Using Cu as a Spacer to Fabricate and Control the Porosity of Titanium Zirconium Based Bulk Metallic Glass Foams for Orthopedic Implant Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Preparation
2.3. Real Porosity Test and Density Test
2.4. Thermal Conductivity Test and Glass-Forming Ability Analysis
2.5. Microstructure Analysis
2.6. Morphology Observation
2.7. Mechanical Property Test and Prediction
2.8. Biocompatibility Test
2.9. Statistical Analysis
3. Results
3.1. Real Porosity
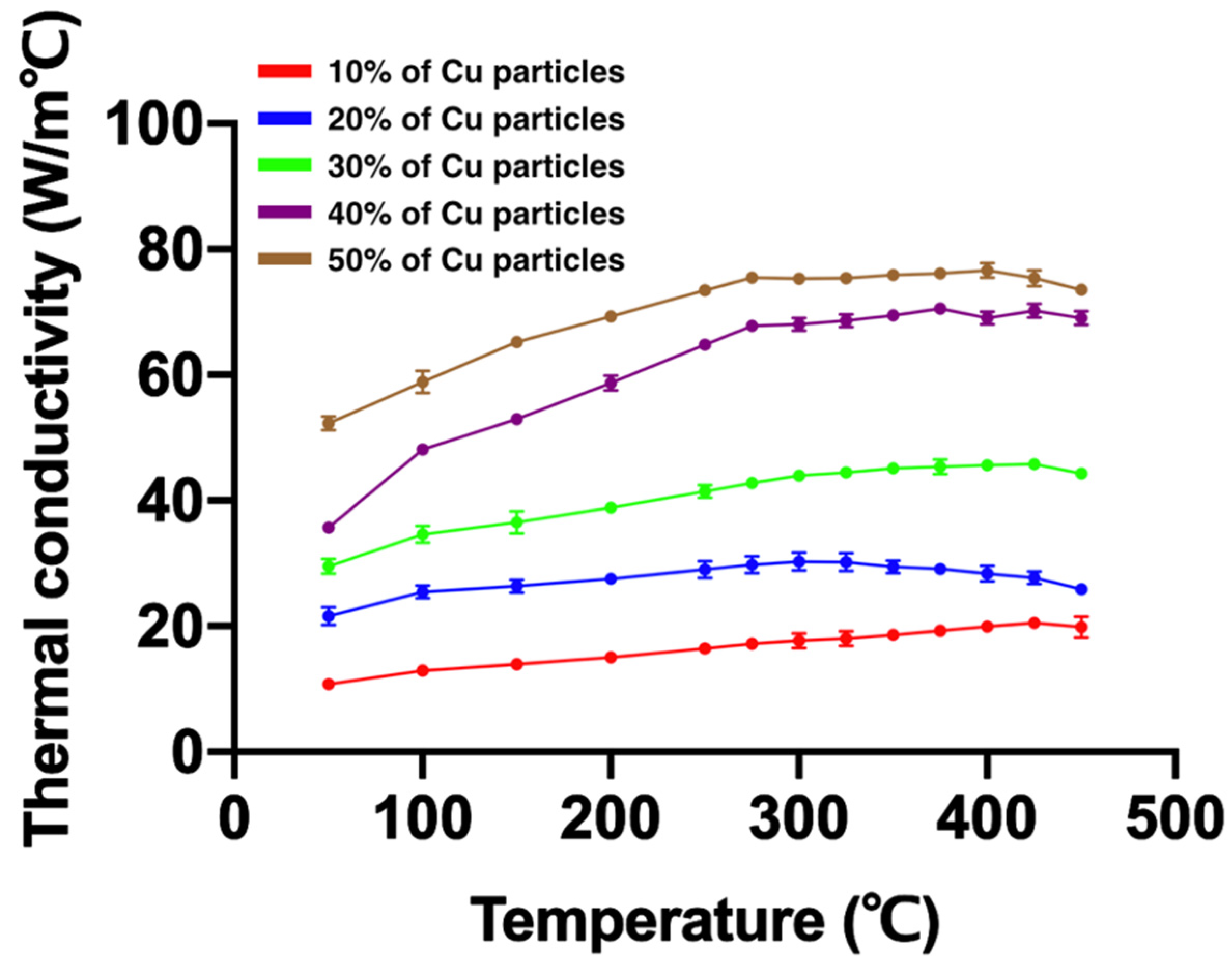
3.2. Thermal Conductivity
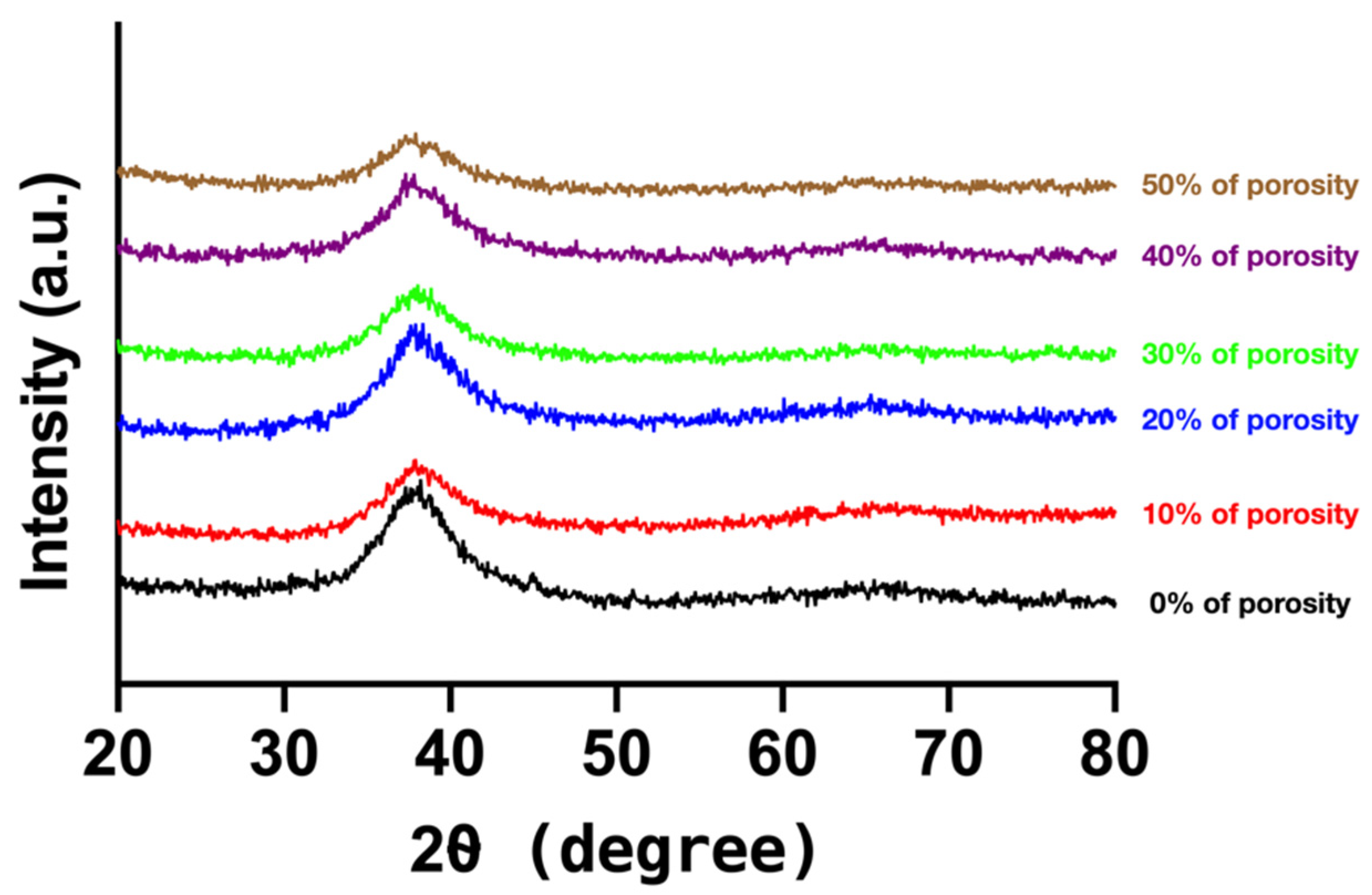
3.3. Glass-Forming Ability
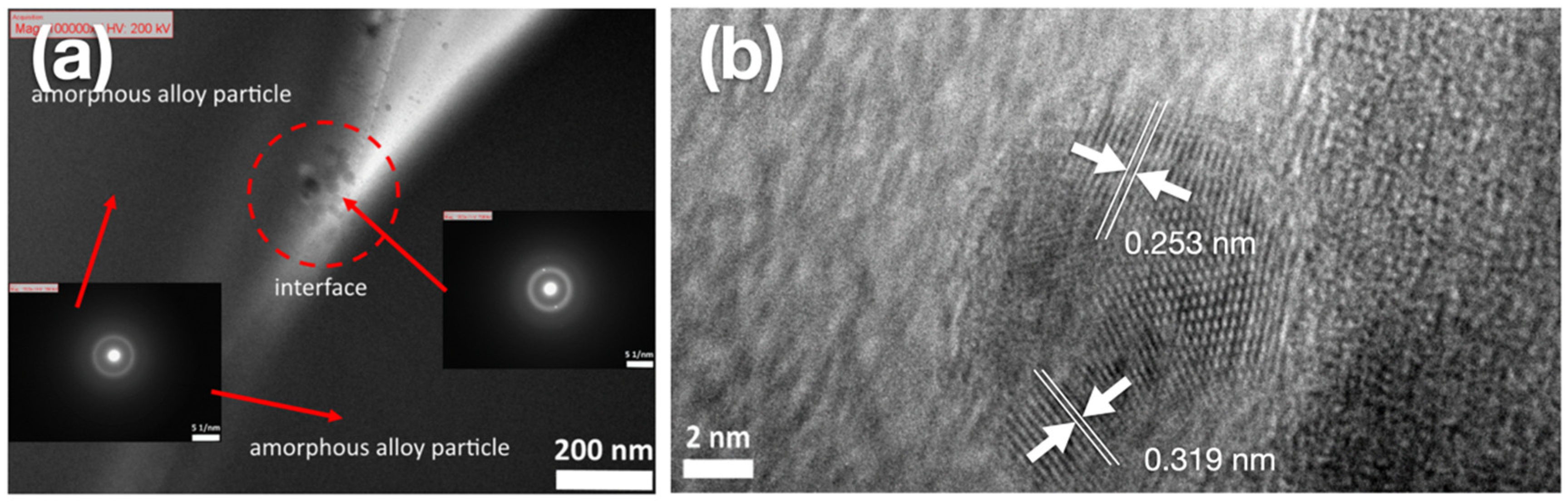
3.4. Microstructure Analysis
3.5. Morphology Observation
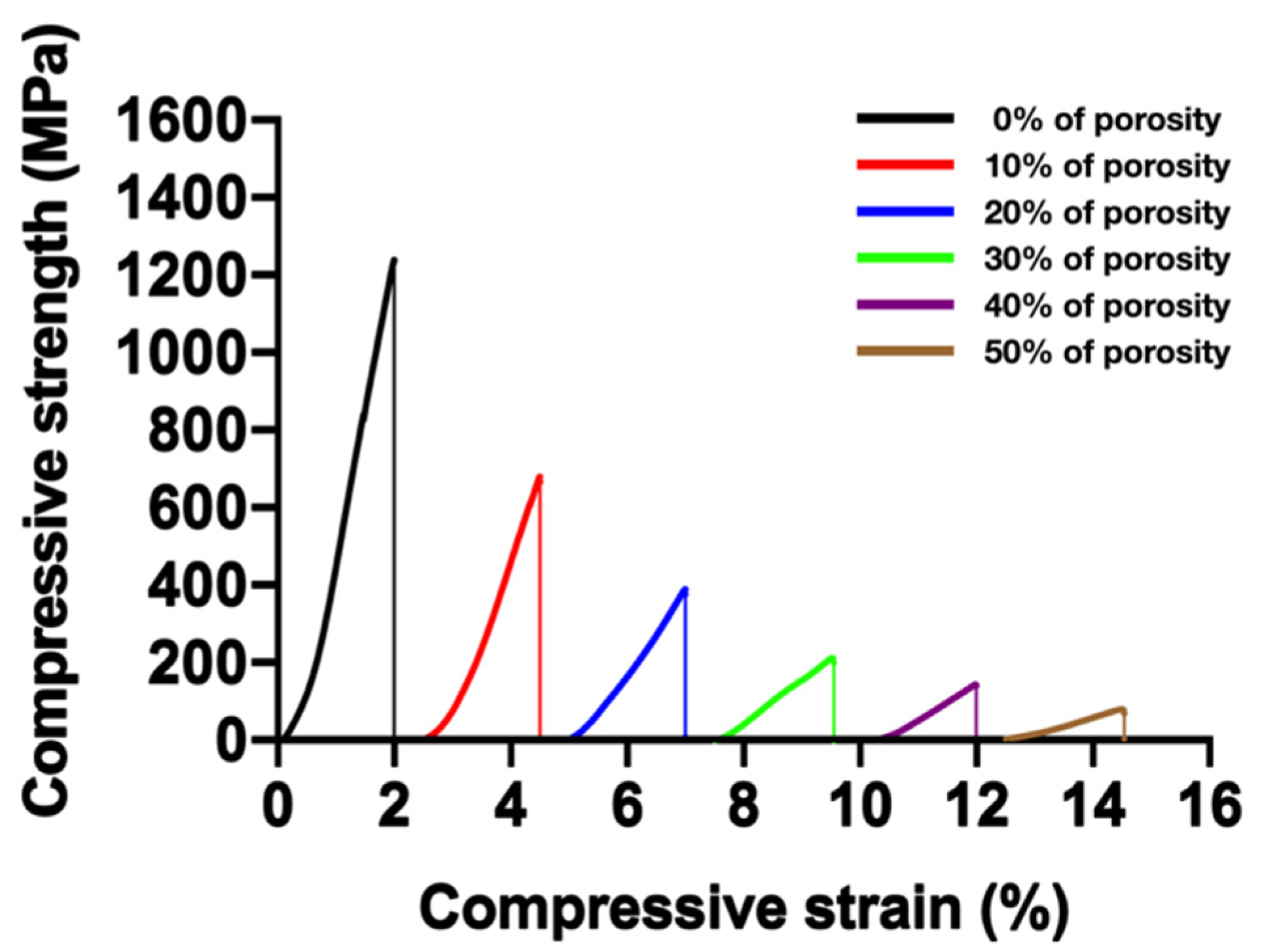
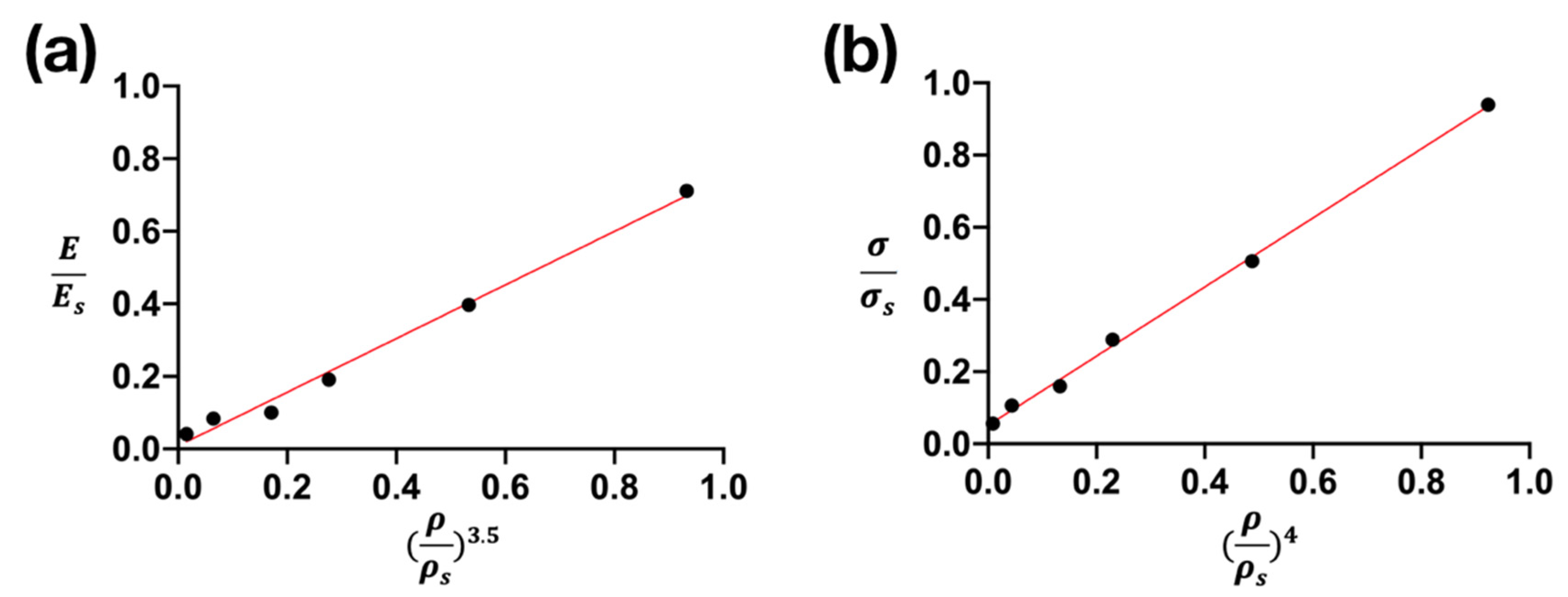
3.6. Mechanical Properties and Their Prediction
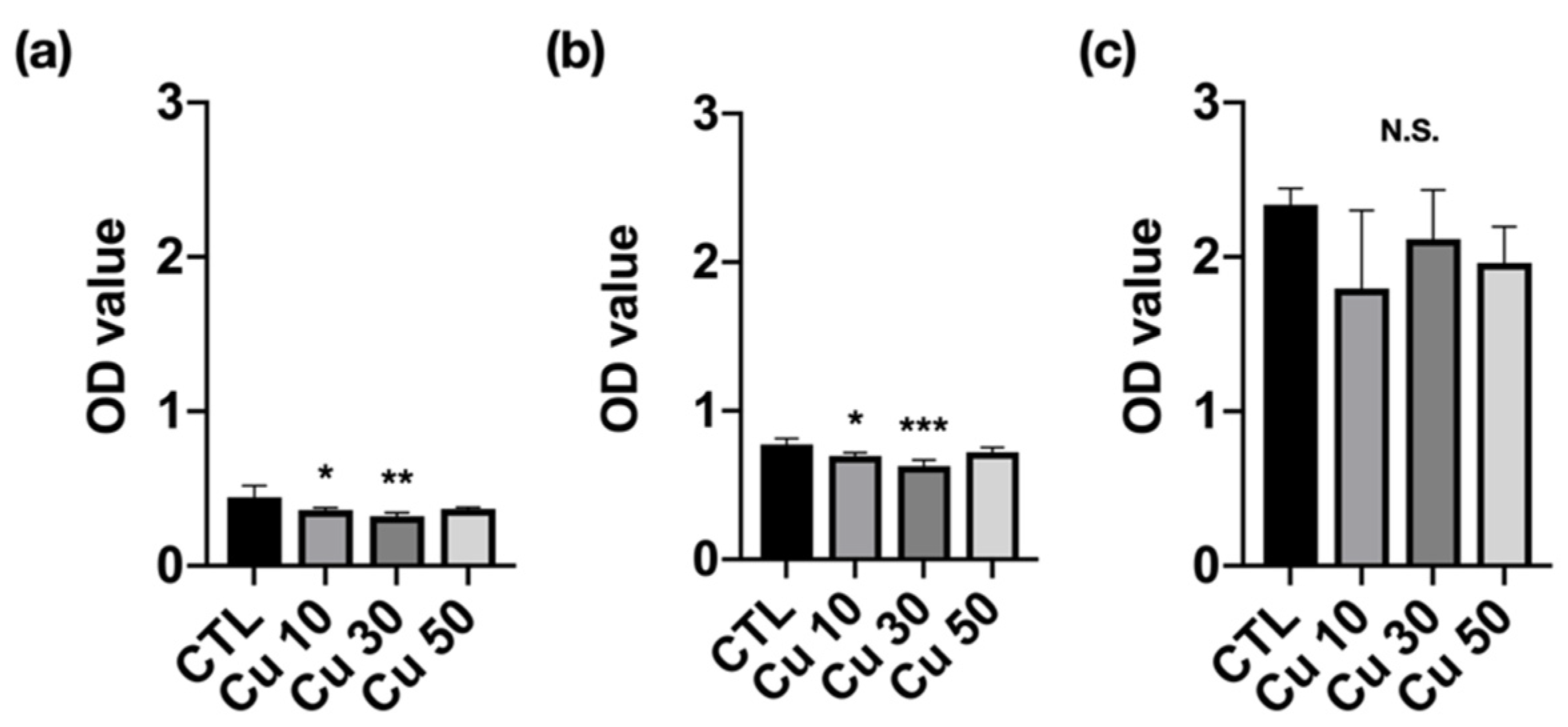
3.7. Cell Viability
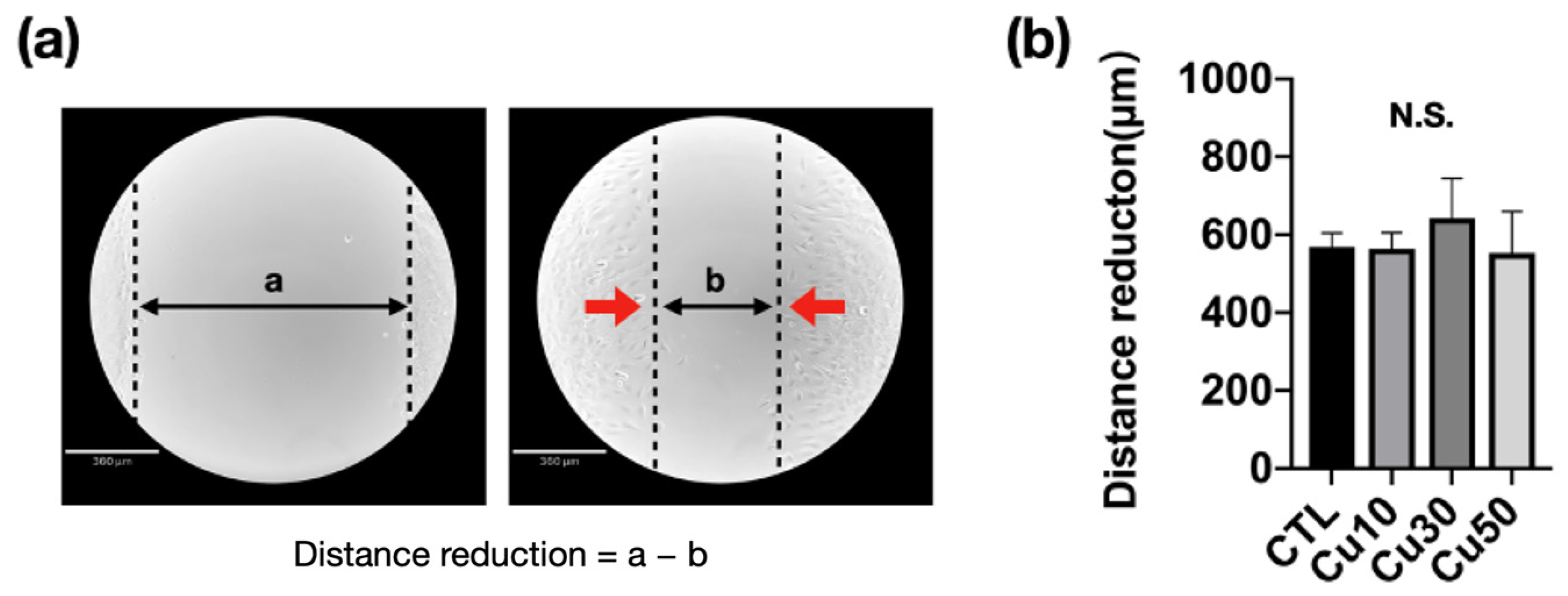
3.8. Cell Migration Capacity
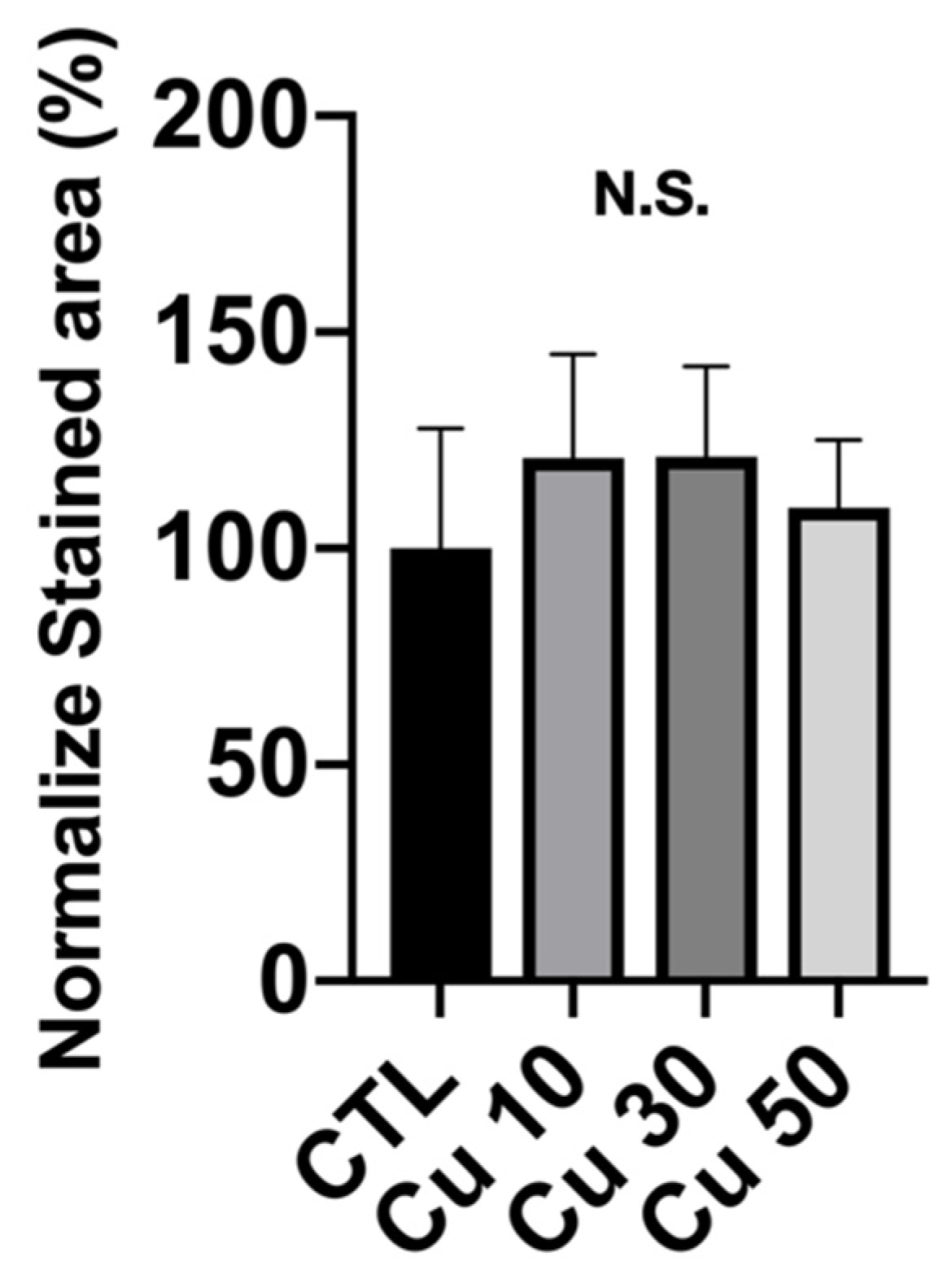
3.9. Calcium Deposition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goodman, S.B.; Yao, Z.; Keeney, M.; Yang, F. The future of biologic coatings for orthopaedic implants. Biomaterials 2013, 34, 3174–3183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borcherding, K.; Schmidmaier, G.; Hofmann, G.O.; Wildemann, B. The rationale behind implant coatings to promote osteointegration, bone healing or regeneration. Injury 2020, 52, S106–S111. [Google Scholar] [CrossRef] [PubMed]
- Yeo, I.-S.L. Modifications of Dental Implant Surfaces at the Micro- and Nano-Level for Enhanced Osseointegration. Materials 2019, 13, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kienapfel, H.; Sprey, C.; Wilke, A.; Griss, P. Implant fixation by bone ingrowth. J. Arthroplast. 1999, 14, 355–368. [Google Scholar] [CrossRef]
- Matassi, F.; Botti, A.; Sirleo, L.; Carulli, C.; Innocenti, M. Porous metal for orthopedics implants. Clin. Cases Miner. Bone Metab. 2013, 10, 111–115. [Google Scholar]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef] [Green Version]
- Matassi, F.; Nistri, L.; Paez, D.C.; Innocenti, M. New biomaterials for bone regeneration. Clin. Cases Miner. Bone Metab. 2011, 8, 21–24. [Google Scholar]
- Woodard, J.R.; Hilldore, A.J.; Lan, S.K.; Park, C.; Morgan, A.W.; Eurell, J.A.C.; Clark, S.G.; Wheeler, M.; Jamison, R.D.; Johnson, A.J.W. The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity. Biomaterials 2007, 28, 45–54. [Google Scholar] [CrossRef]
- Nouri, A.; Hodgson, P.D.; Wen, C. Biomimetic Porous Titanium Scaffolds for Orthopedic and Dental Applications. In Biomimetics Learning from Nature; Intech: Rijeka, Croatia, 2010; Volume 21, pp. 415–450. [Google Scholar]
- Akahori, T.; Niinomi, M.; Nakai, M.; Fukuda, H.; Fukui, H.; Ogawa, M. Bioactive Ceramic Surface Modification of β-Type Ti-Nb-Ta-Zr System Alloy by Alkali Solution Treatment. Mater. Trans. 2007, 48, 293–300. [Google Scholar] [CrossRef] [Green Version]
- Gepreel, M.A.-H.; Niinomi, M. Biocompatibility of Ti-alloys for long-term implantation. J. Mech. Behav. Biomed. Mater. 2013, 20, 407–415. [Google Scholar] [CrossRef]
- Li, T.; Wong, P.; Chang, S.; Tsai, P.; Jang, J.; Huang, J. Biocompatibility study on Ni-free Ti-based and Zr-based bulk metallic glasses. Mater. Sci. Eng. C 2017, 75, 1–6. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Wong, X.P.-C.; Song, S.-M.; Tsai, P.-H.; Jang, J.S.-C.; Tsao, I.-Y.; Lin, C.-H.; Nguyen, V.C. Open-Cell Tizr-Based Bulk Metallic Glass Scaffolds with Excellent Biocompatibility and Suitable Me-chanical Properties for Biomedical Application. J. Funct. Biomater. 2020, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Li, T.H.; Song, S.M.; Liao, Y.C.; Tsai, P.H.; Wong, P.C.; Nguyen, V.C.; Jang, J.S.C. Synthesis of biocompatible TiZr-based bulk metallic glass foams for bio-implant application. Mater. Lett. 2019, 256, 126650. [Google Scholar] [CrossRef]
- Kujala, S.; Ryhänen, J.; Danilov, A.; Tuukkanen, J. Effect of porosity on the osteointegration and bone ingrowth of a weight-bearing nickel–titanium bone graft substitute. Biomaterials 2003, 24, 4691–4697. [Google Scholar] [CrossRef]
- Swieczko-Zurek, B. Porous Materials Used as Inserted Bone Implants. Adv. Mater. Sci. 2009, 9, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Ma, G.-F.; Ali, A.; Verzijl, N.; Hanemaaijer, R.; Tekoppele, J.; Konttinen, Y.T.; Salo, J. Increased collagen degradation around loosened total hip replacement implants. Arthritis Care Res. 2006, 54, 2928–2933. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, B.; Liu, G.; Tang, Y.; Lu, E.; Xie, K.; Lan, C.; Liu, J.; Qin, Z.; Wang, L. Metal Material, Properties and Design Methods of Porous Biomedical Scaffolds for Additive Manufacturing: A Review. Front. Bioeng. Biotechnol. 2021, 9, 194. [Google Scholar] [CrossRef]
- Chen, Z.; Yan, X.; Yin, S.; Liu, L.; Liu, X.; Zhao, G.; Ma, W.; Qi, W.; Ren, Z.; Liao, H.; et al. Influence of the pore size and porosity of selective laser melted Ti6Al4V ELI porous scaffold on cell proliferation, osteogenesis and bone ingrowth. Mater. Sci. Eng. C 2020, 106, 110289. [Google Scholar] [CrossRef]
- Szymczyk-Ziółkowska, P.; Łabowska, M.B.; Detyna, J.; Michalak, I.; Gruber, P. A review of fabrication polymer scaffolds for biomedical applications using additive manufacturing techniques. Biocybern. Biomed. Eng. 2020, 40, 624–638. [Google Scholar] [CrossRef]
- Bose, S.; Ke, D.; Sahasrabudhe, H.; Bandyopadhyay, A. Additive manufacturing of biomaterials. Prog. Mater. Sci. 2017, 93, 45–111. [Google Scholar] [CrossRef]
- Li, X. Additive Manufacturing of Advanced Multi-Component Alloys: Bulk Metallic Glasses and High Entropy Alloys. Adv. Eng. Mater. 2017, 20, 1700874. [Google Scholar] [CrossRef]
- Zhang, P.; Tan, J.; Tian, Y.; Yan, H.; Yu, Z. Research progress on selective laser melting (SLM) of bulk metallic glasses (BMGs): A review. Int. J. Adv. Manuf. Technol. 2021, 118, 1–41. [Google Scholar] [CrossRef]
- Wang, H.-S.; Li, T.-H.; Chen, H.-G.; Pan, J.-H.; Jang, J.S.-C. Microstructural evolution and properties of laser spot-welded Zr Al Co Ta bulk metallic glass under various initial welding temperatures. Intermetallics 2019, 108, 39–44. [Google Scholar] [CrossRef]
- ISO-10993–5; Biological Evaluation of Medical Devices Part 5: Test for Cytotoxicity: In Vitro Methods. ANSI/AAMI: Arlington, VA, USA, 1999.
- Kiran, G.; Suman, K.; Rao, N.; Rao, R. A study on the influence of hot press forming process parameters on mechanical properties of green composites using Taguchi experimental design. Int. J. Eng. Sci. Technol. 2011, 3, 253–263. [Google Scholar] [CrossRef]
- Abreu, A.C.; Tavares, R.R.; Borges, A.; Mergulhão, F.; Simões, M. Current and emergent strategies for disinfection of hospital environments. J. Antimicrob. Chemother. 2013, 68, 2718–2732. [Google Scholar] [CrossRef]
- Song, S.-M.; Wong, P.-C.; Chiang, C.-W.; Tsai, P.-H.; Jang, J.S.C.; Chen, C.-H. A bi-phase core-shell structure of Mg-based bulk metallic glass for application in orthopedic fixation im-plants. Mater Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110783. [Google Scholar] [CrossRef]
- Wong, P.-C.; Tsai, P.-H.; Li, T.-H.; Cheng, C.-K.; Jang, J.; Huang, J. Degradation behavior and mechanical strength of Mg-Zn-Ca bulk metallic glass composites with Ti particles as biodegradable materials. J. Alloys Compd. 2017, 699, 914–920. [Google Scholar] [CrossRef]
- Ternero, F.; Rosa, L.G.; Urban, P.; Montes, J.M.; Cuevas, F.G. Influence of the Total Porosity on the Properties of Sintered Materials—A Review. Metals 2021, 11, 730. [Google Scholar] [CrossRef]
- Unamuno, X.; Imbuluzqueta, E.; Salles, F.; Horcajada, P.; Blanco-Prieto, M. Biocompatible porous metal-organic framework nanoparticles based on Fe or Zr for gentamicin vectorization. Eur. J. Pharm. Biopharm. 2018, 132, 11–18. [Google Scholar] [CrossRef]
- Matsuyama, K.; Hayashi, N.; Yokomizo, M.; Kato, T.; Ohara, K.; Okuyama, T. Supercritical carbon dioxide-assisted drug loading and release from biocompatible porous metal–organic frameworks. J. Mater. Chem. B 2014, 2, 7551–7558. [Google Scholar] [CrossRef]
- Li, Y.J.H.; Zhou, J.; Zadpoor, A.A. Additively manufactured biodegradable porous metals. Acta Biomater. 2020, 115, 29–50. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Tang, Q.; Feng, Q.; Song, J.; Han, X.; Guo, F. Mechanical behaviours and mass transport properties of bone-mimicking scaffolds consisted of gyroid structures manufactured using selective laser melting. J. Mech. Behav. Biomed. Mater. 2019, 93, 158–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, A.; Kohyama, Y.; Kuroda, D.; Hanawa, T. Cytocompatibility evaluation of Ni-free stainless steel manufactured by nitrogen adsorption treatment. Mater. Sci. Eng. C 2004, 24, 737–743. [Google Scholar] [CrossRef]
- Cutolo, A.; Engelen, B.; Desmet, W.; Van Hooreweder, B. Mechanical properties of diamond lattice Ti–6Al–4V structures produced by laser powder bed fusion: On the effect of the load direction. J. Mech. Behav. Biomed. Mater. 2020, 104, 103656. [Google Scholar] [CrossRef]
- Wauthle, R.; van der Stok, J.; Yavari, S.A.; Van Humbeeck, J.; Kruth, J.-P.; Zadpoor, A.A.; Weinans, H.; Mulier, M.; Schrooten, J. Additively manufactured porous tantalum implants. Acta Biomater. 2015, 14, 217–225. [Google Scholar] [CrossRef]
- Yan, C.; Hao, L.; Hussein, A.; Young, P.; Raymont, D. Advanced lightweight 316L stainless steel cellular lattice structures fabricated via selective laser melting. Mater. Des. 2014, 55, 533–541. [Google Scholar] [CrossRef] [Green Version]
- Demir, A.G.; Previtali, B. Additive manufacturing of cardiovascular CoCr stents by selective laser melting. Mater. Des. 2017, 119, 338–350. [Google Scholar] [CrossRef] [Green Version]
- Xue, W.; Krishna, B.V.; Bandyopadhyay, A.; Bose, S. Processing and biocompatibility evaluation of laser processed porous titanium. Acta Biomater. 2007, 3, 1007–1018. [Google Scholar] [CrossRef]

| Volume Fraction of Cu (vol.%) | 0 | 10 | 20 | 30 | 40 | 50 |
|---|---|---|---|---|---|---|
| Real Porosity (%) | 2.0 | 11.1 | 27.6 | 40.4 | 51.0 | 67.9 |
| Volume Fraction of Cu Particles | 10% | 20% | 30% | 40% | 50% | |
|---|---|---|---|---|---|---|
| Temperature (°C) | ||||||
| 50 | 10.78 | 21.62 | 29.54 | 35.68 | 52.29 | |
| 100 | 12.96 | 25.43 | 34.65 | 48.12 | 58.87 | |
| 150 | 13.93 | 26.37 | 36.52 | 52.99 | 65.19 | |
| 200 | 15.03 | 27.56 | 38.85 | 58.70 | 69.28 | |
| 250 | 16.49 | 29.04 | 41.43 | 64.83 | 73.44 | |
| 275 | 17.23 | 29.78 | 42.79 | 67.83 | 75.49 | |
| 300 | 17.73 | 30.32 | 43.99 | 68.01 | 75.33 | |
| 325 | 18.04 | 30.18 | 44.47 | 68.61 | 75.38 | |
| 350 | 18.64 | 29.45 | 45.13 | 69.48 | 75.84 | |
| 375 | 19.26 | 29.15 | 45.39 | 70.52 | 76.11 | |
| 400 | 19.94 | 28.38 | 45.59 | 69.05 | 76.63 | |
| 425 | 20.58 | 27.74 | 45.78 | 70.19 | 75.41 | |
| 450 | 19.89 | 25.90 | 44.28 | 69.05 | 73.57 | |
| Volume Fraction of Cu (vol.%) | |||
|---|---|---|---|
| 0 | 726 | 832 | 106 |
| 10 | 743 | 837 | 94 |
| 20 | 742 | 838 | 96 |
| 30 | 744 | 839 | 95 |
| 40 | 740 | 839 | 99 |
| 50 | 741 | 840 | 99 |
| Volume Fraction of Cu (vol.%) | (GPa) | (GPa) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 79.7 | 1261 | 0.71 | 0.94 | 0.98 | 0.93 | 0.92 |
| 10 | 44.5 | 679 | 0.40 | 0.51 | 0.84 | 0.53 | 0.49 |
| 20 | 21.4 | 388 | 0.19 | 0.29 | 0.69 | 0.28 | 0.23 |
| 30 | 11.2 | 214 | 0.10 | 0.16 | 0.60 | 0.17 | 0.13 |
| 40 | 9.4 | 143 | 0.08 | 0.11 | 0.46 | 0.06 | 0.04 |
| 50 | 4.6 | 76 | 0.04 | 0.06 | 0.30 | 0.02 | 0.009 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, P.-C.; Song, S.-M.; Tsai, P.-H.; Maqnun, M.J.; Wang, W.-R.; Wu, J.-L.; Jang, S.-C. Using Cu as a Spacer to Fabricate and Control the Porosity of Titanium Zirconium Based Bulk Metallic Glass Foams for Orthopedic Implant Applications. Materials 2022, 15, 1887. https://doi.org/10.3390/ma15051887
Wong P-C, Song S-M, Tsai P-H, Maqnun MJ, Wang W-R, Wu J-L, Jang S-C. Using Cu as a Spacer to Fabricate and Control the Porosity of Titanium Zirconium Based Bulk Metallic Glass Foams for Orthopedic Implant Applications. Materials. 2022; 15(5):1887. https://doi.org/10.3390/ma15051887
Chicago/Turabian StyleWong, Pei-Chun, Sin-Mao Song, Pei-Hua Tsai, Muhammad Jauharul Maqnun, Wei-Ru Wang, Jia-Lin Wu, and Shian-Ching (Jason) Jang. 2022. "Using Cu as a Spacer to Fabricate and Control the Porosity of Titanium Zirconium Based Bulk Metallic Glass Foams for Orthopedic Implant Applications" Materials 15, no. 5: 1887. https://doi.org/10.3390/ma15051887







