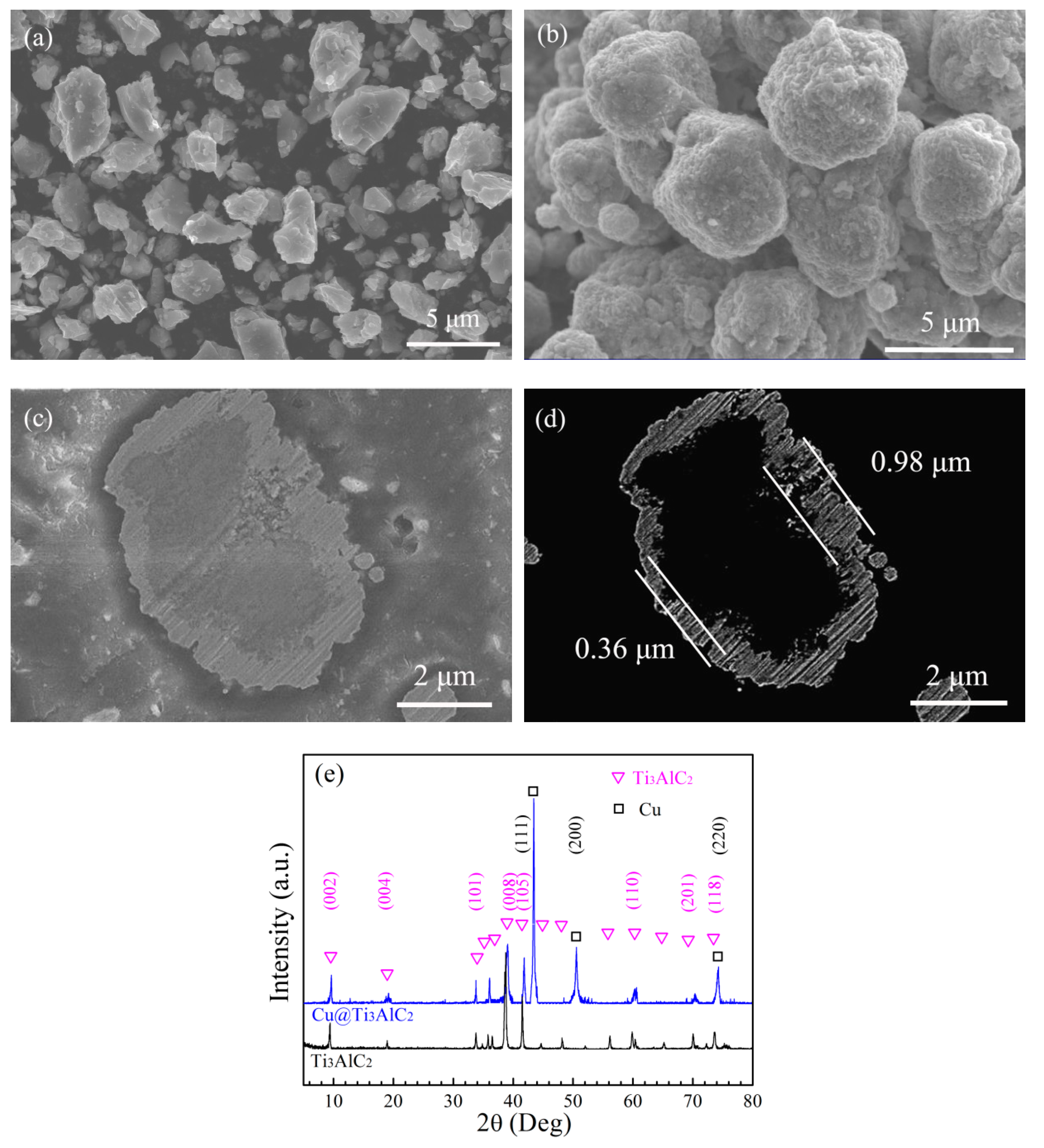
3.1. Electroless Cu Plating on the Surface of Ti3AlC2 Particles
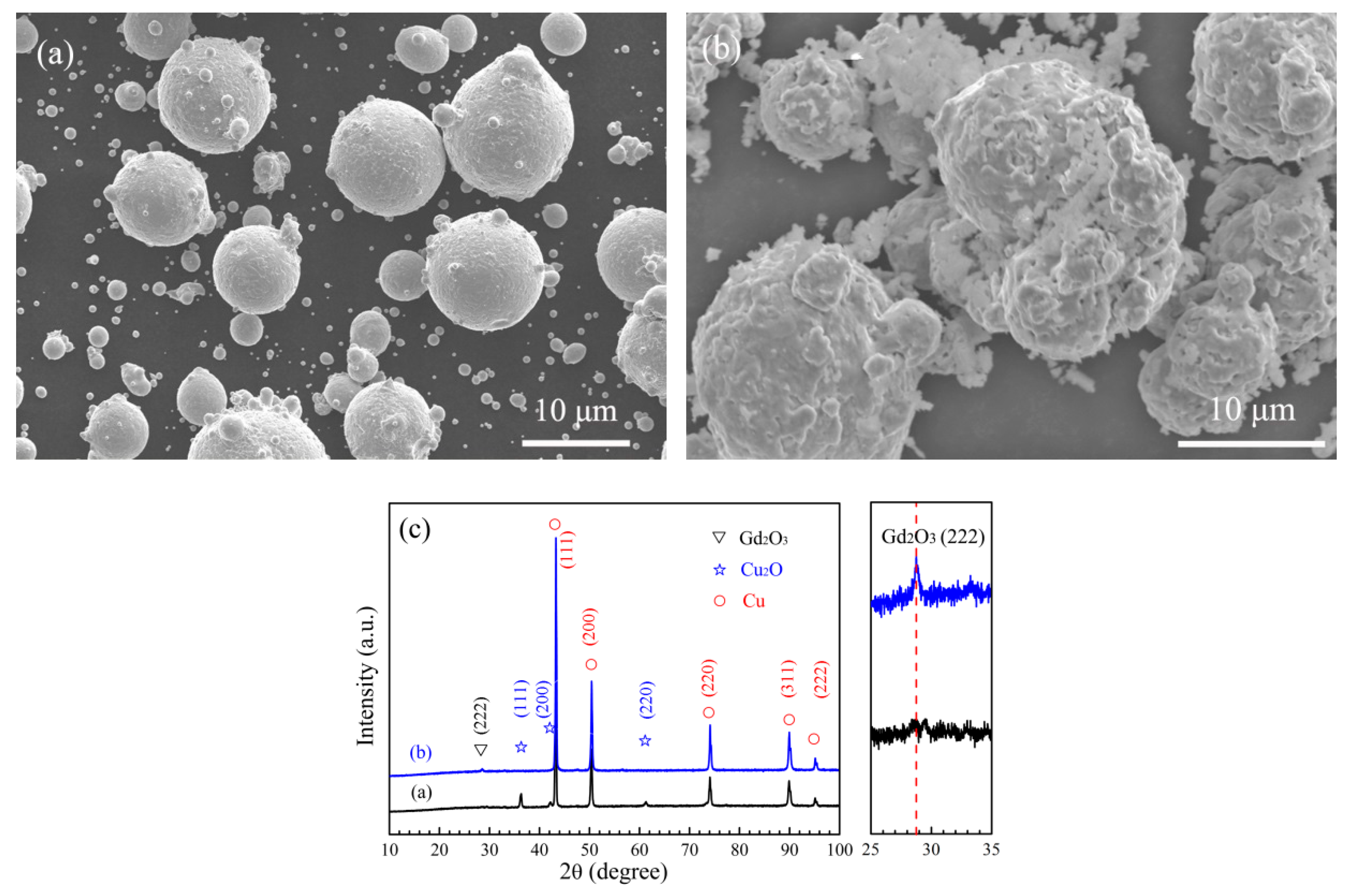
3.2. In Situ Synthesis of Gd2O3
3.3. Microstructure
3.3.1. The Distribution of Ti3AlC2 in the Cu Matrix
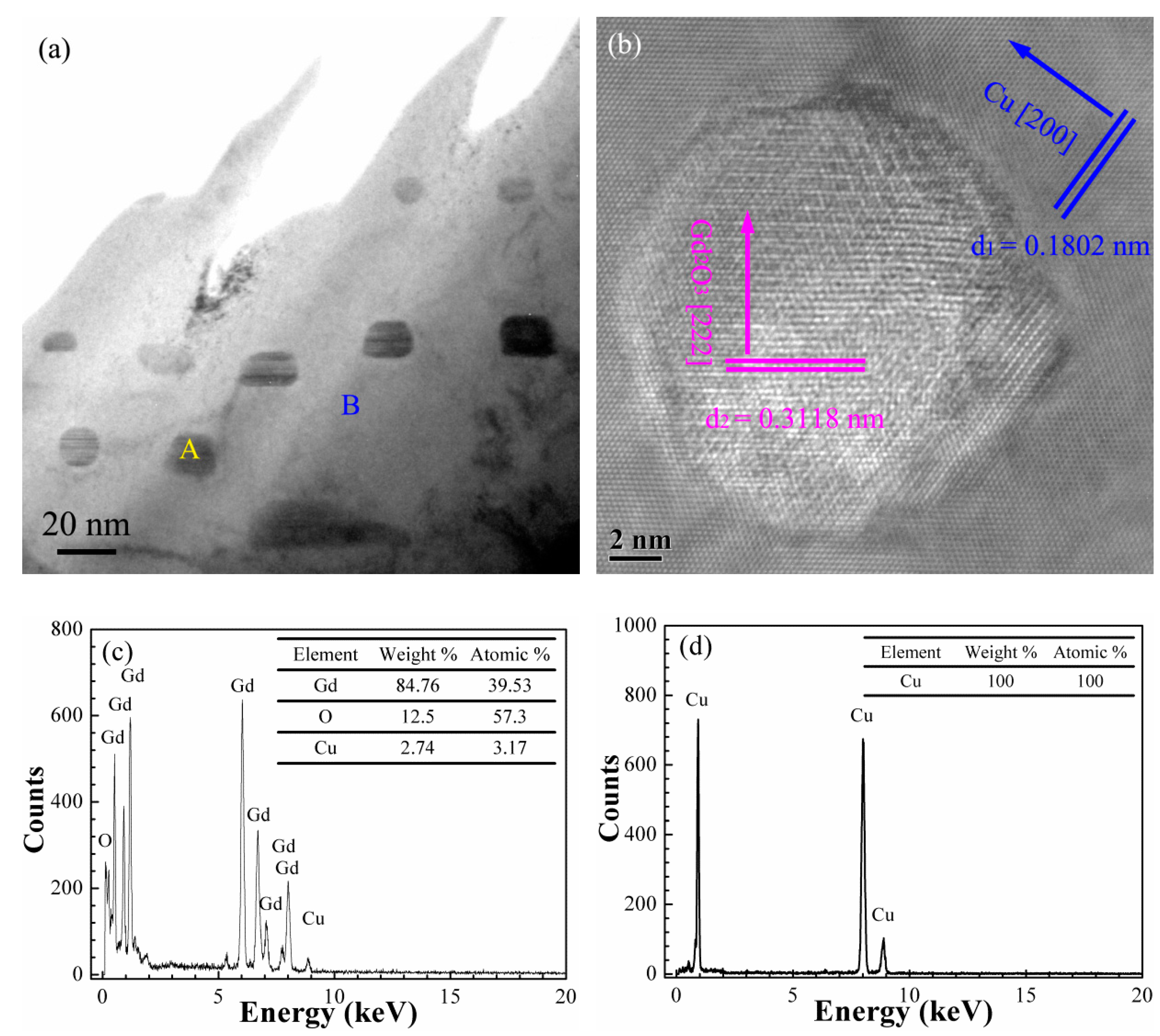
3.3.2. Microstructure of the Gd2O3/Cu Composites
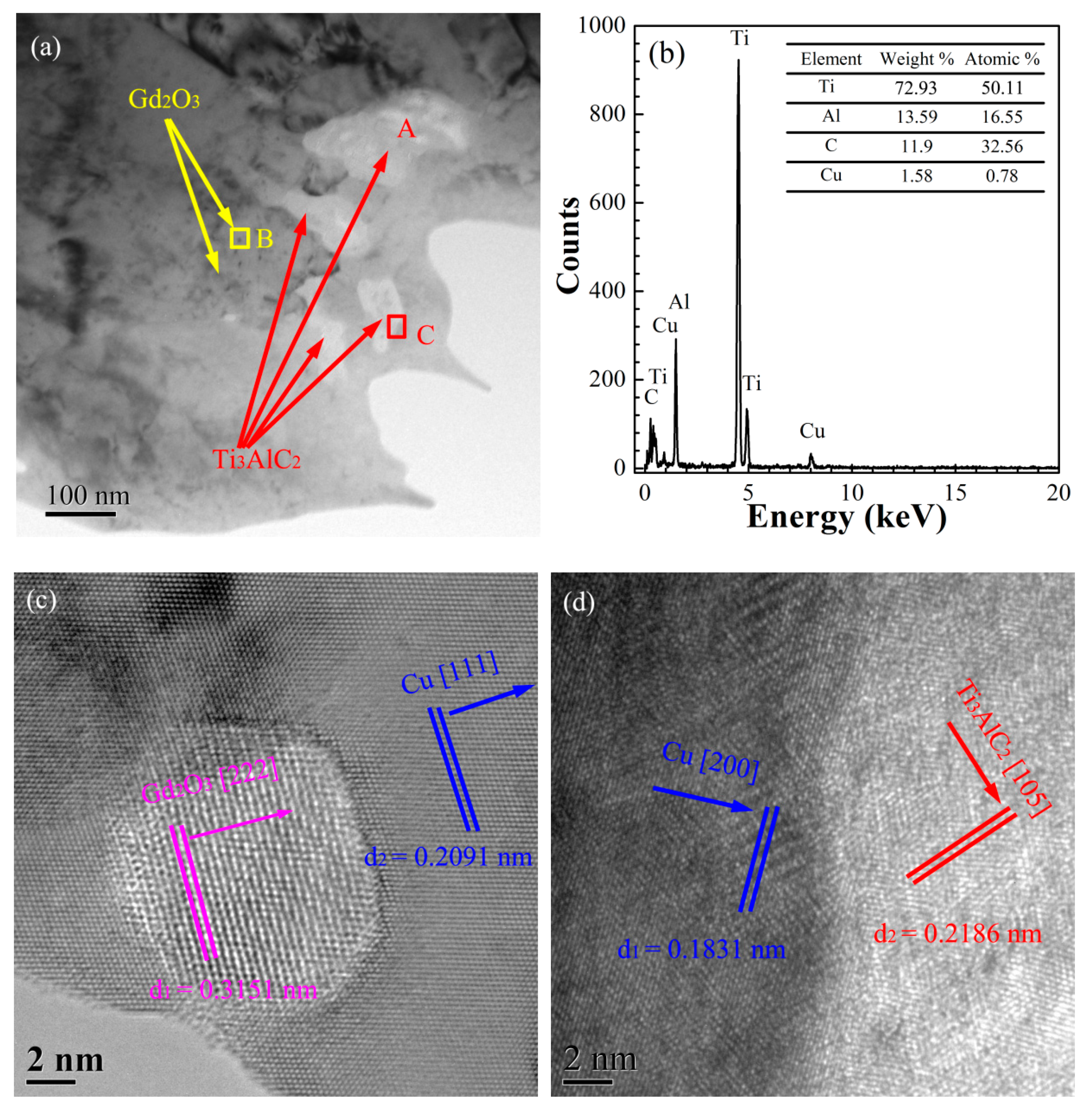
3.3.3. Microstructure of the Ti3AlC2-Gd2O3/Cu Composites
3.3.4. Microstructure of the Cu@Ti3AlC2-Gd2O3/Cu Composites
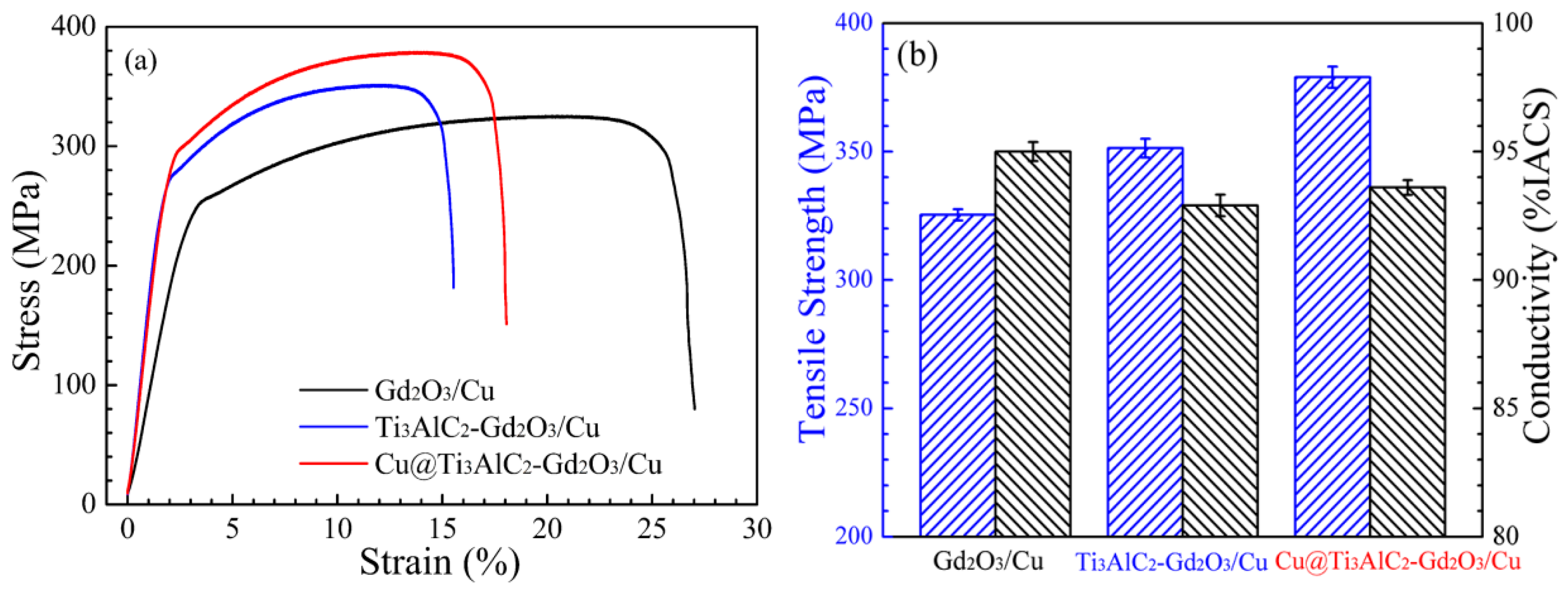
3.4. Tensile Strength and Electrical Conductivity
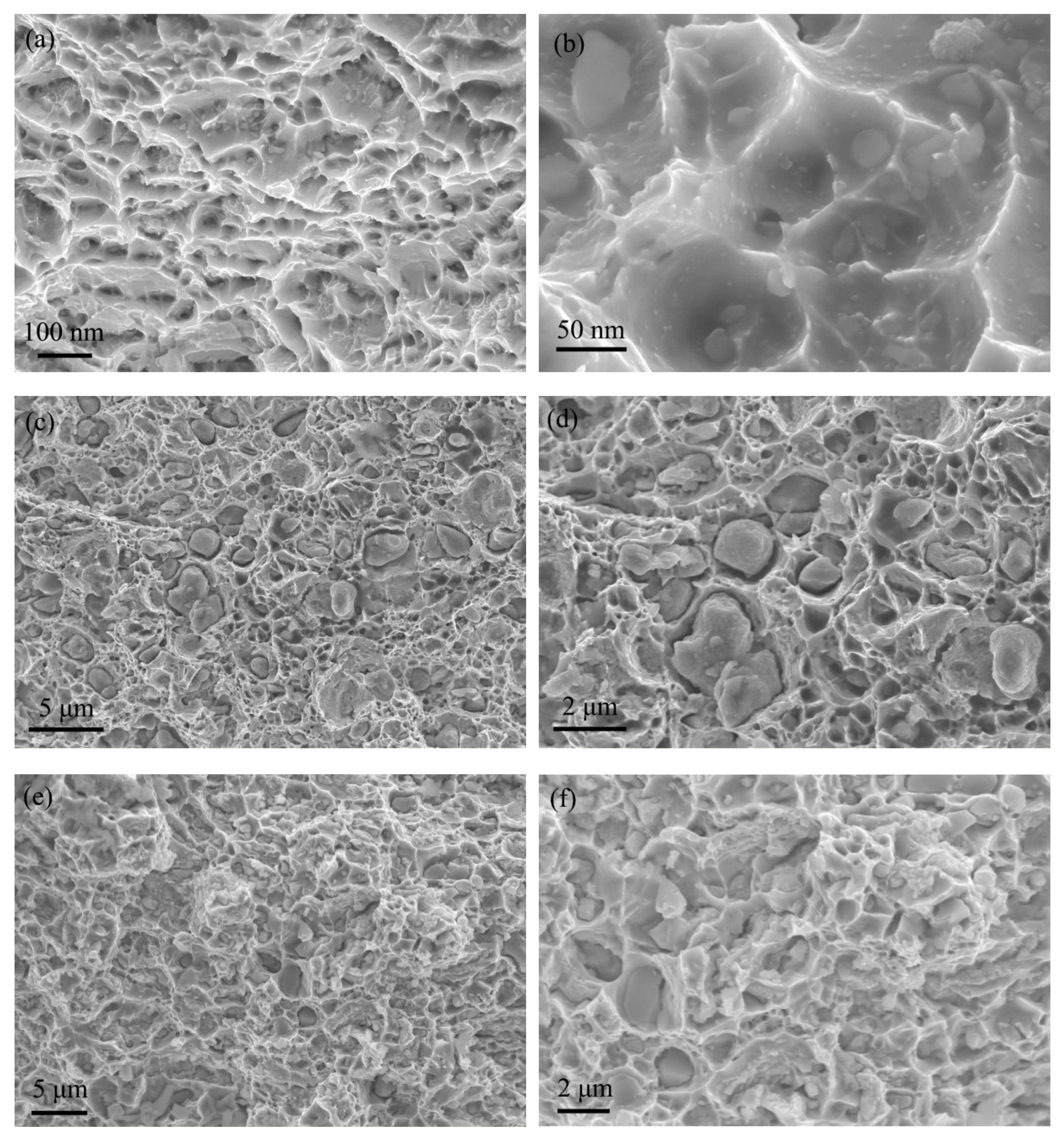
3.5. Fracture Morphology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peng, J.; Chen, B.; Wang, Z.; Guo, J.; Wu, B.; Hao, S.; Zhang, Q.; Gu, L.; Zhou, Q.; Liu, Z.; et al. Surface coordination layer passivates oxidation of copper. Nature 2020, 586, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Kang, H.; Wang, Z.; Guo, E.; Chen, Z.; Wang, T. Microstructure and properties of dual-scale particulate reinforced copper matrix composites with superior comprehensive properties. J. Alloy. Compd. 2021, 860, 157888. [Google Scholar] [CrossRef]
- Ma, B.; Hishinuma, Y.; Noto, H.; Shimada, Y.; Muroga, T. Development of Y2O3 dispersion strengthened Cu alloy using Cu6Y and Cu2O addition through the MA-HIP process. Fusion Eng. Des. 2020, 161, 112045. [Google Scholar] [CrossRef]
- Huang, B.; Ma, B.; Hishinuma, Y.; Noto, H.; Muroga, T. Investigations on the formation of multi-modal size distribution of mechanochemically processed Cu-Y-CuO powders. Fusion Eng. Des. 2020, 158, 111852. [Google Scholar] [CrossRef]
- Cao, H.; Zhan, Z.; Lv, X. Microstructure Evolution and Properties of an In-Situ Nano-Gd2O3/Cu Composite by Powder Metallurgy. Materials 2021, 14, 5021. [Google Scholar] [CrossRef]
- Chandrasekhar, S.B.; Sudhakara Sarma, S.; Ramakrishna, M.; Suresh Babu, P.; Rao, T.N.; Kashyap, B.P. Microstructure and properties of hot extruded Cu-1wt% Al2O3 nano-composites synthesized by various techniques. Mater. Sci. Eng. A 2014, 591, 46–53. [Google Scholar] [CrossRef]
- Güler, O.; Varol, T.; Alver, Ü.; Canakci, A. Effect of Al2O3 content and milling time on the properties of silver coated Cu matrix composites fabricated by electroless plating and hot pressing. Mater. Today Commun. 2020, 24, 101153. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Zhang, Y.; Wang, Y. Improvement of interfacial bonding and mechanical properties of Cu-Al2O3 composite by Cr-nanoparticle-induced interfacial modification. J. Alloy. Compd. 2017, 695, 2124–2130. [Google Scholar] [CrossRef] [Green Version]
- Pan, S.; Zheng, T.; Yao, G.; Chi, Y.; Rosa, I.D.; Li, X. High-strength and high-conductivity in situ Cu–TiB2 nanocomposites. Mater. Sci. Eng. A 2022, 831, 141952. [Google Scholar] [CrossRef]
- Hwang, J.; Yoon, T.; Jin, S.H.; Lee, J.; Kim, T.; Hong, S.H.; Jeon, S. Enhanced Mechanical Properties of Graphene/Copper Nanocomposites Using a Molecular-Level Mixing Process. Adv. Mater. 2013, 25, 6724–6729. [Google Scholar] [CrossRef]
- Zhuo, H.; Tang, J.; Ye, N. A novel approach for strengthening Cu-Y2O3 composites by in situ reaction at liquidus temperature. Mater. Sci. Eng. A 2013, 584, 1–6. [Google Scholar] [CrossRef]
- Kudashov, D.V.; Baum, H.; Martin, U.; Heilmaier, M.; Oettel, H. Microstructure and room temperature hardening of ultra-fine-grained oxide-dispersion strengthened copper prepared by cryomilling. Mater. Sci. Eng. A 2004, 387–389, 768–771. [Google Scholar] [CrossRef]
- Shabadi, R.; Avettand-Fènoël, M.N.; Simar, A.; Taillard, R.; Jain, P.K.; Johnson, R. Thermal conductivity in yttria dispersed copper. Mater. Des. 2015, 65, 869–877. [Google Scholar] [CrossRef]
- Mo, F.; Feng, Y.; Chen, Y.; Wang, Y.; Qian, G.; Dou, Y.; Zhang, X. Effect of La2O3 on electrical friction and wear properties of Cu-graphite composites. J. Rare Earths. 2015, 33, 327–333. [Google Scholar] [CrossRef]
- Groza, J.R.; Gibeling, J.C. Principles of particle selection for dispersion-strengthened copper. Mater. Sci. Eng. A 1993, 171, 115–125. [Google Scholar] [CrossRef]
- Zheng, R.; Zhan, Z.; Wang, W. Wear behavior of Cu-La2O3 composite with or without electrical current. Wear 2010, 268, 72–76. [Google Scholar] [CrossRef]
- Aghamiri, S.M.S.; Oono, N.; Ukai, S.; Kasada, R.; Noto, H.; Hishinuma, Y.; Muroga, T. Microstructure development and high tensile properties of He/H2 milled oxide dispersion strengthened copper. J. Alloy. Compd. 2019, 783, 674–679. [Google Scholar] [CrossRef]
- Huang, F.; Wang, H.; Yang, B.; Liao, T.; Wang, Z. Uniformly dispersed Y2O3 nanoparticles in nanocrystalline copper matrix via multi-step ball milling and reduction process. Mater. Lett. 2019, 242, 119–122. [Google Scholar] [CrossRef]
- Hu, W.; Huang, Z.; Wang, Y.; Li, X.; Zhai, H.; Zhou, Y.; Chen, L. Layered ternary MAX phases and their MX particulate derivative reinforced metal matrix composite:A review. J. Alloy. Compd. 2021, 586, 517313. [Google Scholar] [CrossRef]
- Sobolev, K.; Gorshenkov, M.; Manfrinetti, P.; Peddis, D.; Pazniak, A.; Rodionova, V. Synthesis of phase-pure highly-doped MAX-phase (Cr1-xMnx)2AlC. Ceram. Inf. 2021, 47, 21069–21076. [Google Scholar] [CrossRef]
- Wang, W.; Zhai, H.; Chen, L.; Zhou, Y.; Huang, Z.; Bei, G.; Greil, P. Sintering and properties of mechanical alloyed Ti3AlC2-Cu composites. Mater. Sci. Eng. A 2017, 685, 154–158. [Google Scholar] [CrossRef]
- Griseri, M.; Tunca, B.; Lapauw, T.; Huang, S.; Popescu, L.; Barsoum, M.W.; Lambrinou, K.; Vleugels, J. Synthesis, properties and thermal decomposition of the Ta4AlC3 MAX phase. J. Eur. Ceram. Soc. 2019, 39, 2973–2981. [Google Scholar] [CrossRef] [Green Version]
- Badie1, S.; Dash, A.; Sohn, Y.J.; Vaßen, R.; Guillon, O. Gonzalez-Julian, J. Synthesis, sintering, and effect of surface roughness on oxidation of submicron Ti2AlC ceramics. J. Am. Ceram. Soc. 2021, 104, 1669–1688. [Google Scholar] [CrossRef]
- Was′kowska, A.; Gerward, L.; Olsen, J.S.; Babu, K.R.; Vaitheeswaran, G.; Kanchana, V.; Svane, A.; Filipov, V.B.; Levchenko, G.; Lyaschenko, A. Thermoelastic properties of ScB2, TiB2, YB4 and HoB4: Experimental and theoretical studies. Acta Mater. 2011, 59, 4886–4894. [Google Scholar] [CrossRef]
- Guo, M.; Shen, K.; Wang, M. Relationship between microstructure, properties and reaction conditions for Cu-TiB2 alloys prepared by in situ reaction. Acta Mater. 2009, 57, 4568–4579. [Google Scholar] [CrossRef]
- Magnuson, M.; Wilhelmsson, O.; Palmquist, J.-P.; Jansson, U.; Mattesini, M.; Li, S.; Ahuja, R.; Eriksson, O. Electronic structure and chemical bonding in Ti2AlC investigated by soft x-ray emission spectroscopy. Phys. Rev. B 2006, 74, 195180. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Julian, J. Processing of MAX phases: From synthesis to applications. J. Am. Ceram. Soc. 2021, 104, 659–690. [Google Scholar] [CrossRef]
- Hao, Z.; Hao, Z.; Xie, G.; Liu, X.; Tan, Q.; Wang, R. The precipitation behaviours and strengthening mechanism of a Cu-0.4 wt% Sc alloy. J. Mater. Sci. Technol. 2022, 98, 1–13. [Google Scholar] [CrossRef]
- Ma, K.; Wen, H.; Hu, T.; Topping, T.D.; Isheim, D.; Seidman, D.N.; Lavernia, E.J.; Schoenung, J.M. Mechanical behavior and strengthening mechanisms in ultrafine grain precipitation-strengthened aluminum alloy. Acta Mater. 2014, 62, 141–155. [Google Scholar] [CrossRef]
- Arsenault, R.J.; Shi, N. Dislocation generation due to differences between the coefficients of thermal expansion. Mater. Sci. Eng. 1986, 81, 175–187. [Google Scholar] [CrossRef]
- Dunand, D.C.; Mortensen, A. Reinforced silver chloride as a model material for the study of dislocations in metal matrix composites. Mater. Sci. Eng. A 1991, 144, 179–188. [Google Scholar] [CrossRef]
- Deng, K.; Shi, J.; Wang, C.; Wang, X.; Wu, Y.; Nie, K.; Wu, K. Microstructure and strengthening mechanism of bimodal size particle reinforced magnesium matrix composite. Compos. Part A Appl. Sci. Manuf. 2012, 43, 1280–1284. [Google Scholar] [CrossRef]
- Xiong, B.; Yu, H.; Xu, Z.; Yan, Q.; Cai, C. Fabrication of SiC particulate reinforced AZ91D composite by vacuum-assisted pressure infiltration technology. J. Alloy. Compd. 2011, 509, L279–L283. [Google Scholar] [CrossRef]
- Yan, S.; Dai, S.; Zhang, X.; Yang, C.; Hong, Q.; Chen, J.; Lin, Z. Investigating aluminum alloy reinforced by graphene nanoflakes. Mater. Sci. Eng. A 2014, 612, 440–444. [Google Scholar] [CrossRef]
- Pan, S.; Yuan, J.; Zhang, P.; Sokoluk, M.; Yao, G.; Li, X. Effect of electron concentration on electrical conductivity in in situ Al-TiB2 nanocomposites. Appl. Phys. Lett. 2020, 116, 014102. [Google Scholar] [CrossRef]


| Function | Main Salt | Reductant | PH Regulator | Stabilizer | Complex |
|---|---|---|---|---|---|
| Composition | CuSO4·5H2O | HCHO (37%) | NaOH | 2,2′-Bipyridyl | Na2EDTA |
| Concentration | 18 g/L | 20 mL/L | 8 g/L | 0.05 g/L | 30 g/L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, H.; Zhan, Z.; Lv, X. Effect of Electroless Cu Plating Ti3AlC2 Particles on Microstructure and Properties of Gd2O3/Cu Composites. Materials 2022, 15, 1846. https://doi.org/10.3390/ma15051846
Cao H, Zhan Z, Lv X. Effect of Electroless Cu Plating Ti3AlC2 Particles on Microstructure and Properties of Gd2O3/Cu Composites. Materials. 2022; 15(5):1846. https://doi.org/10.3390/ma15051846
Chicago/Turabian StyleCao, Haiyao, Zaiji Zhan, and Xiangzhe Lv. 2022. "Effect of Electroless Cu Plating Ti3AlC2 Particles on Microstructure and Properties of Gd2O3/Cu Composites" Materials 15, no. 5: 1846. https://doi.org/10.3390/ma15051846
APA StyleCao, H., Zhan, Z., & Lv, X. (2022). Effect of Electroless Cu Plating Ti3AlC2 Particles on Microstructure and Properties of Gd2O3/Cu Composites. Materials, 15(5), 1846. https://doi.org/10.3390/ma15051846






