Leaching of Titanium Dioxide Nanomaterials from Agricultural Soil Amended with Sewage Sludge Incineration Ash: Comparison of a Pilot Scale Simulation with Standard Laboratory Column Elution Experiments
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of ENM Containing Sewage Sludge Incineration Ash (SSA)
2.2. Simulation Experiments
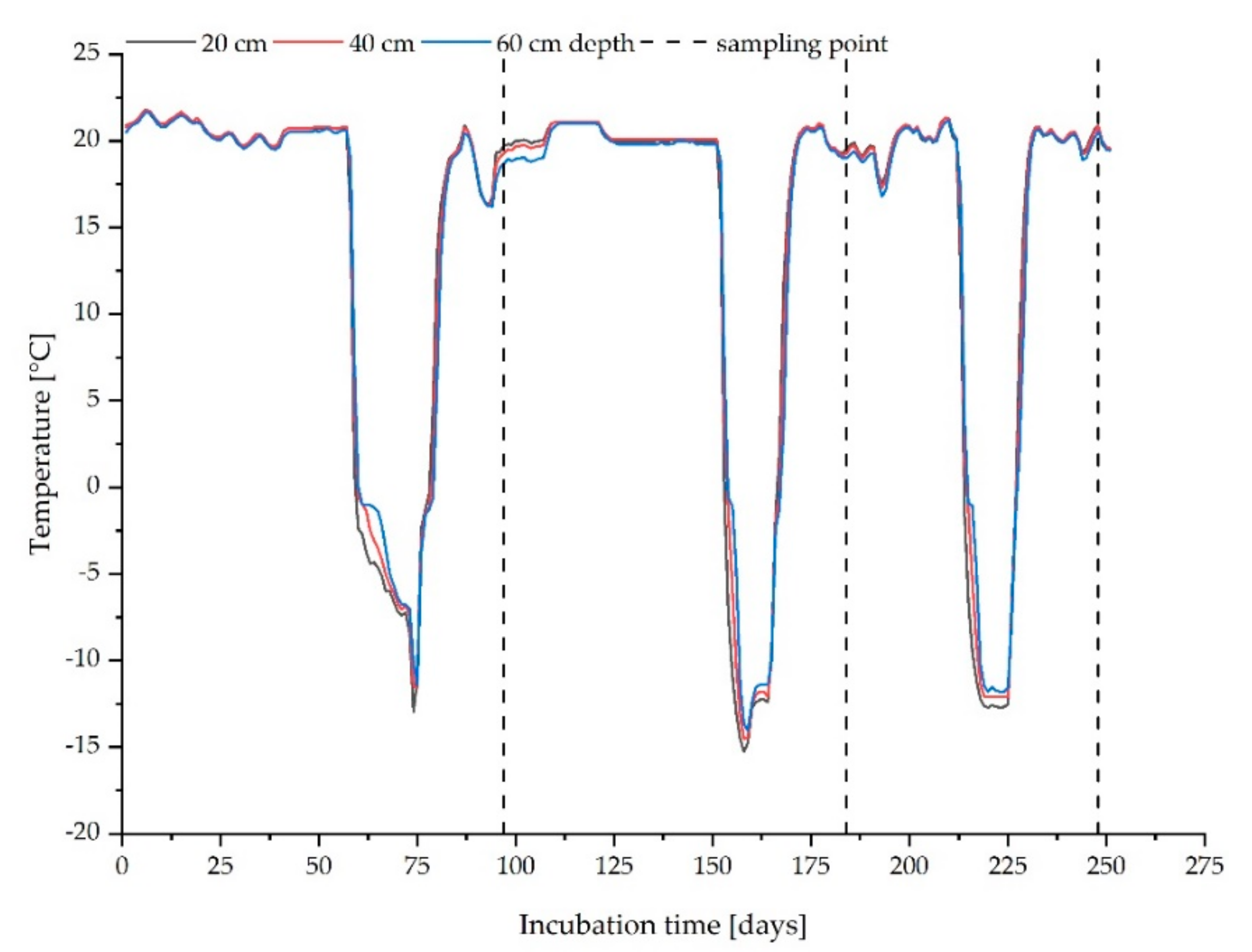
2.2.1. Leaching in Pilot Scale Simulation Reactors
2.2.2. Column Elution Tests
2.3. Chemical Analysis
2.3.1. TiO2 Concentrations in Reactor Leachates and Column Eluates
2.3.2. TiO2 Concentrations in Soil/Ash-Mixed Samples
2.3.3. Single Particle (sp)ICP-MS Analysis of Reactor Leachates and Column Eluates
3. Results
3.1. Characterisation of the Produced SSA
3.2. Leaching from Pilot Scale Simulation Reactors
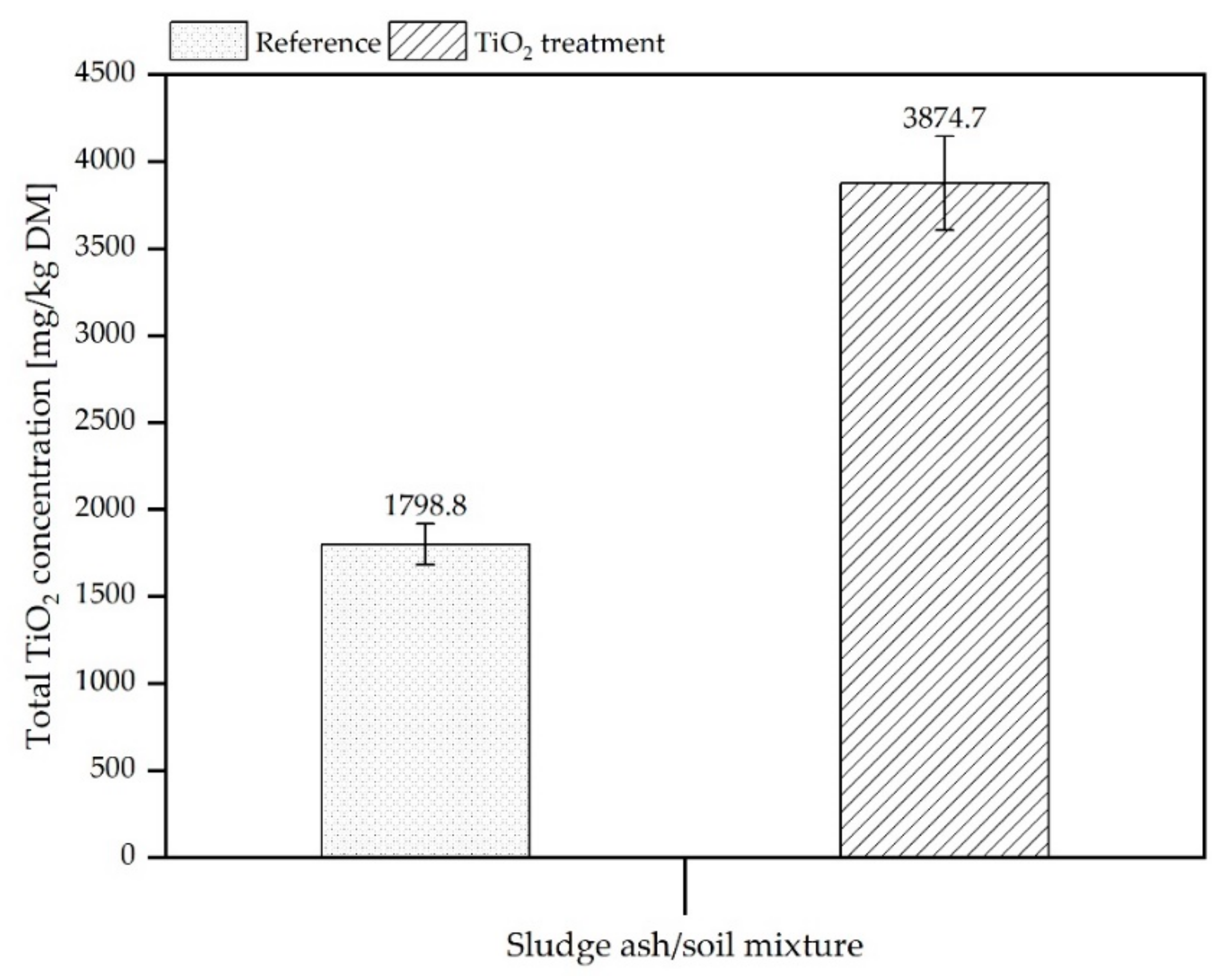
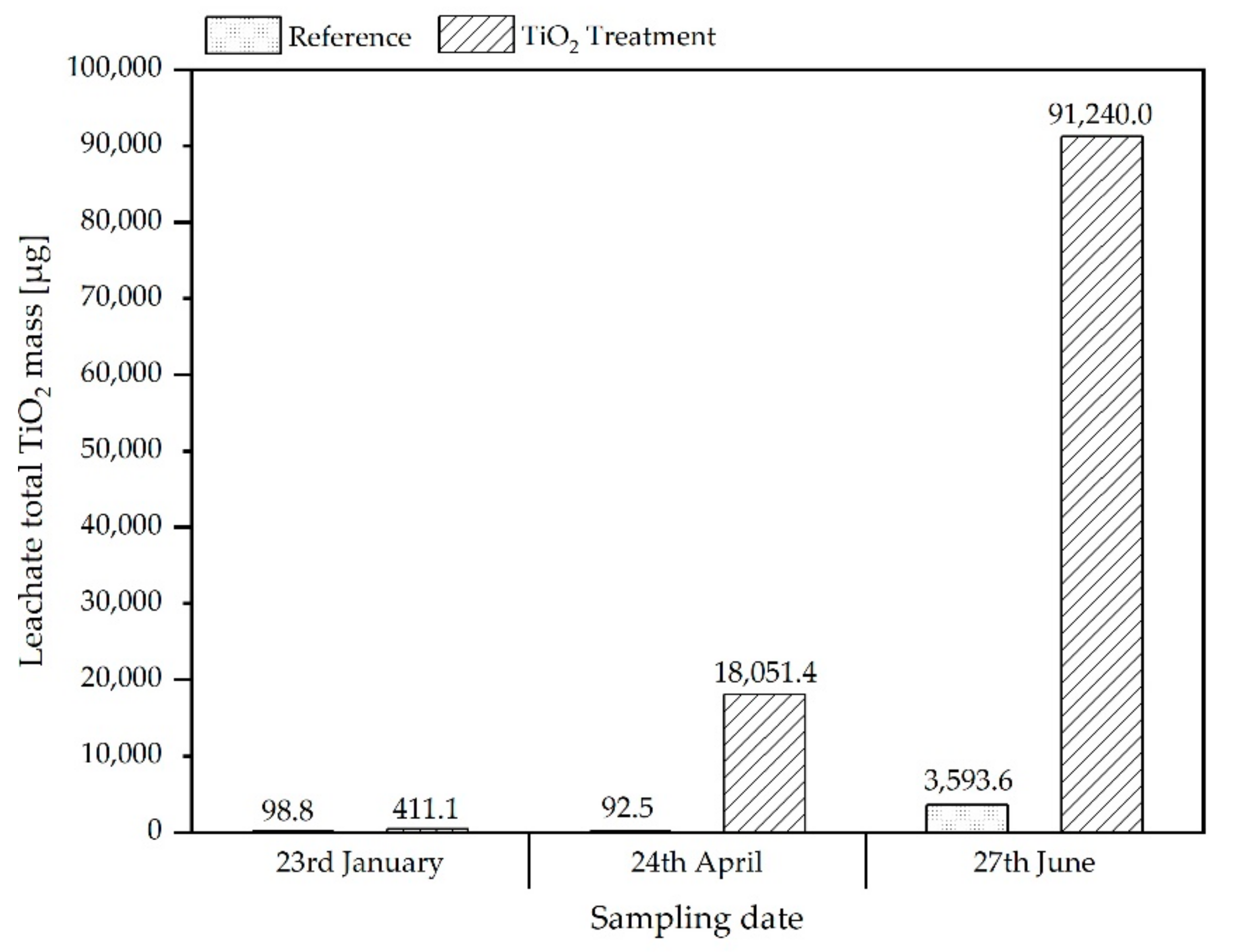
3.2.1. Determination of Total Titanium Content in Mixtures of Soil/SSA and in Leachates
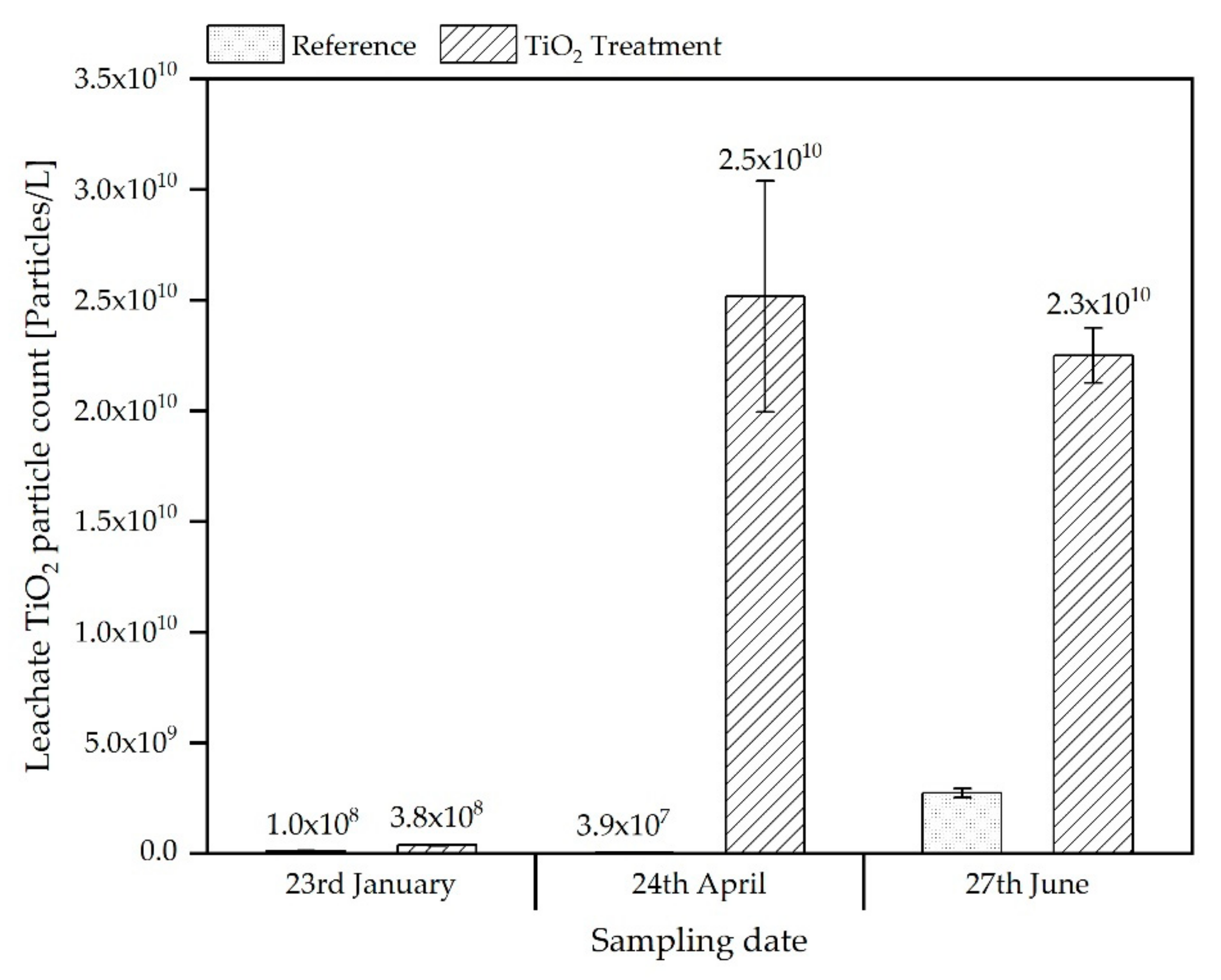
3.2.2. SpICP-MS/MS Analyses of Leachates
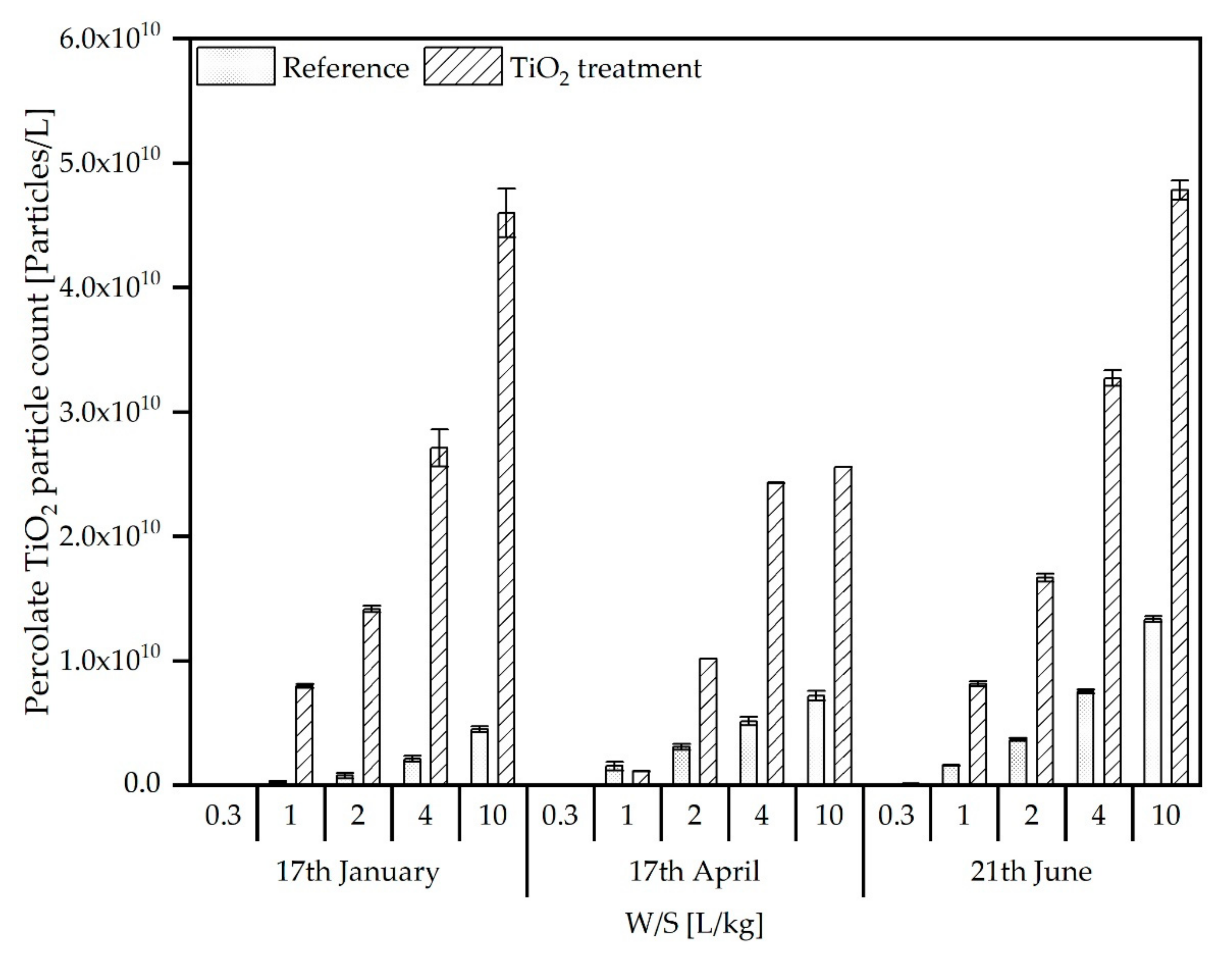
3.3. Column Elution Experiments
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaegi, R.; Sinnet, B.; Zuleeg, S.; Hagendorfer, H.; Mueller, E.; Vonbank, R.; Boller, M.; Burkhardt, M. Release of silver nanoparticles from outdoor facades. Environ. Pollut. 2010, 158, 2900–2905. [Google Scholar] [CrossRef] [PubMed]
- Gondikas, A.P.; von der Kammer, F.; Reed, R.B.; Wagner, S.; Ranville, J.F.; Hofmann, T. Release of TiO2 nanoparticles from sunscreens into surface waters: A one-year survey at the old Danube recreational Lake. Environ. Sci. Technol. 2014, 48, 5415–5422. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.A.; McFerran, S.; Lazareva, A.; Suh, S. Global life cycle releases of engineered nanomaterials. J. Nanopart. Res. 2013, 15, 1692–1709. [Google Scholar] [CrossRef]
- Westerhoff, P.; Song, G.; Hristovski, K.; Kiser, M.A. Occurrence and removal of titanium at full scale wastewater treatment plants: Implications for TiO2 nanomaterials. J. Environ. Monit. 2011, 13, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Kaegi, R.; Voegelin, A.; Sinnet, B.; Zuleeg, S.; Hagendorfer, H.; Burkhardt, M.; Siegrist, H. Behavior of metallic silver nanoparticles in a pilot wastewater treatment plant. Environ. Sci. Technol. 2011, 45, 3902–3908. [Google Scholar] [CrossRef] [PubMed]
- Wiechmann, B.; Dienemann, C.; Kabbe, C.; Brandt, S.; Vogel, I.; Roskosch, A. Sewage Sludge Treatment in Germany; Umweltbundesamt, Dessau-Roßlau. 2015. Available online: https://www.umweltbundesamt.de/sites/default/files/medien/378/publikationen/sewage_sludge_management_in_germany.pdf (accessed on 10 December 2021).
- DüMV, Verordnung über das Inverkehrbringen von Düngemitteln, Bodenhilfsstoffen, Kultursubstraten und Pflanzenhilfsmitteln (Düngemittelverordnung–DüMV) (German Fertilizer Ordinance); Bundesgesetzblatt (Federal Law Gazette) Jahrgang 2012 Teil I Nr. 58. 2012. Available online: https://www.gesetze-im-internet.de/d_mv_2012/ (accessed on 10 December 2021).
- Wielinski, J.; Gogos, A.; Voegelin, A.; Müller, C.R.; Morgenroth, E.; Kaegi, R. Release of gold (Au), silver (Ag) and cerium dioxide (CeO2) nanoparticles from sewage sludge incineration ash. Environ. Sci. Nano 2021, 8, 3220–3232. [Google Scholar] [CrossRef] [PubMed]
- BBodSchV, Bundes-Bodenschutz- und Altlastenverordnung vom 12. Juli 1999 (BGBl. I S. 1554), die Zuletzt durch Artikel 126 der Verordnung vom 19. Juni 2020 (BGBl. I S. 1328) Geändert Worden ist (German Soil Protection Ordinance); Bundesgesetzblatt (Federal Law Gazette) Jahrgang 1999 Teil I, Nr. 36. 1999. Available online: https://www.gesetze-im-internet.de/bbodschv/ (accessed on 10 December 2021).
- Piccinno, F.; Gottschalk, F.; Seeger, S.; Nowack, B. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J. Nanopart. Res. 2012, 14, 1109. [Google Scholar] [CrossRef]
- Foss Hansen, S.; Heggelund, L.R.; Revilla Besora, P.; Mackevica, A.; Boldrin, A.; Baun, A. Nanoproducts–what is actually available to European consumers? Environ. Sci. Nano 2016, 3, 169–180. [Google Scholar] [CrossRef]
- Landi, S.; Carneiro, J.; Soares, O.S.G.P.; Pereira, M.F.R.; Gomes, A.C.; Ribeiro, A.; Fonseca, A.M.; Parpot, P.; Neves, I.C. Photocatalytic performance of N-doped TiO2nano-SiO2-HY nanocomposites immobilized over cotton fabrics. J. Mater. Res. Technol. 2019, 8, 1933–1943. [Google Scholar] [CrossRef]
- Noireaux, J.; López-Sanz, S.; Vidmar, J.; Correia, M.; Devoille, L.; Fisicaro, P.; Loeschner, K. Titanium dioxide nanoparticles in food: Comparison of detection by triple-quadrupole and high-resolution ICP-MS in single-particle mode. J. Nanopart. Res. 2021, 23, 4. [Google Scholar] [CrossRef]
- BioAbfV, Bioabfallverordnung in der Fassung der Bekanntmachung vom 4. April 2013 (BGBl. I S. 658), die Zuletzt durch Artikel 3 Absatz 2 der Verordnung vom 27. September 2017 (BGBl. I S. 3465) Geändert Worden ist. Bundesgesetzblatt (Federal Law Gazette) Jahrgang 1998 Teil I, S. 2955. 1998. Available online: https://www.gesetze-im-internet.de/bioabfv/BJNR295500998.html (accessed on 10 December 2021).
- Praetorius, A.; Tufenkji, N.; Goss, K.-U.; Scheringer, M.; von der Kammer, F.; Elimelech, M. The road to nowhere: Equilibrium partition coefficients for nanoparticles. Environ. Sci. Nano 2014, 1, 317–323. [Google Scholar] [CrossRef]
- Börner, R.; Meiller, M.; Oischinger, J.; Daschner, R. Untersuchung Möglicher Umweltauswirkungen bei der Entsorgung nanomaterialhaltiger Abfälle in Abfallbehandlungsanlagen; Umweltbundesamt: Dessau-Roßlau, Germany, 2016. [Google Scholar]
- Oischinger, J.; Meiller, M.; Daschner, R.; Hornung, A.; Warnecke, R. Fate of nano titanium dioxide during combustion of engineered nanomaterial-containing waste in a municipal solid waste incineration plant. Waste Manag. Res. 2019, 37, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, E. Beispiel einer zukunftsorientierten kommunalen Abwasserreinigung. In Verwertung von Klärschlamm; Holm, O.E., Thomé-Kozmiensky, E., Quicker, P., Kopp-Assenmacher, S., Eds.; Thomé-Kozmiensky Verlag GmbH: Neuruppin, Germany, 2018; pp. 121–130. [Google Scholar]
- Ländergemscinaft Abfall, LAGA PN98 Richtlinie für das Vorgehen bei Physikalischen, Chemischen und Biologischen Untersuchungen im Zusammenhang mit der Verwertung/Beseitigung von Abfällen. Grundregeln für die Entnahme von Proben aus Festen und Stichfesten Abfällen Sowie Abgelagerten Materialien; Ländergemeinschaft Abfall. 2001. Available online: https://www.laga-online.de/documents/m32_laga_pn98_1503993280.pdf (accessed on 10 December 2021).
- Länderarbeitsgemeinschaft Abfall, LAGA-Mitteilung 19, Merkblatt für die Entsorgung von Abfällen aus Verbrennungsanlagen für Siedlungsabfälle; ESV: Berlin, Germany, 1994.
- Chen, G.; Liu, X.; Su, C. Distinct effects of humic acid on transport and retention of TiO2 rutile nanoparticles in saturated sand columns. Environ. Sci. Technol. 2012, 46, 7142–7150. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, B.; Morales, V.L.; Tian, Y.; Wu, L.; Gao, J.; Bai, W.Y.L. Transport of titanium dioxide nanoparticles in saturated porous media under various solution chemistry conditions. J. Nanopart. Res. 2012, 14, 1095–1104. [Google Scholar] [CrossRef]
- DIN 19528:2009-01; Leaching of Solid Materials-Percolation Method for the Joint Examination of the Leaching Behaviour of Inorganic and Organic Substances. Beuth: Berlin, Germany, 2009.
- Montano, M.D.; Olesik, J.W.; Barber, A.G.; Challis, K.; Ranville, J.F. Single Particle ICP-MS: Advances toward routine analysis of nanomaterials. Anal. Bioanal. Chem. 2016, 408, 5053–5074. [Google Scholar] [CrossRef] [PubMed]
- Meermann, B.; Nischwitz, V. ICP-MS for the analysis at the nanoscale–A tutorial review. J. Anal. At. Spectrom. 2018, 33, 1432–1468. [Google Scholar] [CrossRef]
- Oischinger, J.; Meiller, M.; Daschner, R.; Hennecke, D.; Hund-Rinke, K.; Meisterjahn, B.; Schröder, N. Untersuchungen zur Möglichen Freisetzung von Nanopartikeln bei der Ablagerung und Bodenbezogenen Anwendung von Mineralischen Abfällen-Abschlussbericht; Umweltbundesamt: Dessau-Roßlau, Germany, 2020. [Google Scholar]

| Soil Type | Soil Texture (DIN) | Soil Texture | Corg (%) | Ntotal (g/kg) | pHCaCl2 | CECeff (mol/kg) | WHCmax (g/kg) | ||
|---|---|---|---|---|---|---|---|---|---|
| Sand (%) | Silt (%) | Clay (%) | |||||||
| Refesol 04-A | 79.7 | 14.9 | 5.4 | loamy sand | 3.04 | 1.76 | 5.11 | 0.0412 | 346 |
| Soil | Mass per Column | Percolation Rate (mL min−1) | |
|---|---|---|---|
| (g) | Saturation | Percolation | |
| RefeSol 04-A | 1100 | 2.64 | 1.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meisterjahn, B.; Schröder, N.; Oischinger, J.; Hennecke, D.; Weinfurtner, K.; Hund-Rinke, K. Leaching of Titanium Dioxide Nanomaterials from Agricultural Soil Amended with Sewage Sludge Incineration Ash: Comparison of a Pilot Scale Simulation with Standard Laboratory Column Elution Experiments. Materials 2022, 15, 1853. https://doi.org/10.3390/ma15051853
Meisterjahn B, Schröder N, Oischinger J, Hennecke D, Weinfurtner K, Hund-Rinke K. Leaching of Titanium Dioxide Nanomaterials from Agricultural Soil Amended with Sewage Sludge Incineration Ash: Comparison of a Pilot Scale Simulation with Standard Laboratory Column Elution Experiments. Materials. 2022; 15(5):1853. https://doi.org/10.3390/ma15051853
Chicago/Turabian StyleMeisterjahn, Boris, Nicola Schröder, Jürgen Oischinger, Dieter Hennecke, Karlheinz Weinfurtner, and Kerstin Hund-Rinke. 2022. "Leaching of Titanium Dioxide Nanomaterials from Agricultural Soil Amended with Sewage Sludge Incineration Ash: Comparison of a Pilot Scale Simulation with Standard Laboratory Column Elution Experiments" Materials 15, no. 5: 1853. https://doi.org/10.3390/ma15051853
APA StyleMeisterjahn, B., Schröder, N., Oischinger, J., Hennecke, D., Weinfurtner, K., & Hund-Rinke, K. (2022). Leaching of Titanium Dioxide Nanomaterials from Agricultural Soil Amended with Sewage Sludge Incineration Ash: Comparison of a Pilot Scale Simulation with Standard Laboratory Column Elution Experiments. Materials, 15(5), 1853. https://doi.org/10.3390/ma15051853






