Production and Upgrading of Recovered Carbon Black from the Pyrolysis of End-of-Life Tires
Abstract
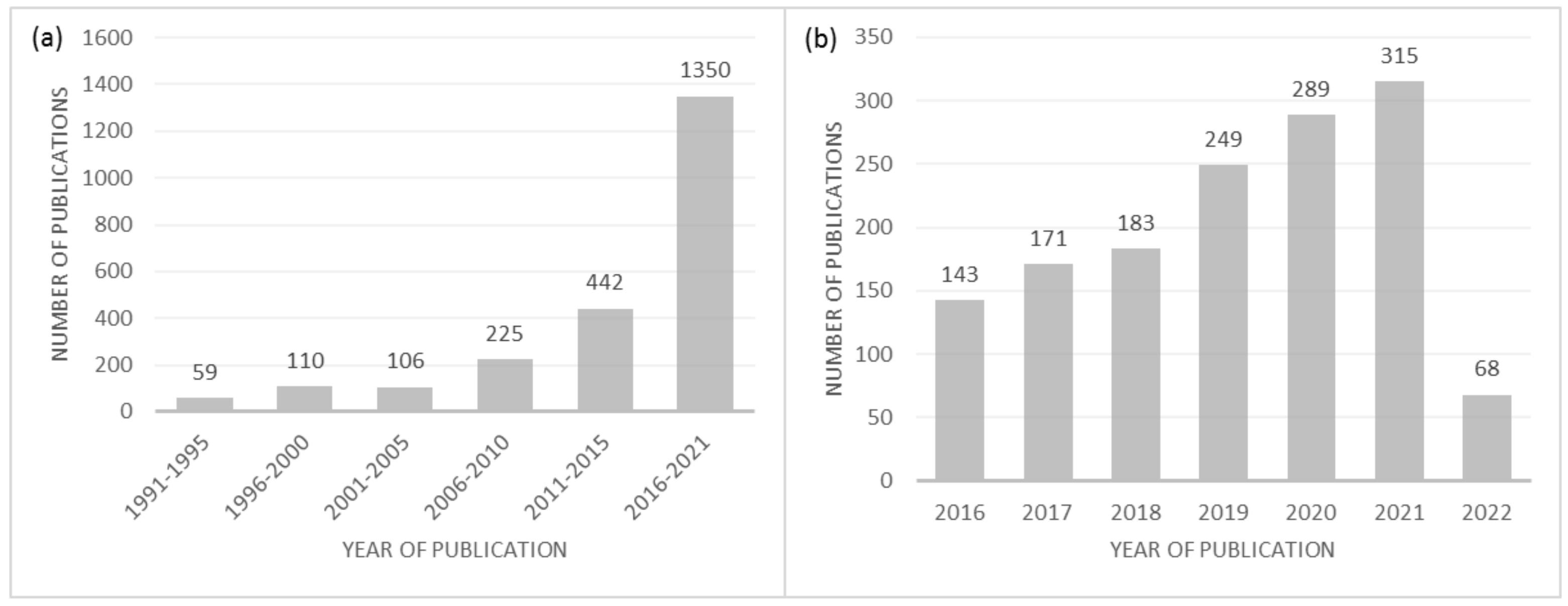
:1. Introduction
2. Pyrolysis of End-of-Life Tires (ELT)
3. ELT Pyrolysis Products
3.1. Gaseous Product
3.2. Liquid Product
3.3. Solid Product
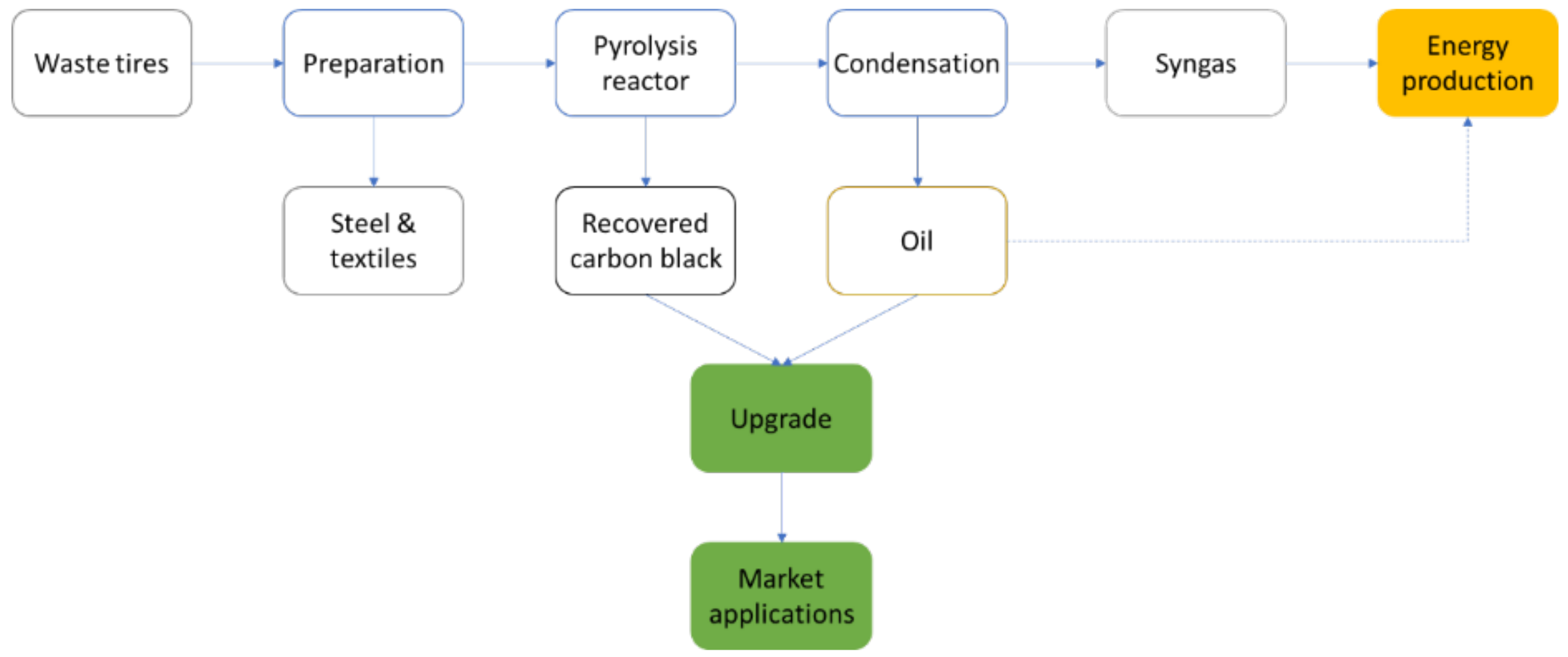
4. Recovered Carbon Black
4.1. Production of rCB by ELT Pyrolysis
4.2. Commercial Applications for rCB
4.2.1. Carbon Black
4.2.2. rCB Applications
4.3. rCB Purification and Modification
4.3.1. rCB Activation
4.3.2. rCB Demineralization
4.3.3. Organic Volatile Contamination
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
References
- Goldstein Market Intelligence. Global Tire Industry Analysis by Product Type And, By Geography & COVID-19 Impact with Market Outlook 2017–2030. 2020. Available online: https://www.goldsteinresearch.com (accessed on 30 November 2021).
- ETRMA—European Tyre & Rubber Manufacturers’ Association. The European Tyre Industry Facts and Figures 2020 Edition; ETRMA: Brussels, Belgium, 2020. [Google Scholar]
- ETRMA—European Tyre & Rubber Manufacturers’ Association. ELT Market Overview. In Proceedings of the Recovered Carbon Black Conference, Amsterdam, The Netherlands, 22–23 November 2021. [Google Scholar]
- European Comission. Landfill of Waste Directive; Council Directive 1999/31/EC; European Comission: Brussels, Belgium, 1999. [Google Scholar]
- Rodriguez, I.M.; Laresgoiti, M.F.; Cabrero, M.A.; Torres, A.; Chomón, M.J.; Caballero, B. Pyrolysis of scrap tyres. Fuel Process. Technol. 2001, 72, 9–22. [Google Scholar] [CrossRef]
- Mugnier, E.; Farhangi, C.; Kley, S.; Aurez, V.; Chhang, A. The Socio-Economic Impact of Truck Tyre Retreading in Europe—The Circular Economy of Tyres in Danger; EY: London, UK, 2016. [Google Scholar]
- Zebala, J.; Ciepka, P.; Reza, A.; Janczur, R. Influence of rubber compound and tread pattern of retreaded tyres on vehicle active safety. Forensic Sci. Int. 2007, 167, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Sienkiewicz, M.; Kucinska-lipka, J.; Janik, H.; Balas, A. Progress in used tyres management in the European Union: A review. Waste Manag. 2012, 32, 1742–1751. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.D.; Puy, N.; Murillo, R.; García, T.; Navarro, M.V.; Mastral, A.M. Waste tyre pyrolysis—A review. Renew. Sustain. Energy Rev. 2013, 23, 179–213. [Google Scholar] [CrossRef]
- Nkosi, N.; Muzenda, E. A Review and Discussion of Waste Tyre Pyrolysis and Derived Products. In Proceedings of the World Congress on Engineering, London, UK, 2–4 July 2014. [Google Scholar]
- Rezaiyan, J.; Cheremisinoff, N.P. Pyrolysis. In Gasification Technologies: A Primer for Engineers and Scientists, 1st ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 145–164. [Google Scholar]
- Hita, I.; Arabiourrutia, M.; Olazar, M.; Bilbao, J.; Arandes, J.M.; Castaño, P. Opportunities and barriers for producing high quality fuels from the pyrolysis of scrap tires. Renew. Sustain. Energy Rev. 2016, 56, 745–759. [Google Scholar] [CrossRef]
- Sharma, V.K.; Fortuna, F.; Mincarini, M.; Berillo, M.; Cornacchia, G. Disposal of waste tyres for energy recovery and safe environment. Appl. Energy 2000, 65, 381–394. [Google Scholar] [CrossRef]
- Lam, S.S.; Liew, R.K.; Jusoh, A.; Chong, C.T.; Ani, F.N.; Chase, H.A. Progress in waste oil to sustainable energy, with emphasis on pyrolysis techniques. Renew. Sustain. Energy Rev. 2016, 53, 741–753. [Google Scholar] [CrossRef]
- Mastral, A.M.; Murillo, R.; Callén, M.S.; García, T. Application of coal conversion technology to tire processing. Fuel 1999, 60, 231–242. [Google Scholar] [CrossRef]
- Williams, P.T.; Besler, S. Pyrolysis-thermogravimetric analysis of tyres and tyre components. Fuel 1995, 74, 1277–1283. [Google Scholar] [CrossRef]
- Evans, A.; Evans, R. The Composition of a Tyre: Typical Components; The Waste and Resources Action Programme: Banbury, UK, 2006; pp. 1–5. [Google Scholar]
- Lewandowski, W.M.; Januszewicz, K.; Kosakowski, W. Efficiency and proportions of waste tyre pyrolysis products depending on the reactor type—A review. J. Anal. Appl. Pyrolysis 2019, 140, 25–53. [Google Scholar] [CrossRef]
- Qureshi, K.M.; Lup, A.N.K.; Khan, S.; Abnisa, F.; Daud, W.M.A.W. A technical review on semi-continuous and continuous pyrolysis process of biomass to bio-oil. J. Anal. Appl. Pyrolysis 2018, 131, 52–75. [Google Scholar] [CrossRef]
- Laresgoiti, M.F.; de Marco, I.; Torres, A.; Caballero, B.; Cabrero, M.A.; Chomón, M.J. Chromatographic analysis of the gases obtained in tyre pyrolysis. J. Anal. Appl. Pyrolysis 2000, 55, 43–54. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Choi, H.S.; Park, H.C.; Hwang, J.G.; Yoo, H.S.; Lee, B.-K.; Upadhyay, M. Influence of process conditions on product yield of waste tyre pyrolysis- A review. Korean J. Chem. Eng. 2016, 33, 2268–2286. [Google Scholar] [CrossRef] [Green Version]
- Leung, D.Y.C.; Yin, X.L.; Zhao, Z.L.; Xu, B.Y.; Chen, Y. Pyrolysis of tire powder: Influence of operation variables on the composition and yields of gaseous product. Fuel Process. Technol. 2002, 79, 141–155. [Google Scholar] [CrossRef]
- López, G.; Olazar, M.; Aguado, R.; Bilbao, J. Continuous pyrolysis of waste tyres in a conical spouted bed reactor. Fuel 2010, 89, 1946–1952. [Google Scholar] [CrossRef]
- Díez, C.; Martínez, O.; Calvo, L.F.; Cara, J.; Morán, A. Pyrolysis of tyres. Influence of the final temperature of the process on emissions and the calorific value of the products recovered. Waste Manag. 2004, 24, 463–469. [Google Scholar] [CrossRef]
- Martínez, J.D.; Murillo, R.; García, T.; Veses, A. Demonstration of the waste tire pyrolysis process on pilot scale in a continuous auger reactor. J. Hazard. Mater. 2013, 261, 637–645. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, T.; Ma, L.; Chang, J. Vacuum pyrolysis of waste tires with basic additives. Waste Manag. 2008, 28, 2301–2310. [Google Scholar] [CrossRef]
- Aylón, E.; Fernández-Colino, A.; Navarro, M.V.; Murillo, R.; García, T.; Mastral, A.M. Waste Tire Pyrolysis: Comparison between Fixed Bed Reactor and Moving Bed Reactor. Ind. Eng. Chem. Res. 2008, 47, 4029–4033. [Google Scholar] [CrossRef]
- Göransson, K.; Söderlind, U.; He, J.; Zhang, W. Review of syngas production via biomass DFBGs. Renew. Sustain. Energy Rev. 2011, 15, 482–492. [Google Scholar] [CrossRef]
- Kaminsky, W.; Mennerich, C.; Zhang, Z. Feedstock recycling of synthetic and natural rubber by pyrolysis in a fluidized bed. J. Anal. Appl. Pyrolysis 2009, 85, 334–337. [Google Scholar] [CrossRef]
- Chaala, A.; Roy, C. Production of coke from scrap tire vacuum pyrolysis oil. Fuel Process. Technol. 1996, 46, 227–239. [Google Scholar] [CrossRef]
- Lopez, G.; Olazar, M.; Amutio, M.; Aguado, R.; Bilbao, J. Influence of Tire Formulation on the Products of Continuous Pyrolysis in a Conical Spouted Bed Reactor. Energy Fuels 2009, 23, 5423–5431. [Google Scholar] [CrossRef]
- Fortuna, F.; Cornacchia, G.; Mincarini, M.; Sharma, V.K. Pilot-scale experimental pyrolysis plant: Mechanical and operational aspects. J. Anal. Appl. Pyrolysis 1997, 40–41, 403–417. [Google Scholar] [CrossRef]
- Yaqoob, H.; Teoh, Y.H.; Jamil, M.A.; Gulzar, M. Potential of tire pyrolysis oil as an alternate fuel for diesel engines: A review. J. Energy Inst. 2021, 96, 205–221. [Google Scholar] [CrossRef]
- Aylón, E.; Murillo, R.; Navarro, M.V.; García, T.; Mastral, A.M. Valorisation of waste tyre by pyrolysis in a moving bed reactor. Waste Manag. 2010, 30, 1220–1224. [Google Scholar] [CrossRef]
- Williams, P.T. Pyrolysis of waste tyres: A review. Waste Manag. 2013, 33, 1714–1728. [Google Scholar] [CrossRef] [Green Version]
- Choi, G.; Oh, S.; Kim, J. Clean pyrolysis oil from a continuous two-stage pyrolysis of scrap tires using in-situ and ex-situ desulfurization. Energy 2017, 141, 2234–2241. [Google Scholar] [CrossRef]
- Murugan, S.; Ramaswamy, M.C.; Nagarajan, G. A comparative study on the performance, emission and combustion studies of a DI diesel engine using distilled tyre pyrolysis oil–diesel blends. Fuel 2008, 87, 2111–2121. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Hossain, F.M.; Rasul, M.G.; Chowdhury, A.A. A Review on the Thermochemical Recycling of Waste Tyres to Oil for Automobile Engine Application. Energies 2021, 14, 3837. [Google Scholar] [CrossRef]
- Danon, B.; van der Gryp, P.; Schwarz, C.E.; Görgens, J.F. A review of dipentene (DL-limonene) production from waste tire pyrolysis. J. Anal. Appl. Pyrolysis 2015, 112, 1–13. [Google Scholar] [CrossRef]
- Pakdel, H.; Pantea, D.M.; Roy, C. Production of dl-limonene by vacuum pyrolysis of used tires. J. Anal. Appl. Pyrolysis 2001, 57, 91–107. [Google Scholar] [CrossRef]
- Pantea, D.; Darmstadt, H.; Kaliaguine, S.; Roy, C. Heat-treatment of carbon blacks obtained by pyrolysis of used tires. Effect on the surface chemistry, porosity and electrical conductivity. J. Anal. Appl. Pyrolysis 2003, 67, 55–76. [Google Scholar] [CrossRef]
- Yazdani, E.; Hashemabadi, S.H.; Taghizadeh, A. Study of waste tire pyrolysis in a rotary kiln reactor in a wide range of pyrolysis temperature. Waste Manag. 2019, 85, 195–201. [Google Scholar] [CrossRef]
- Sharma, A.; Sawant, R.J.; Sharma, A.; Joshi, J.B.; Jain, R.K.; Kasilingam, R. Valorisation of End-of-Life tyres for generating valuable resources under circular economy. Fuel 2022, 314, 123138. [Google Scholar] [CrossRef]
- Kyari, M.; Cunliffe, A.; Williams, P.T. Characterization of Oils, Gases, and Char in Relation to the Pyrolysis of Different Brands of Scrap Automotive Tires. Energy Fuels 2005, 19, 1165–1173. [Google Scholar] [CrossRef]
- Li, S.Q.; Yao, Q.; Chi, Y.; Yan, J.H.; Cen, K.F. Pilot-Scale Pyrolysis of Scrap Tires in a Continuous Rotary Kiln Reactor. Ind. Eng. Chem. Res. 2004, 43, 5133–5145. [Google Scholar] [CrossRef]
- Galvagno, S.; Casu, S.; Casabianca, T.; Calabrese, A.; Cornacchia, G. Pyrolysis process for the treatment of scrap tyres: Preliminary experimental results. Waste Manag. 2002, 22, 917–923. [Google Scholar] [CrossRef]
- Lopez, G.; Alvarez, J.; Amutio, M.; Mkhize, N.M.; Danon, B.; van der Gryp, P.; Görgens, J.F.; Bilbao, J.; Olazar, M. Waste truck-tyre processing by flash pyrolysis in a conical spouted bed reactor. Energy Convers. Manag. 2017, 142, 523–532. [Google Scholar] [CrossRef]
- Murillo, R.; Aylón, E.; Navarro, M.V.; Callén, M.S.; Aranda, A.; Mastral, A.M. The application of thermal processes to valorise waste tyre. Fuel Process. Technol. 2006, 87, 143–147. [Google Scholar] [CrossRef]
- Martínez, J.D.; Campuzano, F.; Agudelo, A.F.; Cardona-Uribe, N.; Arenas, C.N. Chemical recycling of end-of-life tires by intermediate pyrolysis using a twin-auger reactor: Validation in a laboratory environment. J. Anal. Appl. Pyrolysis 2021, 159, 105298. [Google Scholar] [CrossRef]
- Mikulova, Z.; Sedenkova, I.; Matejova, L.; Vecer, M.; Dombek, V. Study of carbon black obtained by pyrolysis of waste scrap tyres. J. Therm. Anal. Calorim. 2013, 111, 1475–1481. [Google Scholar] [CrossRef]
- Darmstadt, H.; Roy, C.; Kaliaguine, S. Characterization of pyrolytic carbon blacks from commercial tire pyrolysis plants. Carbon 1995, 33, 1449–1455. [Google Scholar] [CrossRef]
- Huang, K.; Gao, Q.H.; Tang, L.H.; Zhu, Z.B.; Zhang, C.F. A comparison of surface morphology and chemistry of pyrolytic carbon blacks with commercial carbon blacks. Powder Technol. 2005, 160, 190–193. [Google Scholar] [CrossRef]
- Hess, W.H.; Herd, C.R. Microstructure, Morphology and General Physical Properties. In Carbon Black: Science and Technology, 2nd ed.; Donnet, J.-B., Bansal, R.C., Wang, M.-J., Eds.; CRC Press Marcel Dekker, Inc.: New York, NY, USA, 1993. [Google Scholar]
- Rodgers, B.; Waddell, W. The Science of Rubber Compounding. In The Science and Technology of Rubber, 4th ed.; Mark, J.E., Erman, B., Roland, C.M., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 417–471. [Google Scholar]
- Kuhner, G.; Voll, M. Manufacture of Carbon Black. In Carbon Black: Science and Technology, 2nd ed.; Donnet, J.-B., Bansal, R.C., Wang, M.-J., Eds.; CRC Press Marcel Dekker, Inc.: New York, NY, USA, 1993; pp. 1–66. [Google Scholar]
- ASTM International. ASTM D1765-21 Standard Classification System for Carbon Blacks Used in Rubber Products; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar]
- Orion Engineered Carbons. What is Carbon Black? Orion Engineered Carbons: Senningerberg, Luxembourg, 2015. [Google Scholar]
- Vohler, O.; Nutsch, G.; Collin, G.; von Sturm, F.; Wege, E.; Frohs, W.; Henning, K.-D.; von Kienle, H.; Voll, M.; Kleinschmit, P.; et al. Carbon. In Ullmann’s Encyclopedia of Industrial Chemistry, 7th ed.; Wiley-VCH: Hoboken, NJ, USA, 2002. [Google Scholar]
- Liu, L.; Shen, Z.; Zhang, X.; Ma, H. Highly conductive graphene/carbon black screen printing inks for flexible electronics. J. Colloid Interface Sci. 2021, 582, 12–21. [Google Scholar] [CrossRef]
- Claypole, A.; Claypole, J.; Kilduff, L.; Gethin, D.; Claypole, T. Stretchable Carbon and Silver Inks for Wearable Applications. Nanomaterials 2021, 11, 1200. [Google Scholar] [CrossRef]
- Kaspar, P.; Sobola, D.; Částková, K.; Knápek, A.; Burda, D.; Orudzhev, F.; Dallaev, R.; Tofel, P.; Trčka, T.; Grmela, L.; et al. Characterization of polyvinylidene fluoride (PVDF) electrospun fibers doped by carbon flakes. Polymers 2020, 12, 2766. [Google Scholar] [CrossRef]
- Uranbey, L.; Unal, H.I.; Calis, G.; Gumus, O.Y.; Katmer, S.; Karatas, C. One-Pot Preparation of Electroactive Shape Memory Polyurethane/Carbon Black Blend. J. Mater. Eng. Perform. 2021, 30, 1665–1673. [Google Scholar] [CrossRef]
- Xia, G.; Ye, J.; Zheng, Z.; Li, X.; Chen, C.; Hu, C. Catalytic FeP decorated carbon black as a multifunctional conducting additive for high-performance lithium-sulfur batteries. Carbon 2021, 172, 96–105. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Carbon Black. In IARC Monographs on the Identification of Carcinogenic Hazards to Humans; International Agency for Research on Cancer: Lyon, France, 2010; Volume 93, pp. 43–192. [Google Scholar]
- Cardona, N.; Campuzano, F.; Betancur, M.; Jaramillo, L.; Martínez, J.D. Possibilities of carbon black recovery from waste tyre pyrolysis to be used as additive in rubber goods—A review. IOP Conf. Ser. Mater. Sci. Eng. 2018, 437, 012012. [Google Scholar] [CrossRef]
- Roy, C.; Chaala, A.; Darmstadt, H.; Caumia, B.; Pakdel, H.; Yang, J. Conversion of Used Tires to Carbon Black and Oil by Pyrolysis. In Rubber Recycling; De, S.K., Isayev, A.I., Khait, K., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2005; pp. 429–467. [Google Scholar]
- Xu, J.; Yu, J.; He, W.; Huang, J.; Xu, J.; Li, G. Wet compounding with pyrolytic carbon black from waste tyre for manufacture of new tyre—A mini review. Waste Manag. Res. 2021, 39, 1440–1450. [Google Scholar] [CrossRef]
- Doja, S.; Pillari, L.K.; Bichler, L. Processing and activation of tire-derived char: A review. Renew. Sustain. Energy Rev. 2022, 155, 111860. [Google Scholar] [CrossRef]
- Xu, J.; Yu, J.; Xu, J.; Sun, C.; He, W.; Huang, J.; Li, G. High-value utilization of waste tires: A review with focus on modified carbon black from pyrolysis. Sci. Total Environ. 2020, 742, 140235. [Google Scholar] [CrossRef]
- Hofman, M.; Pietrzak, R. Adsorbents obtained from waste tires for NO2 removal under dry conditions at room temperature. Chem. Eng. J. 2011, 170, 202–208. [Google Scholar] [CrossRef]
- Jankovská, Z.; Večeř, M.; Koutník, I.; Matějová, L. A Case Study of Waste Scrap Tyre-Derived Carbon Black Tested for Nitrogen, Carbon Dioxide, and Cyclohexane Adsorption. Molecules 2020, 25, 4445. [Google Scholar] [CrossRef]
- Kuśmierek, K.; Świątkowski, A.; Kotkowski, T.; Cherbański, R.; Molga, E. Adsorption on activated carbons from end-of-life tyre pyrolysis for environmental applications. Part I. preparation of adsorbent and adsorption from gas phase. J. Anal. Appl. Pyrolysis 2021, 157, 105205. [Google Scholar] [CrossRef]
- Kuśmierek, K.; Świątkowski, A.; Kotkowski, T.; Cherbański, R.; Molga, E. Adsorption on activated carbons from end-of-life tyre pyrolysis for environmental applications. Part II. Adsorption from aqueous phase. J. Anal. Appl. Pyrolysis 2021, 158, 105206. [Google Scholar] [CrossRef]
- Mui, E.L.K.; Cheung, W.H.; McKay, G. Tyre char preparation from waste tyre rubber for dye removal from effluents. J. Hazard. Mater. 2010, 175, 151–158. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, Q.; Wang, D.; Xia, D.; Zheng, X.; Li, Z.; Hwang, J.Y. Preparation of Pyrolytic Carbon from Waste Tires for Methylene Blue Adsorption. JOM 2019, 71, 3658–3666. [Google Scholar] [CrossRef]
- Frikha, K.; Limousy, L.; Claret, J.P.; Vaulot, C.; Pérez, K.F.; Garcia, B.C.; Bennici, S. Potential Valorization of Waste Tires as Activated Carbon-Based Adsorbent for Organic Contaminants Removal. Materials 2022, 15, 1099. [Google Scholar] [CrossRef]
- Gupta, H.; Gupta, B. Vehicular Tire as Potential Adsorbent for the Removal of Polycyclic Aromatic Hydrocarbons. Polycycl. Aromat. Compd. 2018, 38, 354–368. [Google Scholar] [CrossRef]
- Kuśmierek, K.; Świątkowski, A.; Kotkowski, T.; Cherbański, R.; Molga, E. Adsorption Properties of Activated Tire Pyrolysis Chars for Phenol and Chlorophenols. Chem. Eng. Technol. 2020, 43, 770–780. [Google Scholar] [CrossRef]
- Jusoh, N.W.C.; Choo, T.Y.; Masudi, A.; Ali, R.R. Waste Tire Carbon Adsorbent for Active Removal of Paracetamol in Aqueous Solution. J. Phys. Conf. Ser. 2020, 1447, 012050. [Google Scholar] [CrossRef]
- Ji, J.; Chen, G.; Zhao, J.; Wei, Y. Efficient removal of Pb (II) by inexpensive magnetic adsorbents prepared from one-pot pyrolysis of waste tyres involved magnetic nanoparticles. Fuel 2020, 282, 118715. [Google Scholar] [CrossRef]
- Shahrokhi-Shahraki, R.; Benally, C.; El-Din, M.G.; Park, J. High efficiency removal of heavy metals using tire-derived activated carbon vs commercial activated carbon: Insights into the adsorption mechanisms. Chemosphere 2021, 264, 128455. [Google Scholar] [CrossRef]
- Abbasi, S.; Foroutan, R.; Esmaeili, H.; Esmaeilzadeh, F. Preparation of activated carbon from worn tires for removal of Cu(II), Ni(II) and Co(II) ions from synthetic wastewater. Desalination Water Treat. 2019, 141, 269–278. [Google Scholar] [CrossRef]
- Veldevi, T.; Raghu, S.; Kalaivani, R.A.; Shanmugharaj, A.M. Waste tire derived carbon as potential anode for lithium-ion batteries. Chemosphere 2022, 288, 132438. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, L.; Li, J.; Han, N.; Li, X.; Liu, G.; Jia, D.; Ma, Z.; Song, G.; Zhu, X.; et al. The Positive Effect of ZnS in Waste Tire Carbon as Anode for Lithium-Ion Batteries. Materials 2021, 14, 2178. [Google Scholar] [CrossRef]
- Djuandhi, L.; Gaikwad, V.; Cowie, B.C.C.; Sahajwalla, V.; Sharma, N. Repurposing Waste Tires as Tunable Frameworks for Use in Sodium-Ion and Lithium-Sulfur Batteries. ACS Sustain. Chem. Eng. 2021, 9, 6972–6990. [Google Scholar] [CrossRef]
- Dell’Era, A.; Pasquali, M.; Tarquini, G.; Scaramuzzo, F.A.; de Gasperis, P.; Prosini, P.P.; Mezzi, A.; Tuffi, R.; Cafiero, L. Carbon powder material obtained from an innovative high pressure water jet recycling process of tires used as anode in alkali ion (Li, Na) batteries. Solid State Ion. 2018, 324, 20–27. [Google Scholar] [CrossRef]
- Jiang, G.; Guo, J.; Sun, Y.; Liu, X.; Pan, J. Pyrolytic carbon black-derived porous carbon with spherical skeleton as recovered and enduring electrode material for supercapacitor. J. Energy Storage 2021, 44, 103372. [Google Scholar] [CrossRef]
- Husár, J.; Haydary, J.; Šuhaj, P.; Steltenpohl, P. Potential of tire pyrolysis char as tar-cracking catalyst in solid waste and biomass gasification. Chem. Pap. 2019, 73, 2091–2101. [Google Scholar] [CrossRef]
- Al-Rahbi, A.S.; Williams, P.T. Hydrogen-rich syngas production and tar removal from biomass gasification using sacrificial tyre pyrolysis char. Appl. Energy 2017, 190, 501–509. [Google Scholar] [CrossRef]
- González-González, R.B.; González, L.T.; Madou, M.; Leyva-Porras, C.; Martinez-Chapa, S.O.; Mendoza, A. Synthesis, Purification, and Characterization of Carbon Dots from Non-Activated and Activated Pyrolytic Carbon Black. Nanomaterials 2022, 12, 298. [Google Scholar] [CrossRef]
- González-González, R.B.; González, L.T.; Iglesias-González, S.; González-González, E.; Martinez-Chapa, S.O.; Madou, M.; Alvarez, M.M.; Mendoza, A. Characterization of chemically activated pyrolytic carbon black derived from waste tires as a candidate for nanomaterial precursor. Nanomaterials 2020, 10, 2213. [Google Scholar] [CrossRef]
- Tang, L.; Huang, H. Thermal plasma pyrolysis of used tires for carbon black recovery. J. Mater. Sci. 2005, 40, 3817–3819. [Google Scholar] [CrossRef]
- Xu, J.; Yu, J.; He, W.; Huang, J.; Xu, J.; Li, G. Replacing commercial carbon black by pyrolytic residue from waste tire for tire processing: Technically feasible and economically reasonable. Sci. Total Environ. 2021, 793, 148597. [Google Scholar] [CrossRef]
- Sagar, M.; Nibedita, K.; Manohar, N.; Kumar, K.R.; Suchismita, S.; Pradnyesh, A.; Reddy, A.B.; Sadiku, E.R.; Gupta, U.N.; Lachit, P.; et al. A potential utilization of end-of-life tyres as recycled carbon black in EPDM rubber. Waste Manag. 2018, 74, 110–122. [Google Scholar] [CrossRef]
- Urrego-Yepes, W.; Cardona-Uribe, N.; Vargas-Isaza, C.A.; Martínez, J.D. Incorporating the recovered carbon black produced in an industrial-scale waste tire pyrolysis plant into a natural rubber formulation. J. Environ. Manag. 2021, 287, 112292. [Google Scholar] [CrossRef]
- Berki, P.; Göbl, R.; Karger-Kocsis, J. Structure and properties of styrene-butadiene rubber (SBR) with pyrolytic and industrial carbon black. Polym. Test. 2017, 61, 404–415. [Google Scholar] [CrossRef] [Green Version]
- Martínez, J.D.; Carbona-Uribe, N.; Murillo, R.; García, T.; López, J.M. Carbon black recovery from waste tire pyrolysis by demineralization: Production and application in rubber compounding. Waste Manag. 2019, 85, 574–584. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Xu, J.; Li, Z.; He, W.; Huang, J.; Xu, J.; Li, G. Upgrading pyrolytic carbon-blacks (CBp) from end-of-life tires: Characteristics and modification methodologies. Front. Environ. Sci. Eng. 2020, 14, 19. [Google Scholar] [CrossRef]
- Mui, E.L.K.; Ko, D.C.K.; McKay, G. Production of active carbons from waste tyres—A review. Carbon 2004, 42, 2789–2805. [Google Scholar] [CrossRef]
- Miguel, G.S.; Fowler, G.D.; Sollars, C.J. A study of the characteristics of activated carbons produced by steam and carbon dioxide activation of waste tyre rubber. Carbon 2003, 41, 1009–1016. [Google Scholar] [CrossRef]
- Choi, G.G.; Jung, S.H.; Oh, S.J.; Kim, J.S. Total utilization of waste tire rubber through pyrolysis to obtain oils and CO2 activation of pyrolysis char. Fuel Process. Technol. 2014, 123, 57–64. [Google Scholar] [CrossRef]
- González, J.F.; Encinar, J.M.; González-García, C.M.; Sabio, E.; Ramiro, A.; Canito, J.L.; Gañán, J. Preparation of activated carbons from used tyres by gasification with steam and carbon dioxide. Appl. Surf. Sci. 2006, 252, 5999–6004. [Google Scholar] [CrossRef]
- Cunliffe, A.M.; Williams, P.T. Influence of Process Conditions on the Rate of Activation of Chars Derived from Pyrolysis of Used Tires. Energy Fuels 1999, 13, 166–175. [Google Scholar] [CrossRef]
- Hamadi, N.K.; Chen, X.D.; Farid, M.M.; Lu, M.G.Q. Adsorption kinetics for the removal of chromium(VI) from aqueous solution by adsorbents derived from used tyres and sawdust. Chem. Eng. J. 2001, 84, 95–105. [Google Scholar] [CrossRef]
- Allen, J.L.; Gatz, J.L.; Eklund, P.C. Applications for activated carbons from used tires: Butane working capacity. Carbon 1999, 37, 1485–1489. [Google Scholar] [CrossRef]
- Lehmann, C.M.B.; Rostam-Abadi, M.; Rood, M.J.; Sun, J. Reprocessing and Reuse of Waste Tire Rubber to Solve Air-Quality Related Problems. Energy Fuels 1998, 12, 1095–1099. [Google Scholar] [CrossRef]
- Chan, O.S.; Cheung, W.H.; McKay, G. Single and multicomponent acid dye adsorption equilibrium studies on tyre demineralised activated carbon. Chem. Eng. J. 2012, 191, 162–170. [Google Scholar] [CrossRef]
- Budinova, T.; Ekinci, E.; Yardim, F.; Grimm, A.; Björnbom, E.; Minkova, V.; Goranova, M. Characterization and application of activated carbon produced by H3PO4 and water vapor activation. Fuel Process. Technol. 2006, 87, 899–905. [Google Scholar] [CrossRef]
- Zabaniotou, A.A.; Stavropoulos, G. Pyrolysis of used automobile tires and residual char utilization. J. Anal. Appl. Pyrolysis 2003, 70, 711–722. [Google Scholar] [CrossRef]
- Arabiourrutia, M.; Lopez, G.; Artetxe, M.; Alvarez, J.; Bilbao, J.; Olazar, M. Waste tyre valorization by catalytic pyrolysis—A review. Renew. Sustain. Energy Rev. 2020, 129, 109932. [Google Scholar] [CrossRef]
- Antoniou, N.; Stavropoulos, G.; Zabaniotou, A. Activation of end of life tyres pyrolytic char for enhancing viability of pyrolysis—Critical review, analysis and recommendations for a hybrid dual system. Renew. Sustain. Energy Rev. 2014, 39, 1053–1073. [Google Scholar] [CrossRef]
- Gong, G.-Z.; Xie, Q.; Zheng, Y.-F.; Ye, S.-F.; Chen, Y.-F. Regulation of pore size distribution in coal-based activated carbon. New Carbon Mater. 2009, 24, 141–146. [Google Scholar] [CrossRef]
- Acosta, R.; Fierro, V.; de Yuso, A.M.; Nabarlatz, D.; Celzard, A. Tetracycline adsorption onto activated carbons produced by KOH activation of tyre pyrolysis char. Chemosphere 2016, 149, 168–176. [Google Scholar] [CrossRef]
- Gupta, V.K.; Ganjali, M.R.; Nayak, A.; Bhushan, B.; Agarwal, S. Enhanced heavy metals removal and recovery by mesoporous adsorbent prepared from waste rubber tire. Chem. Eng. J. 2012, 197, 330–342. [Google Scholar] [CrossRef]
- Cunliffe, A.M.; Williams, P.T. Properties of Chars and Activated Carbons Derived from the Pyrolysis of Used Tyres. Environ. Technol. 1998, 19, 1177–1190. [Google Scholar] [CrossRef]
- Suuberg, E.M.; Aarna, I. Kinetics of tire derived fuel (TDF) char oxidation and accompanying changes in surface area. Fuel 2009, 88, 179–186. [Google Scholar] [CrossRef]
- Ucar, S.; Karagoz, S.; Ozkan, A.R.; Yanik, J. Evaluation of two different scrap tires as hydrocarbon source by pyrolysis. Fuel 2005, 84, 1884–1892. [Google Scholar] [CrossRef]
- López, F.A.; Centeno, T.A.; Rodríguez, O.; Alguacil, F.J. Preparation and characterization of activated carbon from the char produced in the thermolysis of granulated scrap tyres. J. Air Waste Manag. Assoc. 2013, 63, 534–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Li, H.; Cao, Q.; Jin, L.; Wang, F. Upgrading pyrolytic residue from waste tires to commercial carbon black. Waste Manag. Res. 2018, 5, 436–444. [Google Scholar] [CrossRef] [Green Version]
- Cardona-uribe, N.; Betancur, M.; Martínez, J.D. Towards the chemical upgrading of the recovered carbon black derived from pyrolysis of end-of-life tires. Sustain. Mater. Technol. 2021, 28, e00287. [Google Scholar] [CrossRef]
- Department of Health & Human Services. Polycyclic Aromatic Hydrocarbons (PAHs); ToxFAQs: Atlanta, GA, USA, 1996; pp. 1–2. [Google Scholar]
- European Chemicals Agency (ECHA). Annex XVII to REACH-Entry 50-Polycyclic-Aromatic Hydrocarbons (PAH); ECHA: Helsinki, Finland, 2013. [Google Scholar]
- ASTM International. ASTM D8143-17 Standard Test Method for Determination of the EU-8 List of PAH Compounds in Carbon Black; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar]
- Cataldo, F. On the characterisation of carbon black from tire pyrolysis. Fullerenes Nanotub. Carbon Nanostruct. 2020, 28, 368–376. [Google Scholar] [CrossRef]
- Jonker, M.T.O.; Koelmans, A.A. Extraction of Polycyclic Aromatic Hydrocarbons from Soot and Sediment: Solvent Evaluation and Implications for Sorption Mechanism. Environ. Sci. Technol. 2002, 36, 4107–4113. [Google Scholar] [CrossRef]
- Yardim, M.F.; Ekinci, E.; Minkova, V.; Razvigorova, M.; Budinova, T.; Petrov, N.; Goranova, M. Formation of porous structure of semicokes from pyrolysis of Turkish coals in different atmospheres. Fuel 2003, 82, 459–463. [Google Scholar] [CrossRef]
- Helleur, R.; Popovic, N.; Ikura, M.; Stanciulescu, M.; Liu, D. Characterization and potential applications of pyrolytic char from ablative pyrolysis of used tires. J. Anal. Appl. Pyrolysis 2001, 58–59, 813–824. [Google Scholar] [CrossRef]
- ASTM International. ASTM D1618-18 Standard Test Method for Carbon Black Extractables—Transmittance of Toluene Extract; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar]
- Bridgestone Corporation. Looking ahead to the World in 2050; Bridgestone Group Environmental Report 2015; Bridgestone Corporation: Tokyo, Japan, 2016. [Google Scholar]
- Continental AG. 2017 Sustainability Report; Continental AG: Hanover, Germany, 2018. [Google Scholar]
- Rodat, S.; Abanades, S.; Flamant, G. Co-production of hydrogen and carbon black from solar thermal methane splitting in a tubular reactor prototype. Sol. Energy 2011, 85, 645–652. [Google Scholar] [CrossRef]
- Okoye, C.O.; Zhu, M.; Jones, I.; Zhang, J.; Zhang, Z.; Zhang, D. Preparation and characterization of carbon black (CB) using heavy residue fraction of spent tyre pyrolysis oil. J. Environ. Chem. Eng. 2021, 9, 106561. [Google Scholar] [CrossRef]
- Okoye, C.O.; Jones, I.; Zhu, M.; Zhang, Z.; Zhang, D. Manufacturing of carbon black from spent tyre pyrolysis oil—A literature review. J. Clean. Prod. 2021, 279, 123336. [Google Scholar] [CrossRef]


| rCB Yield (wt.%) | Reactor (1) | T (°C) | Proximate Analysis (2) (wt.%) | Elemental Analysis on a Dry Basis (wt.%) | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| VM | FC | A | M | C | H | N | S | ||||
| 38 | FBR | 600 | 2.51 | 83.41 | 13.82 | 0.26 | 81.57 | 0.84 | 0.33 | 2.95 | [27] |
| 38 | AR | 600 | 3.50 | 82.09 | 13.17 | 1.24 | 82.10 | 0.97 | 0.35 | 3.41 | [27] |
| 37.9 | FBR | 500 | - | - | - | - | 82.7 | 0.4 | <0.1 | 2.2 | [44] |
| 39.3 | RKR | 600 | 5.86 | 77.93 | 14.30 | 1.98 | 81.00 | 1.38 | 0.51 | 2.53 | [45] |
| 39.9 | RKR | 550 | 6.92 | 77.22 | 14.58 | 1.28 | 80.82 | 1.46 | 0.53 | 2.41 | |
| 41.3 | RKR | 500 | 16.14 | 69.19 | 12.32 | 2.35 | 82.17 | 2.28 | 0.61 | 2.32 | |
| 48.86 | RKR | 680 | 5.24 | 82.98 | 11.78 | 1.44 | 85.16 | 0.93 | 0.22 | 2.57 | [46] |
| 47.40 | RKR | 600 | 10.75 | 76.06 | 13.19 | 3.01 | 85.56 | 1.33 | 0.28 | 2.32 | |
| 49.09 | RKR | 550 | 12.78 | 71.89 | 15.33 | 3.57 | 85.31 | 1.77 | 0.34 | 2.13 | |
| 38.30 | CSBR (truck tire) | 600 | - | - | - | - | 87.24 | 0.73 | 0.39 | 3.37 | [31] |
| 36.92 | 500 | - | - | - | - | 87.36 | 0.91 | 0.44 | 3.29 | ||
| 35.36 | 425 | - | - | - | - | 86.19 | 1.25 | 0.45 | 3.06 | ||
| 35.81 | CSBR (car tire) | 600 | - | - | - | - | 86.57 | 7.66 | 0.44 | 2.13 | [31] |
| 34.05 | 500 | - | - | - | - | 86.62 | 1.39 | 0.75 | 2.24 | ||
| 33.91 | 425 | - | - | - | - | 86.46 | 0.7 | 0.34 | 3.59 | ||
| 40.5 | AR | 550 | 4.7 | 79.3 | 12.4 | 3.6 | 84.4 | 1.3 | 0.5 | 2.3 | [25] |
| 35.9 | CSBR | 575 | 2.72 | 87.66 | 9.62 | - | 84.98 | 0.83 | 0.69 | 3.63 | [47] |
| 35.9 | CSBR | 475 | 3.17 | 87.54 | 9.29 | - | 85.71 | 0.86 | 0.67 | 3.28 | |
| 37.9 | CSBR | 425 | 13.86 | 77.1 | 9.04 | - | 83.81 | 1.99 | 0.65 | 2.96 | |
| 33.0 | FBR | 550 | 1.2 | 81.3 | 16.5 | 1.0 | 80.1 | 0.4 | 0.2 | 2.8 | [24] |
| 38.0 | FBR | 500 | 0.67 | 90.8 | 8.41 | 0.09 | 90.27 | 0.26 | 0.16 | 1.22 | [48] |
| 41.3 | AR | 475 | 4.0 | 75.5 | 18.5 | 2.0 | 76.6 | 1.4 | 0.3 | 3.3 | [49] |
| First Digit (ASTM Grade) | Particle Size (nm) | Surface Area (m2/g) |
|---|---|---|
| 0 | 0–10 | >150 |
| 1 | 11–19 | 121–150 |
| 2 | 20–25 | 100–120 |
| 3 | 26–30 | 70–99 |
| 4 | 31–39 | 50–69 |
| 5 | 40–48 | 40–49 |
| 6 | 49–60 | 33–39 |
| 7 | 61–100 | 21–32 |
| 8 | 101–200 | 11–20 |
| 9 | 201–500 | 0–10 |
| Grade | IV (g/kg) | DBPA (mL/100 g) | c-DBPA (mL/100 g) | NSA (m2/g) | STSA (m2/g) | Properties | Applications |
|---|---|---|---|---|---|---|---|
| N110 | 145 | 113 | 97 | 127 | 115 | High reinforcement and abrasion resistance | Special and off-road tires |
| N220 | 121 | 114 | 98 | 114 | 106 | High reinforcement and tear strength | Special and off-road tires |
| N330 | 82 | 102 | 88 | 76 | 75 | Medium–high reinforcement; high elongation; good tear and fatigue resistance | Tire tread, carcass and sidewall; bicycle tires |
| N550 | 43 | 121 | 85 | 40 | 39 | Medium–high reinforcement; high modulus and hardness | Tire inner liners, carcass and sidewall; hoses and tubing |
| N660 | 36 | 90 | 74 | 35 | 34 | Medium reinforcement and modulus; good flex and fatigue resistance; low heat build-up | Tire inner liners, carcass and sidewall; sealing rings; cable jackets; hoses and tubing |
| N762 | 27 | 65 | 59 | 29 | 28 | Medium reinforcement; high elongation and resilience; low compression set | Mechanical rubber goods (e.g., extruded profiles and moldings); footwear; rubber flooring |
| N774 | 29 | 72 | 63 | 30 | 29 | Medium reinforcement; high loading capacity; low hysteresis | Tire inner liners; footwear; belts and hoses |
| N990 | - | 38 | 37 | 8 | 8 | Low reinforcement; low modulus, hardness, hysteresis, and tensile strength; high elongation and loading capacity | Tire inner liners; wire insulation and jackets; footwear; belts, hoses, gaskets and O-rings |
| Benzo(a)pyrene |  | Benzo(b)fluoranthene |  |
| Benzo(e)pyrene |  | Benzo(j)fluoranthene |  |
| Benz(a)anthracene |  | Benzo(k)fluoranthene |  |
| Chrysene |  | Dibenz(a,h)anthracene |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, S.M.R.; Fowler, D.; Carreira, G.A.; Portugal, I.; Silva, C.M. Production and Upgrading of Recovered Carbon Black from the Pyrolysis of End-of-Life Tires. Materials 2022, 15, 2030. https://doi.org/10.3390/ma15062030
Costa SMR, Fowler D, Carreira GA, Portugal I, Silva CM. Production and Upgrading of Recovered Carbon Black from the Pyrolysis of End-of-Life Tires. Materials. 2022; 15(6):2030. https://doi.org/10.3390/ma15062030
Chicago/Turabian StyleCosta, Sebastião M. R., David Fowler, Germano A. Carreira, Inês Portugal, and Carlos M. Silva. 2022. "Production and Upgrading of Recovered Carbon Black from the Pyrolysis of End-of-Life Tires" Materials 15, no. 6: 2030. https://doi.org/10.3390/ma15062030






