Effect of Phase Composition on Leaching Behavior and Mechanical Properties of Ceramics from Ferrochrome Slag and Tundish Slag
Abstract
:1. Introduction
2. Experimental
2.1. Raw Materials and Analysis
2.2. Formula Design
2.3. Experimental Procedure
2.4. Physical Properties and Element Leaching Rates
3. Results and Discussion
3.1. Typical Samples
3.1.1. Crystal Evolution during Sintering Process
3.1.2. Physical Properties and Cr/Mn Leaching Concentrations
3.2. FS and TS Series
3.2.1. Cr/Mn Leaching Rates and Mechanical Properties
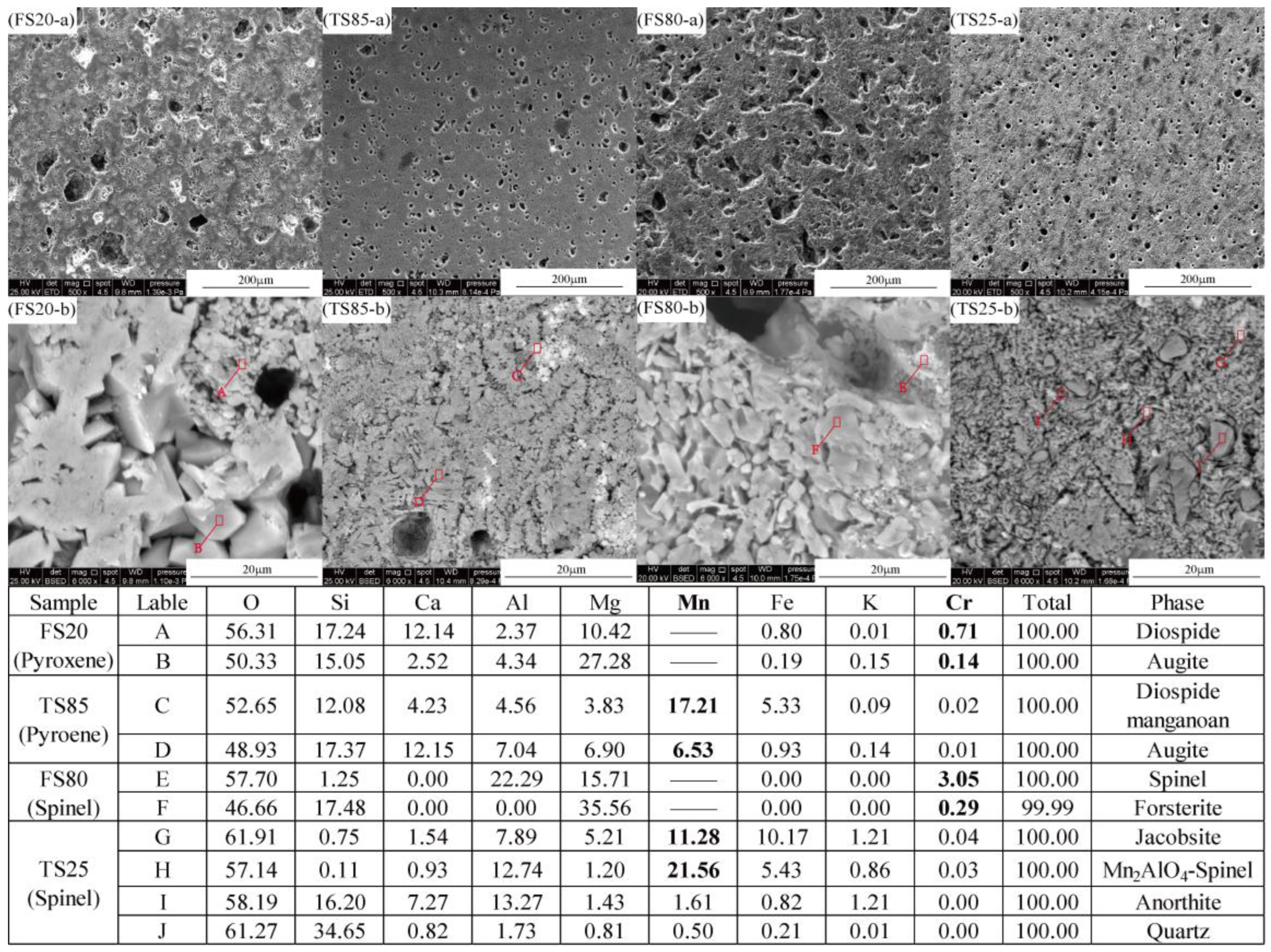
3.2.2. Comparison of Crystals Phases
3.2.3. Relationship between Crystals Containing Cr/ Mn and Its Retention Ability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fares, A.I.; Sohel, K.; Al-Jabri, K.; Al-Mamun, A. Characteristics of Ferrochrome Slag Aggregate and Its Uses as a Green Material in Concrete—A Review. Constr. Build. Mater. 2021, 294, 123552. [Google Scholar] [CrossRef]
- Kumar, P.H.; Srivastava, A.; Kumar, V.; Singh, V.K. Implementation of Industrial Waste Ferrochrome Slag in Conventional and Low Cement Castables: Effect of Calcined Alumina. J. Asian Ceram. Soc. 2014, 2, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Kurtulus, C.; Kurtulus, R.; Kavas, T. Foam Glass Derived from Ferrochrome Slag and Waste Container Glass: Synthesis and Extensive Characterizations. Ceram. Int. 2021, 47, 24997–25008. [Google Scholar] [CrossRef]
- Islam, M.Z.; Sohel, K.M.A.; Al-Jabri, K.; Al Harthy, A. Properties of Concrete with Ferrochrome Slag as a Fine Aggregate at Elevated Temperatures. Case Stud. Constr. Mater. 2021, 15, e00599. [Google Scholar] [CrossRef]
- Erdem, M.; Altundoğan, H.S.; Turan, M.D.; Tümen, F. Hexavalent Chromium Removal by Ferrochromium Slag. J. Hazard. Mater. 2005, 126, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Moshtaghioun, B.M.; Monshi, A. Hot Corrosion Mechanism of Tundish Plaster with Steel Slags in Continuous Casting. J. Mater. Sci. 2007, 42, 6720–6728. [Google Scholar] [CrossRef]
- Zhao, L.; Li, Y.; Zhang, L.; Cang, D. Effects of CaO and Fe2O3 on the Microstructure and Mechanical Properties of SiO2–CaO–MgO–Fe2O3 Ceramics from Steel Slag. ISIJ Int. 2016, 57, 4–22. [Google Scholar] [CrossRef] [Green Version]
- Pei, D.; Li, Y.; Hua, S.; Li, S.; Jiang, F.; Yao, J. In Situ XRD Study on Function Mechanism of Pyroxene and Anorthite in Si-Ca Ceramics from Ferronickel Slag. Mater. Lett. 2021, 305, 130839. [Google Scholar] [CrossRef]
- Pei, D.; Li, Y.; Cang, D. In Situ XRD Study on Sintering Mechanism of SiO2-Al2O3-CaO-MgO Ceramics from Red Mud. Mater. Lett. 2019, 240, 229–232. [Google Scholar] [CrossRef]
- Jiang, F.; Li, Y.; Zhao, L.; Cang, D. Novel Ceramics Prepared from Inferior Clay Rich in CaO and Fe2O3: Properties, Crystalline Phases Evolution and Densification Process. Appl. Clay Sci. 2017, 143, 199–204. [Google Scholar] [CrossRef]
- Pei, D.; Li, Y.; Cang, D. Na+-Solidification Behavior of SiO2-Al2O3-CaO-MgO (10 wt.%) Ceramics Prepared from Red Mud. Ceram. Int. 2017, 43, 16936–16942. [Google Scholar] [CrossRef]
- Burja, J.; Tehovnik, F.; Medved, J.; Godec, M.; Knap, M. Chromite Spinel Formation in Steelmaking Slags. Mater. Tehnol. 2014, 48, 753–756. [Google Scholar]
- Barella, S.; Gruttadauria, A.; Magni, F.; Mapelli, C.; Mombelli, D. Survey about Safe and Reliable Use of EAF Slag. ISIJ Int. 2012, 52, 2295–2302. [Google Scholar] [CrossRef] [Green Version]
- Van Weert, G.; Boering, M. Selective Pressure Leaching of Zinc and Manganese from Natural and Man-Made Spinels Using Nitric Acid. Hydrometallurgy 1995, 39, 201–213. [Google Scholar] [CrossRef]
- Stahleisen, V.; Eisenhüttenleute, V.D. Slag Atlas; Verlag Stahleisen: Düsseldorf, Germany, 1995. [Google Scholar]






| Raw Materials | CaO | SiO2 | Al2O3 | MgO | Fe2O3 | MnO | TiO2 | K2O | Na2O | Cr2O3 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ferrochrome slag | 3.48 | 32.32 | 19.19 | 38.32 | 1.48 | 0.00 | 0.54 | 0.15 | 0.16 | 3.88 | 99.53 |
| Tundish slag | 16.08 | 42.79 | 8.78 | 7.55 | 2.32 | 18.69 | 1.50 | 1.39 | 0.44 | 0.00 | 99.54 |
| Sample | Corresponding Slag | Clay | Dolomite | Quartz | Feldspar |
|---|---|---|---|---|---|
| FS20 | 20 | 40 | 30 | 5 | 5 |
| FS40 | 40 | 30 | 20 | 5 | 5 |
| FS60 | 60 | 20 | 10 | 5 | 5 |
| FS80 | 80 | 10 | 0 | 5 | 5 |
| TS25 | 25 | 50 | — | — | 25 |
| TS40 | 40 | 40 | — | — | 20 |
| TS55 | 55 | 30 | — | — | 15 |
| TS70 | 70 | 20 | — | — | 10 |
| TS85 | 85 | 10 | — | — | 5 |
| Properties | Pyroxene Ceramics | Spinel Ceramics | ||
|---|---|---|---|---|
| FS20 | TS85 | FS80 | TS25 | |
| Bending strength (highest) | 114.52 MPa | 124.61 MPa | 77.97 MPa | 95.14 MPa |
| Water absorption (lowest) | 0.56% | 0.12% | 2.11% | 0.21% |
| Sintering temperature | 1240 °C | 1100–1120 °C | 1240–1270 °C | 1080–1110 °C |
| Cr/Mn leaching concentration | 0.836 mg/L (Cr) | 0.121 mg/L (Mn) | 1.148 mg/L (Cr) | 0.016 mg/L (Mn) |
| Cr/Mn leaching rate | 0.15% (Cr) | 0.98% (Mn) | 0.05% (Cr) | 0.43% (Mn) |
| Sample | FS | Sample Sintered at Optimal Temperature | Limitations | |||
|---|---|---|---|---|---|---|
| FS80 | FS60 | FS40 | FS20 | |||
| Cr | 0.645 | 1.148 | 1.028 | 0.911 | 0.836 | 1.5 |
| Sample | TS | Sample Sintered at Optimal Temperature | Limitations | ||||
|---|---|---|---|---|---|---|---|
| TS25 | TS40 | TS55 | TS70 | TS85 | |||
| Mn | 0.164 | 0.016 | 0.034 | 0.059 | 0.082 | 0.121 | 2.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, D.; Li, Y.; Duan, X.; Cang, D.; Yang, Y.; McLean, A.; Guo, Z.; Xu, C. Effect of Phase Composition on Leaching Behavior and Mechanical Properties of Ceramics from Ferrochrome Slag and Tundish Slag. Materials 2022, 15, 1993. https://doi.org/10.3390/ma15061993
Pei D, Li Y, Duan X, Cang D, Yang Y, McLean A, Guo Z, Xu C. Effect of Phase Composition on Leaching Behavior and Mechanical Properties of Ceramics from Ferrochrome Slag and Tundish Slag. Materials. 2022; 15(6):1993. https://doi.org/10.3390/ma15061993
Chicago/Turabian StylePei, Dejian, Yu Li, Xiangjie Duan, Daqiang Cang, Yindong Yang, Alex McLean, Zhancheng Guo, and Chuanhua Xu. 2022. "Effect of Phase Composition on Leaching Behavior and Mechanical Properties of Ceramics from Ferrochrome Slag and Tundish Slag" Materials 15, no. 6: 1993. https://doi.org/10.3390/ma15061993
APA StylePei, D., Li, Y., Duan, X., Cang, D., Yang, Y., McLean, A., Guo, Z., & Xu, C. (2022). Effect of Phase Composition on Leaching Behavior and Mechanical Properties of Ceramics from Ferrochrome Slag and Tundish Slag. Materials, 15(6), 1993. https://doi.org/10.3390/ma15061993







