Enhanced Switching Reliability of Sol–Gel-Processed Y2O3 RRAM Devices Based on Y2O3 Surface Roughness-Induced Local Electric Field
Abstract
:1. Introduction
2. Materials and Methods
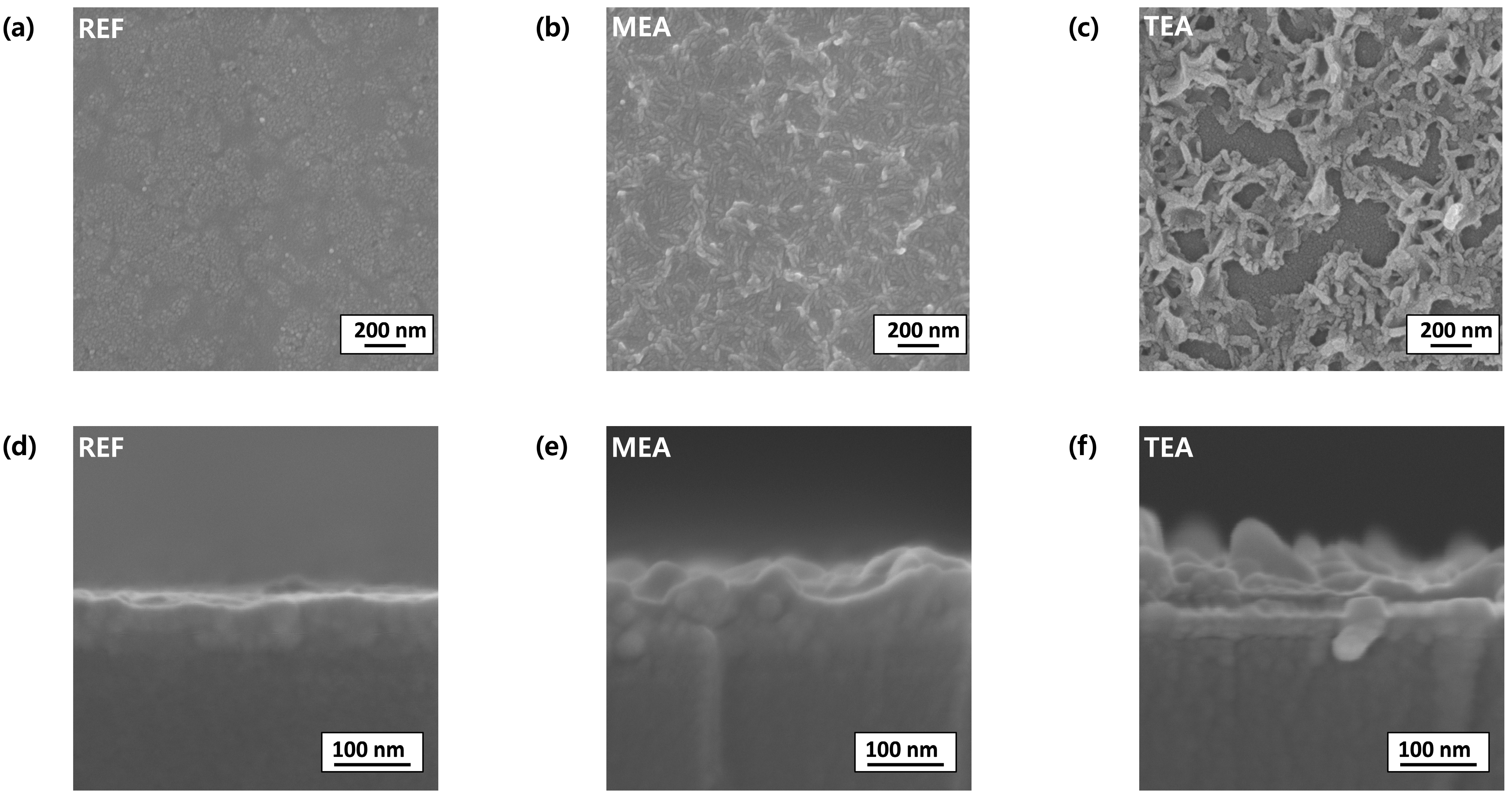
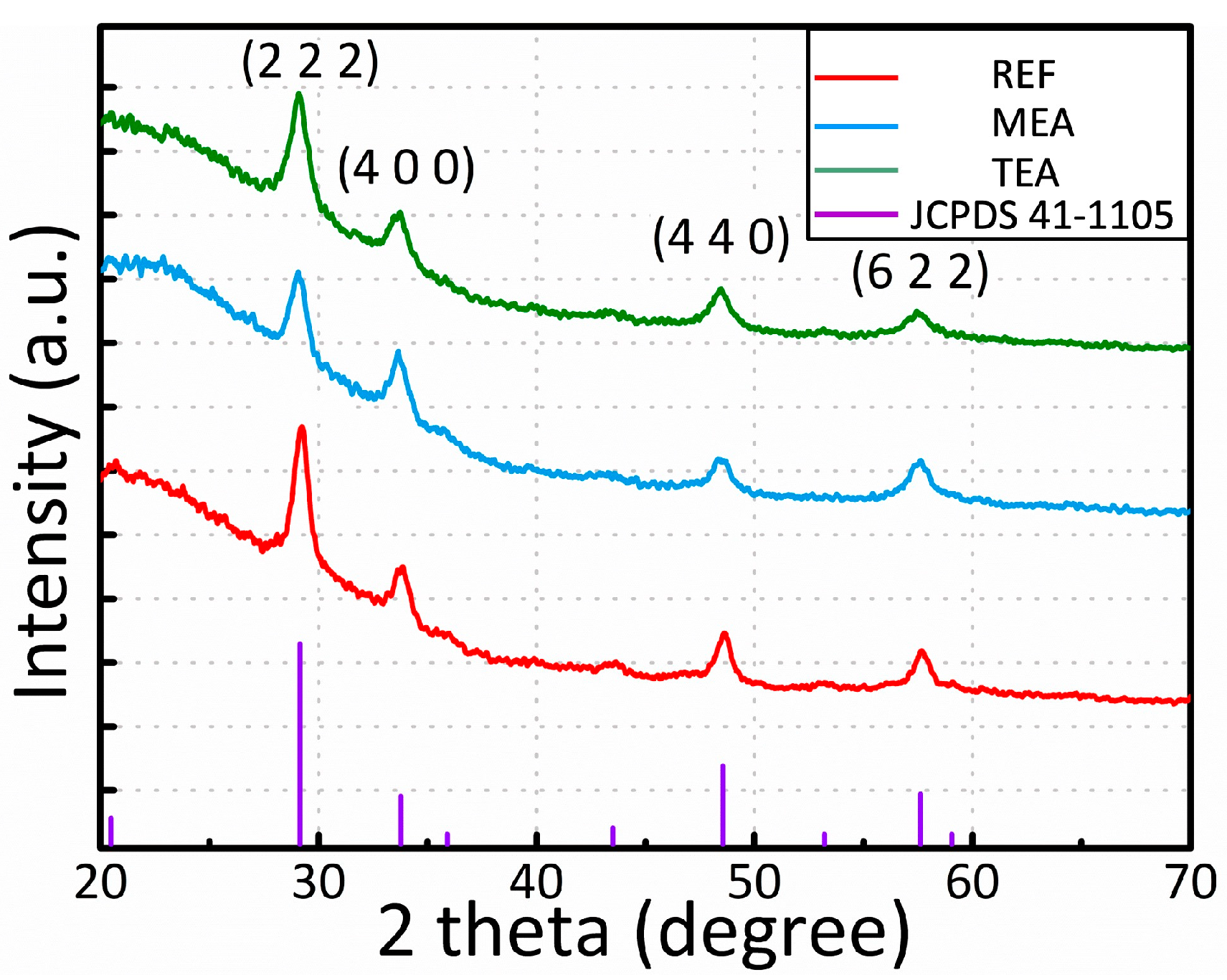
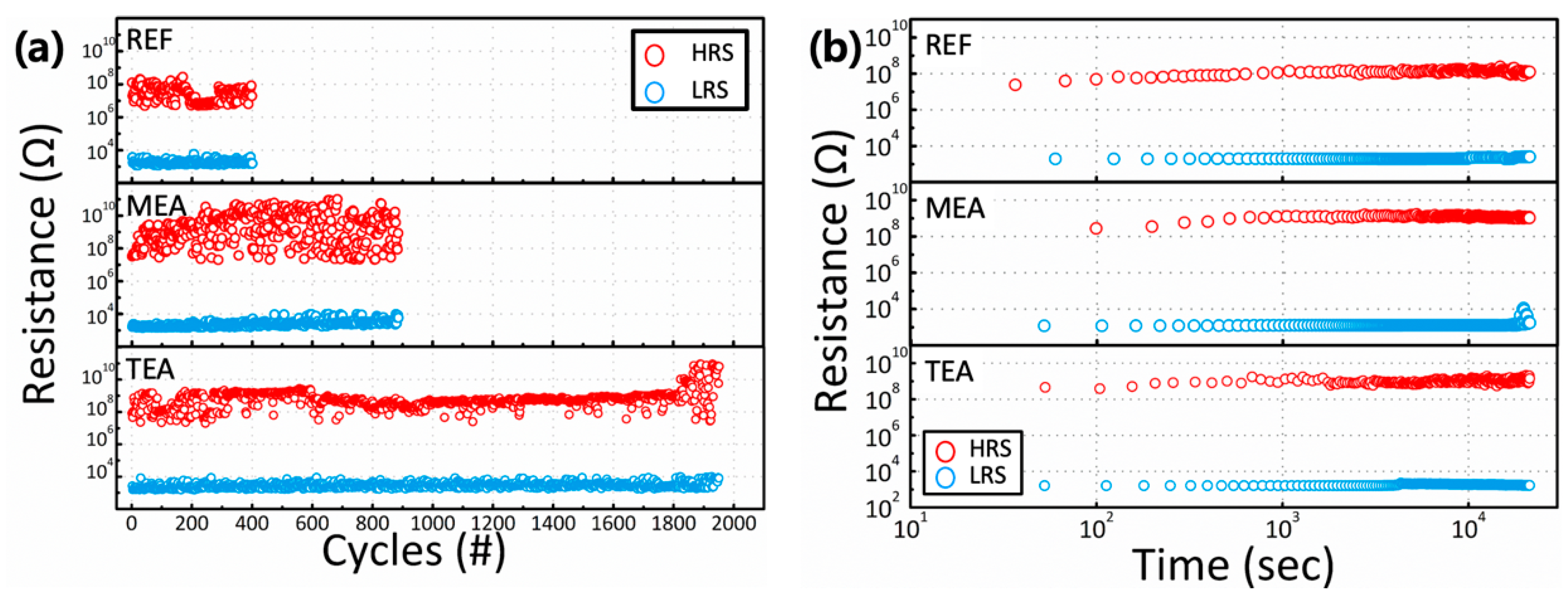
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Joshi, S.; Savel’ev, S.E.; Jiang, H.; Midya, R.; Lin, P.; Hu, M.; Ge, N.; Strachan, J.P.; Li, Z.; et al. Memristors with diffusive dynamics as synaptic emulators for neuromorphic computing. Nat. Mater. 2017, 16, 101–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, J.H.; Wang, Z.; Kim, K.M.; Wu, H.; Ravichandran, V.; Xia, Q.; Hwang, C.S.; Yang, J.J. An artificial nociceptor based on a diffusive memristor. Nat. Commun. 2018, 9, 417. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.S.; Hwang, C.S. Nonvolatile Memory Materials for Neuromorphic Intelligent Machines. Adv. Mater. 2018, 30, 1704729. [Google Scholar] [CrossRef]
- Jang, J.; Subramanian, V. Effect of electrode material on resistive switching memory behavior of solution-processed resistive switches: Realization of robust multi-level cell. Thin Solid Films 2017, 625, 87–92. [Google Scholar] [CrossRef]
- Smith, J.; Chung, S.; Jang, J.; Biaou, C.; Subramanian, V. Solution-processed complementary resistive switching arrays for associative memory. IEEE Trans. Electron Devices 2017, 64, 4310–4316. [Google Scholar] [CrossRef]
- Lee, S.; Kim, T.; Jang, B.; Lee, W.Y.; Song, K.C.; Kim, H.S.; Do, G.Y.; Hwang, S.B.; Chung, S.; Jang, J. Impact of Device Area and Film Thickness on Performance of Sol-gel Processed ZrO2 RRAM. IEEE Electron Device Lett. 2018, 39, 668–671. [Google Scholar] [CrossRef]
- Ha, S.; Lee, H.; Lee, W.Y.; Jang, B.; Kwon, H.J.; Kim, K.; Jang, J. Effect of Annealing Environment on the Performance of Sol–Gel-Processed ZrO2 RRAM. Electronics 2019, 8, 947. [Google Scholar] [CrossRef] [Green Version]
- Ding, Z.; Feng, Y.; Huang, P.; Liu, L.; Kang, J. Low-Power Resistive Switching Characteristic in HfO2/TiOx Bi-Layer Resistive Random-Access Memory. Nanoscale Res. Lett. 2018, 14, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piros, E.; Petzold, S.; Zintler, A.; Kaiser, N.; Vogel, T.; Eilhardt, R.; Wenger, C.; Luna, L.M.; Alff, L. Enhanced thermal stability of yttrium oxide-based RRAM devices with inhomogeneous Schottky-barrier. Appl. Phys. Lett. 2020, 177, 013504. [Google Scholar] [CrossRef]
- Petzold, S.; Piros, E.; Sharath, S.U.; Zintler, A.; Hildebrandt, E.; Molina-Luna, L.; Wenger, C.; Alff, L. Gradual reset and set characteristics in yttrium oxide based resistive random access memory. Semicond. Sci. Technol. 2019, 34, 075008. [Google Scholar] [CrossRef]
- Chiam, S.Y.; Chim, W.K.; Pi, C.; Huan, A.C.H.; Wang, S.J.; Pan, J.S.; Turner, S.; Zhang, J. Band alignment of yttrium oxide on various relaxed and strained semiconductor substrates. J. Appl. Phys. 2008, 103, 083702. [Google Scholar] [CrossRef]
- Rushchanskii, K.Z.; Blügel, S.; Ležaić, M. Ab initio phase diagrams of Hf-O, Zr-O and Y-O: A comparative study. Faraday Discuss. 2019, 213, 321–337. [Google Scholar] [CrossRef]
- Das, M.; Kumar, A.; Singh, R.; Mukherjee, S. Realization of synaptic learning and memory functions in Y2O3 based memristive device fabricated by dual ion beam sputtering. Nanotechnology 2018, 29, 055203–055211. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, C.; Lee, W.Y.; Kim, D.W.; Kim, H.J.; Lee, S.H.; Bae, J.H.; Kang, I.M.; Jang, J. Enhanced switching ratio of sol–gel-processed Y2O3 RRAM device by suppressing oxygen vacancy formation at high annealing temperatures. Semicond. Sci. Technol. 2022, 37, 015007–105013. [Google Scholar] [CrossRef]
- Kumar, A.; Das, M.; Garg, V.; Sengar, B.S.; Htay, M.T.; Kumar, S.; Kranti, A.; Mukherjee, S. Forming-free high- endurance Al/ZnO/Al memristor fabricated by dual ion beam sputtering. Appl. Phys. Lett. 2017, 110, 253509. [Google Scholar] [CrossRef]
- Zou, H.; Hu, Y.; Zhu, X.; Song, Z. Simultaneously high thermal stability and ultra-fast phase change speed based on samarium-doped antimony thin films. RSC Adv. 2017, 7, 31110–31114. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.Q.; Xu, H.Y.; Li, X.H.; Yu, H.; Liu, Y.C.; Zhu, X.J. Synaptic learning and memory functions achieved using oxygen ion migration/diffusion in an amorphous InGaZnO memristor. Adv. Funct. Mater. 2012, 22, 2759–2765. [Google Scholar] [CrossRef]
- Lo, C.L.; Hou, T.H.; Chen, M.C.; Huang, J.J. Dependence of read margin on pull-up schemes in high-density one selector–one resistor crossbar array. IEEE Trans. Electron Devices 2013, 60, 420–426. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, P.; Chen, B.; Yang, X.; Gao, B.; Wang, J.; Zeng, L.; Du, G.; Kang, J. RRAM, Crossbar array with cell selection device: A device and circuit interaction study. IEEE Trans. Electron Devices 2013, 60, 719–726. [Google Scholar] [CrossRef]
- Lee, C.; Lee, W.Y.; Kim, H.J.; Bae, J.H.; Kang, I.M.; Lim, D.; Kim, K.; Jang, J. Extremely bias stress stable enhancement mode sol–gel-processed SnO2 thin-film transistors with Y2O3 passivation layers. Appl. Surf. Sci. 2021, 559, 149971. [Google Scholar] [CrossRef]
- Niraula, D.; Karpov, V. Comprehensive numerical modeling of filamentary RRAM devices including voltage ramp-rate and cycle-to-cycle variations. J. Appl. Phys. 2018, 124, 174502. [Google Scholar] [CrossRef] [Green Version]
- Karpov, V.G.; Niraula, D.; Karpov, I.V.; Kotlyar, R. Thermodynamics of phase transitions and bipolar filamentary switching in resistive random-access memory. Phys. Rev. Appl. 2018, 8, 024028. [Google Scholar] [CrossRef] [Green Version]
- Waser, R.; Aono, M. Nanoionics-Based Resistive Switching Memories. Nat. Mater. 2007, 6, 833–840. [Google Scholar] [CrossRef]
- Yang, Y.C.; Pan, F.; Liu, Q.; Liu, M.; Zeng, F. Fully Room- Temperature-Fabricated Nonvolatile Resistive Memory for Ultrafast and High-Density Memory Application. Nano Lett. 2009, 9, 1636–1643. [Google Scholar] [CrossRef]
- Choi, H.H.; Kim, M.; Jang, J.; Lee, K.H.; Jho, J.Y.; Park, J.H. Tip-enhanced electric field-driven efficient charge injection and transport in organic material-based resistive memories. Appl. Mater. Today 2020, 20, 100746. [Google Scholar] [CrossRef]
- Park, J.; Jung, S.; Lee, W.; Kim, S.; Shin, J.; Lee, D.; Woo, J.; Hwang, H. Improved Switching Variability and Stability by Activating a Single Conductive Filament. IEEE Electron Device Lett. 2012, 33, 646–648. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, T.H.; Yoon, K.J.; Seong, W.M.; Jeon, J.W.; Kwon, Y.J.; Kim, Y.; Kwon, D.E.; Kim, G.S.; Ha, T.J.; et al. Fabrication of a Cu-Cone-Shaped Cation Source Inserted Conductive Bridge Random Access Memory and Its Improved Switching Reliability. Adv. Funct. Mater. 2019, 29, 1806278. [Google Scholar] [CrossRef]
- Liu, Q.; Long, S.; Lv, H.; Wang, W.; Niu, J.; Huo, Z.; Chen, J.; Liu, M. Controllable Growth of Nanoscale Conductive Filaments in Solid- Electrolyte-Based ReRAM by Using a Metal Nanocrystal Covered Bottom Electrode. ACS Nano 2010, 4, 6162–6168. [Google Scholar] [CrossRef]
- Shin, K.Y.; Kim, Y.; Antolinez, F.V.; Ha, J.S.; Lee, S.S.; Park, J.H. Controllable Formation of Nanofilaments in Resistive Memories via Tip-Enhanced Electric Fields. Adv. Electron. Mater. 2016, 2, 1–8. [Google Scholar] [CrossRef]
- Jang, J.; Kang, H.; Chakravarthula, H.C.N.; Subramanian, V. Fully inkjet-printed transparent oxide thin film transistors using a fugitive wettability switch. Adv. Electron. Mater. 2015, 1, 1500086. [Google Scholar] [CrossRef]
- Scheideler, W.J.; Jang, J.; Karim, M.A.U.; Kitsomboonloha, R.; Zeumault, Z.; Subramanian, V. Gravure-Printed Sol–Gels on Flexible Glass: A Scalable Route to Additively Patterned Transparent Conductors. ACS Appl. Mater. Interfaces 2015, 7, 12679–12687. [Google Scholar] [CrossRef] [PubMed]
- Birnie, D.P. Rational solvent selection strategies to combat striation formation during spin coating of thin films. J. Mater. Res. 2001, 16, 1145–1154. [Google Scholar] [CrossRef]
- Birnie, D.P. A Model for Drying Control Cosolvent Selection for Spin-Coating Uniformity: The Thin Film Limit. Langmuir 2013, 29, 9072–9078. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, H.; Matsui, T.; Kozuka, H. Spontaneous Pattern Formation Induced by Bénard−Marangoni Convection for Sol−Gel-Derived Titania Dip-Coating Films: Effect of Co-solvents with a High Surface Tension and Low Volatility. Langmuir 2015, 31, 12497–12504. [Google Scholar] [CrossRef] [PubMed]
- Houng, B.; Huang, C.L.; Tsai, S.Y. Effect of the pH on the growth and properties of sol–gel derived boron-doped ZnO transparent conducting thin film. J. Cryst. Growth 2007, 307, 328–333. [Google Scholar] [CrossRef]
- Ghosh, G.; Orlowski, M.K. Correlation between set and reset voltages in resistive RAM cells. Curr. Appl. Phys. 2015, 15, 1124–1129. [Google Scholar] [CrossRef]
- Menzel, S.; Waser, R. Analytical analysis of the generic SET and RESET characteristics of electrochemical metallization memory cells. Nanoscale 2013, 5, 11003–11010. [Google Scholar] [CrossRef] [Green Version]
- Laurentis, S.; Nardi, F.; Balatti, S.; Gilmer, D.C.; Ielemini, D. Resistive switching by voltage-driven ion migration in bipolar RRAMdPart II: Modeling. IEEE Trans. Elect. Dev. 2012, 59, 2468–2475. [Google Scholar] [CrossRef]



| Name (Abbreviation) | Surface Tension (N/m) at 25 °C | Boiling Point (°C) | Vapor Pressure (mm Hg) at 25 °C |
|---|---|---|---|
| 2-methoxyethanol (2-ME) | 42.8 | 124.0 | 9.5 |
| Ethanolamine (MEA) | 48.3 | 170.0 | 0.4 |
| Triethylamine (TEA) | 22.02 | 88.8 | 57.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-W.; Kim, H.-J.; Lee, W.-Y.; Kim, K.; Lee, S.-H.; Bae, J.-H.; Kang, I.-M.; Kim, K.; Jang, J. Enhanced Switching Reliability of Sol–Gel-Processed Y2O3 RRAM Devices Based on Y2O3 Surface Roughness-Induced Local Electric Field. Materials 2022, 15, 1943. https://doi.org/10.3390/ma15051943
Kim D-W, Kim H-J, Lee W-Y, Kim K, Lee S-H, Bae J-H, Kang I-M, Kim K, Jang J. Enhanced Switching Reliability of Sol–Gel-Processed Y2O3 RRAM Devices Based on Y2O3 Surface Roughness-Induced Local Electric Field. Materials. 2022; 15(5):1943. https://doi.org/10.3390/ma15051943
Chicago/Turabian StyleKim, Do-Won, Hyeon-Joong Kim, Won-Yong Lee, Kyoungdu Kim, Sin-Hyung Lee, Jin-Hyuk Bae, In-Man Kang, Kwangeun Kim, and Jaewon Jang. 2022. "Enhanced Switching Reliability of Sol–Gel-Processed Y2O3 RRAM Devices Based on Y2O3 Surface Roughness-Induced Local Electric Field" Materials 15, no. 5: 1943. https://doi.org/10.3390/ma15051943







