A Sustainable Technique to Prepare High-Purity Vanadium Pentoxide via Purification with Low Ammonium Consumption
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Experimental Steps
2.2.1. Hydrolyzed Vanadium Precipitation
2.2.2. Ammonium Salt Purification
3. Characterizations and Analysis
4. Results and Discussion
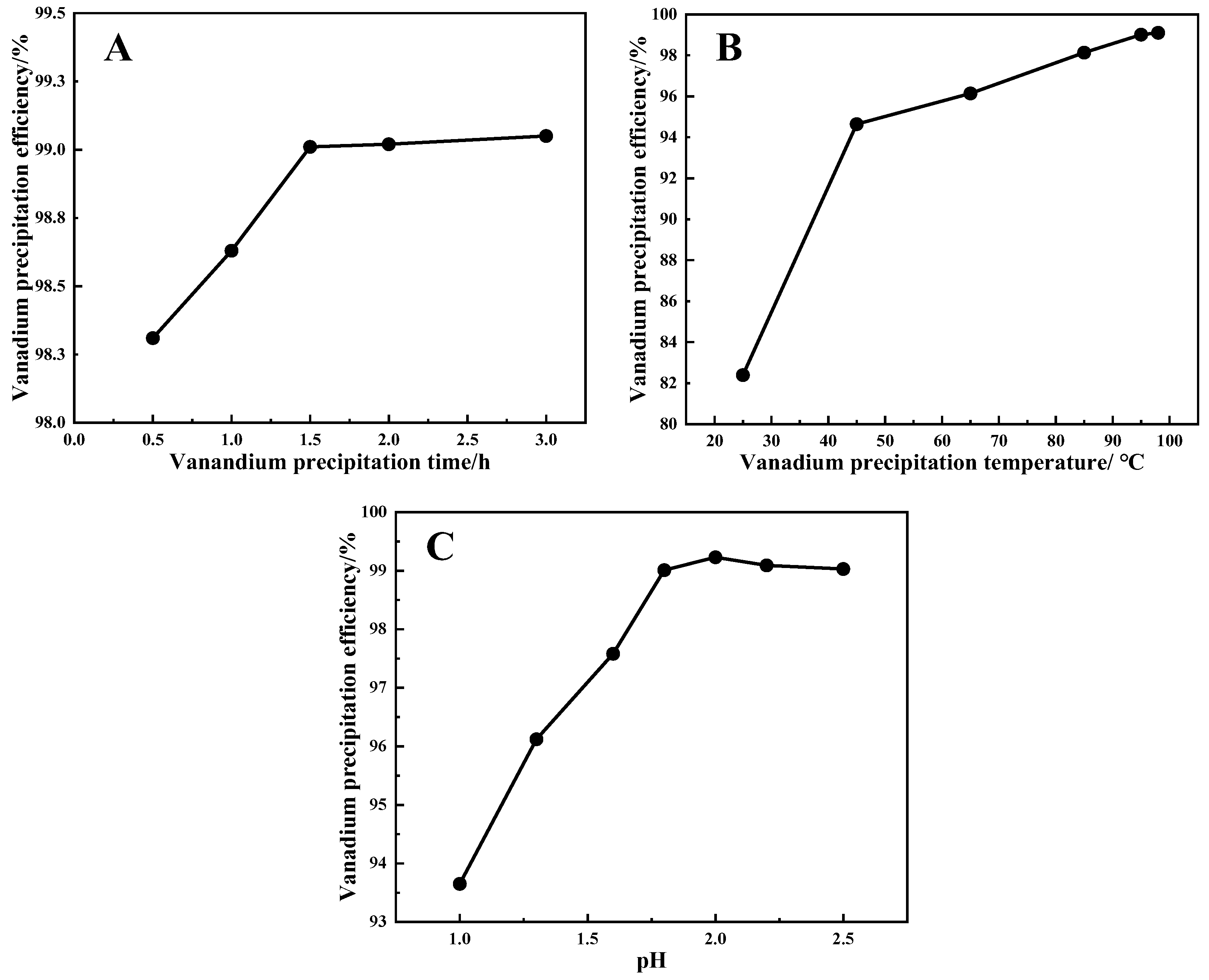
4.1. Effects of Hydrolyzed Vanadium Precipitation Conditions on Precipitation Efficiency
4.2. Ammonium Salt Purification
4.2.1. Effect of Detergent on Ammonium Salt Purification
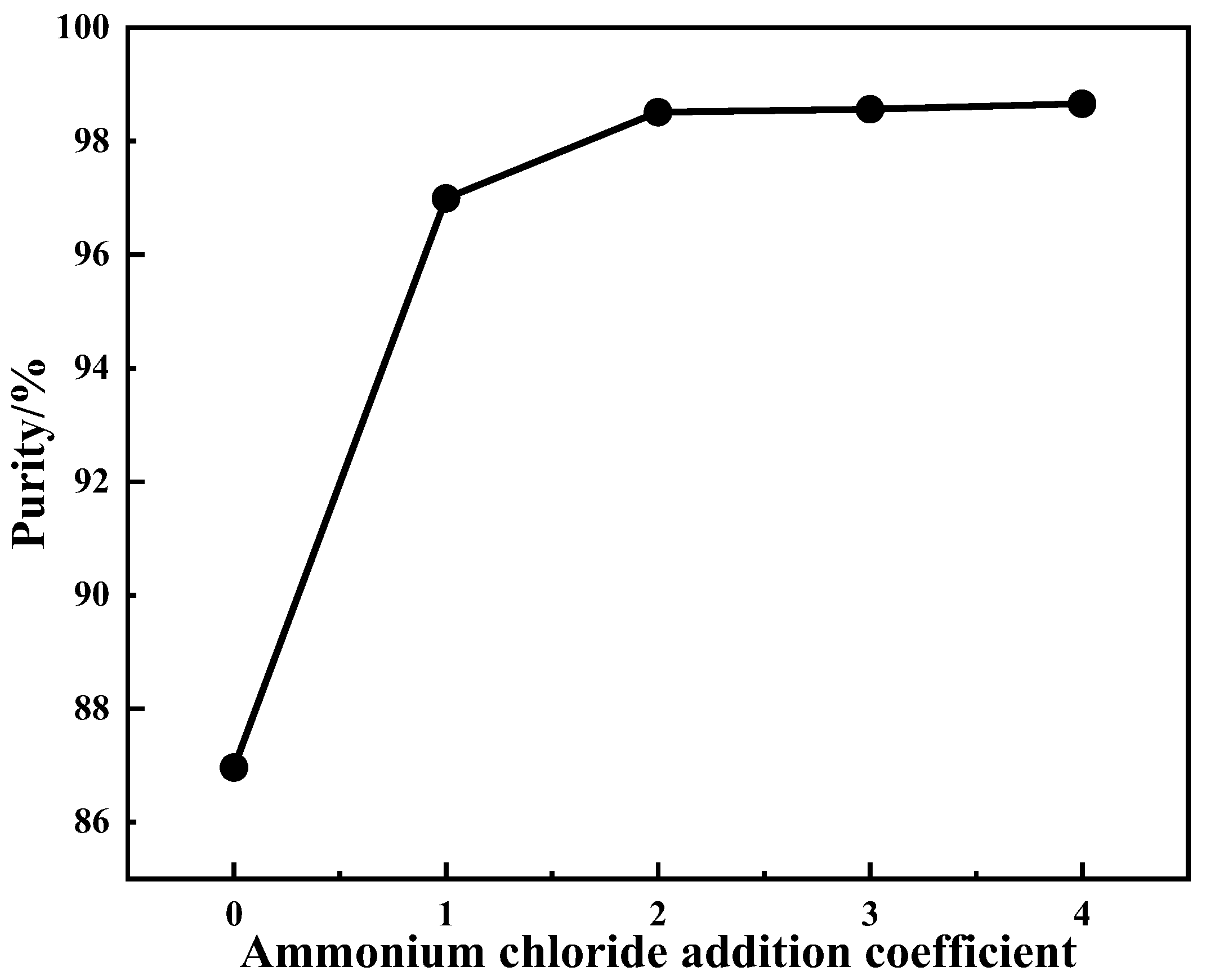
4.2.2. Effect of Ammonium Addition Coefficient on Product Purity
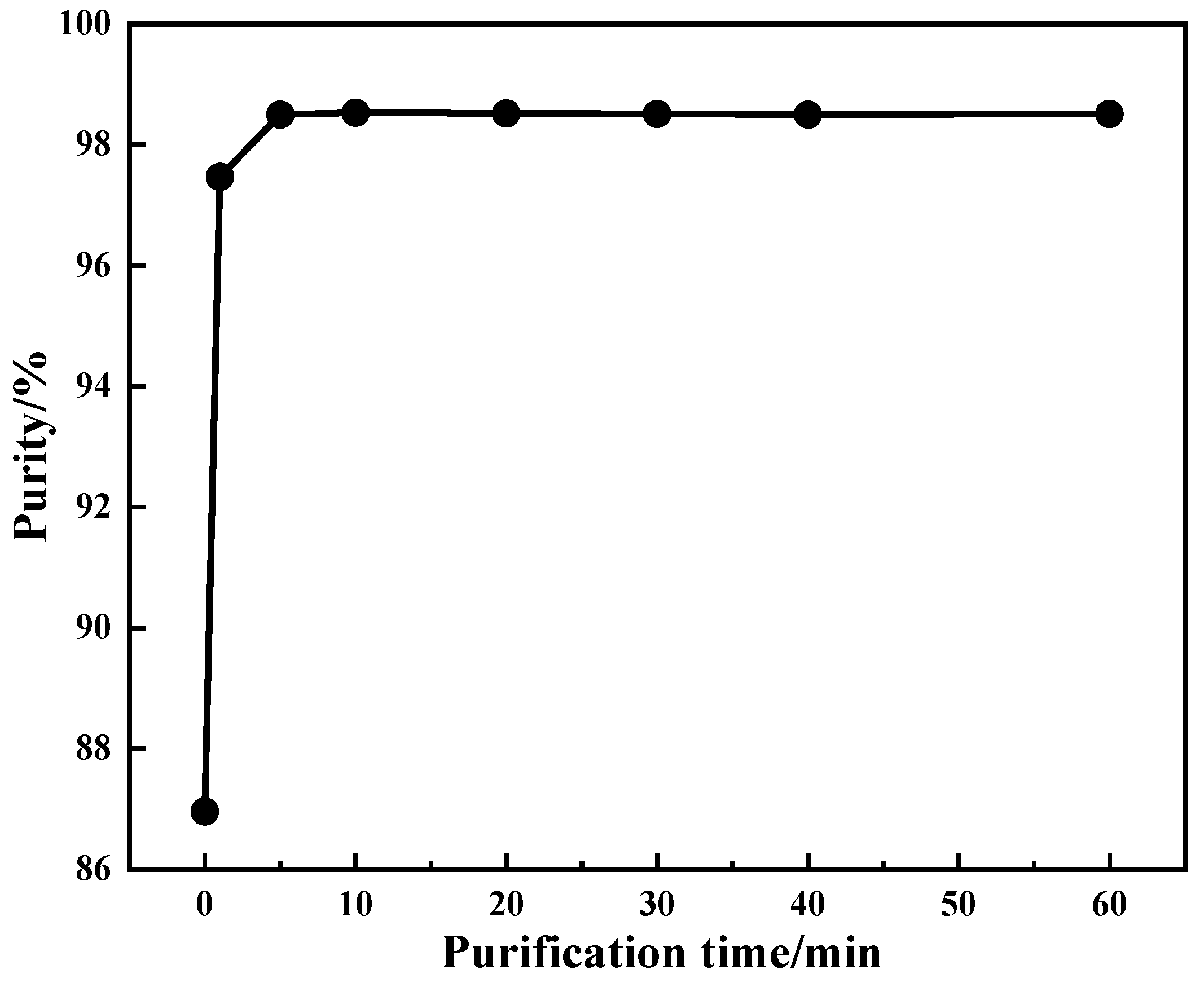
4.2.3. Effect of Purification Time on Product Purity
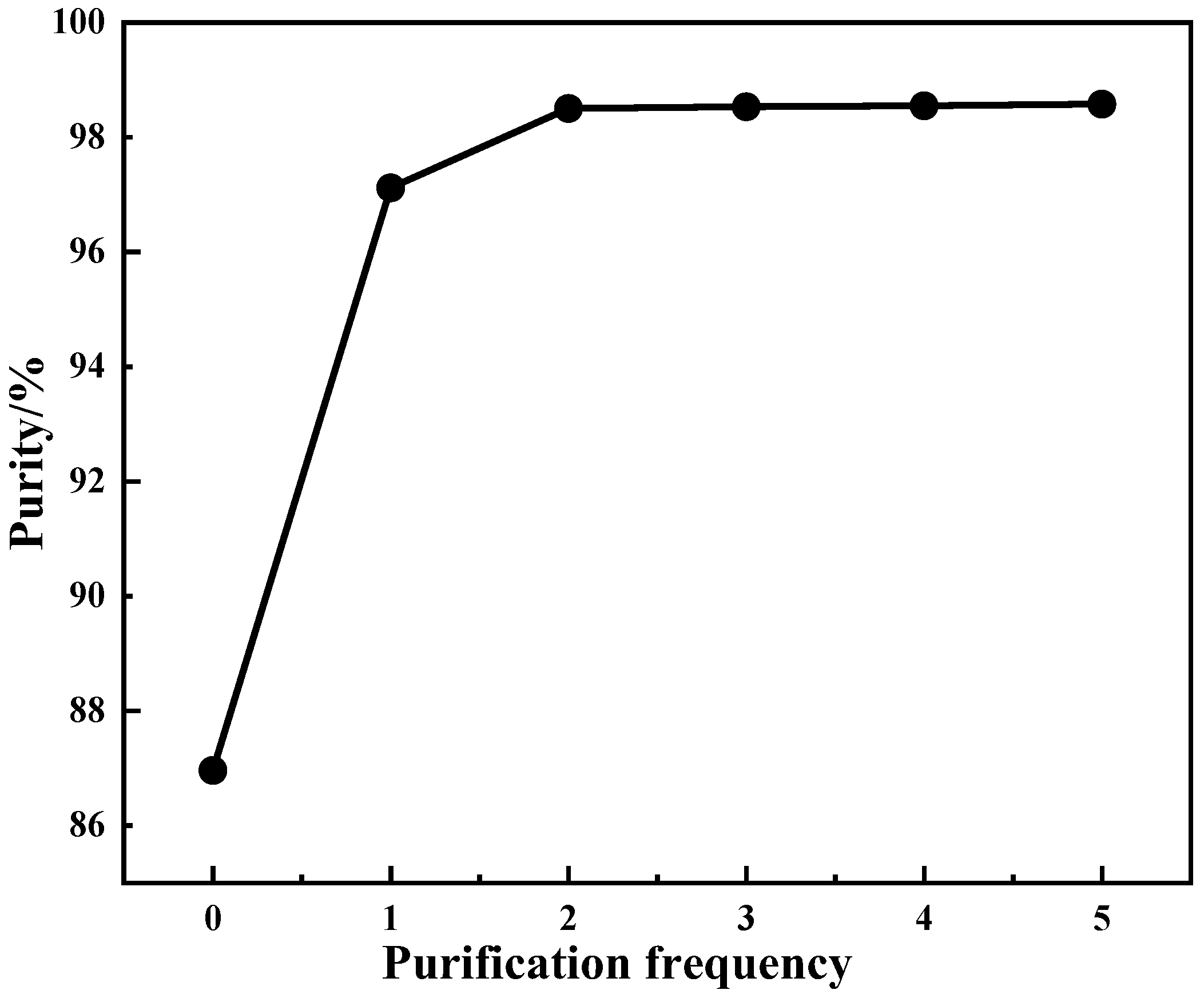
4.2.4. Effect of Purification Frequency on Product Purity
4.2.5. Effect of Purification Temperature on Product Purity
4.3. Characterization of V2O5 Product
4.4. Comparison of Vanadium Preparation Processes
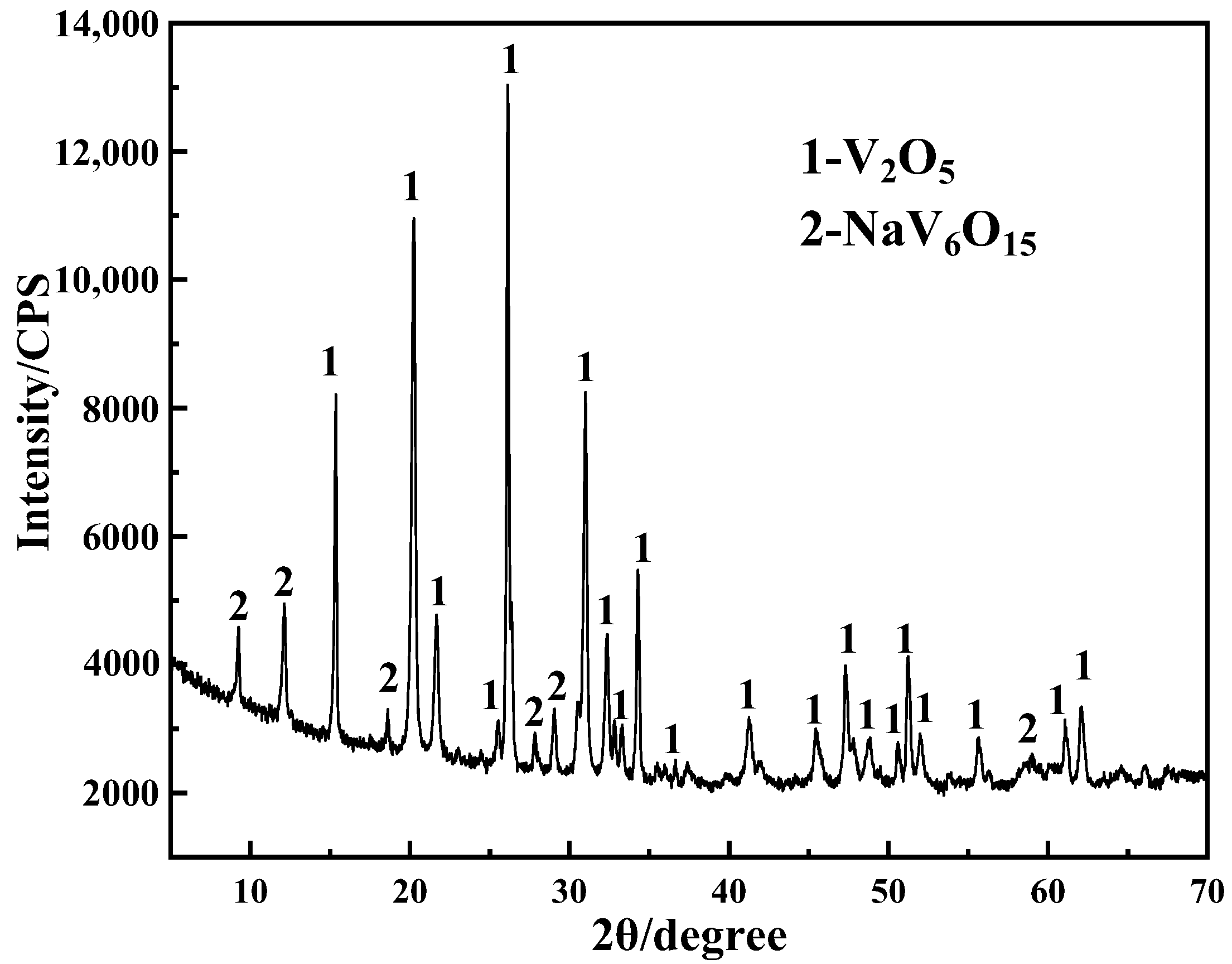
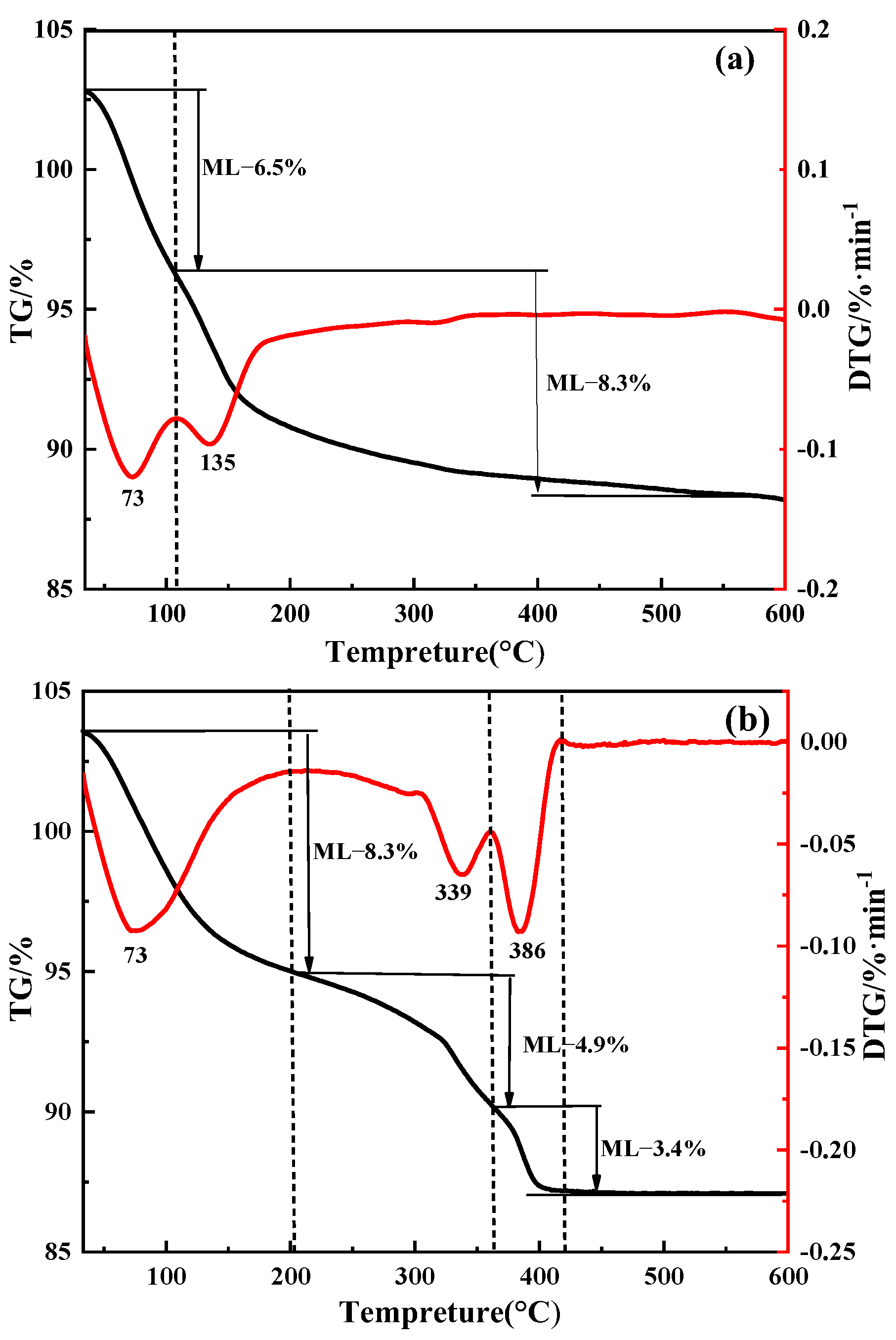
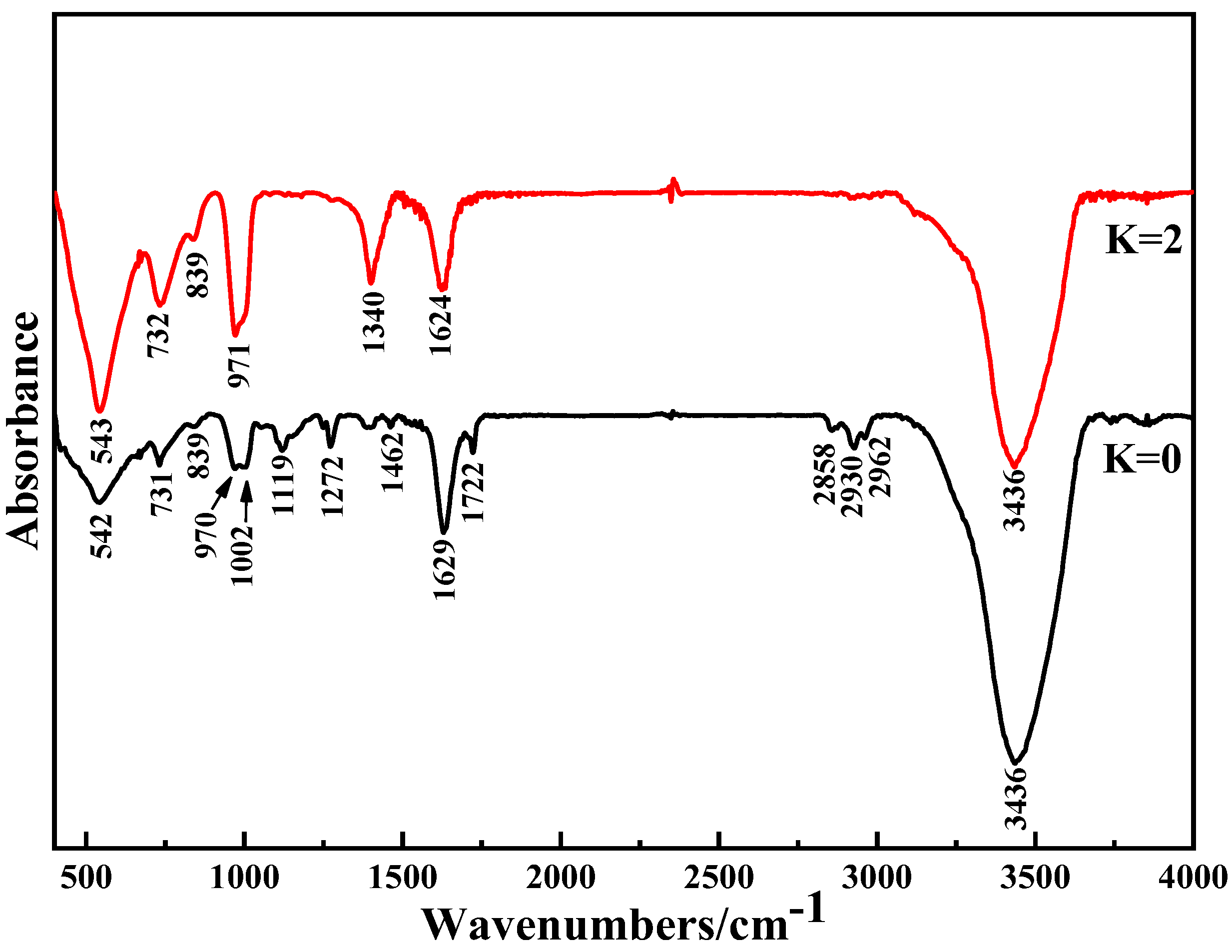
4.5. Composition and Structure of Purified Products
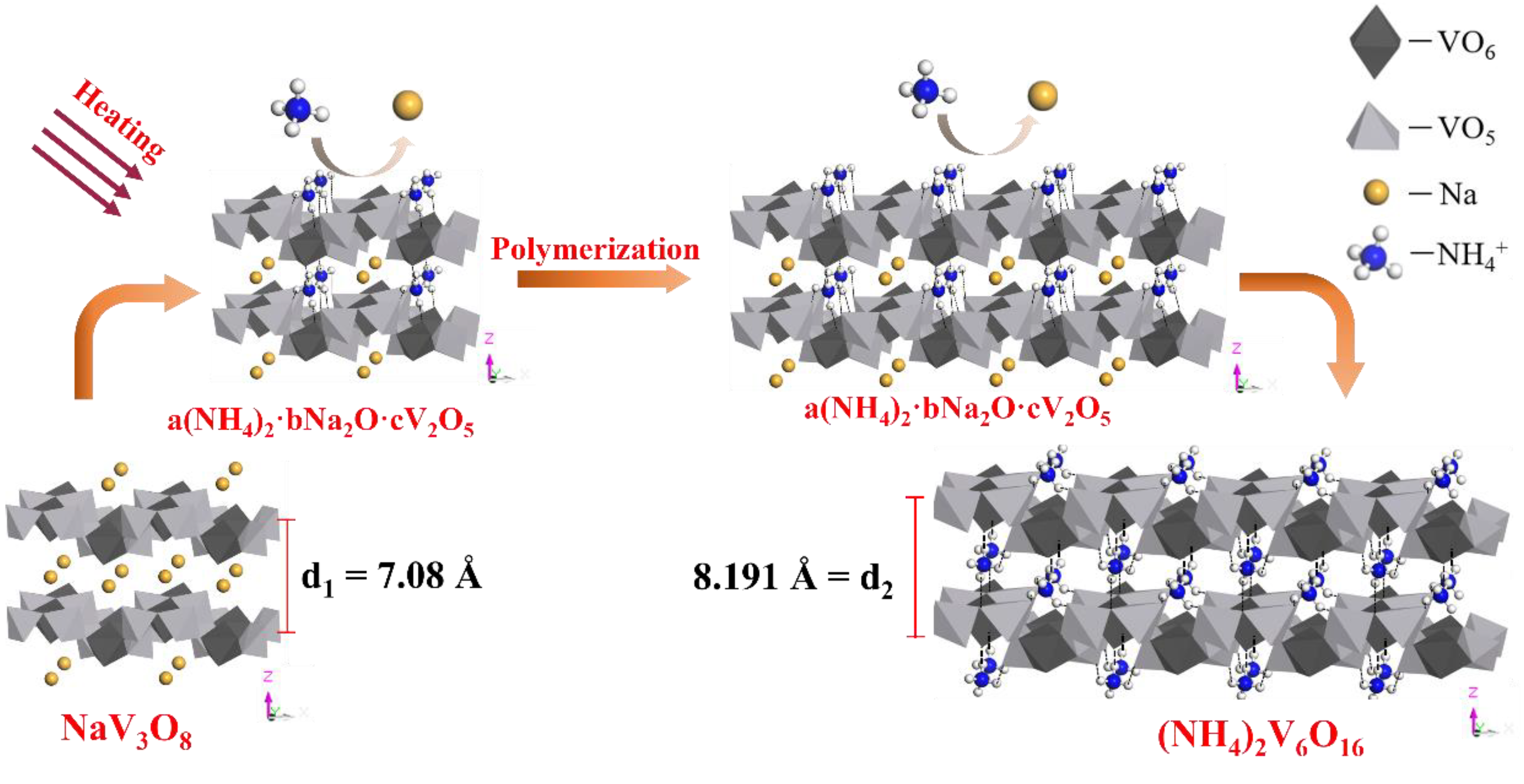
Crystal Purification Mechanism
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, P.; Zhang, Y. Mechanism of vanadium selective separation from iron in shale under an environmentally friendly oxalate ligand system. Sep. Purif. Technol. 2021, 276, 119269. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Bao, S.X.; Liu, T.; Chen, T.J.; Huang, J. The technology of extracting vanadium from stone coal in China: History, current status and future prospects. Hydrometallurgy 2011, 109, 116–124. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, H.; Zhang, B.; Li, G.; Zhu, H.; Ren, Y.; Geng, H.; Yang, Y.; Liu, Q.; Li, C.C. Tuning the Kinetics of Zinc-Ion Insertion/Extraction in V2O5 by In Situ Polyaniline Intercalation Enables Improved Aqueous Zinc-Ion Storage Performance. Adv. Mater. 2020, 32, e2001113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, Y.; Bao, S.; Huang, J.; Zhang, L. A Novel Eco-Friendly Vanadium Precipitation Method by Hydrothermal Hydrogen Reduction Technology. Minerals 2017, 7, 182. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Q.; Zhang, Y.; Liu, T.; Huang, J.; Xue, N.; Shi, Q. Optimal Location of Vanadium in Muscovite and Its Geometrical and Electronic Properties by DFT Calculation. Minerals 2017, 7, 32. [Google Scholar] [CrossRef] [Green Version]
- Skyllas Kazacos, M.; Cao, L.; Kazacos, M.; Kausar, N.; Mousa, A. Vanadium Electrolyte Studies for the Vanadium Redox Battery-A Review. ChemSusChem 2016, 9, 1521–1543. [Google Scholar] [CrossRef]
- Thangarasu, R.; Kulathuraan, K.; Chang, J.H.; Subramani, S.; Chandar, N.R.; Balasundaram, O.N.; Mohanraj, K.; Shkir, M.; Ali, A.M. Influence of zirconium ions on the key characteristics of V2O5 nanorods and current–voltage features of the n-ZrxV2O5/p-Si photodetector. J Mater Sci. Mater Electron 2022, 33, 2932–2948. [Google Scholar] [CrossRef]
- Ravinder, G.; Sreelatha, C.J.; Ganesh, V.; Shkir, M.; Anis, M.; Rao, C.R. Thickness-dependent structural, spectral, linear, nonlinear and z-scan optical studies of V2O5 thin films prepared by a low-cost sol-gel spin coating technique. Mater. Res. Express 2019, 6, 096403. [Google Scholar] [CrossRef]
- Ubaidullah, M.; AlEnizi, A.M.; Shaikh, S.; Ghanem, M.A.; Mane, R.S. Waste PET plastic derived ZnO@NMC nanocomposite via MOF-5 construction for hydrogen and oxygen evolution reactions. J. King Saud Univ. Sci. 2020, 32, 2397–2405. [Google Scholar] [CrossRef]
- Gilligan, R.; Nikoloski, A.N. The extraction of vanadium from titanomagnetites and other sources. Miner. Eng. 2019, 146, 106106. [Google Scholar] [CrossRef]
- Peng, H. A literature review on leaching and recovery of vanadium. J. Environ. Chem. Eng. 2019, 7, 103313. [Google Scholar] [CrossRef]
- Xiong, P.; Zhang, Y.; Bao, S.; Huang, J. Precipitation of vanadium using ammonium salt in alkaline and acidic media and the effect of sodium and phosphorus. Hydrometallurgy 2018, 180, 113–120. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, Y.; Song, S. Improved extraction of vanadium from a Chinese vanadium-bearing stone coal using a modified roast-leach process. Asia-Pacific J. Chem. Eng. 2009, 5, 778–784. [Google Scholar] [CrossRef]
- Zhang, Y.M. Extracting Vanadium from Stone Coal, 1st ed.; Science Press: Beijing, China, 2014; pp. 185–197. [Google Scholar]
- Kang, Q.; Zhang, Y.; Bao, S. An environmentally friendly hydrothermal method of vanadium precipitation with the application of oxalic acid. Hydrometallurgy 2019, 185, 125–132. [Google Scholar] [CrossRef]
- F, Z.Y.; Zhang, Y.M.; Liu, T.; Huang, J.; Yang, X.K. Impact of Impurity Ions on Vanadium Precipitation Process of Stripping Solution from Vanadium Extraction of Stone Coal. Chinese J. Rare Metals 2016, 40, 1060–1065. [Google Scholar]
- Wang, X.; Wang, H.; Gao, D.; Chen, B.; Meng, Y.; Wang, M. A clean technology to separate and recover vanadium and chromium from chromate solutions. Hydrometallurgy 2018, 177, 94–99. [Google Scholar] [CrossRef]
- Chithaiah, P.; Vijaya kumar, G.; Nagabhushana, G.P.; Nagaraju, G.; Chandrappa, G.T. Synthesis of single crystalline (NH4)2V6O16·1.5H2O nest-like structures. Phys. E Low-Dimens. Syst. Nanostructures 2014, 59, 218–222. [Google Scholar] [CrossRef] [Green Version]
- Elias, A.; Abdellah, Z.N.; Villemin, D.; Didi, M.A. Effect of microwave irradiations on the sorption of alkylimidazolium salts on bentonite. Chem. Pap. 2016, 71, 59–65. [Google Scholar] [CrossRef]
- Guo, J.K. Preparation of Ammonium Polyvanadate Using High Concentration of Vanadium Solution Containing Sodium. Iron Steel Vanadium Titanium. 2017, 35, 13–20. [Google Scholar]
- Zhu, L.; Li, W.; Xie, L.; Yang, Q.; Cao, X. Rod-like NaV3O8 as cathode materials with high capacity and stability for sodium storage. Chem. Eng. J. 2019, 372, 1056–1065. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.H.; Park, C.; Kim, H.S.; Park, J.H.; Chung, K.I.; Ahn, H. Controlling Vanadate Nanofiber Interlayer via Intercalation with Conducting Polymers: Cathode Material Design for Rechargeable Aqueous Zinc Ion Batteries. Adv. Funct. Mater. 2021, 31, 2100005. [Google Scholar] [CrossRef]
- Wang, H.; Ren, Y.; Wang, W.; Huang, X.; Huang, K.; Wang, Y.; Liu, S. NH4V3O8 nanorod as a high performance cathode material for rechargeable Li-ion batteries. J. Power Sources 2012, 199, 315–321. [Google Scholar] [CrossRef]
- Harreld, J.; Wong, H.; Dave, B.; Dunn, B.; Nazar, L. Synthesis and properties of polypyrrole–vanadium oxide hybrid aerogels. J. Non-Crystalline Solids 1998, 225, 319–324. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, J.; Wang, Z.; Zhang, Z.; Ding, Z. The phase transition of W-doped VO2 nanoparticles synthesized by an improved thermolysis method. J. Nanosci. Nanotechnol. 2013, 13, 1543–1548. [Google Scholar] [CrossRef]
- Ksiksi, R.; Abdelkafi Koubaa, Z.; Mlayah Bellalouna, S.; Aissaoui, D.; Marrakchi, N.; Srairi-Abid, N.; Zid, M.F.; Graia, M. Synthesis, structural characterization and antitumoral activity of (NH4)4Li2V10O28∙10H2O compound. J. Mol. Struct. 2020, 1229, 129492. [Google Scholar] [CrossRef]
- Gupta, S.; Singh, K. Structural and optical properties of melt quenched barium doped bismuth vanadate. Phys. B Condens. Matter 2013, 431, 89–93. [Google Scholar] [CrossRef]
- Lian, Z.; Zhang, J.; Gu, Y.; Wang, T.; Lou, T. Synthesis and crystal structures of two inorganic–organic hybrid vanadium selenites with layered structures: (DABCOH2) [(VO2) (SeO3)]2 · 1.25H2O and (pipeH2) [(VO)2(C2O4)(SeO3)]. J. Mol. Struct. 2009, 919, 122–127. [Google Scholar] [CrossRef]
- Doadrio, A.L.; Sotelo, J.; Fernández Ruano, A. Synthesis and characterization of oxovanadium (IV) dithiocarbamates with pyridine. Quim. Nova 2002, 25, 525–528. [Google Scholar] [CrossRef]
- Jin, A.; Chen, W.; Zhu, Q.; Yang, Y.; Volkov, V.L.; Zakharova, G.S. Electrical and electrochemical characterization of poly (ethylene oxide)/V2O5 xerogel electrochromic films. Solid State Ion. 2008, 179, 1256–1262. [Google Scholar] [CrossRef]
- Rani, B.J.; Ravi, G.; Yuvakkumar, R. Solvothermal optimization of V2O5 nanostructures for electrochemical energy production. AIP Conf. Proc. 2020, 030619. [Google Scholar] [CrossRef]
- Martynova, S.A.; Plyusnin, P.E.; Asanova, T.I.; Asanov, I.P.; Pishchur, D.P.; Korenev, S.V.; Kosheev, S.V.; Floquet, S.; Cadot, E.; Yusenko, K.V. New Exothermal effects in the thermal decomposition of [IrCl6]2−-containing salts with [M(NH3)5Cl]2+ cations: [M(NH3)5Cl] [IrCl6] (M = Co, Cr, Ru, Rh, Ir) †‡. New J. Chem. 2018, 42, 1762–1770. [Google Scholar] [CrossRef] [Green Version]
- Novák, P.; Shklover, V.; Nesper, R. Magnesium Insertion in Vanadium Oxides: A Structural Study. Z. Für Phys. Chem. 1994, 185, 51–68. [Google Scholar] [CrossRef] [Green Version]
- Fuentes, G.; Hernández, Y.; Campos, Y.; López, N.; Rojas, M.L.; Peón, E.; Almirall, A.; Delgado, J.A. COMPOSITION INFLUENCE ON PROPERTIES OF ACRYLIC COMPOSITES LOADED WITH SYNTHETIC HYDROXYAPATITE. Lat Am. Appl. Res. 2008, 38, 105–112. [Google Scholar]
- Spahr, M.E.; Novák, P.; Scheifele, W.; Haas, O.; Nesper, R. Electrochemistry of Chemically Lithiated NaV3O8: A Positive Electrode Material for Use in Rechargeable Lithium-Ion Batteries. J. Electrochem. Soc. 1998, 145, 421–427. [Google Scholar] [CrossRef]
- Oka, Y.; Yao, T.; Sato, S.; Yamamoto, N. Hydrothermal Synthesis and Crystal Structure of Barium Hewettite: BaV6O16·nH2O. J. Solid State Chem. 1998, 140, 219–225. [Google Scholar] [CrossRef]
- Lin, B.Z.; Liu, S.X. Ammonium trivanadate (V), NH4V3O8. Acta Crystallogr. Sect. C Cryst. Struct. 1999, 55, 1961–1963. [Google Scholar] [CrossRef]
- Wang, J. Study on a Strengthened Process and Mechanism of Vanadium Leaching from Shale with Microwave Intervening. PhD Dissertation, Wuhan University of Science and Technology, Wuhan, China, 2018. Available online: https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CDFDLAST2019&filename=1019068807.nh (accessed on 19 January 2022).
- Wei, L.; Lian, R.; Wang, D.; Zhao, Y.; Yang, D.; Zhao, H.; Wang, Y.; Chen, G.; Wei, Y. Magnesium Ion Storage Properties in a Layered (NH4)2V6O16·1.5H2O Nanobelt Cathode Material Activated by Lattice Water. ACS Appl. Mater. Interfaces 2021, 13, 30625–30632. [Google Scholar] [CrossRef]
- Fu, Z.Y.; Zhang, Y.M.; Liu, T.; Huang, J.; Shi, Q.H.; Zhang, G.B. Vanadium Precipitation with Acidic Ammonium Salt of Stripping Solution from One-Step Vanadium Extraction of Stone Coal. Chin. J. Rare Met. 2015, 39, 462–467. [Google Scholar]

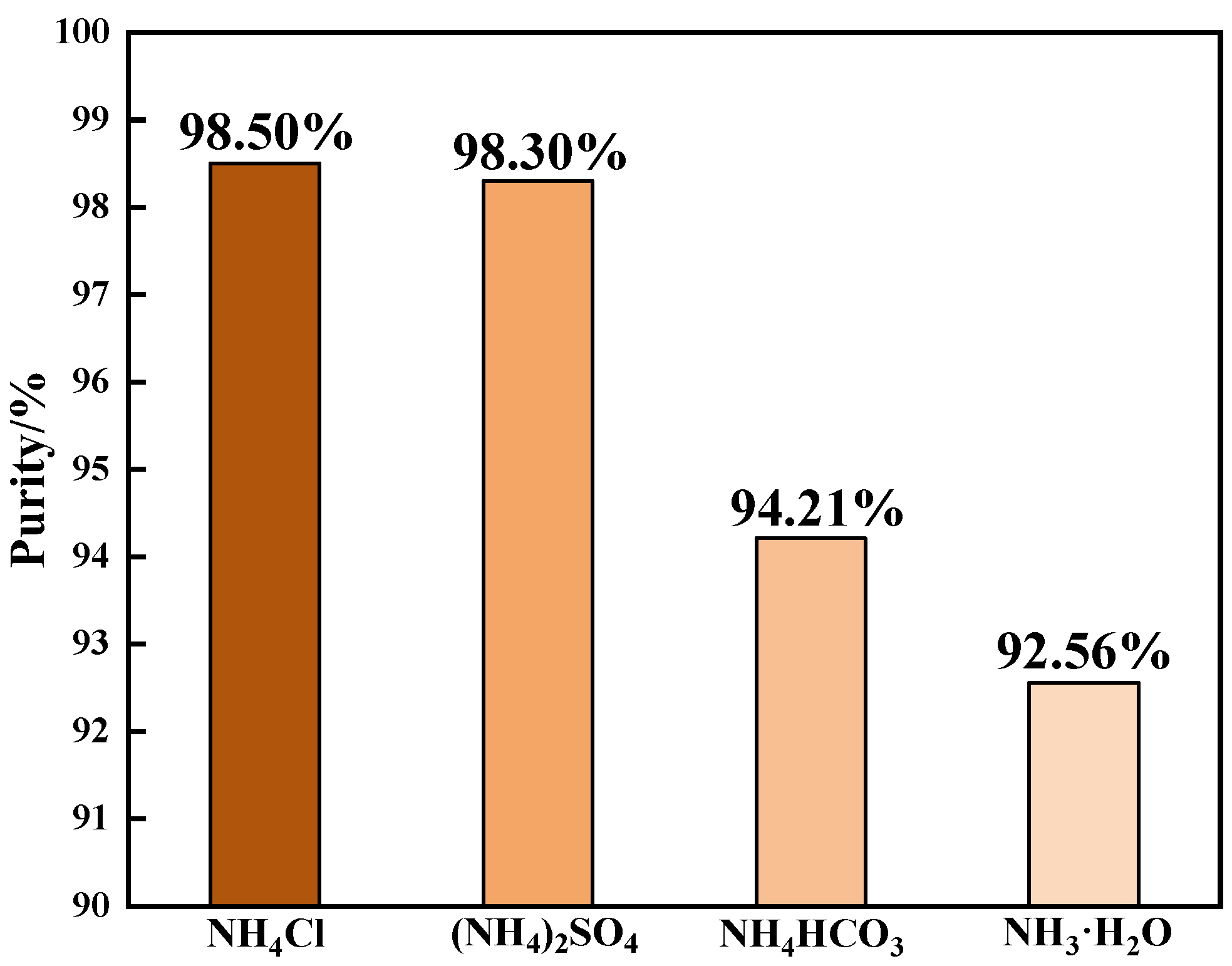


| Element | V | K | Ca | Na | Mg | Al | Si | P | As | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| Content | 42.27 | 0.37 | 0.44 | 0.42 | 0.08 | 8.80 | 0.26 | 0.48 | 0.37 | 0.12 |
| Item | V2O5 | Si | Fe | P | S | As | Na2O + K2O |
|---|---|---|---|---|---|---|---|
| V2O5 sample | 99.05 | <0.01 | 0.075 | 0.026 | 0.01 | <0.01 | 0.74 |
| Standard sample | ≥99.00 | ≤0.08 | ≤0.08 | ≤0.03 | ≤0.08 | ≤0.01 | ≤0.8 |
| Item | Precipitation by NH3·H2O | Precipitation by NH4Cl | Purification by NH4Cl |
|---|---|---|---|
| K | 9.9 | 10 | 2.0 |
| efficiency | 99.41% | 99.12% | 99.23% |
| purity | 98.35% | 98.04% | 99.05% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, G.; Huang, J.; Zhang, Y.; Hu, P. A Sustainable Technique to Prepare High-Purity Vanadium Pentoxide via Purification with Low Ammonium Consumption. Materials 2022, 15, 1945. https://doi.org/10.3390/ma15051945
Lin G, Huang J, Zhang Y, Hu P. A Sustainable Technique to Prepare High-Purity Vanadium Pentoxide via Purification with Low Ammonium Consumption. Materials. 2022; 15(5):1945. https://doi.org/10.3390/ma15051945
Chicago/Turabian StyleLin, Guoce, Jing Huang, Yimin Zhang, and Pengcheng Hu. 2022. "A Sustainable Technique to Prepare High-Purity Vanadium Pentoxide via Purification with Low Ammonium Consumption" Materials 15, no. 5: 1945. https://doi.org/10.3390/ma15051945
APA StyleLin, G., Huang, J., Zhang, Y., & Hu, P. (2022). A Sustainable Technique to Prepare High-Purity Vanadium Pentoxide via Purification with Low Ammonium Consumption. Materials, 15(5), 1945. https://doi.org/10.3390/ma15051945




