Flexible Sol-Gel—Processed Y2O3 RRAM Devices Obtained via UV/Ozone-Assisted Photochemical Annealing Process
Abstract
:1. Introduction
2. Materials and Methods
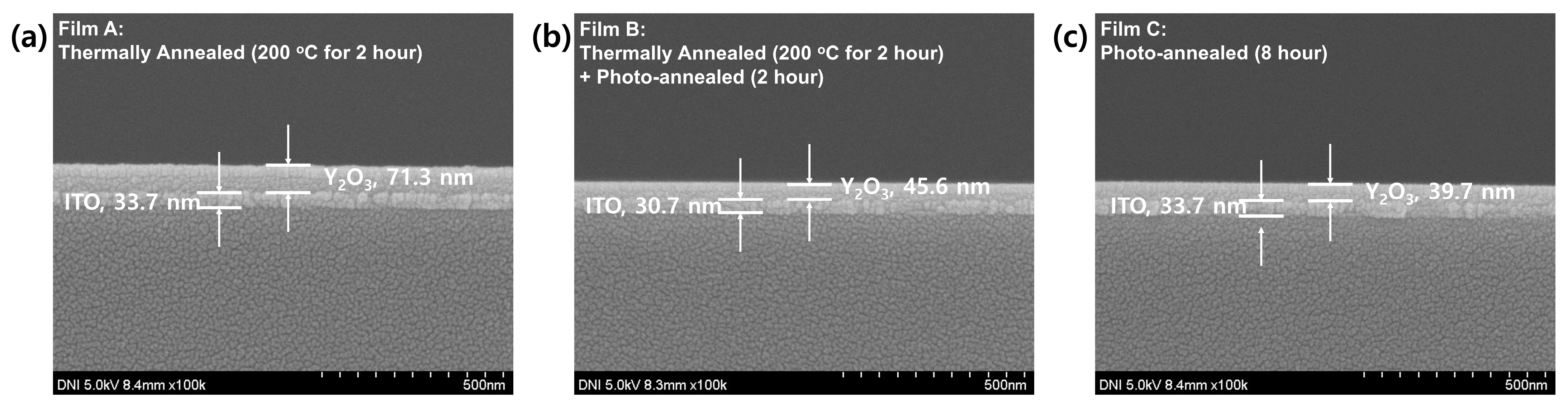
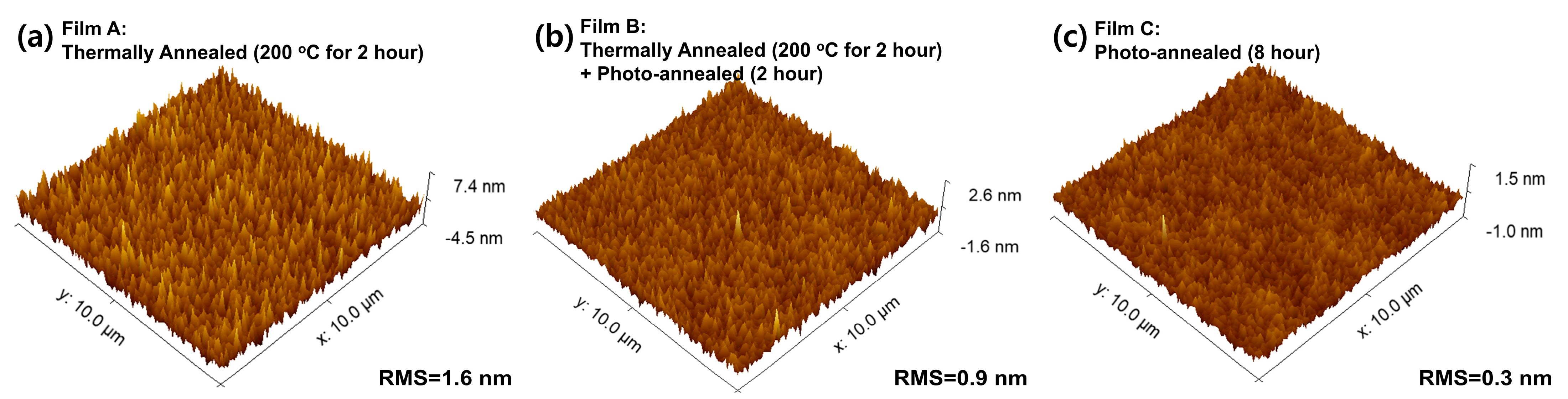
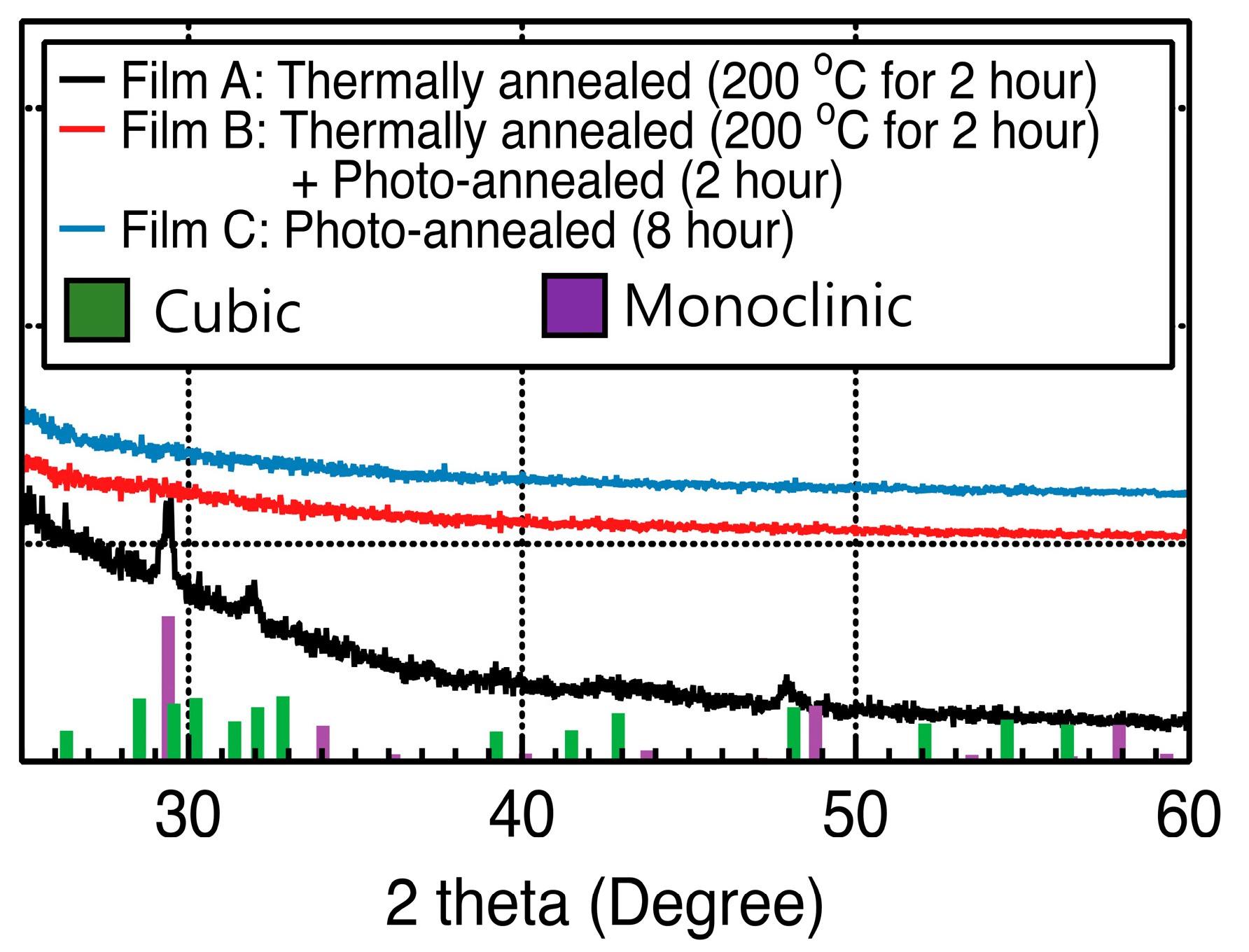
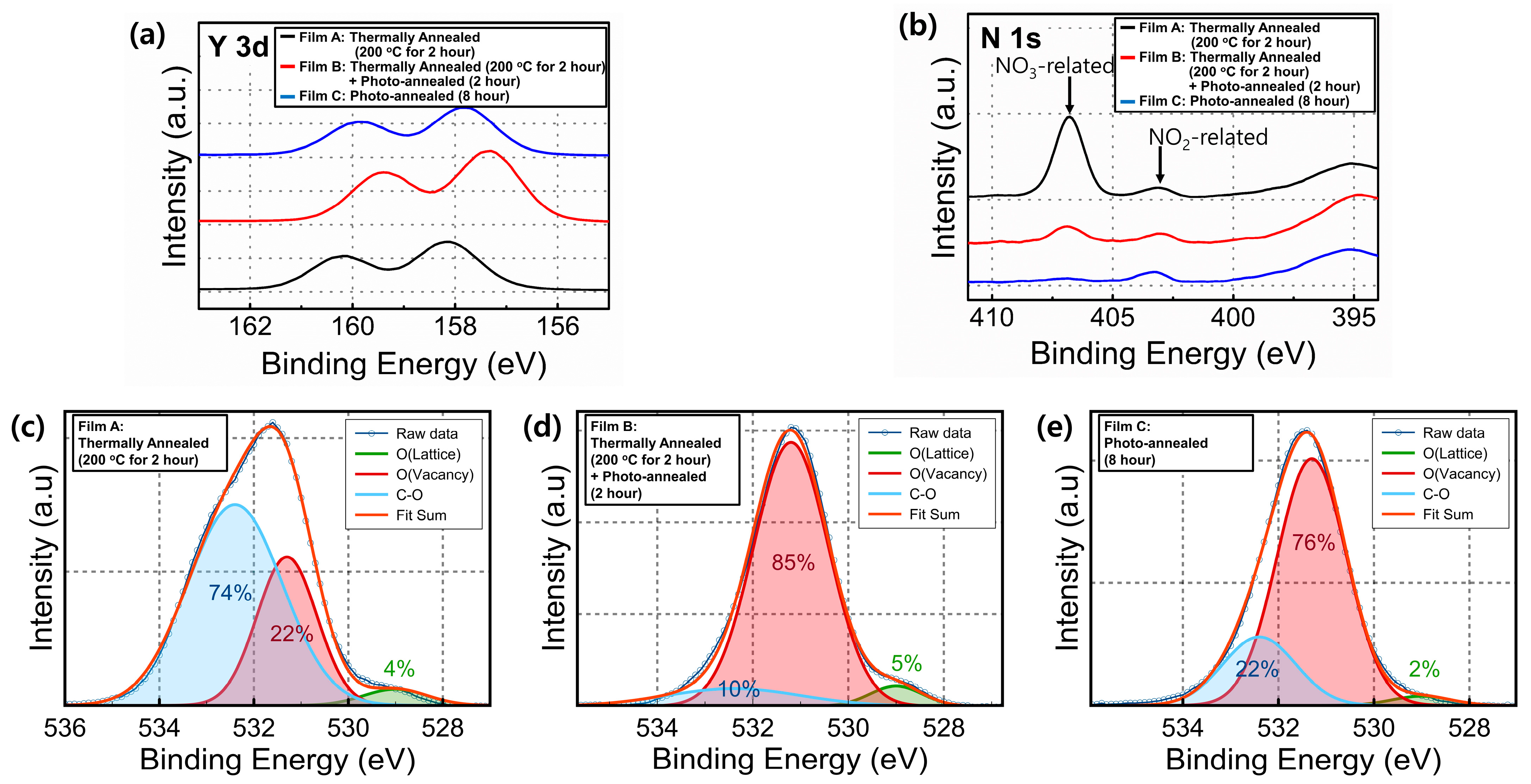
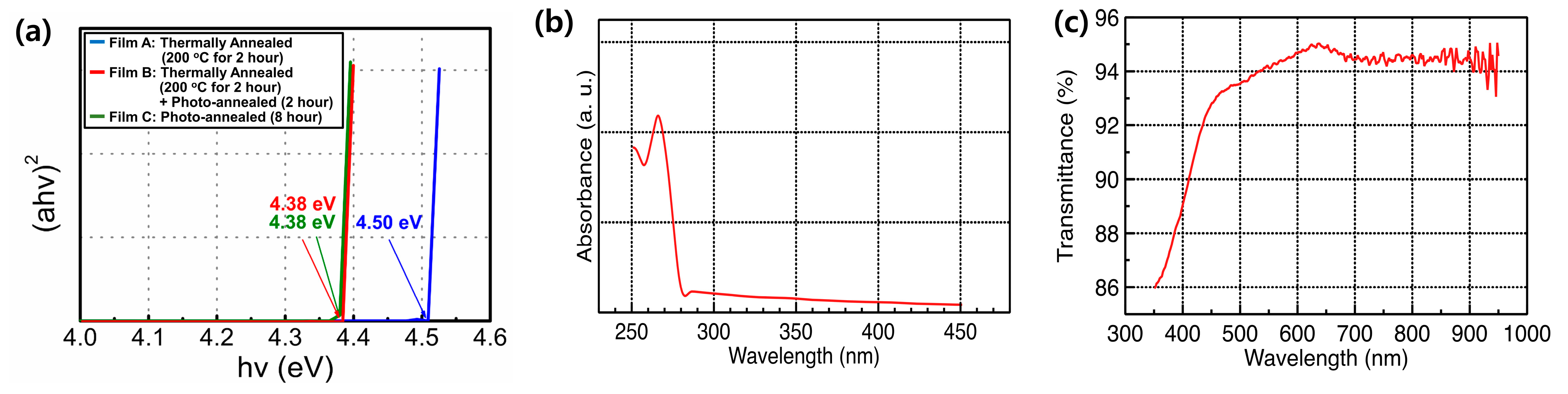
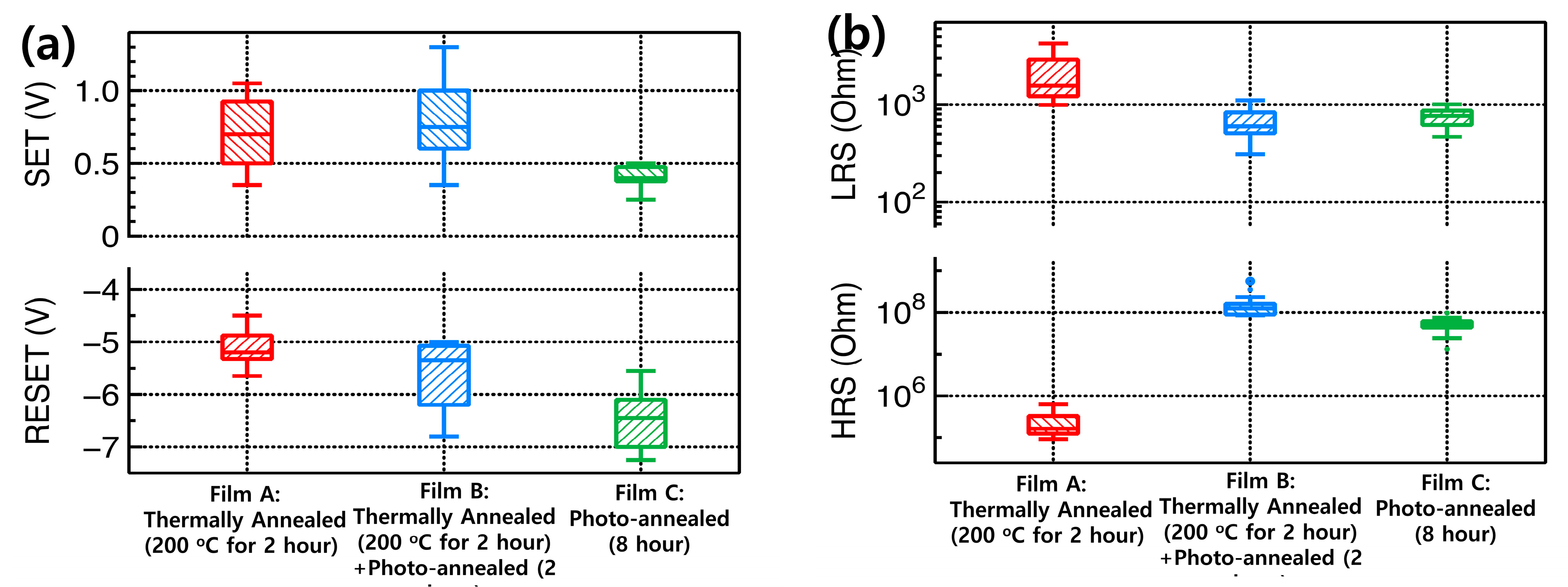
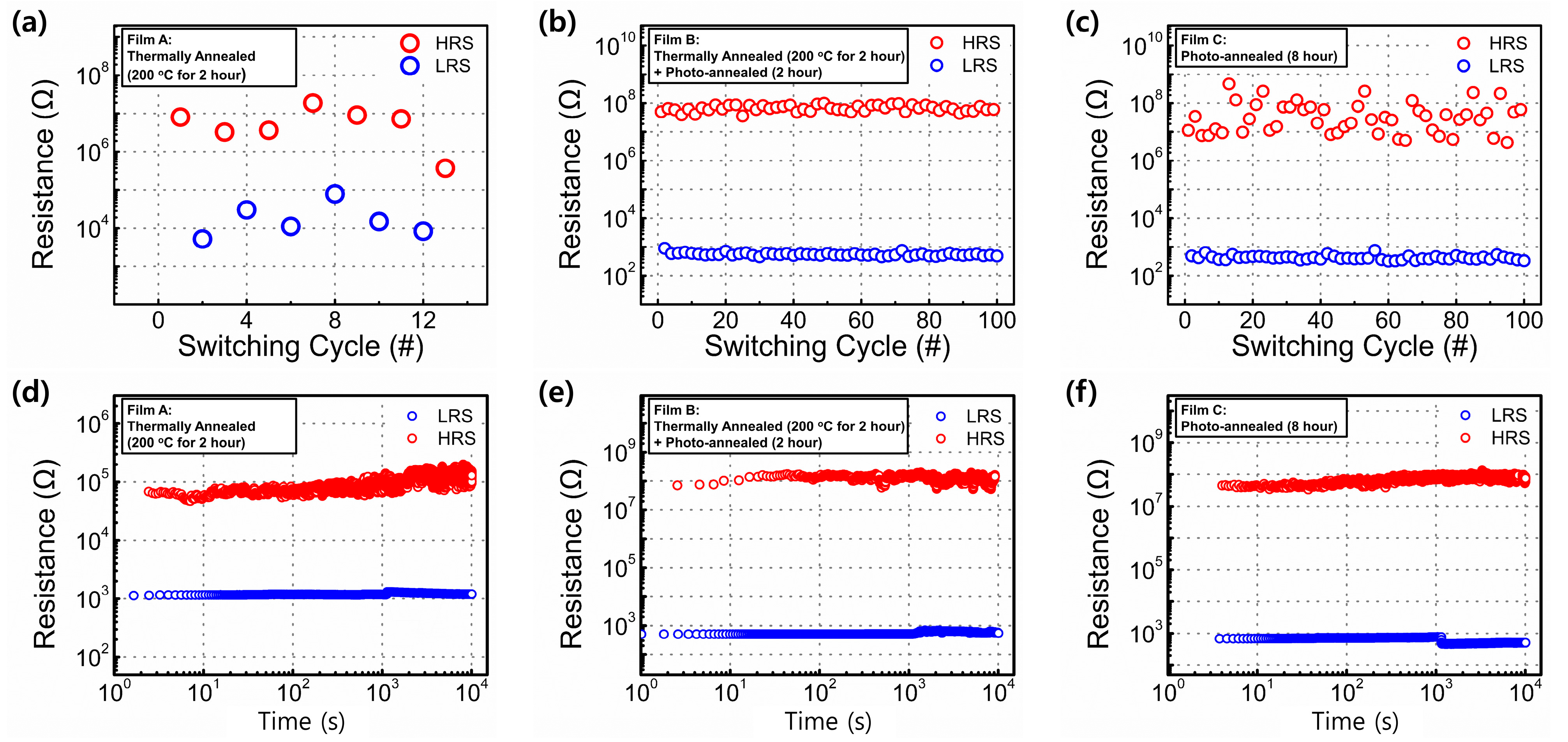
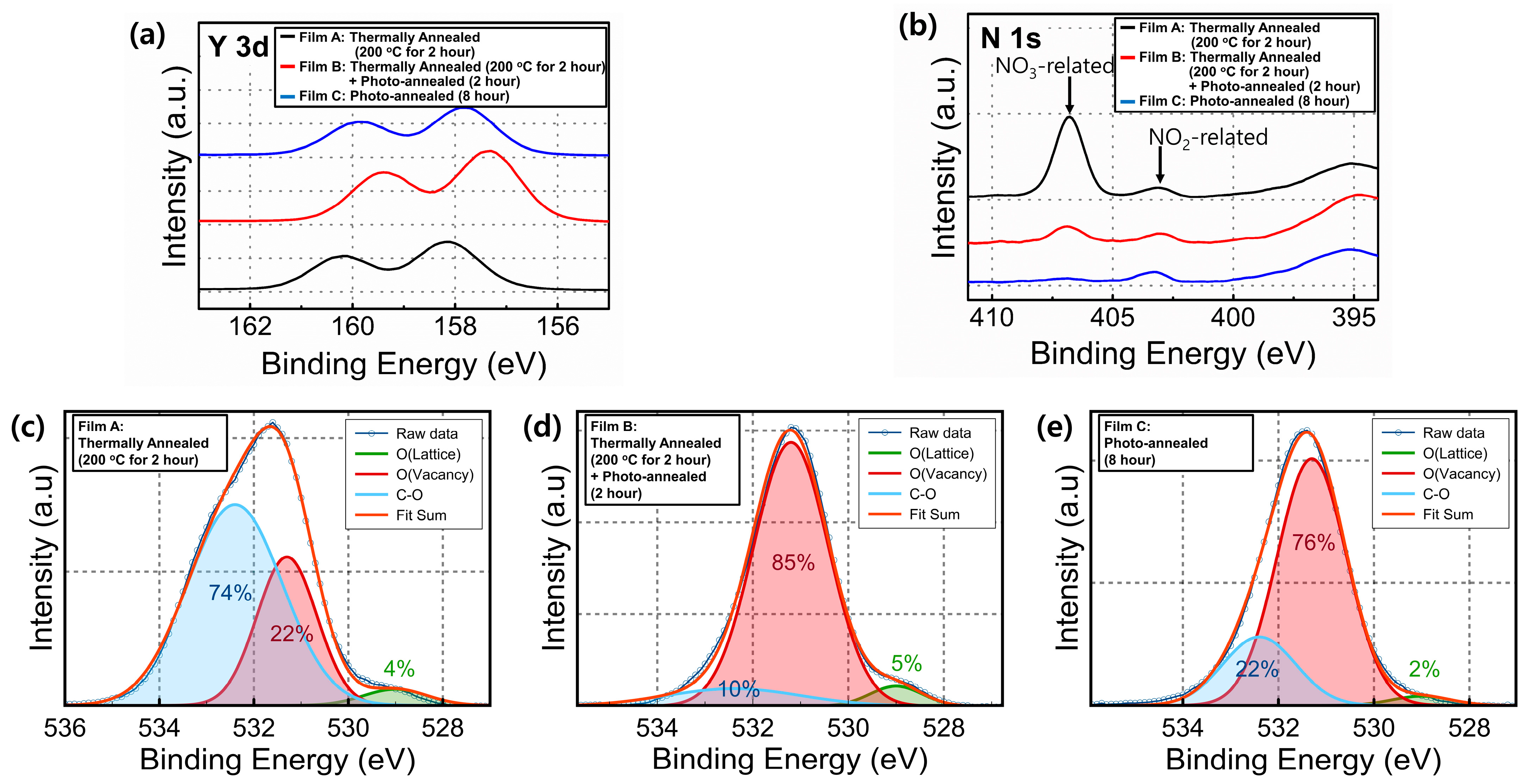
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Joshi, S.; Savel’ev, S.E.; Jiang, H.; Midya, R.; Lin, P.; Hu, M.; Ge, N.; Strachan, J.P.; Li, Z.; et al. Memristors with diffusive dynamics as synaptic emulators for neuromorphic computing. Nat. Mater. 2017, 16, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Jeong, D.S.; Hwang, C.S. Nonvolatile Memory Materials for Neuromorphic Intelligent Machines. Adv. Mater. 2018, 30, 1704729. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Wang, Z.; Kim, K.M.; Wu, H.; Ravichandran, V.; Xia, Q.; Hwang, C.S.; Yang, J.J. An artificial nociceptor based on a diffusive memristor. Nat. Commun. 2018, 9, 417. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Subramanian, V. Effect of electrode material on resistive switching memory behavior of solution-processed resistive switches: Realization of robust multi-level cell. Thin Solid Film. 2017, 625, 87–92. [Google Scholar] [CrossRef]
- Smith, J.; Chung, S.; Jang, J.; Biaou, C.; Subramanian, V. Solution-processed complementary resistive switching arrays for associative memory. IEEE Trans. Electron. Devices 2017, 64, 4310–4316. [Google Scholar] [CrossRef]
- Lee, S.; Kim, T.; Jang, B.; Lee, W.Y.; Song, K.C.; Kim, H.S.; Do, G.Y.; Hwang, S.B.; Chung, S.; Jang, J. Impact of device area and film thickness on performance of sol-gel Processed ZrO2 RRAM. IEEE Electron. Device Lett. 2018, 39, 668–671. [Google Scholar] [CrossRef]
- Ha, S.; Lee, H.; Lee, W.Y.; Jang, B.; Kwon, H.J.; Kim, K.; Jang, J. Effect of annealing environment on the performance of sol-gel-processed ZrO2 RRAM. Electronics 2019, 8, 947. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Hong, W.; Lee, C.; Lee, C.Y.; Kim, H.J.; Kwon, H.J.; Kang, H.; Jang, J. Sol-gel-processed amorphous-phase ZrO2 based resistive random access memory. Mater. Res. Express 2021, 8, 116301. [Google Scholar] [CrossRef]
- Ding, Z.; Feng, Y.; Huang, P.; Liu, L.; Kang, J. Low-power resistive switching characteristic in HfO2/TiOx bi-layer resistive random-access memory. Nanoscale Res. Lett. 2018, 14, 157. [Google Scholar] [CrossRef] [Green Version]
- Piros, E.; Petzold, S.; Zintler, A.; Kaiser, N.; Vogel, T.; Eilhardt, R.; Wenger, C.; Luna, L.M.; Alff, L. Enhanced thermal stability of yttrium oxide-based RRAM devices with inhomogeneous Schottky-barrier. Appl. Phys. Lett. 2020, 177, 013504. [Google Scholar] [CrossRef]
- Kim, K.; Lee, C.; Lee, W.Y.; Kim, D.W.; Kim, H.J.; Lee, S.H.; Bae, J.H.; Kang, I.M.; Jang, J. Enhanced switching ratio of sol-gel-processed Y2O3 RRAM device by suppressing oxygen vacancy formation at high annealing temperatures. Semicond. Sci. Technol. 2022, 37, 015007. [Google Scholar] [CrossRef]
- Petzold, S.; Piros, E.; Sharath, S.U.; Zintler, A.; Hildebrandt, E.; Molina-Luna, L.; Wenger, C.; Alff, L. Gradual reset and set characteristics in yttrium oxide based resistive random access memory. Semicond. Sci. Technol. 2019, 34, 075008. [Google Scholar] [CrossRef]
- Chiam, S.Y.; Chim, W.K.; Pi, C.; Huan, A.C.H.; Wang, S.J.; Pan, J.S.; Turner, S.; Zhang, J. Band alignment of yttrium oxide on various relaxed and strained semiconductor substrates. J. Appl. Phys. 2008, 103, 083702. [Google Scholar] [CrossRef]
- Cranton, W.; Spink, D.; Stevens, R.; Thomas, C. Growth and dielectric characterization of yttrium oxide thin films deposited on Si by RF-magnetron sputtering. Thin Solid Films 1993, 226, 156–160. [Google Scholar] [CrossRef]
- Lo, C.L.; Hou, T.H.; Chen, M.C.; Huang, J.J. Dependence of read margin on pull-up schemes in high-density one selector–one resistor crossbar array. IEEE Trans. Electron. Devices 2013, 60, 420–426. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, P.; Chen, B.; Yang, X.; Gao, B.; Wang, J.; Zeng, L.; Du, G.; Kang, J. RRAM, Crossbar array with cell selection device: A device and circuit interaction study. IEEE Trans. Electron. Devices 2013, 60, 719–726. [Google Scholar] [CrossRef]
- Lee, C.; Lee, W.Y.; Kim, H.J.; Bae, J.H.; Kang, I.M.; Lim, D.; Kim, K.; Jang, J. Extremely bias stress stable enhancement mode sol-gel-processed SnO2 thin-film transistors with Y2O3 passivation layers. Appl. Surf. Sci. 2021, 559, 149971. [Google Scholar] [CrossRef]
- Casa Branca, L.; Deuermeier, J.; Martins, J.; Carlos, E.; Pereira, M.; Martins, R.; Fortunato, E.; Kiazadeh, A. 2D resistive switching based on amorphous zinc-tin oxide schottky diodes. Adv. Electron. Mater. 2020, 6, 1900958. [Google Scholar] [CrossRef]
- Chen, A.; Haddad, S.; Wu, Y.C.; Fang, T.N.; Lan, Z.; Avanzino, S.; Pangrle, S.; Buynoski, M.; Rathor, M.; Cai, W.; et al. Non-volatile resistive switching for advanced memory applications. In Proceedings of the IEEE International Electron Devices Meeting. IEDM Technical Digest, Washington, DC, USA, 5 December 2005; pp. 746–749. [Google Scholar]
- Tang, G.S.; Zeng, F.; Chen, C.; Gao, S.; Fu, H.D.; Song, C.; Wang, G.Y.; Pan, F. Resistive switching behaviour of a tantalum oxide nanolayer fabricated by plasma oxidation. Phys. Status Solidi RRL 2013, 7, 282–284. [Google Scholar] [CrossRef]
- Choi, B.J.; Jeong, D.S.; Kim, S.K.; Rohde, C.; Choi, S.; Oh, J.H.; Kim, H.J.; Hwang, C.S.; Szot, K.; Waser, R.; et al. Resistive switching mechanism of TiO2 thin films grown by atomic-layer deposition. J. Appl. Phys. 2005, 98, 033715. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.; Kang, H.; Chakravarthula, H.C.N.; Subramanian, V. Fully inkjet-printed transparent oxide thin film transistors using a fugitive wettability switch. Adv. Electron. Mater. 2015, 1, 1500086. [Google Scholar] [CrossRef]
- Scheideler, W.J.; Jang, J.; Karim, M.A.U.; Kitsomboonloha, R.; Zeumault, Z.; Subramanian, V. Gravure-printed sol–gels on flexible glass: A scalable route to additively patterned transparent conductors. ACS Appl. Mater. Interfaces 2015, 7, 12679–12687. [Google Scholar] [CrossRef] [PubMed]
- Carlos, E.; Branquinho, R.; Martins, R.; Kiazadeh, A.; Fortunato, E. Recent progress in solution-based metal oxide resistive switching devices. Adv. Mater. 2021, 33, 2004328. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Heo, J.S.; Kim, T.H.; Park, S.; Yoon, M.H.; Kim, J.; Oh, M.S.; Yi, G.R.; Noh, Y.Y.; Park, S.K. Flexible metal-oxide devices made by room-temperature photochemical activation of sol-gel films. Nature 2012, 489, 128–132. [Google Scholar] [CrossRef]
- Jang, B.; Kang, H.; Lee, W.Y.; Bae, J.H.; Kang, I.N.; Kim, K.; Kwon, H.J.; Jang, J. Enhancement mode flexible SnO2 thin film transistors via a UV/Ozone-assisted sol-gel approach. IEEE Access 2020, 8, 123013–123018. [Google Scholar] [CrossRef]
- Kim, J.H.; Ma, J.; Lee, S.; Jo, S.; Kim, C.S. Effect of ultraviolet–ozone treatment on the properties and antibacterial activity of zinc oxide sol-gel film. Materials 2019, 12, 2422. [Google Scholar] [CrossRef] [Green Version]
- Park, H.L.; Lee, Y.; Kim, N.; Seo, D.G.; Go, G.T.; Lee, T.W. Flexible neuromorphic electronics for computing, soft robotics, and neuroprosthetics. Adv. Mater. 2020, 32, 1903558. [Google Scholar] [CrossRef]
- Giraldi, T.R.; Escote, M.T.; Bernard, M.I.B.; Bouquet, V.; Leite, E.R.; Longo, E.; Varel, J.A. Effect of thickness on the electrical and optical properties of Sb doped sno2 (ATO) thin films. J. Electroceram. 2004, 13, 159–165. [Google Scholar] [CrossRef]
- Khedmia, N.; Ben Rabeha, M.; Kanzari, M. Thickness dependent structural and optical properties of vacuum evaporated CuIn5S8 thin films. Energy Procedia 2014, 44, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Park, I.S.; Jung, Y.C.; Seong, S.; Ahn, J.; Kang, J.; Noh, W.; Lansalot-Matrasd, C. Atomic layer deposition of Y2O3 films using heteroleptic liquid (iPrCp)2Y(iPr-amd) precursor. J. Mater. Chem. C 2014, 2, 9240–9247. [Google Scholar] [CrossRef]
- Panz, T.M.; Lee, J.D. Physical and electrical properties of yttrium oxide gate dielectrics on si substrate with NH3 plasma treatment. J. Electrochem. Soc. 2007, 154, H698–H703. [Google Scholar]
- Lei, P.; Zhu, J.; Zhu, Y.; Jiang, C.; Yin, X. Yttrium oxide thin films prepared under different oxygen-content atmospheres: Microstructure and optical properties. Appl. Phys. A 2012, 108, 621–628. [Google Scholar] [CrossRef]
- Ghosh, G.; Orlowski, M.K. Correlation between set and reset voltages in resistive RAM cells. Curr. Appl. Phys. 2015, 15, 1124–1129. [Google Scholar] [CrossRef]
- Menzel, S.; Waser, R. Analytical analysis of the generic SET and RESET characteristics of electrochemical metallization memory cells. Nanoscale 2013, 5, 11003–11010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laurentis, S.; Nardi, F.; Balatti, S.; Gilmer, D.C.; Ielemini, D. Resistive switching by voltage-driven ion migration in bipolar RRAM, Part II: Modeling. IEEE Trans. Electron. Devices 2012, 59, 2468–2475. [Google Scholar] [CrossRef]
- Zhu, X.; Zhuge, F.; Li, M.; Yin, K.; Liu, Y.; Zuo, Z.; Chen, B.; Li, R.W. Microstructure dependence of leakage and resistive switching behaviours in Ce-doped BiFeO3 thin films. J. Phys. D Appl. Phys. 2011, 44, 415104–415110. [Google Scholar] [CrossRef]
- Ram Kumar, K.; Satyam, M. Carrier mobility in polycrystalline semiconductors. Appl. Phys. Lett. 1998, 39, 898. [Google Scholar] [CrossRef]
- Steinhauser, J.; Faÿ, S.; Oliveira, N.; Vallat-Sauvain, E.; Ballif, C. Transition between grain boundary and intragrain scattering transport mechanisms in boron-doped zinc oxide thin films. Appl. Phys. Lett. 2007, 90, 142107. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.; Shin, D.; Lee, Y.; Pak, J.J. Fabrication of solution-processed SnO2–Based flexible ReRAM using laser-induced graphene transferred onto PDMS. Curr. Appl. Phys. 2021, 25, 70–74. [Google Scholar] [CrossRef]
- Lee, Y.; Jung, J.; Shin, D.; Pak, J.J. Effect of UV irradiation on the resistive switching characteristics of low-temperature solution-processed ZrO2 RRAM. Semicond. Sci. Technol. 2021, 36, 085004. [Google Scholar]
- Shen, Z.; Qi, Y.; Mitrovic, I.Z.; Zhao, C.; Hall, S.; Yang, Y.; Luo, T.; Huang, Y.; Zhao, C. Effect of annealing temperature for Ni/AlOx/Pt RRAM devices fabricated with solution-based dielectric. Micromachines 2019, 10, 446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yen, T.J.; Chin, A.; Gritsenko, V. High performance all nonmetal SiNx resistive random-access memory with strong process dependence. Sci. Rep. 2020, 10, 2807. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Reference | Material System | Process Temperature (°C) | VSET/VRESET | HRS/LRS | Forming | Endurance (Cycle)/Retention (s) | Conductive Filament Type |
|---|---|---|---|---|---|---|---|
| [40] | Au/Graphene/SnO2 | 80 | +2.5 V/+1.0 V | ~10 | No | ~1 × 102/1.8 × 103 | Oxygen vacancy |
| [41] | ITO/ZrO2/W | 45 | +1.0 V/−1.5 V | ~10 | No | ~2 × 102/104 | Oxygen vacancy |
| [42] | Al/Pt/AlOx/Ni | 300 | +2.5 V/−0.75 V | ~10 | No | ~1 × 102/~104 | Oxygen vacancy |
| [43] | P+/SiNx/N+–Si | 300 | +3.0 V/−1.5 V | ~102 | Yes | ~105/~104 | Nitride vacancy/ Si dangling bonds |
| This work | Ag/Y2O3/ITO | RT | +0.5 V/−7.0 V | ~104 | No | 102/104 | ECM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-J.; Kim, D.-W.; Lee, W.-Y.; Kim, K.; Lee, S.-H.; Bae, J.-H.; Kang, I.-M.; Kim, K.; Jang, J. Flexible Sol-Gel—Processed Y2O3 RRAM Devices Obtained via UV/Ozone-Assisted Photochemical Annealing Process. Materials 2022, 15, 1899. https://doi.org/10.3390/ma15051899
Kim H-J, Kim D-W, Lee W-Y, Kim K, Lee S-H, Bae J-H, Kang I-M, Kim K, Jang J. Flexible Sol-Gel—Processed Y2O3 RRAM Devices Obtained via UV/Ozone-Assisted Photochemical Annealing Process. Materials. 2022; 15(5):1899. https://doi.org/10.3390/ma15051899
Chicago/Turabian StyleKim, Hyeon-Joong, Do-Won Kim, Won-Yong Lee, Kyoungdu Kim, Sin-Hyung Lee, Jin-Hyuk Bae, In-Man Kang, Kwangeun Kim, and Jaewon Jang. 2022. "Flexible Sol-Gel—Processed Y2O3 RRAM Devices Obtained via UV/Ozone-Assisted Photochemical Annealing Process" Materials 15, no. 5: 1899. https://doi.org/10.3390/ma15051899







