Fabricated Flexible Composite for a UV-LED Color Filter and Anti-Counterfeiting Application of Calcium Molybdate Phosphor Synthesized at Room Temperature
Abstract
:1. Introduction
2. Materials and Method
2.1. Synthesis of CaMoO4 and CaMoO4:RE3+ via Co-Precipitation at Room Temperature
2.2. Fabricated of a Flexible Composite
2.3. Characterization
3. Results and Discussion
3.1. Structure and Surface Morphology of Crystalline CaMoO4
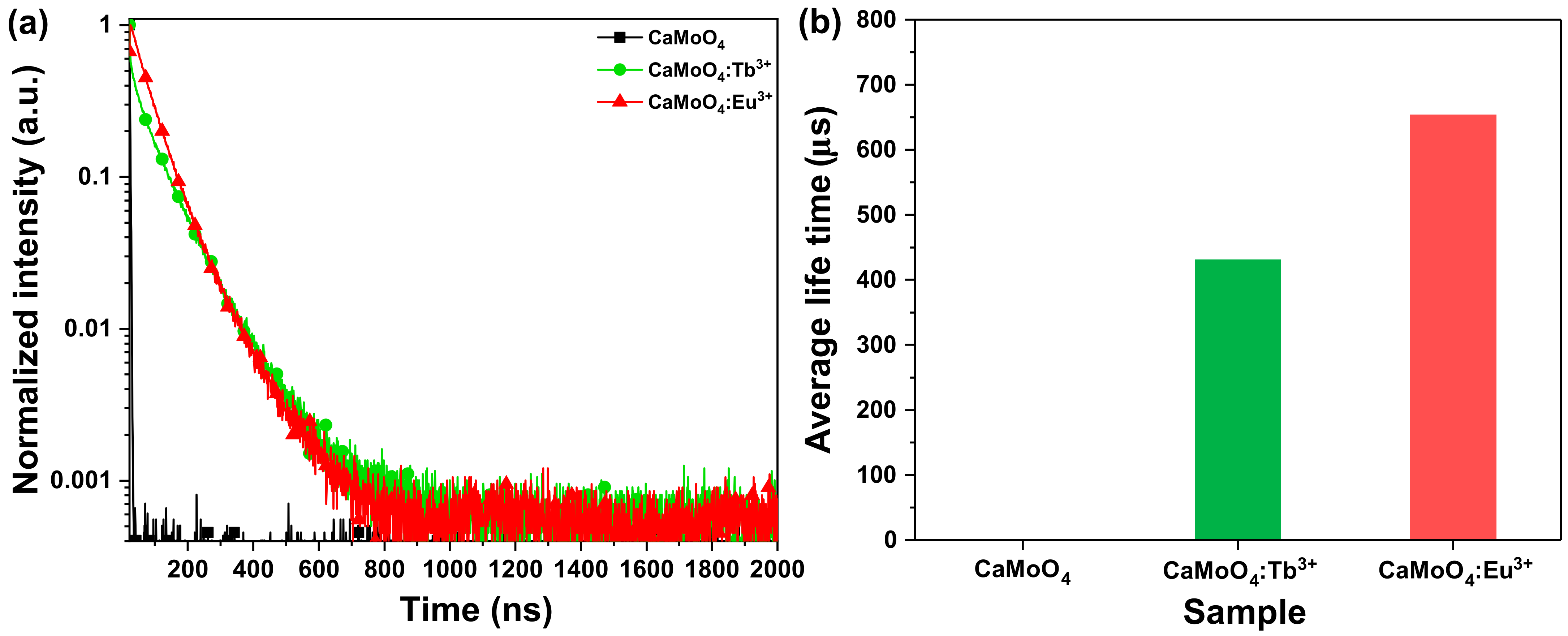
3.2. Chemical States and Luminescence Properties of the CaMoO4 Phosphor
3.3. Flexible Composite with a UV-LED Color Filter Applied for Anti-Counterfeiting
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Takahashi, H.; Matsushima, Y. Investigation on Luminescent Properties of Rare Earth Doped Mullite Phosphors and the Occupation Site of the Doped Rare Earths. J. Electrochem. Soc. 2019, 166, B3209–B3217. [Google Scholar] [CrossRef]
- Wang, X.; Xu, J.; Yu, J.; Bu, Y.; Marques-Hueso, J.; Yan, X. Morphology control, spectrum modification and extended optical applications of rare earth ion doped phosphors. Phys. Chem. Chem. Phys. PCCP 2020, 22, 1512–15162. [Google Scholar] [CrossRef]
- Secu, M.; Secu, C.; Bartha, C. Optical Properties of Transparent Rare-Earth Doped Sol-Gel Derived Nano-Glass Ceramics. Materials 2021, 14, 6871. [Google Scholar] [CrossRef]
- Verma, S.; Verma, K.; Kumar, D.; Chaudhary, B.; Som, S.; Sharma, V.; Kumar, V.; Swart, H.C. Recent advances in rare earth doped alkali-alkaline earth borates for solid state lighting applications. Phys. B Condens. Matter 2018, 535, 106–113. [Google Scholar] [CrossRef]
- Wang, M.; Liu, J.; Zhang, Y.; Hou, W.; Wu, X.; Xu, S. Two-phase solvothermal synthesis of rare-earth doped NaYF4 upconversion fluorescent nanocrystals. Mater. Lett. 2009, 63, 325–327. [Google Scholar] [CrossRef]
- Maia, A.S.; Stefani, R.; Kodaira, C.A.; Felinto, M.C.F.C.; Teotonio, E.E.S.; Brito, H.F. Luminescent nanoparticles of MgAl2O4:Eu, Dy prepared by citrate sol–gel method. Opt. Mater. 2008, 31, 440–444. [Google Scholar] [CrossRef]
- Jain, U. Structural and Photoluminescence Characterization of MgAl2O4:Eu Phosphor Synthesized by Combustion Method. Int. J. Adv. Res. 2017, 5, 1887–1890. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Yang, J.; Wang, X.; Gan, S.; Li, L. Solvent directed morphologies and enhanced luminescent properties of BaWO4:Tm3+, Dy 3+ for white light emitting diodes. Solid State Sci. 2018, 79, 85–92. [Google Scholar] [CrossRef]
- Botelho, G.; Nogueira, I.C.; Moraes, E.; Longo, E. Study of structural and optical properties of CaMoO4 nanoparticles synthesized by the microwave-assisted solvothermal method. Mater. Chem. Phys. 2016, 183, 110–120. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, F.K.F.; Oliveira, M.C.; Gracia, L.; Tranquilin, R.L.; Paskocimas, C.A.; Motta, F.V.; Longo, E.; Andrés, J.; Bomio, M.R.D. Experimental and theoretical study to explain the morphology of CaMoO4 crystals. J. Phys. Chem. Solids 2018, 114, 141–152. [Google Scholar] [CrossRef]
- Kim, M.; Huh, Y. Synthesis and optical properties of CaMoO4:Eu3+, Na+ nanophosphors and a transparent CaMoO4:Eu3+, Na+ suspension. Opt. Mater. 2012, 35, 263–267. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, J.; Tao, J.; Zhu, X.; Zhou, J.; Zhao, Z.; Xie, L.; Tian, H. Low temperature synthesis of CaMoO4 nanoparticles. Ceram. Int. 2007, 33, 693–695. [Google Scholar] [CrossRef]
- Lim, C.S. Cyclic MAM synthesis and upconversion photoluminescence properties of CaMoO4:Er3+/Yb3+ particles. Mater. Res. Bull. 2012, 47, 4220–4225. [Google Scholar] [CrossRef]
- Xia, Z.; Chen, D. Synthesis and Luminescence Properties of BaMoO4:Sm3+ Phosphors. J. Am. Ceram. Soc. 2010, 93, 1397–1401. [Google Scholar] [CrossRef]
- Pereira, P.F.S.; Nogueira, I.C.; Longo, E.; Nassar, E.J.; Rosa, I.L.V.; Cavalcante, L.S. Rietveld refinement and optical properties of SrWO4:Eu3+ powders prepared by the non-hydrolytic sol-gel method. J. Rare Earths 2015, 33, 113. [Google Scholar] [CrossRef]
- Liu, S.; Kang, L.; Hu, J.; Jung, E.; Zhang, J.; Jun, S.C.; Yamauchi, Y. Unlocking the Potential of Oxygen-Deficient Copper-Doped Co3O4 Nanocrystals Confined in Carbon as an Advanced Electrode for Flexible Solid-State Supercapacitors. ACS Energy Lett. 2021, 6, 3011. [Google Scholar] [CrossRef]
- Thongtem, T.; Kungwankunakorn, S.; Kuntalue, B.; Phuruangrat, A.; Thongtem, S. Luminescence and absorbance of highly crystalline CaMoO4, SrMoO4, CaWO4 and SrWO4 nanoparticles synthesized by co-precipitation method at room temperature. J. Alloys Compd. 2010, 506, 475–481. [Google Scholar] [CrossRef]
- Kacher, J.; Landon, C.; Adams, B.L.; Fullwood, D. Bragg’s Law diffraction simulations for electron backscatter diffraction analysis. Ultramicroscopy 2009, 109, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Khanna, A.; Singh, M.N.; Sinha, A.K. Structural and optical characterization of Eu and Dy doped CaWO4 nanoparticles for white light emission. J. Alloys Compd. 2020, 834, 154804. [Google Scholar] [CrossRef]
- He, P.Y.; Zhang, Y.J.; Chen, H.; Liu, L.C. Development of an eco-efficient CaMoO4/electroconductive geopolymer composite for recycling silicomanganese slag and degradation of dye wastewater. J. Clean. Prod. 2019, 208, 1476–1487. [Google Scholar] [CrossRef]
- Gou, Y.; Liu, Q.; Shi, X.; Asiri, A.; Hu, J.; Sun, X. CaMoO4 nanosheet array for efficient and durable water oxidation electrocatalysis under alkaline conditions. RSC Chem. Commun. 2018, 54, 5066. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Som, S.; Kunti, A.K.; Kumar, V.; Sharma, S.K.; Swart, H.C.; Visser, H.G. Structural and luminescence responses of CaMoO4 nano phosphors synthesized by hydrothermal route to swift heavy ion irradiation: Elemental and spectral stability. Acta Mater. 2017, 124, 109–119. [Google Scholar] [CrossRef]
- Gupta, S.K.; Sahu, M.; Ghosh, P.S.; Tyagi, D.; Saxena, M.K.; Kadam, R.M. Energy transfer dynamics and luminescence properties of Eu3+ in CaMoO4 and SrMoO4. Dalton Trans. Int. J. Inorg. Chem. 2015, 44, 18957–18969. [Google Scholar] [CrossRef]
- Bharat, L.K.; Raju, G.S.R.; Yu, J.S. Red and green colors emitting spherical-shaped calcium molybdate nanophosphors for enhanced latent fingerprint detection. Sci. Rep. 2017, 7, 11571. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.P.; Maheshwary, M.; Ramakrishna, P.V.; Singh, S.; Sonu, V.K.; Singh, S.; Singh, P.; Bahadur, A.; Singh, R.A.; Rai, S.B. Improved photo-luminescence behaviour of Eu3+ activated CaMoO4 nanoparticles via Zn2+ incorporation. RSC Adv. 2015, 5, 55977–55985. [Google Scholar] [CrossRef]
- Ryu, J.H.; Choi, B.G.; Yoon, J.-W.; Shim, K.B.; Machi, K.; Hamada, K. Synthesis of nanoparticles by pulsed laser ablation in deionized water and optical properties. J. Lumin. 2007, 124, 67–70. [Google Scholar] [CrossRef]
- Ansari, A.A.; Parchur, A.K.; Alam, M.; Azzeer, A. Structural and photoluminescence properties of Tb-doped CaMoO4 nanoparticles with sequential surface coatings. Mater. Chem. Phys. 2014, 147, 715–721. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, J.; Zhu, Y.; Nie, S.; Wang, W.; Shi, J.; Miu, Y. Brightly luminescent and color-tunable CaMoO4:RE3+ nanofibers synthesized through a facile route for efficient light-emitting diodes. J. Mater. Sci. 2018, 53, 4861. [Google Scholar] [CrossRef]
- Parchur, A.K.; Prasad, A.I.; Ansari, A.A.; Rai, S.B.; Ningthoujam, R.S. Luminescence properties of Tb3+-doped CaMoO4 nanoparticles: Annealing effect, polar medium dispersible, polymer film and core–shell formation. Dalton Trans. Int. J. Inorg. Chem. 2012, 41, 11032–11045. [Google Scholar] [CrossRef]
- Liu, J.; Lian, H.; Shi, C. Improved optical photoluminescence by charge compensation in the phosphor system CaMoO4:Eu3+. Opt. Mater. 2007, 29, 1591–1594. [Google Scholar] [CrossRef]
- Ansari, A.A.; Parchur, A.K.; Alam, M.; Azzeer, A. Effect of surface coating on optical properties of Eu3+-doped CaMoO4 nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 131, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Tranquilin, R.L.; Lovisa, L.X.; Almeida, C.R.R.; Paskocimas, C.A.; Li, M.S.; Oliveira, M.C.; Gracia, L.; Andres, J.J.; Longo, E.; Motta, F.V.; et al. Understanding the White-Emitting CaMoO4 Co-Doped Eu3+, Tb3+, and Tm3+ Phosphor through Experiment and Computation. J. Phys. Chem. C 2019, 123, 18536. [Google Scholar] [CrossRef]
- Letswalo, M.L.A.; Reddy, L.; Balakrishna, A.; Swart, H.C.; Ntwaeaborwa, O.M. Influence of SO42− anionic group substitution on the enhanced photoluminescence behaviour of red emitting CaMoO4:Eu3+ phosphor. J. Alloys Compd. 2021, 854, 157022. [Google Scholar] [CrossRef]
- Chance, B.; Leigh, J.S.; Miyake, H.; Smith, D.S.; Nioka, S.; Greenfeld, R.; Finander, M.; Kaufmann, K.; Levy, W.; Young, M. Comparison of Time-Resolved and -Unresolved Measurements of Deoxyhemoglobin in Brain. Proc. Natl. Acad. Sci. USA 1988, 85, 4971–4975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chukhutsina, V.U.; Holzwarth, A.R.; Croce, R. Time-resolved fluorescence measurements on leaves: Principles and recent developments. Photosynth. Res. 2019, 140, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Wang, Z.; Ba, Z.; Li, X.; Dong, J.; Fang, Y.; Zhang, Q.; Zhao, X. 3D MXene-holey graphene hydrogel for supercapacitor with superior energy storage. J. Energy Storage 2022, 47, 103911. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, J.-Y. Fabricated Flexible Composite for a UV-LED Color Filter and Anti-Counterfeiting Application of Calcium Molybdate Phosphor Synthesized at Room Temperature. Materials 2022, 15, 2078. https://doi.org/10.3390/ma15062078
Jung J-Y. Fabricated Flexible Composite for a UV-LED Color Filter and Anti-Counterfeiting Application of Calcium Molybdate Phosphor Synthesized at Room Temperature. Materials. 2022; 15(6):2078. https://doi.org/10.3390/ma15062078
Chicago/Turabian StyleJung, Jae-Yong. 2022. "Fabricated Flexible Composite for a UV-LED Color Filter and Anti-Counterfeiting Application of Calcium Molybdate Phosphor Synthesized at Room Temperature" Materials 15, no. 6: 2078. https://doi.org/10.3390/ma15062078
APA StyleJung, J.-Y. (2022). Fabricated Flexible Composite for a UV-LED Color Filter and Anti-Counterfeiting Application of Calcium Molybdate Phosphor Synthesized at Room Temperature. Materials, 15(6), 2078. https://doi.org/10.3390/ma15062078





