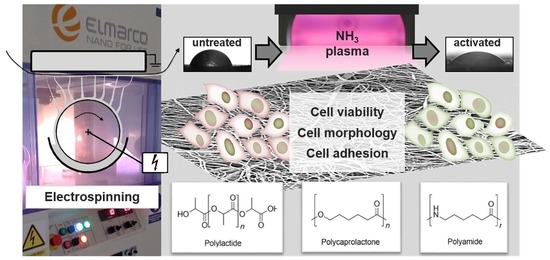
Accelerated Endothelialization of Nanofibrous Scaffolds for Biomimetic Cardiovascular Implants
Abstract
:1. Introduction
2. Materials and Methods
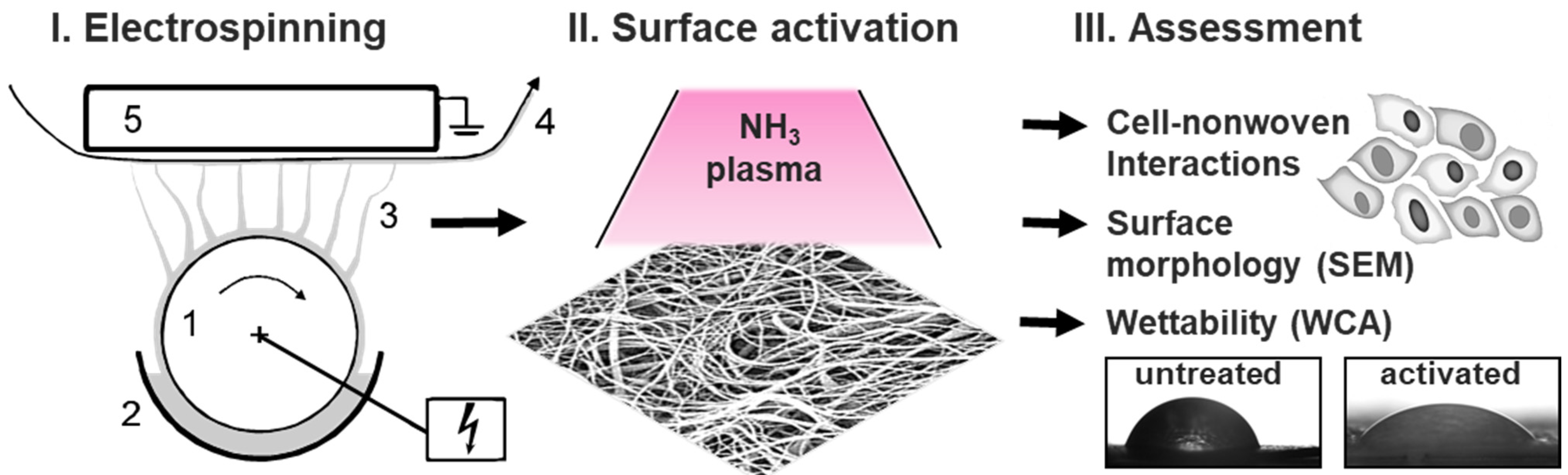
2.1. Fabrication of Polymeric Nanofiber Nonwovens by Electrospinning
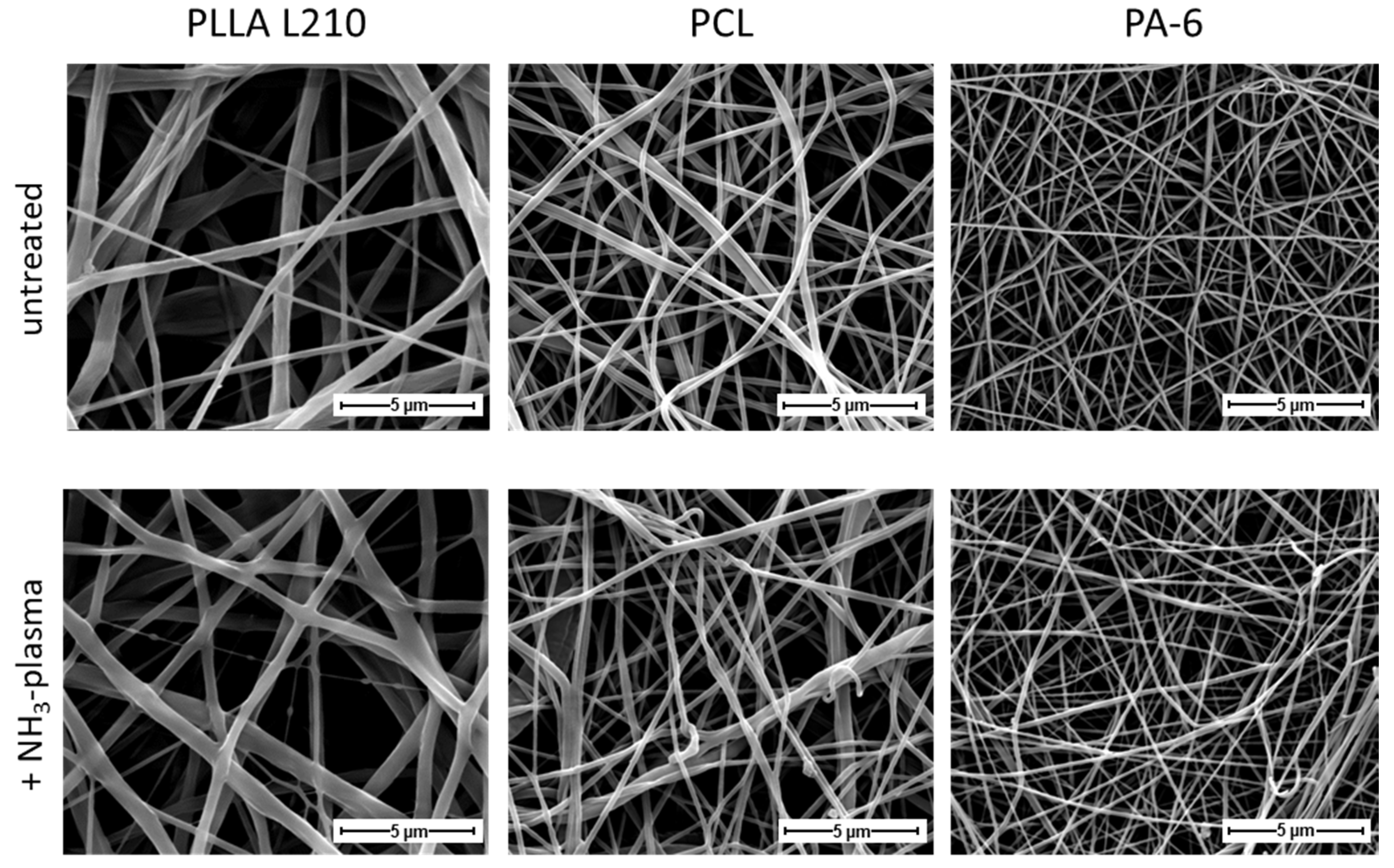
2.2. Nonwoven Characterization by Scanning Electron Microscopy
2.3. Surface Plasma Modification of Nonwovens
2.4. Water Contact Angle and Surface Free Energy
2.5. Cell Culture
2.6. Cell Viability Assay
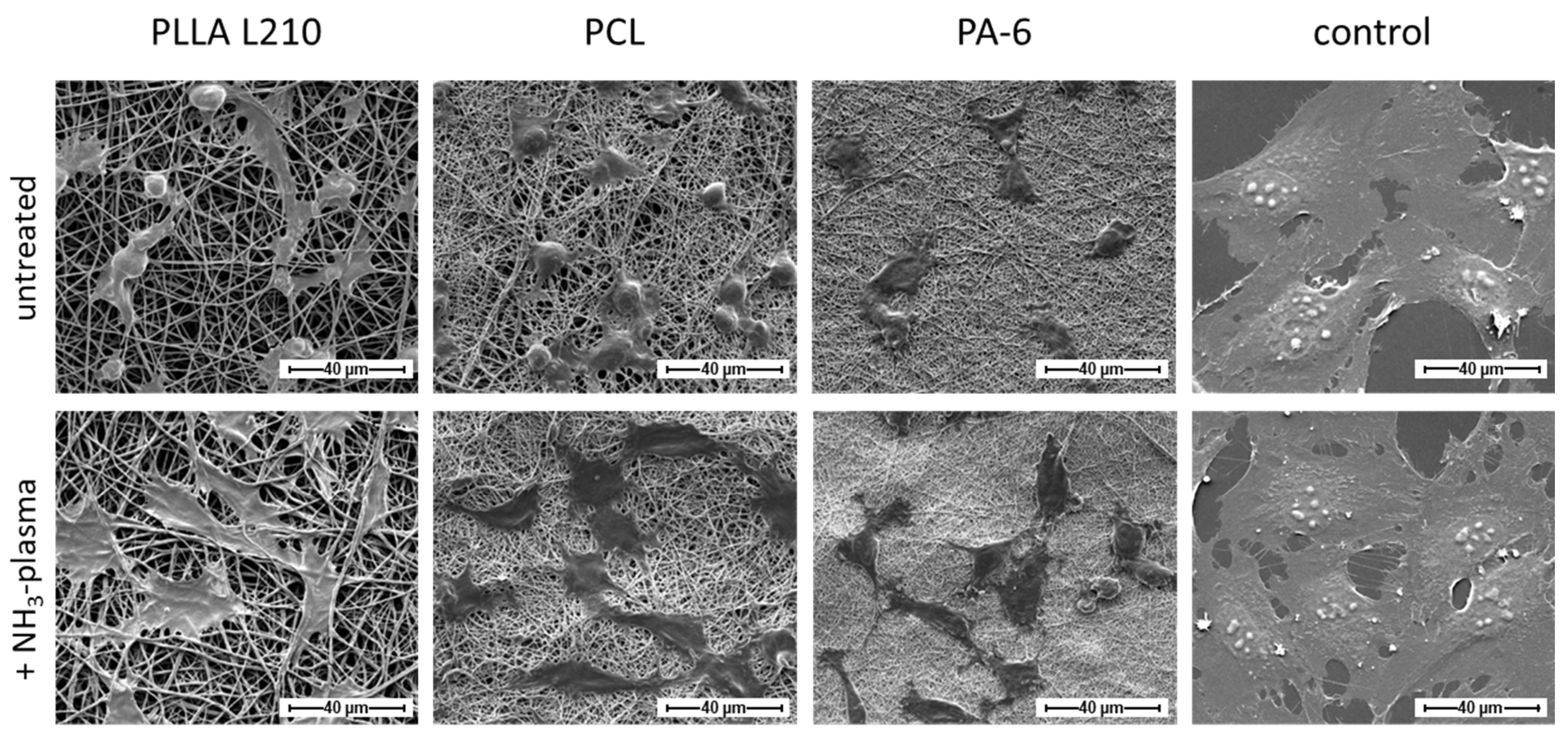
2.7. Cell Morphology Analysis by Scanning Electron Microscopy
2.8. Endothelialization Analysis by Cell Spreading and Cell Shape Index
2.9. Immunofluorescence of PECAM-1 (CD31) and Actin Cytoskeleton
2.10. Statistical Analysis
3. Results
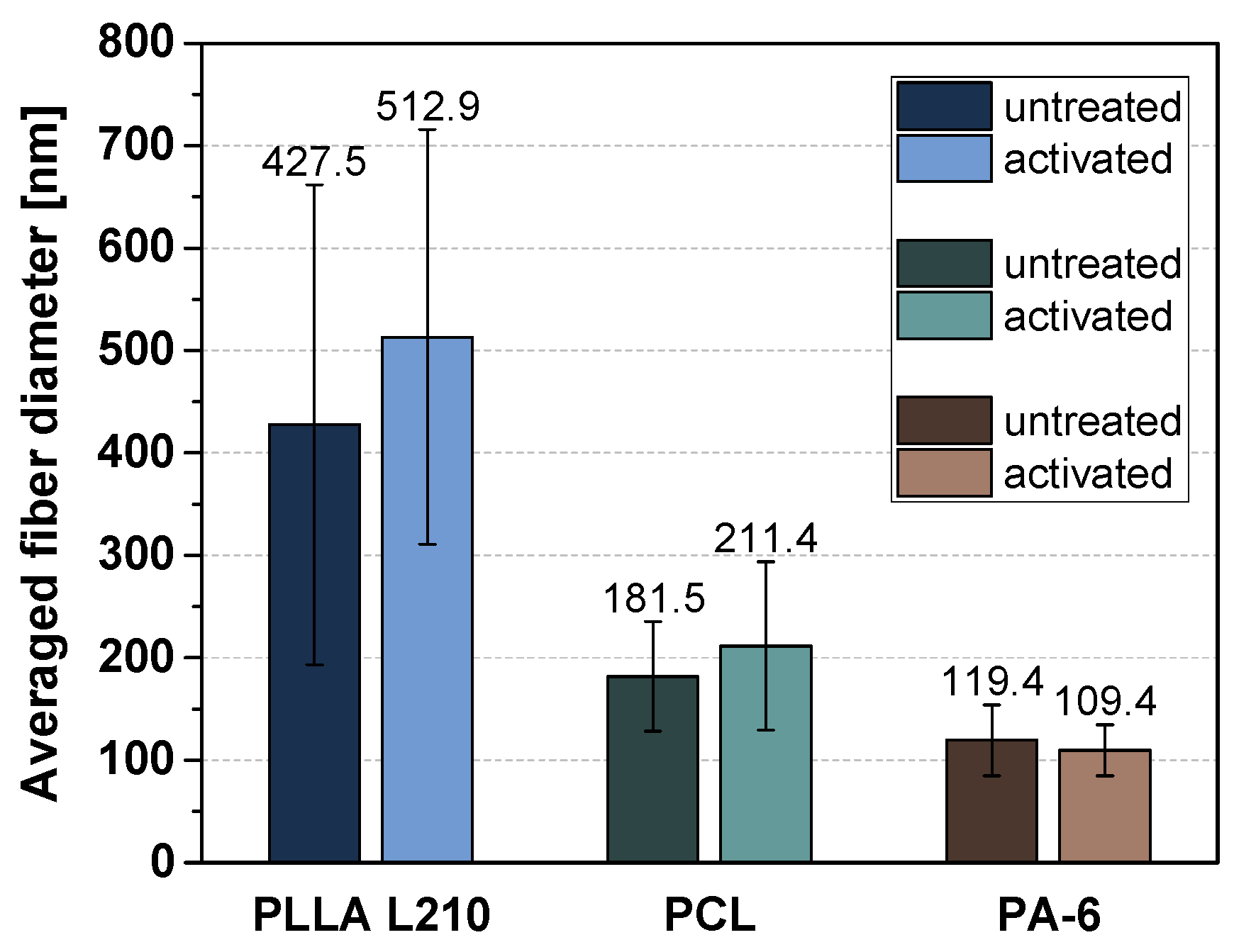
3.1. Morphology of Polymeric Nonwovens
3.2. Wettability Analysis and Surface Free Energy of Polymeric Nonwovens
3.3. Biocompatibility of Polymeric Nonwovens Assessed by Cell Viability
3.4. Influence of Nanofiber Scaffolds on Cell Attachment and Morphology
3.5. Quantification of Endothelialization
3.6. Endothelial Actin Cytoskeleton Formation on Polymeric Nonwovens
3.7. Expression of Endothelial Cell-Specific PECAM-1 Marker
4. Discussion
4.1. Surface Characterization and Chemical Modification by Plasma Treatment
4.2. Evaluation of Cell Physiology and Cell Phenotype Maintenance on Nanofibrous Polymer Scaffolds
4.3. Plasma Functionalization of Nanofibrous Polymer Scaffolds towards Improved Biocompatibility and Cellular Growth Patterns
4.4. Limitations and Potential of Plasma-Activated Nonwovens for Cardiovascular Applications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mensah, G.A.; Roth, G.A.; Fuster, V. The Global Burden of Cardiovascular Diseases and Risk Factors: 2020 and Beyond. J. Am. Coll. Cardiol. 2019, 74, 2529–2532. [Google Scholar] [CrossRef] [PubMed]
- Fioretta, E.S.; Motta, S.E.; Lintas, V.; Loerakker, S.; Parker, K.K.; Baaijens, F.P.T.; Falk, V.; Hoerstrup, S.P.; Emmert, M.Y. Next-generation tissue-engineered heart valves with repair, remodelling and regeneration capacity. Nat. Rev. Cardiol. 2021, 18, 92–116. [Google Scholar] [CrossRef] [PubMed]
- Roshandel, M.; Dorkoosh, F. Cardiac tissue engineering, biomaterial scaffolds, and their fabrication techniques. Polym. Adv. Technol. 2021, 32, 2290–2305. [Google Scholar] [CrossRef]
- Finn, A.V.; Joner, M.; Nakazawa, G.; Kolodgie, F.; Newell, J.; John, M.C.; Gold, H.K.; Virmani, R. Pathological correlates of late drug-eluting stent thrombosis: Strut coverage as a marker of endothelialization. Circulation 2007, 115, 2435–2441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wessely, R. New drug-eluting stent concepts. Nat. Rev. Cardiol. 2010, 7, 194–203. [Google Scholar] [CrossRef]
- Finn, A.V.; Nakazawa, G.; Joner, M.; Kolodgie, F.D.; Mont, E.K.; Gold, H.K.; Virmani, R. Vascular responses to drug eluting stents: Importance of delayed healing. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1500–1510. [Google Scholar] [CrossRef] [PubMed]
- Dangas, G.D.; Claessen, B.E.; Caixeta, A.; Sanidas, E.A.; Mintz, G.S.; Mehran, R. In-stent restenosis in the drug-eluting stent era. J. Am. Coll. Cardiol. 2010, 56, 1897–1907. [Google Scholar] [CrossRef] [Green Version]
- Hytönen, J.P.; Taavitsainen, J.; Tarvainen, S.; Ylä-Herttuala, S. Biodegradable coronary scaffolds: Their future and clinical and technological challenges. Cardiovasc. Res. 2018, 114, 1063–1072. [Google Scholar] [CrossRef]
- Kilic, I.D.; Fabris, E.; Serdoz, R.; Caiazzo, G.; Foin, N.; Abou-Sherif, S.; Di Mario, C. Coronary covered stents. EuroIntervention 2016, 12, 1288–1295. [Google Scholar] [CrossRef] [Green Version]
- Montoya, Y.; Cardenas, J.; Bustamante, J.; Valencia, R. Effect of sequential electrospinning and co-electrospinning on morphological and fluid mechanical wall properties of polycaprolactone and bovine gelatin scaffolds, for potential use in small diameter vascular grafts. Biomater. Res. 2021, 25, 38. [Google Scholar] [CrossRef]
- Capulli, A.K.; MacQueen, L.A.; Sheehy, S.P.; Parker, K.K. Fibrous scaffolds for building hearts and heart parts. Adv. Drug Deliv. Rev. 2016, 96, 83–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpentier, A. Hemodynamic factors affecting the fate of valvular bioprosthesis. Circulation 2010, 121, 2083–2084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoerstrup, S.P.; Sodian, R.; Daebritz, S.; Wang, J.; Bacha, E.A.; Martin, D.P.; Moran, A.M.; Guleserian, K.J.; Sperling, J.S.; Kaushal, S.; et al. Functional living trileaflet heart valves grown in vitro. Circulation 2000, 102, III44-9. [Google Scholar] [CrossRef] [PubMed]
- Stock, A.M.; Robinson, V.L.; Goudreau, P.N. Two-component signal transduction. Annu. Rev. Biochem. 2000, 69, 183–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davoudi, P.; Assadpour, S.; Derakhshan, M.A.; Ai, J.; Solouk, A.; Ghanbari, H. Biomimetic modification of polyurethane-based nanofibrous vascular grafts: A promising approach towards stable endothelial lining. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 80, 213–221. [Google Scholar] [CrossRef]
- Emmert, S.; Pantermehl, S.; Foth, A.; Waletzko-Hellwig, J.; Hellwig, G.; Bader, R.; Illner, S.; Grabow, N.; Bekeschus, S.; Weltmann, K.-D.; et al. Combining Biocompatible and Biodegradable Scaffolds and Cold Atmospheric Plasma for Chronic Wound Regeneration. Int. J. Mol. Sci. 2021, 22, 9199. [Google Scholar] [CrossRef]
- Ramakrishna, S. An introduction to Electrospinning and Nanofibers; World Scientific: Singapore; Hackensack, NJ, USA, 2005; ISBN 9789812567611. [Google Scholar]
- Xu, C.; Inai, R.; Kotaki, M.; Ramakrishna, S. Electrospun nanofiber fabrication as synthetic extracellular matrix and its potential for vascular tissue engineering. Tissue Eng. 2004, 10, 1160–1168. [Google Scholar] [CrossRef]
- Khorshidi, S.; Solouk, A.; Mirzadeh, H.; Mazinani, S.; Lagaron, J.M.; Sharifi, S.; Ramakrishna, S. A review of key challenges of electrospun scaffolds for tissue-engineering applications. J. Tissue Eng. Regen. Med. 2016, 10, 715–738. [Google Scholar] [CrossRef]
- Murugan, R.; Ramakrishna, S. Nano-featured scaffolds for tissue engineering: A review of spinning methodologies. Tissue Eng. 2006, 12, 435–447. [Google Scholar] [CrossRef]
- Singhvi, R.; Stephanopoulos, G.; Wang, D.I. Effects of substratum morphology on cell physiology. Biotechnol. Bioeng. 1994, 43, 764–771. [Google Scholar] [CrossRef]
- Hashi, C.K.; Zhu, Y.; Yang, G.-Y.; Young, W.L.; Hsiao, B.S.; Wang, K.; Chu, B.; Li, S. Antithrombogenic property of bone marrow mesenchymal stem cells in nanofibrous vascular grafts. Proc. Natl. Acad. Sci. USA 2007, 104, 11915–11920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnell, E.; Klinkhammer, K.; Balzer, S.; Brook, G.; Klee, D.; Dalton, P.; Mey, J. Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-epsilon-caprolactone and a collagen/poly-epsilon-caprolactone blend. Biomaterials 2007, 28, 3012–3025. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Hussain, M.; Mao, J.J. Continuing differentiation of human mesenchymal stem cells and induced chondrogenic and osteogenic lineages in electrospun PLGA nanofiber scaffold. Biomaterials 2007, 28, 316–325. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.; Feng, Y.; Guo, J.; Wang, H.; Li, Q.; Yang, J.; Hao, X.; Lv, J.; Ma, N.; Li, W. Surface modification and endothelialization of biomaterials as potential scaffolds for vascular tissue engineering applications. Chem. Soc. Rev. 2015, 44, 5680–5742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badami, A.S.; Kreke, M.R.; Thompson, M.S.; Riffle, J.S.; Goldstein, A.S. Effect of fiber diameter on spreading, proliferation, and differentiation of osteoblastic cells on electrospun poly(lactic acid) substrates. Biomaterials 2006, 27, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Anselme, K.; Ploux, L.; Ponche, A. Cell/Material Interfaces: Influence of Surface Chemistry and Surface Topography on Cell Adhesion. J. Adhes. Sci. Technol. 2010, 24, 831–852. [Google Scholar] [CrossRef]
- Matschegewski, C.; Staehlke, S.; Loeffler, R.; Lange, R.; Chai, F.; Kern, D.P.; Beck, U.; Nebe, B.J. Cell architecture–cell function dependencies on titanium arrays with regular geometry. Biomaterials 2010, 31, 5729–5740. [Google Scholar] [CrossRef]
- Xu, H.; Nguyen, K.T.; Brilakis, E.S.; Yang, J.; Fuh, E.; Banerjee, S. Enhanced endothelialization of a new stent polymer through surface enhancement and incorporation of growth factor-delivering microparticles. J. Cardiovasc. Transl. Res. 2012, 5, 519–527. [Google Scholar] [CrossRef]
- Chung, T.-W.; Lu, Y.-F.; Wang, H.-Y.; Chen, W.-P.; Wang, S.-S.; Lin, Y.-S.; Chu, S.-H. Growth of human endothelial cells on different concentrations of Gly-Arg-Gly-Asp grafted chitosan surface. Artif. Organs 2003, 27, 155–161. [Google Scholar] [CrossRef]
- Tabares, F.L.; Junkar, I. Cold Plasma Systems and their Application in Surface Treatments for Medicine. Molecules 2021, 26, 1903. [Google Scholar] [CrossRef]
- Sultana, A.; Zare, M.; Luo, H.; Ramakrishna, S. Surface Engineering Strategies to Enhance the In Situ Performance of Medical Devices Including Atomic Scale Engineering. Int. J. Mol. Sci. 2021, 22, 11788. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.-S.; Choi, S.-H.; Choi, E.H.; Kim, K.-M.; Chu, P.K. Enhanced Osteogenic Differentiation of Human Mesenchymal Stem Cells on Amine-Functionalized Titanium Using Humidified Ammonia Supplied Nonthermal Atmospheric Pressure Plasma. Int. J. Mol. Sci. 2020, 21, 6085. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-W.; Wu, J.-Y.; Liu, C.-T.; Liao, G.-C.; Huang, H.-Y.; Hsu, R.-Q.; Chiang, M.-H.; Wu, J.-S. Fast incorporation of primary amine group into polylactide surface for improving C₂C₁₂ cell proliferation using nitrogen-based atmospheric-pressure plasma jets. J. Biomed. Mater. Res. A 2014, 102, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Ehtesabi, H.; Massah, F. Improvement of hydrophilicity and cell attachment of polycaprolactone scaffolds using green synthesized carbon dots. Mater. Today Sustain. 2021, 13, 100075. [Google Scholar] [CrossRef]
- Wang, W.; Caetano, G.; Ambler, W.S.; Blaker, J.J.; Frade, M.A.; Mandal, P.; Diver, C.; Bártolo, P. Enhancing the Hydrophilicity and Cell Attachment of 3D Printed PCL/Graphene Scaffolds for Bone Tissue Engineering. Materials 2016, 9, 992. [Google Scholar] [CrossRef]
- Cho, S.J.; Jung, S.M.; Kang, M.; Shin, H.S.; Youk, J.H. Preparation of hydrophilic PCL nanofiber scaffolds via electrospinning of PCL/PVP-b-PCL block copolymers for enhanced cell biocompatibility. Polymer 2015, 69, 95–102. [Google Scholar] [CrossRef]
- Baek, H.S.; Park, Y.H.; Ki, C.S.; Park, J.-C.; Rah, D.K. Enhanced chondrogenic responses of articular chondrocytes onto porous silk fibroin scaffolds treated with microwave-induced argon plasma. Surf. Coat. Technol. 2008, 202, 5794–5797. [Google Scholar] [CrossRef]
- Park, H.; Lee, K.Y.; Lee, S.J.; Park, K.E.; Park, W.H. Plasma-treated poly(lactic-co-glycolic acid) nanofibers for tissue engineering. Macromol. Res. 2007, 15, 238–243. [Google Scholar] [CrossRef]
- Martins, A.; Pinho, E.D.; Faria, S.; Pashkuleva, I.; Marques, A.P.; Reis, R.L.; Neves, N.M. Surface modification of electrospun polycaprolactone nanofiber meshes by plasma treatment to enhance biological performance. Small 2009, 5, 1195–1206. [Google Scholar] [CrossRef] [Green Version]
- Prabhakaran, M.P.; Venugopal, J.; Chan, C.K.; Ramakrishna, S. Surface modified electrospun nanofibrous scaffolds for nerve tissue engineering. Nanotechnology 2008, 19, 455102. [Google Scholar] [CrossRef]
- Rudolph, A.; Teske, M.; Illner, S.; Kiefel, V.; Sternberg, K.; Grabow, N.; Wree, A.; Hovakimyan, M. Surface Modification of Biodegradable Polymers towards Better Biocompatibility and Lower Thrombogenicity. PLoS ONE 2015, 10, e0142075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, H.S.; Kim, T.G.; Park, T.G. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv. Drug Deliv. Rev. 2009, 61, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Barry, J.J.A.; Silva, M.M.C.G.; Shakesheff, K.M.; Howdle, S.M.; Alexander, M.R. Using Plasma Deposits to Promote Cell Population of the Porous Interior of Three-Dimensional Poly(D,L-Lactic Acid) Tissue-Engineering Scaffolds. Adv. Funct. Mater. 2005, 15, 1134–1140. [Google Scholar] [CrossRef]
- Cheng, Q.; Lee, B.L.-P.; Komvopoulos, K.; Yan, Z.; Li, S. Plasma surface chemical treatment of electrospun poly(L-lactide) microfibrous scaffolds for enhanced cell adhesion, growth, and infiltration. Tissue Eng. Part A 2013, 19, 1188–1198. [Google Scholar] [CrossRef] [Green Version]
- Park, K.; Ju, Y.M.; Son, J.S.; Ahn, K.-D.; Han, D.K. Surface modification of biodegradable electrospun nanofiber scaffolds and their interaction with fibroblasts. J. Biomater. Sci. Polym. Ed. 2007, 18, 369–382. [Google Scholar] [CrossRef]
- Gugala, Z.; Gogolewski, S. Attachment, growth, and activity of rat osteoblasts on polylactide membranes treated with various low-temperature radiofrequency plasmas. J. Biomed. Mater. Res. A 2006, 76, 288–299. [Google Scholar] [CrossRef]
- Slepička, P.; Malá, Z.; Rimpelová, S.; Slepičková Kasálková, N.; Švorčík, V. Plasma treatment of the surface of poly(hydroxybutyrate) foil and non-woven fabric and assessment of the biological properties. React. Funct. Polym. 2015, 95, 71–79. [Google Scholar] [CrossRef]
- Wulf, K.; Teske, M.; Löbler, M.; Luderer, F.; Schmitz, K.-P.; Sternberg, K. Surface functionalization of poly(ε-caprolactone) improves its biocompatibility as scaffold material for bioartificial vessel prostheses. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 98, 89–100. [Google Scholar] [CrossRef]
- Guex, A.G.; Frobert, A.; Valentin, J.; Fortunato, G.; Hegemann, D.; Cook, S.; Carrel, T.P.; Tevaearai, H.T.; Giraud, M.N. Plasma-functionalized electrospun matrix for biograft development and cardiac function stabilization. Acta Biomater. 2014, 10, 2996–3006. [Google Scholar] [CrossRef] [Green Version]
- Teske, M.; Sternberg, K. Surface functionalization of poly(ε-caprolactone) and poly(3-hydroxybutyrate) with VEGF. BioNanoMaterials 2017, 18. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Malek, A.M.; Izumo, S. Mechanism of endothelial cell shape change and cytoskeletal remodeling in response to fluid shear stress. J. Cell Sci. 1996, 109 Pt 4, 713–726. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Lee, J.; Choi, S.-O.; Kim, J. Interaction of Surface Energy Components between Solid and Liquid on Wettability, and Its Application to Textile Anti-Wetting Finish. Polymers 2019, 11, 498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, M.; Ramos, T.; Wieringa, P.; van Blitterswijk, C.; de Boer, J.; Moroni, L. Geometric constraints of endothelial cell migration on electrospun fibres. Sci. Rep. 2018, 8, 6386. [Google Scholar] [CrossRef]
- Del Gaudio, C.; Bianco, A.; Folin, M.; Baiguera, S.; Grigioni, M. Structural characterization and cell response evaluation of electrospun PCL membranes: Micrometric versus submicrometric fibers. J. Biomed. Mater. Res. A 2009, 89, 1028–1039. [Google Scholar] [CrossRef]
- Hasan, A.; Memic, A.; Annabi, N.; Hossain, M.; Paul, A.; Dokmeci, M.R.; Dehghani, F.; Khademhosseini, A. Electrospun scaffolds for tissue engineering of vascular grafts. Acta Biomater. 2014, 10, 11–25. [Google Scholar] [CrossRef] [Green Version]
- Ko, Y.-G.; Park, J.H.; Lee, J.B.; Oh, H.H.; Park, W.H.; Cho, D.; Kwon, O.H. Growth behavior of endothelial cells according to electrospun poly(D,L-lactic-co-glycolic acid) fiber diameter as a tissue engineering scaffold. Tissue Eng. Regen. Med. 2016, 13, 343–351. [Google Scholar] [CrossRef]
- Masaeli, E.; Morshed, M.; Tavanai, H. Study of the wettability properties of polypropylene nonwoven mats by low-pressure oxygen plasma treatment. Surf. Interface Anal. 2007, 39, 770–774. [Google Scholar] [CrossRef]
- Bico, J.; Tordeux, C.; Quéré, D. Rough wetting. Europhys. Lett. 2001, 55, 214–220. [Google Scholar] [CrossRef] [Green Version]
- El-Saber, M.; Abou-Gabal, H.; Aloufy, A.; El Saghir, A. Enhancing the Wetting Properties of Polypropylene and Polyethylene Nonwoven Fabrics Using a Cost-Effective Surface Dielectric Barrier Discharge. KEM 2018, 786, 258–266. [Google Scholar] [CrossRef]
- Palasantzas, G.; de Hosson, J. Wetting on rough surfaces. Acta Mater. 2001, 49, 3533–3538. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Perwuelz, A.; Lewandowski, M.; Campagne, C. Wetting behavior of thermally bonded polyester nonwoven fabrics: The importance of porosity. J. Appl. Polym. Sci. 2006, 102, 387–394. [Google Scholar] [CrossRef]
- Arima, Y.; Iwata, H. Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials 2007, 28, 3074–3082. [Google Scholar] [CrossRef]
- Harnett, E.M.; Alderman, J.; Wood, T. The surface energy of various biomaterials coated with adhesion molecules used in cell culture. Colloids Surf. B Biointerfaces 2007, 55, 90–97. [Google Scholar] [CrossRef]
- Cousins, B.G.; Zekonyte, J.; Doherty, P.J.; Garvey, M.J.; Williams, R.L. Manufacturing a nanometre scale surface topography with varying surface chemistry to assess the combined effect on cell behaviour. IJNBM 2008, 1, 320. [Google Scholar] [CrossRef]
- Mahmoodi, M.; Zamanifard, M.; Safarzadeh, M.; Bonakdar, S. In vitro evaluation of collagen immobilization on polytetrafluoroethylene through NH3 plasma treatment to enhance endothelial cell adhesion and growth. Biomed. Mater. Eng. 2017, 28, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.T.; Tan, E.P.S.; Ng, S.Y. Effects of crystalline morphology on the tensile properties of electrospun polymer nanofibers. Appl. Phys. Lett. 2008, 92, 141908. [Google Scholar] [CrossRef]
- Abdal-Hay, A.; Abdelrazek Khalil, K.; Al-Jassir, F.F.; Gamal-Eldeen, A.M. Biocompatibility properties of polyamide 6/PCL blends composite textile scaffold using EA.hy926 human endothelial cells. Biomed. Mater. 2017, 12, 35002. [Google Scholar] [CrossRef]
- Garipcan, B.; Maenz, S.; Pham, T.; Settmacher, U.; Jandt, K.D.; Zanow, J.; Bossert, J. Image Analysis of Endothelial Microstructure and Endothelial Cell Dimensions of Human Arteries—A Preliminary Study. Adv. Eng. Mater. 2011, 13, B54–B57. [Google Scholar] [CrossRef]
- Lu, Z.J.; Ren, Y.Q.; Wang, G.P.; Song, Q.; Li, M.; Jiang, S.S.; Ning, T.; Guan, Y.S.; Yang, J.L.; Luo, F. Biological behaviors and proteomics analysis of hybrid cell line EAhy926 and its parent cell line A549. J. Exp. Clin. Cancer Res. 2009, 28, 16. [Google Scholar] [CrossRef] [Green Version]
- Pinevich, A.A.; Vartanyan, N.L.; Terekhina, L.A.; Krutetskaya, I.Y.; Shashkova, O.A.; Smirnov, I.V.; Samoylovich, M.P. Endoglin Expression and Surface Renewal in Mesenchymal Stem Cells and Endothelial Cells. Cell Tiss. Biol. 2021, 15, 107–119. [Google Scholar] [CrossRef]
- Zhao, J.; Feng, Y. Surface Engineering of Cardiovascular Devices for Improved Hemocompatibility and Rapid Endothelialization. Adv. Healthc. Mater. 2020, 9, e2000920. [Google Scholar] [CrossRef] [PubMed]
- Gapizov, S.S.; Petrovskaya, L.E.; Shingarova, L.N.; Svirschevskaya, E.V.; Dolgikh, D.A.; Kirpichnikov, M.P. The effect of tnf and VEGF on the properties of EA. Hy926 endothelial cells in a model of multi-cellular spheroids. Acta Nat. 2018, 10, 34–42. [Google Scholar] [CrossRef]

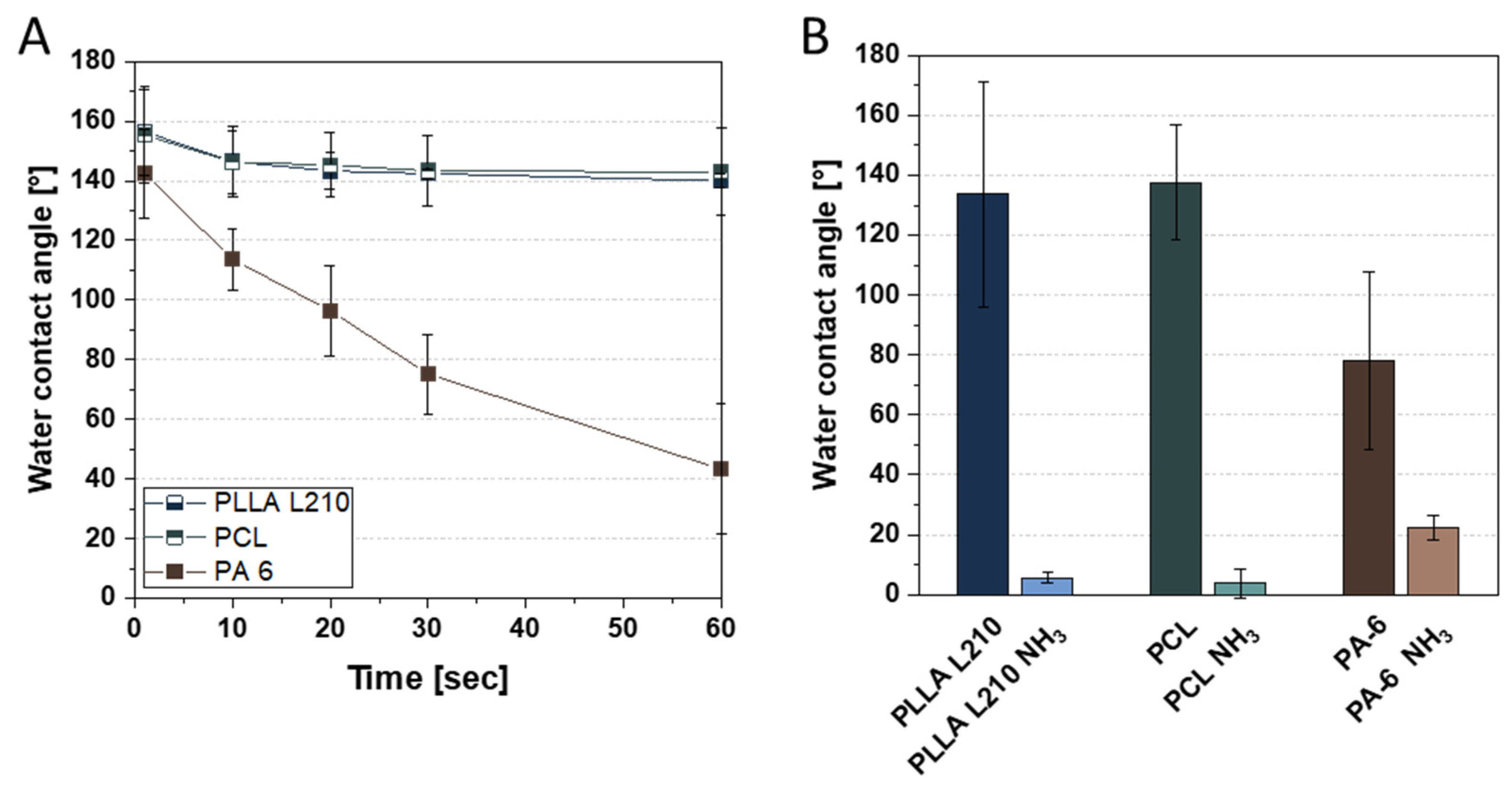





| Untreated Nonwovens | NH3-Plasma-Activated Nonwovens | |||||
|---|---|---|---|---|---|---|
| PLLA L210 * | PCL | PA-6 | PLLA L210 * | PCL | PA-6 | |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Water [°] | 133.6 ± 5.6 | 137.6 ± 3.8 | 78.0 ± 22.2 | 37.8 ± 1.7 | 19.3 ± 4.8 | 29.8 ± 4.1 |
| Diiodomethane [°] | 18.1 ± 2.4 | 21.6 ± 11.1 | 26.8 ± 7.6 | 64.6 ± 0.3 | 25.9 ± 1.0 | 31.6 ± 3.3 |
| Surface free energy [mN/m] | 57.1 ± 2.8 | 57.3 ± 5.3 | 48.5 ± 9.6 | 59.5 ± 1.3 | 75.9 ± 1.9 | 70.7 ± 3.5 |
| Disperse fraction [mN/m] | 48.3 ± 0.7 | 47.3 ± 3.5 | 45.5 ± 2.9 | 25.9 ± 0.2 | 45.8 ± 0.4 | 43.6 ± 1.4 |
| Polar fraction [mN/m] | 8.8 ± 2.1 | 10.0 ± 1.8 | 3.1 ± 6.8 | 33.6 ± 1.0 | 30.1 ± 1.6 | 27.1 ± 2.0 |
| Control of | Selected Parameters | Selected Limitations | |
|---|---|---|---|
| Plasma →← Electrospinning | Fiber topography | Adjustable fiber diameters ranging from nano- to micrometers | No electrospinning of chemical inert polymers, complex 3D scaffolds |
| Biological response | Biofilm inhibition and endothelial cell enhancement | Polymer specific nonwovens, long-term stability of plasma activation | |
| Tribological properties | Surface roughness, sliding vs. adhesive | Specific hydrophilicity | |
| Local- or side-specific modifications | Layer structure, composites, and top vs. bottom | Possible material defects, e.g., delamination, etching or radiation | |
| Patient individuality | Easy and fast plasma modification, e.g., during interventions | Transferability, e.g., no defined electrospinning and plasma parameters | |
| Surface chemistry | Specific functional groups for graftings, coatings or drugs | Material and plasma dependence |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matschegewski, C.; Kohse, S.; Markhoff, J.; Teske, M.; Wulf, K.; Grabow, N.; Schmitz, K.-P.; Illner, S. Accelerated Endothelialization of Nanofibrous Scaffolds for Biomimetic Cardiovascular Implants. Materials 2022, 15, 2014. https://doi.org/10.3390/ma15062014
Matschegewski C, Kohse S, Markhoff J, Teske M, Wulf K, Grabow N, Schmitz K-P, Illner S. Accelerated Endothelialization of Nanofibrous Scaffolds for Biomimetic Cardiovascular Implants. Materials. 2022; 15(6):2014. https://doi.org/10.3390/ma15062014
Chicago/Turabian StyleMatschegewski, Claudia, Stefanie Kohse, Jana Markhoff, Michael Teske, Katharina Wulf, Niels Grabow, Klaus-Peter Schmitz, and Sabine Illner. 2022. "Accelerated Endothelialization of Nanofibrous Scaffolds for Biomimetic Cardiovascular Implants" Materials 15, no. 6: 2014. https://doi.org/10.3390/ma15062014
APA StyleMatschegewski, C., Kohse, S., Markhoff, J., Teske, M., Wulf, K., Grabow, N., Schmitz, K.-P., & Illner, S. (2022). Accelerated Endothelialization of Nanofibrous Scaffolds for Biomimetic Cardiovascular Implants. Materials, 15(6), 2014. https://doi.org/10.3390/ma15062014