Aminopropyltriethoxysilane (APTES)-Modified Nanohydroxyapatite (nHAp) Incorporated with Iron Oxide (IO) Nanoparticles Promotes Early Osteogenesis, Reduces Inflammation and Inhibits Osteoclast Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Iron Oxide Nanoparticles (IO)
2.2. Synthesis of Hydroxyapatite Nanoparticles (nHAp)
2.3. Synthesis of Nanohydroxyapatite–Iron Oxide Composite (nHAp/IO)
2.4. Surface Modification of nHAp, nHAp/IO and IO Using APTES
2.5. Characterization of Fabricated nHAp@APTES, IO@APTES and nHAp/IO@APTES
2.6. Cell Lines
2.7. In Vitro Toxicology Assay
2.8. Mitochondria Network Evaluation
2.9. Western Blot
2.10. Gene Expression Evaluation
2.11. Osteoblasts and Osteoclast Coculturing
2.12. Statistical Analysis
3. Results
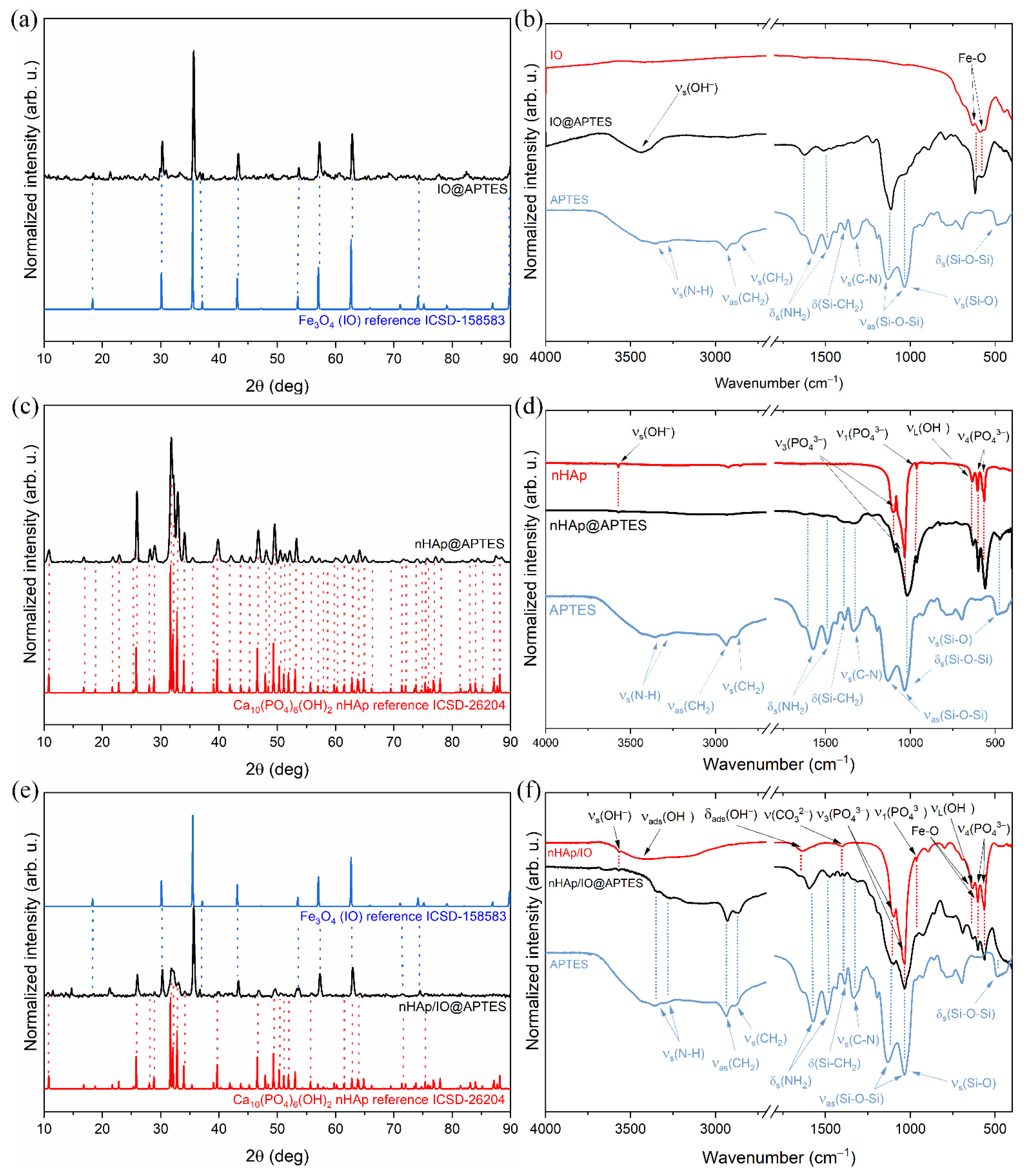
3.1. Structure of the Obtained Materials
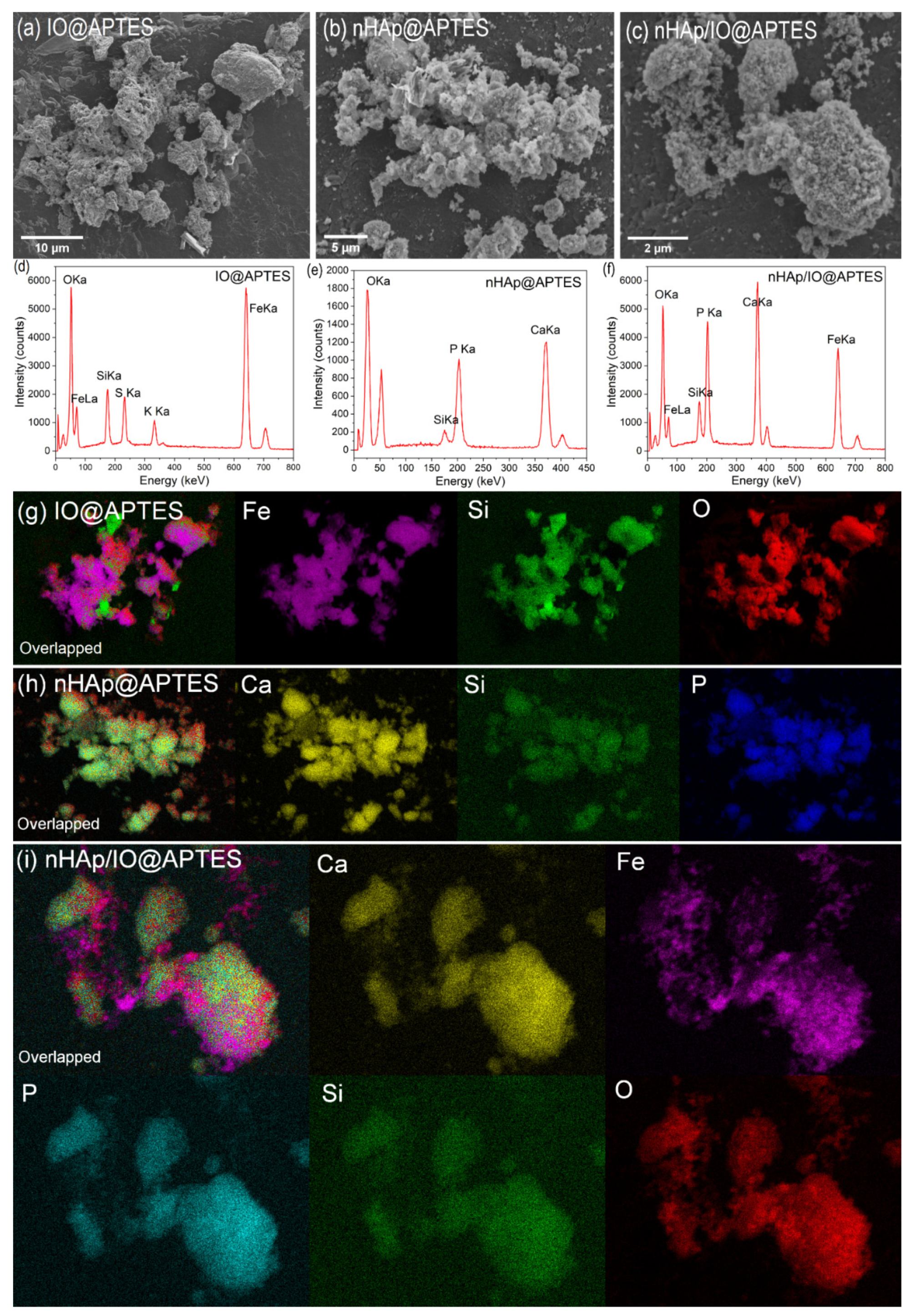
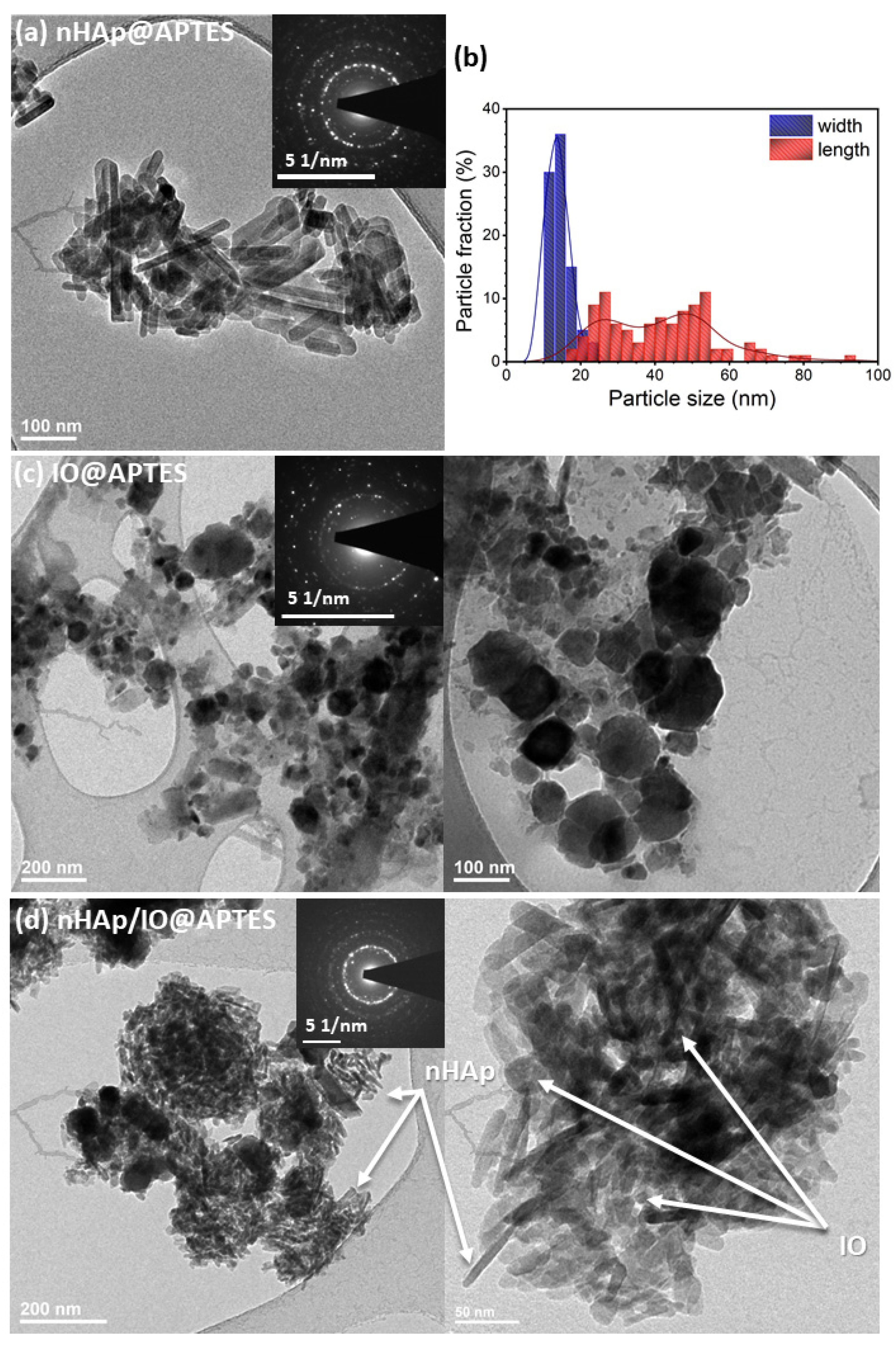
3.2. Quantitative Analysis, Morphology and Particle Size of the Obtained Materials
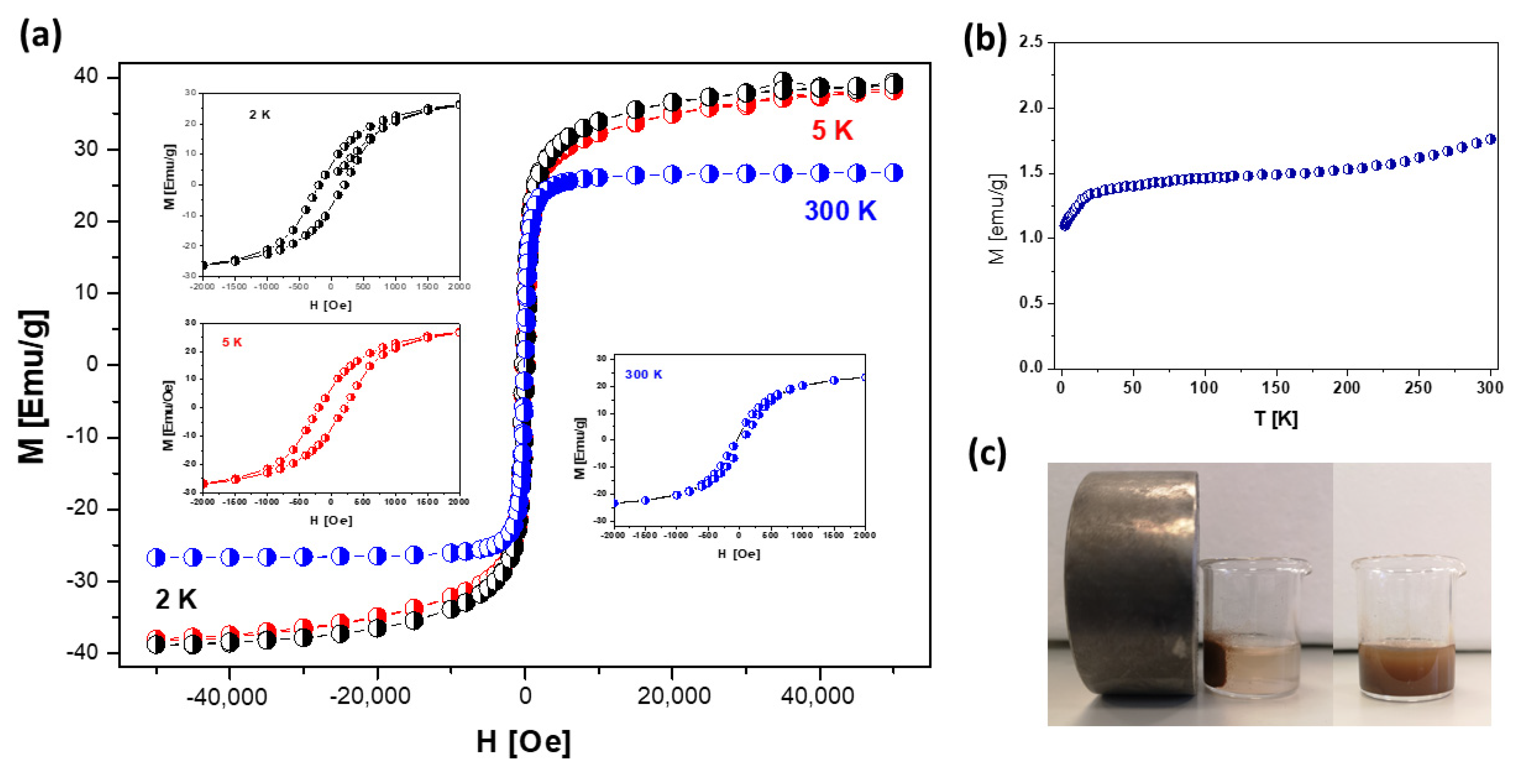
3.3. Magnetic Properties of nHAp/IO@APTES
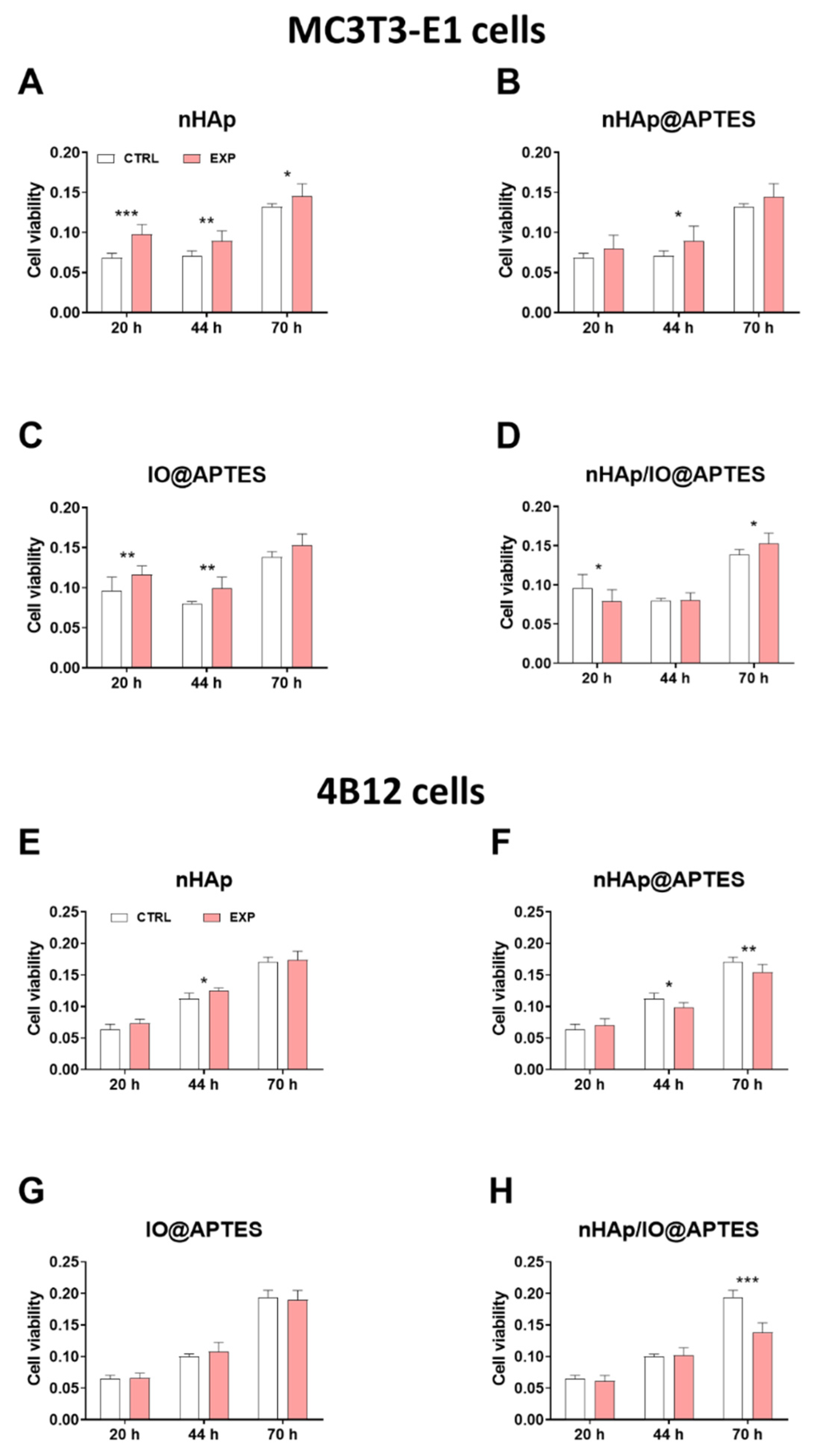
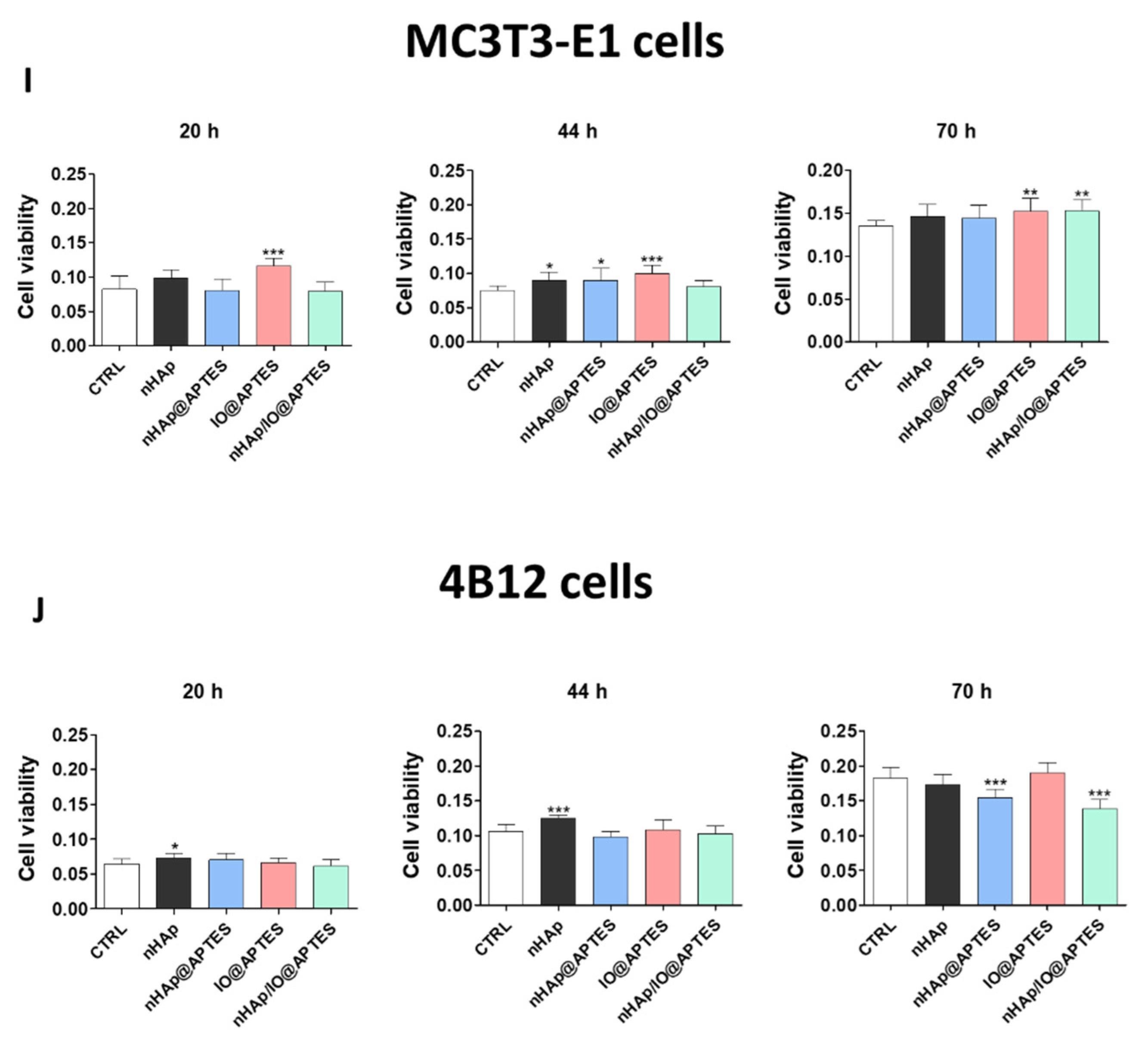
3.4. Cytotoxicity
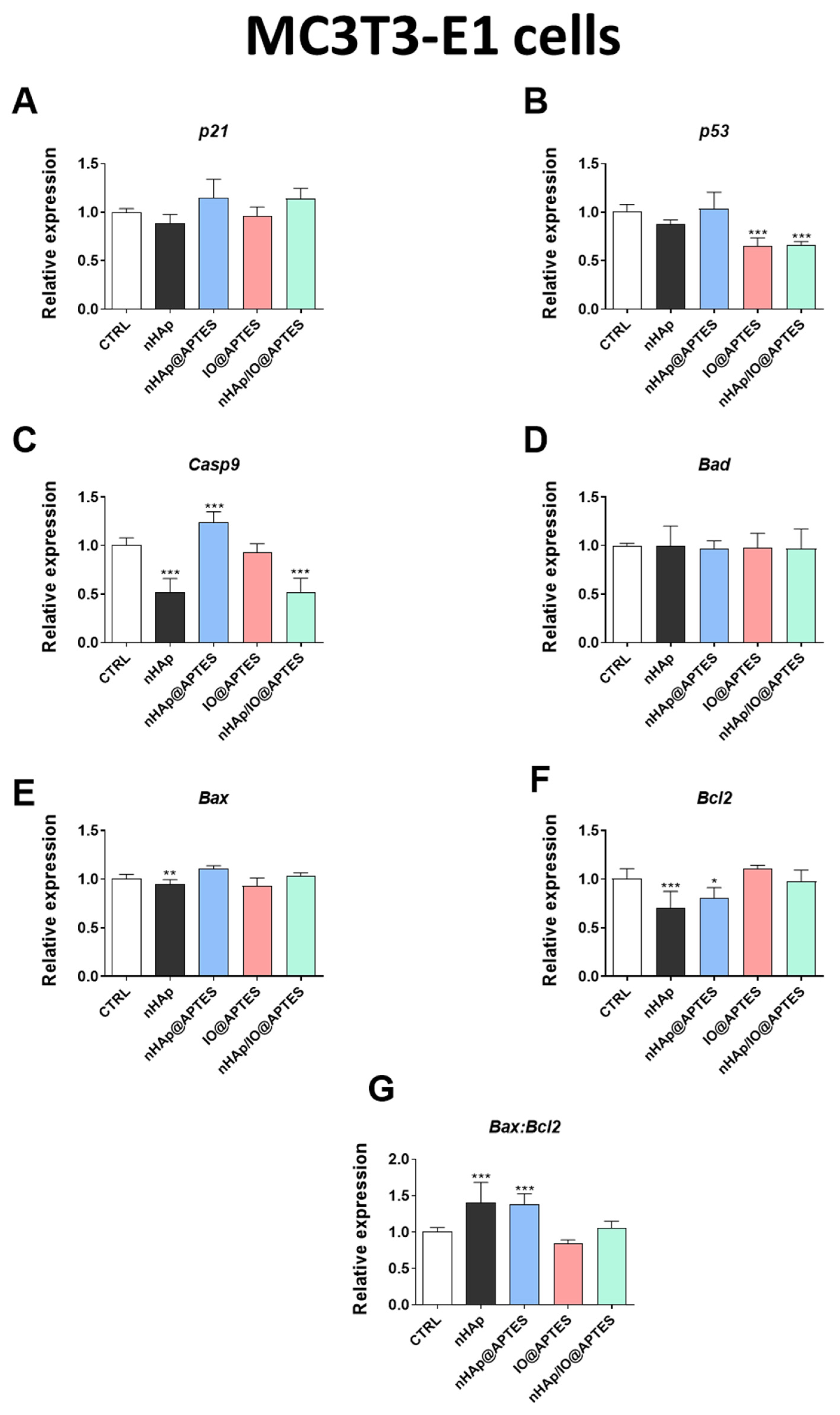
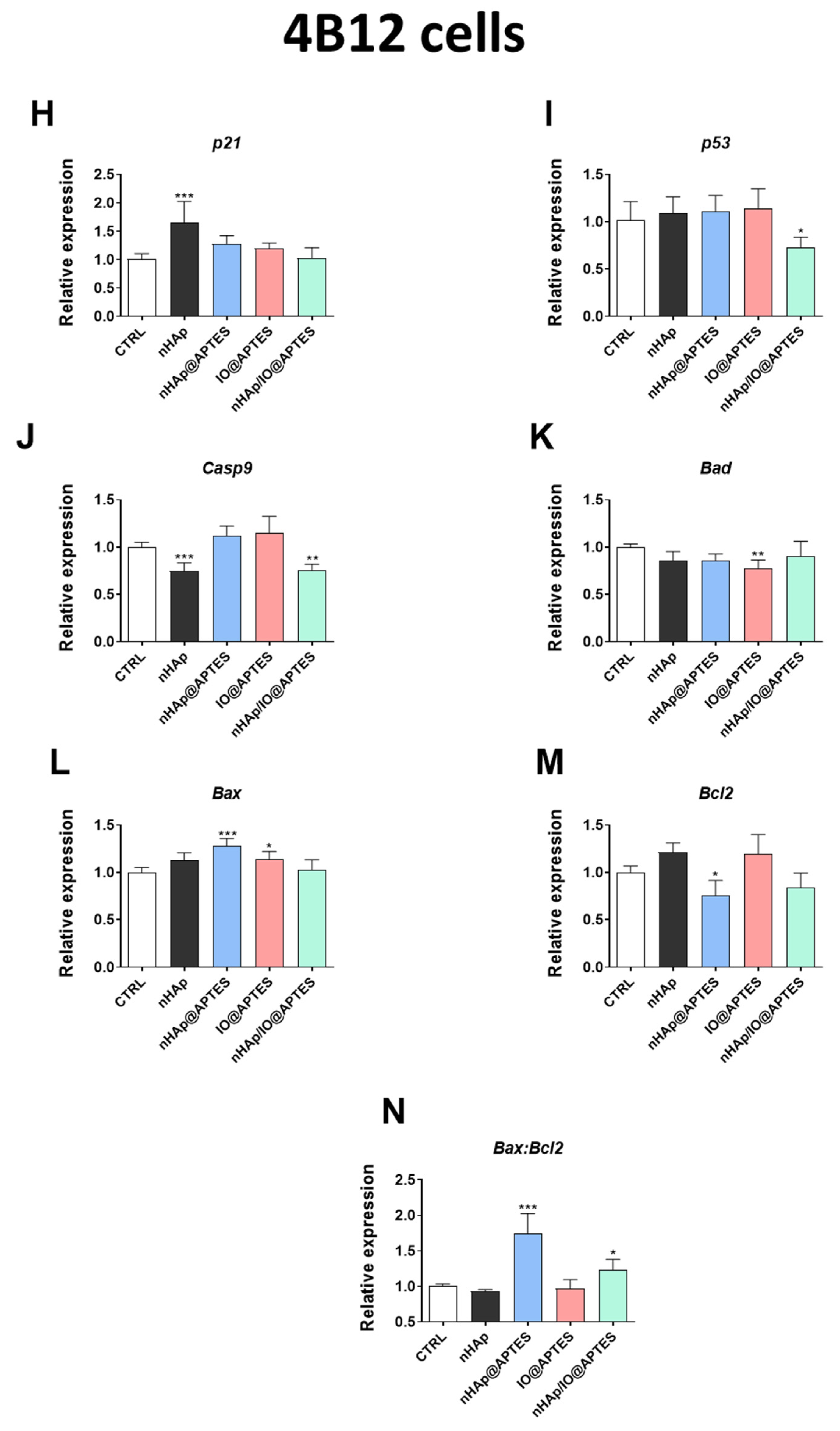
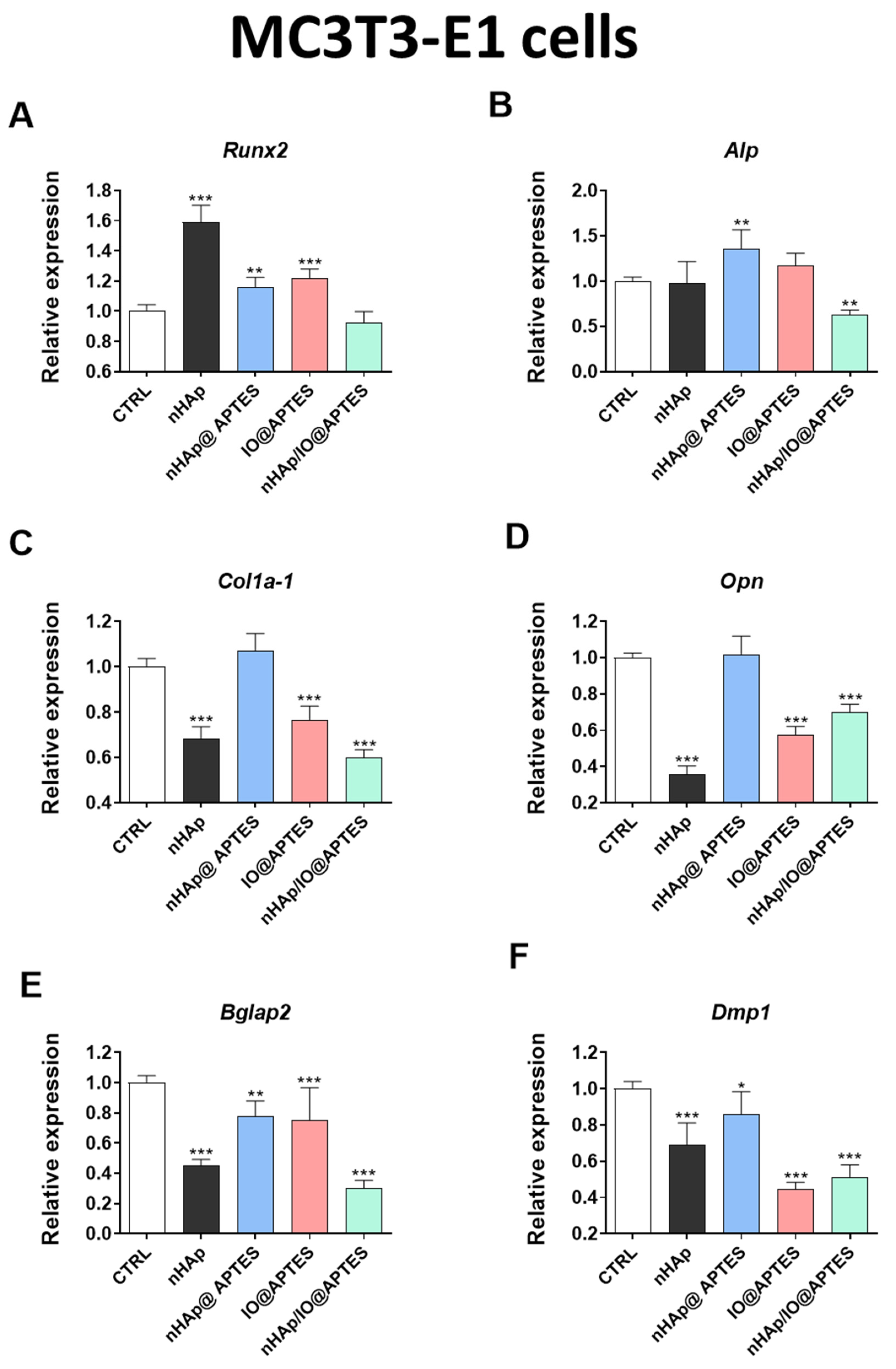
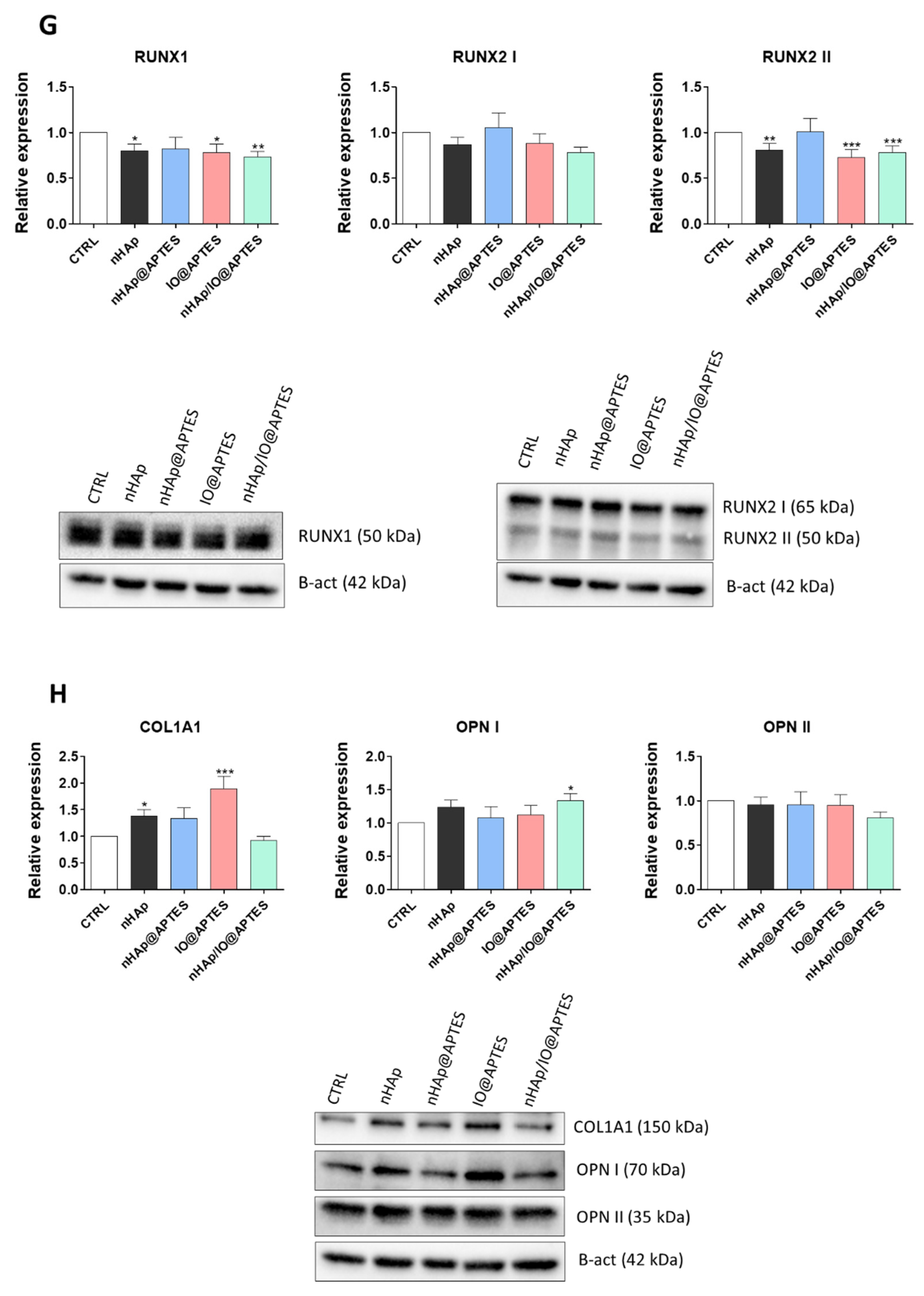
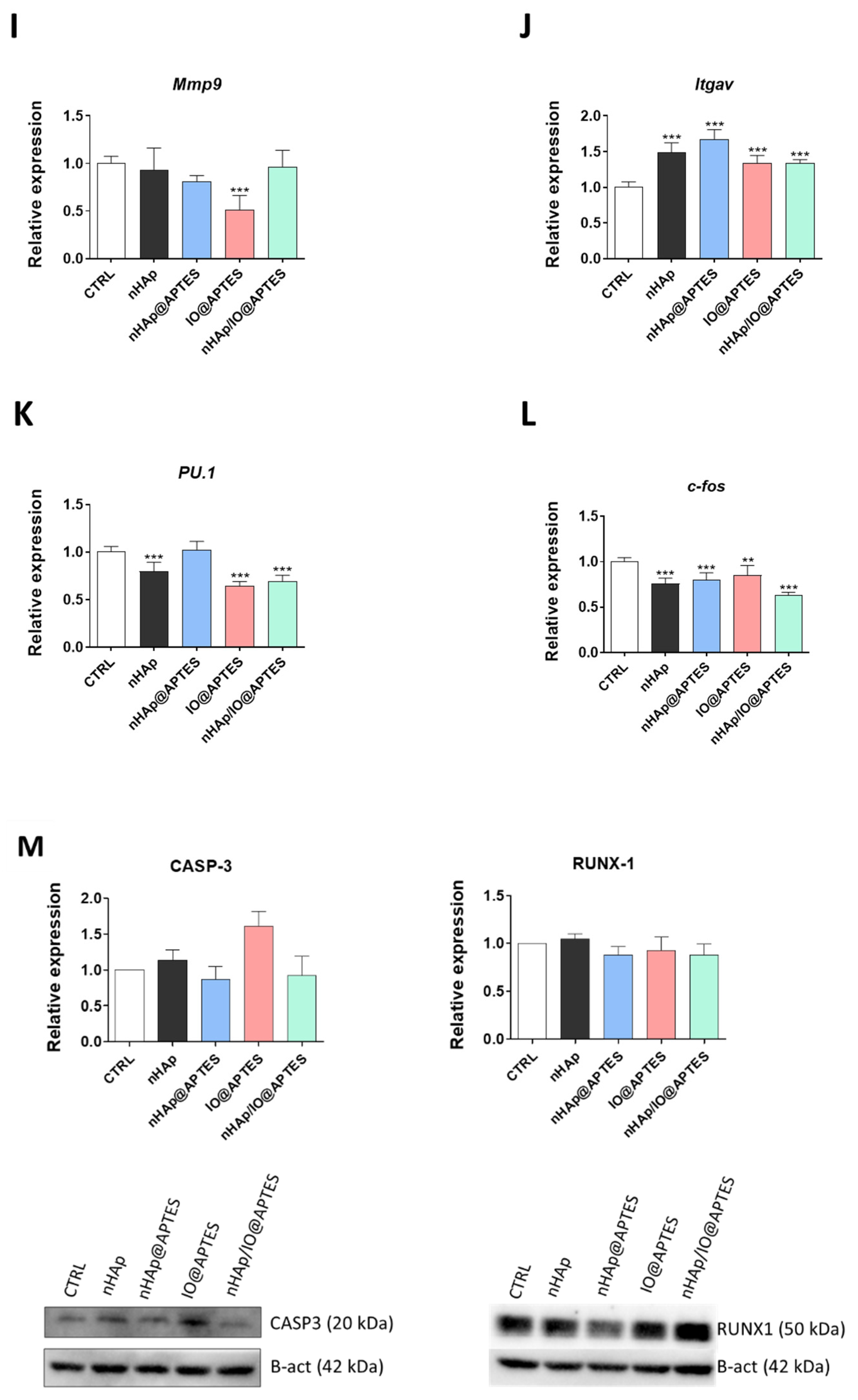
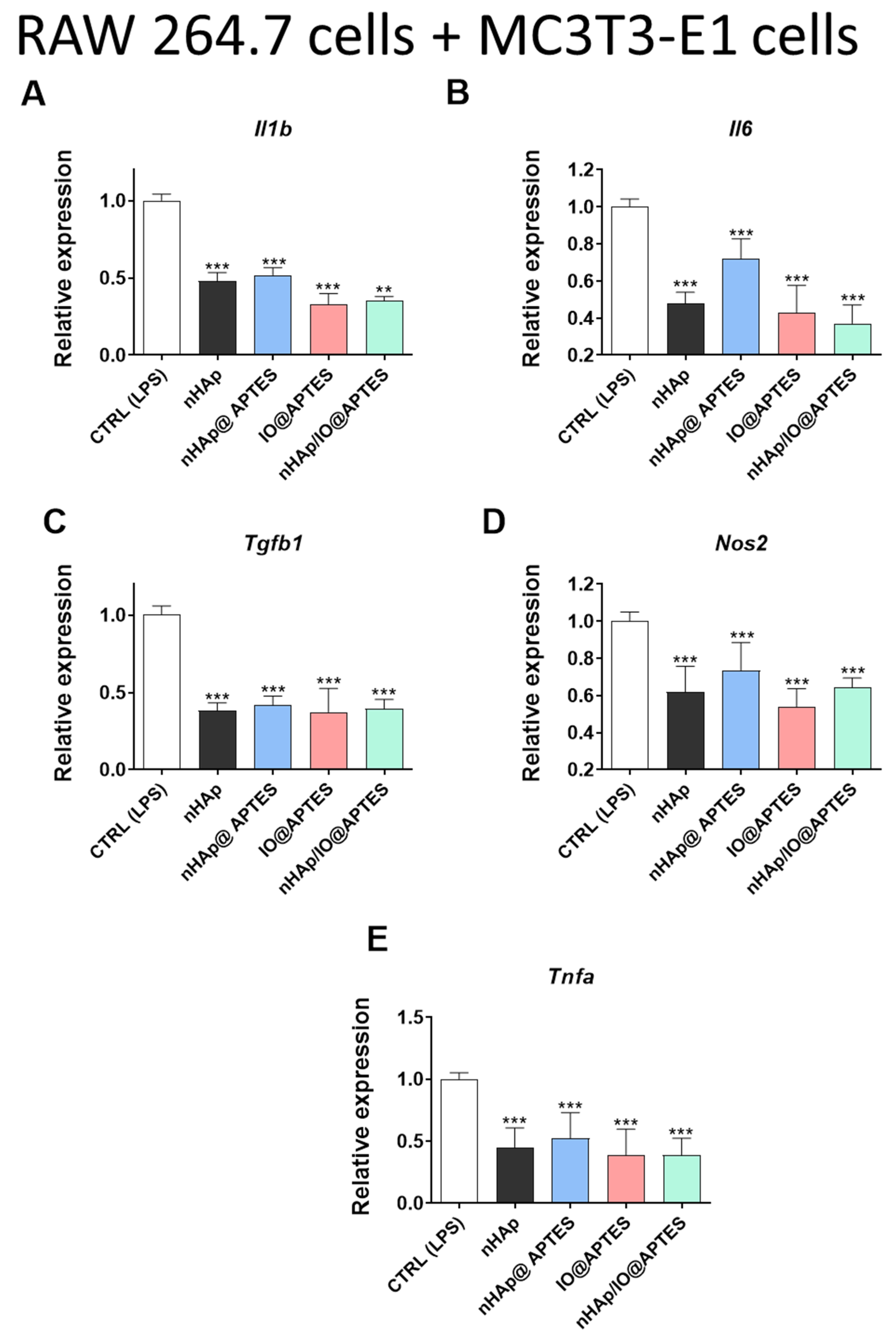
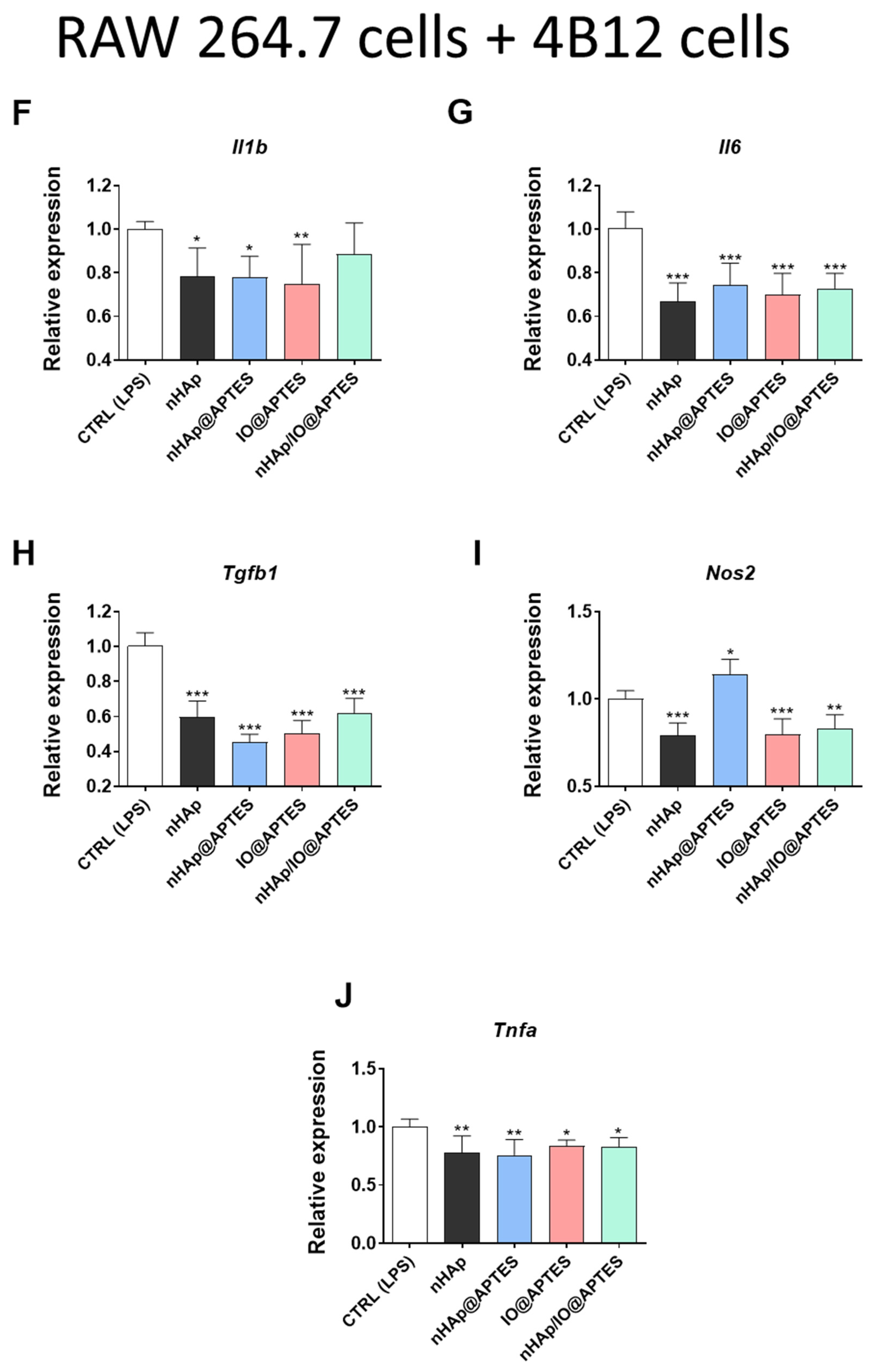
3.5. Gene Expression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cosman, F.; De Beur, S.J.; LeBoff, M.S.; Lewiecki, E.M.; Tanner, B.; Randall, S.; Lindsay, R. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos. Int. 2014, 25, 2359–2381. [Google Scholar] [CrossRef] [PubMed]
- Sozen, T.; Ozisik, L.; Basaran, N.C. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Akkawi, I.; Zmerly, H. Osteoporosis: Current Concepts. Joints 2018, 6, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Langdahl, B.; Ferrari, S.L.; Dempster, D.W. Bone modeling and remodeling: Potential as therapeutic targets for the treatment of osteoporosis. Ther. Adv. Musculoskelet. Dis. 2016, 8, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Regulation of Proliferation, Differentiation and Functions of Osteoblasts by Runx2. Int. J. Mol. Sci. 2019, 20, 1694. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, N.; Rosenberg, O.; Soudry, M. Osteoblasts in Bone Physiology—Mini Review. Rambam Maimonides Med. J. 2012, 3, e0013. [Google Scholar] [CrossRef]
- Ginaldi, L.; Di Benedetto, M.C.; De Martinis, M. Osteoporosis, inflammation and ageing. Immun. Ageing 2005, 2, 14. [Google Scholar] [CrossRef]
- Goonasekera, C.S.; Jack, K.S.; Cooper-White, J.J.; Grøndahl, L. Attachment of poly(acrylic acid) to 3-aminopropyltriethoxysilane surface-modified hydroxyapatite. J. Mater. Chem. B 2013, 1, 5842–5852. [Google Scholar] [CrossRef]
- Kim, J.; Seidler, P.; Wan, L.S.; Fill, C. Formation, structure, and reactivity of amino-terminated organic films on silicon substrates. J. Colloid Interface Sci. 2009, 329, 114–119. [Google Scholar] [CrossRef]
- Michelot, A.; Sarda, S.; Audin, C.; Deydier, E.; Manoury, E.; Poli, R.; Rey, C. Spectroscopic characterisation of hydroxyapatite and nanocrystalline apatite with grafted aminopropyltriethoxysilane: Nature of silane–surface interaction. J. Mater. Sci. 2015, 50, 5746–5757. [Google Scholar] [CrossRef]
- Durrieu, M.-C.; Pallu, S.; Guillemot, F.; Bareille, R.; Amédée, J.; Baquey, C.; Labrugère, C.; Dard, M. Grafting RGD containing peptides onto hydroxyapatite to promote osteoblastic cells adhesion. J. Mater. Sci. Mater. Med. 2004, 15, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Qiu, F.; Zhang, Y.; Cao, W.; Wu, Z.; Nian, S.; Zhou, N. Novel vaterite-containing tricalcium silicate bone cement by surface functionalization using 3-aminopropyltriethoxysilane: Setting behavior, in vitro bioactivity and cytocompatibility. Biomed. Mater. 2017, 12, 65007. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, N.; Moratti, S.C.; Dias, G.J. Hydroxyapatite-polymer biocomposites for bone regeneration: A review of current trends. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2046–2057. [Google Scholar] [CrossRef] [PubMed]
- Gomes, D.S.; Santos, A.M.C.; Neves, G.A.; Menezes, R. A brief review on hydroxyapatite production and use in biomedicine. Cerâmica 2019, 65, 282–302. [Google Scholar] [CrossRef]
- Khajuria, D.K.; Vasireddi, R.; Trebbin, M.; Karasik, D.; Razdan, R. Novel therapeutic intervention for osteoporosis prepared with strontium hydroxyapatite and zoledronic acid: In vitro and pharmacodynamic evaluation. Mater. Sci. Eng. C 2017, 71, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Khajuria, D.K.; Kumar, V.B.; Gigi, D.; Gedanken, A.; Karasik, D. Accelerated Bone Regeneration by Nitrogen-Doped Carbon Dots Functionalized with Hydroxyapatite Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 19373–19385. [Google Scholar] [CrossRef] [PubMed]
- Jegatheeswaran, S.; Sundrarajan, M. PEGylation of novel hydroxyapatite/PEG/Ag nanocomposite particles to improve its antibacterial efficacy. Mater. Sci. Eng. C 2015, 51, 174–181. [Google Scholar] [CrossRef]
- Gao, C.; Deng, Y.; Feng, P.; Mao, Z.; Li, P.; Yang, B.; Deng, J.; Cao, Y.; Shuai, C.; Peng, S. Current Progress in Bioactive Ceramic Scaffolds for Bone Repair and Regeneration. Int. J. Mol. Sci. 2014, 15, 4714–4732. [Google Scholar] [CrossRef]
- Alaribe, F.N.; Manoto, S.L.; Motaung, S.C.K.M. Scaffolds from biomaterials: Advantages and limitations in bone and tissue engineering. Biologia 2016, 71, 353–366. [Google Scholar] [CrossRef]
- Song, B.; Xu, Q.; Wang, C.; Xu, S.; Zhang, H. Fabrication of polymer/drug-loaded hydroxyapatite particle composite fibers for drug sustained release. J. Appl. Polym. Sci. 2015, 133. [Google Scholar] [CrossRef]
- Yusoff, A.H.M.; Salimi, M.N.; Jamlos, M.F. Synthesis of Superparamagnetic Hydroxyapatite Core-Shell Nanostructure by a Rapid Sol-Gel Route. J. Surf. Sci. Nanotechnol. 2017, 15, 121–126. [Google Scholar] [CrossRef]
- Arruebo, M.; Fernández-Pacheco, R.; Ibarra, M.R.; Santamaría, J. Magnetic nanoparticles for drug delivery. Nano Today 2007, 2, 22–32. [Google Scholar] [CrossRef]
- Chen, R.; Jia, Z.-Y. Efficient nano iron particle-labeling and noninvasive MR imaging of mouse bone marrow-derived endothelial progenitor cells. Int. J. Nanomed. 2011, 6, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, J.; Hakata, T.; Kato, R.; Yamashita, N.; Morino, T.; Kobayashi, T.; Honda, H. Size dependent heat generation of magnetite nanoparticles under AC magnetic field for cancer therapy. Biomagn. Res. Technol. 2008, 6, 4. [Google Scholar] [CrossRef]
- Tang, I.-M.; Krishnamra, N.; Charoenphandhu, N.; Hoonsawat, R.; Pon-On, W. Biomagnetic of Apatite-Coated Cobalt Ferrite: A Core–Shell Particle for Protein Adsorption and pH-Controlled Release. Nanoscale Res. Lett. 2010, 6, 19. [Google Scholar] [CrossRef]
- Özgür, M.E.; Ulu, A.; Balcioglu, S.; Özcan, I.; Köytepe, S.; Ateş, B. The Toxicity Assessment of Iron Oxide (Fe3O4) Nanoparticles on Physical and Biochemical Quality of Rainbow Trout Spermatozoon. Toxics 2018, 6, 62. [Google Scholar] [CrossRef]
- Marycz, K.; Sobierajska, P.; Wiglusz, R.; Idczak, R.; Nedelec, J.-M.; Fal, A.; Kornicka-Garbowska, K. Fe3O4 Magnetic Nanoparticles Under Static Magnetic Field Improve Osteogenesis via RUNX-2 and Inhibit Osteoclastogenesis by the Induction of Apoptosis. Int. J. Nanomed. 2020, 15, 10127–10148. [Google Scholar] [CrossRef]
- Sobierajska, P.; Wiglusz, R.J. Influence of Li+ ions on the physicochemical properties of nanocrystalline calcium–strontium hydroxyapatite doped with Eu3+ ions. New J. Chem. 2019, 43, 14908–14916. [Google Scholar] [CrossRef]
- Karthickraja, D.; Karthi, S.; Kumar, G.A.; Sardar, D.K.; Dannangoda, G.C.; Martirosyan, K.S.; Girija, E.K. Fabrication of core–shell CoFe2O4@HAp nanoparticles: A novel magnetic platform for biomedical applications. New J. Chem. 2019, 43, 13584–13593. [Google Scholar] [CrossRef]
- Elayaraja, K.; Rajesh, P.; Joshy, M.A.; Chandra, V.S.; Suganthi, R.; Kennedy, J.V.; Kulriya, P.; Sulania, I.; Asokan, K.; Kanjilal, D.; et al. Enhancement of wettability and antibiotic loading/release of hydroxyapatite thin film modified by 100MeV Ag7+ ion irradiation. Mater. Chem. Phys. 2012, 134, 464–477. [Google Scholar] [CrossRef]
- Yamanaka, T.; Shimazu, H.; Ota, K. Electric conductivity of Fe 2 SiO 4 -Fe 3 O 4 spinel solid solutions. Phys. Chem. Miner. 2001, 28, 110–118. [Google Scholar] [CrossRef]
- Ansari, A.; Vahedi, S.; Tavakoli, O.; Khoobi, M.; Faramarzi, M.A. Novel Fe3 O4 /hydroxyapatite/β-cyclodextrin nanocomposite adsorbent: Synthesis and application in heavy metal removal from aqueous solution. Appl. Organomet. Chem. 2019, 33, e4634. [Google Scholar] [CrossRef]
- Yao, S.; Yan, X.; Zhao, Y.; Li, B.; Sun, L. Selective binding and magnetic separation of histidine-tagged proteins using Ni2+-decorated Fe3O4/hydroxyapatite composite nanoparticles. Mater. Lett. 2014, 126, 97–100. [Google Scholar] [CrossRef]
- Wei, Y.; Han, B.; Hu, X.; Lin, Y.; Wang, X.; Deng, X. Synthesis of Fe3O4 Nanoparticles and their Magnetic Properties. Procedia Eng. 2012, 27, 632–637. [Google Scholar] [CrossRef]
- Russo, L.; Taraballi, F.; Lupo, C.; Poveda, A.; Jimenez-Barbero, J.; Sandri, M.; Tampieri, A.; Nicotra, F.; Cipolla, L. Carbonate hydroxyapatite functionalization: A comparative study towards (bio)molecules fixation. Interface Focus 2014, 4, 20130040. [Google Scholar] [CrossRef]
- Majoul, N.; Aouida, S.; Bessaïs, B. Progress of porous silicon APTES-functionalization by FTIR investigations. Appl. Surf. Sci. 2015, 331, 388–391. [Google Scholar] [CrossRef]
- Shi, X.; Wang, S.; Wen, S.; Shen, M.; Guo, R.; Cao, X. Aminopropyltriethoxysilane-mediated surface functionalization of hydroxyapatite nanoparticles: Synthesis, characterization, and in vitro toxicity assay. Int. J. Nanomed. 2011, 6, 3449–3459. [Google Scholar] [CrossRef]
- Raya, I.; Mayasari, E.; Yahya, A.; Syahrul, M.; Latunra, A. Shynthesis and Characterizations of Calcium Hydroxyapatite Derived from Crabs Shells (Portunus pelagicus) and Its Potency in Safeguard against to Dental Demineralizations. Int. J. Biomater. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Zawisza, K.; Sobierajska, P.; Nowak, N.; Kedziora, A.; Korzekwa, K.; Poźniak, B.; Tikhomirov, M.; Miller, J.; Mrówczyńska, L.; Wiglusz, R.J. Preparation and preliminary evaluation of bio-nanocomposites based on hydroxyapatites with antibacterial properties against anaerobic bacteria. Mater. Sci. Eng. C 2020, 106, 110295. [Google Scholar] [CrossRef]
- Gheisari, H.; Karamian, E.; Abdellahi, M. A novel hydroxyapatite—Hardystonite nanocomposite ceramic. Ceram. Int. 2015, 41, 5967–5975. [Google Scholar] [CrossRef]
- Astala, R.; Stott, M.J. First Principles Investigation of Mineral Component of Bone: CO3 Substitutions in Hydroxyapatite. Chem. Mater. 2005, 17, 4125–4133. [Google Scholar] [CrossRef]
- Bang, L.T.; Long, B.D.; Othman, R. Carbonate Hydroxyapatite and Silicon-Substituted Carbonate Hydroxyapatite: Synthesis, Mechanical Properties, and Solubility Evaluations. Sci. World J. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Landi, E.; Tampieri, A.; Celotti, G.; Vichi, L.; Sandri, M. Influence of synthesis and sintering parameters on the characteristics of carbonate apatite. Biomaterials 2004, 25, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- Gibson, I.R.; Bonfield, W. Novel synthesis and characterization of an AB-type carbonate-substituted hydroxyapatite. J. Biomed. Mater. Res. 2002, 59, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Wang, Y.; Jones, S.K.; Hawkett, B.; Warr, G.G. Optimized Steric Stabilization of Aqueous Ferrofluids and Magnetic Nanoparticles. Langmuir 2010, 26, 4465–4472. [Google Scholar] [CrossRef] [PubMed]
- Woo, K.; Hong, J.; Choi, S.; Lee, H.-W.; Ahn, J.-P.; Kim, C.S.; Lee, S.W. Easy Synthesis and Magnetic Properties of Iron Oxide Nanoparticles. Chem. Mater. 2004, 16, 2814–2818. [Google Scholar] [CrossRef]
- Raynaud, S.; Champion, E.; Bernache-Assollant, D.; Thomas, P. Calcium phosphate apatites with variable Ca/P atomic ratio I. Synthesis, characterisation and thermal stability of powders. Biomaterials 2002, 23, 1065–1072. [Google Scholar] [CrossRef]
- Singh, A.K.; Srivastava, O.N.; Singh, K. Shape and Size-Dependent Magnetic Properties of Fe3O4 Nanoparticles Synthesized Using Piperidine. Nanoscale Res. Lett. 2017, 12, 1–7. [Google Scholar] [CrossRef]
- Ghosh, B.; Kumar, S.; Poddar, A.; Mazumdar, C.; Banerjee, S.; Reddy, V.R.; Gupta, A. Spin glasslike behavior and magnetic enhancement in nanosized Ni–Zn ferrite system. J. Appl. Phys. 2010, 108, 34307. [Google Scholar] [CrossRef]
- Bhowmik, R.N.; Ranganathan, R.; Nagarajan, R.; Ghosh, B.; Kumar, S. Role of strain-induced anisotropy on magnetic enhancement in mechanically alloyedCo0.2Zn0.8Fe2O4nanoparticle. Phys. Rev. B 2005, 72, 94405. [Google Scholar] [CrossRef]
- Gao, C.; Peng, S.; Feng, P.; Shuai, C. Bone biomaterials and interactions with stem cells. Bone Res. 2017, 5, 17059. [Google Scholar] [CrossRef] [PubMed]
- Ishack, S.; Mediero, A.; Wilder, T.; Ricci, J.L.; Cronstein, B.N. Bone regeneration in critical bone defects using three-dimensionally printed β-tricalcium phosphate/hydroxyapatite scaffolds is enhanced by coating scaffolds with either dipyridamole or BMP-2. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Sterling, J.A.; Guelcher, S.A. Biomaterial Scaffolds for Treating Osteoporotic Bone. Curr. Osteoporos. Rep. 2014, 12, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Bal, Z.; Kaito, T.; Korkusuz, F.; Yoshikawa, H. Bone regeneration with hydroxyapatite-based biomaterials. Emerg. Mater. 2019, 3, 1–24. [Google Scholar] [CrossRef]
- Khajuria, D.K.; Disha, C.; Vasireddi, R.; Razdan, R.; Mahapatra, D.R. Risedronate/zinc-hydroxyapatite based nanomedicine for osteoporosis. Mater. Sci. Eng. C 2016, 63, 78–87. [Google Scholar] [CrossRef]
- Khajuria, D.K.; Razdan, R.; Mahapatra, D.R. Development, in vitro and in vivo characterization of zoledronic acid functionalized hydroxyapatite nanoparticle based formulation for treatment of osteoporosis in animal model. Eur. J. Pharm. Sci. 2015, 66, 173–183. [Google Scholar] [CrossRef]
- Zhang, H.; Li, S.; Liu, Y.; Yu, Y.; Lin, S.; Wang, Q.; Miao, L.; Wei, H.; Sun, W. Fe3O4@GO magnetic nanocomposites protect mesenchymal stem cells and promote osteogenic differentiation of rat bone marrow mesenchymal stem cells. Biomater. Sci. 2020, 8, 5984–5993. [Google Scholar] [CrossRef]
- Govindan, R.; Karthi, S.; Kumar, G.S.; Girija, E.K. Development of Fe3O4 integrated polymer/phosphate glass composite scaffolds for bone tissue engineering. Mater. Adv. 2020, 1, 3466–3475. [Google Scholar] [CrossRef]
- Syga, Ł.; Spakman, D.; Punter, C.M.; Poolman, B. Method for immobilization of living and synthetic cells for high-resolution imaging and single-particle tracking. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Dadfar, S.M.; Camozzi, D.; Darguzyte, M.; Roemhild, K.; Varvarà, P.; Metselaar, J.; Banala, S.; Straub, M.; Güvener, N.; Engelmann, U.; et al. Size-isolation of superparamagnetic iron oxide nanoparticles improves MRI, MPI and hyperthermia performance. J. Nanobiotechnol. 2020, 18, 1–13. [Google Scholar] [CrossRef]
- Balogh, E.; Paragh, G.; Jeney, V. Influence of Iron on Bone Homeostasis. Pharmaceuticals 2018, 11, 107. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, I.; Terracciano, M.; Martucci, N.M.; De Stefano, L.; Migliaccio, N.; Tatè, R.; Rendina, I.; Arcari, P.; Lamberti, A.; Rea, I. Diatomite silica nanoparticles for drug delivery. Nanoscale Res. Lett. 2014, 9, 329. [Google Scholar] [CrossRef]
- Kamarudin, N.; Jalil, A.A.; Triwahyono, S.; Salleh, N.F.M.; Karim, A.; Mukti, R.; Hameed, B.H.; Ahmad, A. Role of 3-aminopropyltriethoxysilane in the preparation of mesoporous silica nanoparticles for ibuprofen delivery: Effect on physicochemical properties. Microporous Mesoporous Mater. 2013, 180, 235–241. [Google Scholar] [CrossRef]
- Śmieszek, A.; Marycz, K.; Szustakiewicz, K.; Kryszak, B.; Targonska, S.; Zawisza, K.; Watras, A.; Wiglusz, R.J. New approach to modification of poly (l-lactic acid) with nano-hydroxyapatite improving functionality of human adipose-derived stromal cells (hASCs) through increased viability and enhanced mitochondrial activity. Mater. Sci. Eng. C 2019, 98, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Marycz, K.; Marędziak, M.; Grzesiak, J.; Lis, A.; Śmieszek, A. Biphasic Polyurethane/Polylactide Sponges Doped with Nano-Hydroxyapatite (nHAp) Combined with Human Adipose-Derived Mesenchymal Stromal Stem Cells for Regenerative Medicine Applications. Polymers 2016, 8, 339. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Chan, W.-I.; Yu, N.; Gan, J.; Dong, L.; Wang, C. APTES-modified nanosilica -- but neither APTES nor nanosilica -- inhibits endothelial cell growth via arrest of cell cycle at G1 phase. J. Biomater. Appl. 2015, 30, 608–617. [Google Scholar] [CrossRef]
- Chaves, A.V.; Freire, R.M.; Feitosa, V.P.; Ricardo, N.M.P.S.; DeNardin, J.C.; Neto, D.M.A.; Fechine, P.B.A. Hydroxyapatite-Based Magnetic Bionanocomposite as Pharmaceuticals Carriers in Chitosan Scaffolds. J. Compos. Sci. 2021, 5, 37. [Google Scholar] [CrossRef]
- Mohammadi-Aghdam, S.; Valinezhad-Saghezi, B.; Mortazavi, Y.; Ghoreishi, S.M. Modified Fe3O4/HAp Magnetically Nanoparticles as the Carrier for Ibuprofen: Adsorption and Release Study. Drug Res. 2018, 69, 93–99. [Google Scholar] [CrossRef]
- Zheltova, V.; Vlasova, A.; Bobrysheva, N.; Abdullin, I.; Semenov, V.; Osmolowsky, M.; Voznesenskiy, M.; Osmolovskaya, O. Fe3O4@HAp core–shell nanoparticles as MRI contrast agent: Synthesis, characterization and theoretical and experimental study of shell impact on magnetic properties. Appl. Surf. Sci. 2020, 531, 147352. [Google Scholar] [CrossRef]
- Mollazadeh, S.; Bazzaz, B.S.F.; Kerachian, M.A. Role of apoptosis in pathogenesis and treatment of bone-related diseases. J. Orthop. Surg. Res. 2015, 10, 1–7. [Google Scholar] [CrossRef]
- Dobson, P.F.; Dennis, E.P.; Hipps, D.; Reeve, A.; Laude, A.; Bradshaw, C.; Stamp, C.; Smith, A.; Deehan, D.J.; Turnbull, D.M.; et al. Mitochondrial dysfunction impairs osteogenesis, increases osteoclast activity, and accelerates age related bone loss. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yang, X.; Zhao, Y.; Li, Y.; Guo, L. Effects of 17β-Estradiol on Mitophagy in the Murine MC3T3-E1 Osteoblast Cell Line is Mediated via G Protein-Coupled Estrogen Receptor and the ERK1/2 Signaling Pathway. Med. Sci. Monit. 2018, 24, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.-P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2010, 7, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Ruscitti, P.; Cipriani, P.; Carubbi, F.; Liakouli, V.; Zazzeroni, F.; Di Benedetto, P.; Berardicurti, O.; Alesse, E.; Giacomelli, R. The Role of IL-1βin the Bone Loss during Rheumatic Diseases. Mediat. Inflamm. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Cardoso, P.R.G.; Fragoso, T.S.; Barbosa, A.D.; Rêgo, M.J.B.D.M.; Pitta, I.D.R.; Duarte, A.L.B.P.; Marques, C.D.L.; Pitta, M.G.D.R. Cytokine measurements in Brazilian postmenopausal osteoporosis patients reveal high levels of IL-8. Sci. Med. 2016, 26, 23399. [Google Scholar] [CrossRef]
| Protein | Dilution | Manufacturer, Catalog No. |
|---|---|---|
| CASP3 | 1:500 | Sigma Aldrich, C8487 |
| COL1A1 | 1:50 | Sigma Aldrich, A2066 |
| RUNX1 | 1:200 | Santa Cruz, sc-365644 |
| RUNX2 | 1:100 | Santa Cruz, sc-390351 |
| OPN | 1:1000 | Abcam, ab8448 |
| Β-ACT | 1:2500 | Sigma Aldrich, A5441 |
| Gene | Primers (5′→3′) | Length of Amplicon | Accession No. |
|---|---|---|---|
| Alp | F: TTCATAAGCAGGCGGGGGAG R: TGAGATTCGTCCCTCGCTGG | 198 | NM_007431.3 |
| Bad | F: ACATTCATCAGCAGGGACGG R: ATCCCTTCATCCTCCTCGGT | 115 | NM_001285453.1 |
| Bax | F: AGGACGCATCCACCAAGAAGC R: GGTTCTGATCAGCTCGGGCA | 251 | NM_007527.3 |
| Bcl2 | F: GGATCCAGGATAACGGAGGC R: ATGCACCCAGAGTGATGCAG | 141 | NM_009741.5 |
| Bglap2 | F: CTCCTGAGAGTCTGACAAAGCCTT R: GCTGTGACATCCATTACTTGC | 100 | NM_001032298.3 |
| Casp9 | F: CCGGTGGACATTGGTTCTGG R: GCCATCTCCATCAAAGCCGT | 278 | NM_001355176.1 |
| c-fos | F: CCAGTCAAGAGCATCAGCAA R: TAAGTAGTGCAGCCCGGAGT | 248 | NM_010234.3 |
| Col1a-1 | F: CCAGCCGCAAAGAGTCTACA R: CAGGTTTCCACGTCTCACCA | 175 | NM_007742.4 |
| Dmp1 | F: CCCAGAGGCACAGGCAAATA R: TCCTCCCCAATGTCCTTCTT | 211 | NM_001359013.1 |
| Gapdh | F: TGCACCACCAACTGCTTAG R: GGATGCAGGGATGATGTTC | 177 | NM_001289726.1 |
| Il-1b | F: TGCCACCTTTTGACAGTGATG R: TGATGTGCTGCTGCGAGATT | 138 | NM_008361.4 |
| Il6 | F: GAGGATACCACTCCCAACAGACC R: AAGTGCATCATCGTTGTTCATACA | 141 | NM_001314054.1 |
| Itgav | F: ACAATGTAAGCCCAGTTGTGTCT R: TTTGTAAGGCCACTGGAGATTTA | 236 | NM_008402.3 |
| Mff | F: TCACATTTGGTGAGTGGGGC R: TTTTCCGGGACCCTCATTCG | 125 | NM_001372412.1 |
| Mfn1 | F: ATCACTGCAATCTTCGGCCA R: AGCAGTTGGTTGTGTGACCA | 283 | NM_024200.4 |
| Mmp9 | F: TTGCCCCTACTGGAAGGTATTAT R: GAGAATCTCTGAGCAATCCTTGA | 172 | NM_013599.4 |
| Nos2 | F: GACAAGCTGCATGTGACATC R: GCTGGTAGGTTCCTGTTGTT | 325 | NM_001313922.1 |
| Opn | F: AGACCATGCAGAGAGCGAG R: GCCCTTTCCGTTGTTGTCCT | 340 | NM_001204203.1 |
| p21 | F: TGTTCCACACAGGAGCAAAG R: AACACGCTCCCAGACGTAGT | 175 | NM_001111099.2 |
| p53 | F: AGTCACAGCACATGACGGAGG R: GGAGTCTTCCAGTGTGATGATGG | 287 | NM_001127233.1 |
| Pink1 | F: CCCCAGTGCGGTAATTGACT R: CTAGAAGATGCTCGCCCCAG | 281 | NM_026880.2 |
| PU.1 | F: GAGAAGCTGATGGCTTGGAG R: TTGTGCTTGGACGAGAACTG | 175 | NM_001378899.1 |
| Runx2 | F: TCCGAAATGCCTCTGCTGTT R: GCCACTTGGGGAGGATTTGT | 130 | NM_001271630.1 |
| Tgfb1 | F: GGAGAGCCCTGGATACCAAC R: CAACCCAGGTCCTTCCTAAA | 94 | NM_011577.2 |
| Tnfa | F: ACAGAAAGCATGATCCGCGA R: CTTGGTGGTTTGCTACGACG | 295 | NM_013693.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marycz, K.; Kornicka-Garbowska, K.; Patej, A.; Sobierajska, P.; Kotela, A.; Turlej, E.; Kepska, M.; Bienko, A.; Wiglusz, R.J. Aminopropyltriethoxysilane (APTES)-Modified Nanohydroxyapatite (nHAp) Incorporated with Iron Oxide (IO) Nanoparticles Promotes Early Osteogenesis, Reduces Inflammation and Inhibits Osteoclast Activity. Materials 2022, 15, 2095. https://doi.org/10.3390/ma15062095
Marycz K, Kornicka-Garbowska K, Patej A, Sobierajska P, Kotela A, Turlej E, Kepska M, Bienko A, Wiglusz RJ. Aminopropyltriethoxysilane (APTES)-Modified Nanohydroxyapatite (nHAp) Incorporated with Iron Oxide (IO) Nanoparticles Promotes Early Osteogenesis, Reduces Inflammation and Inhibits Osteoclast Activity. Materials. 2022; 15(6):2095. https://doi.org/10.3390/ma15062095
Chicago/Turabian StyleMarycz, Krzysztof, Katarzyna Kornicka-Garbowska, Adrian Patej, Paulina Sobierajska, Andrzej Kotela, Eliza Turlej, Martyna Kepska, Alina Bienko, and Rafal J. Wiglusz. 2022. "Aminopropyltriethoxysilane (APTES)-Modified Nanohydroxyapatite (nHAp) Incorporated with Iron Oxide (IO) Nanoparticles Promotes Early Osteogenesis, Reduces Inflammation and Inhibits Osteoclast Activity" Materials 15, no. 6: 2095. https://doi.org/10.3390/ma15062095








