Accelerated Curing for Glass-Based Mortars Using Water at 80 °C
Abstract
:1. Introduction
2. Materials and Methods
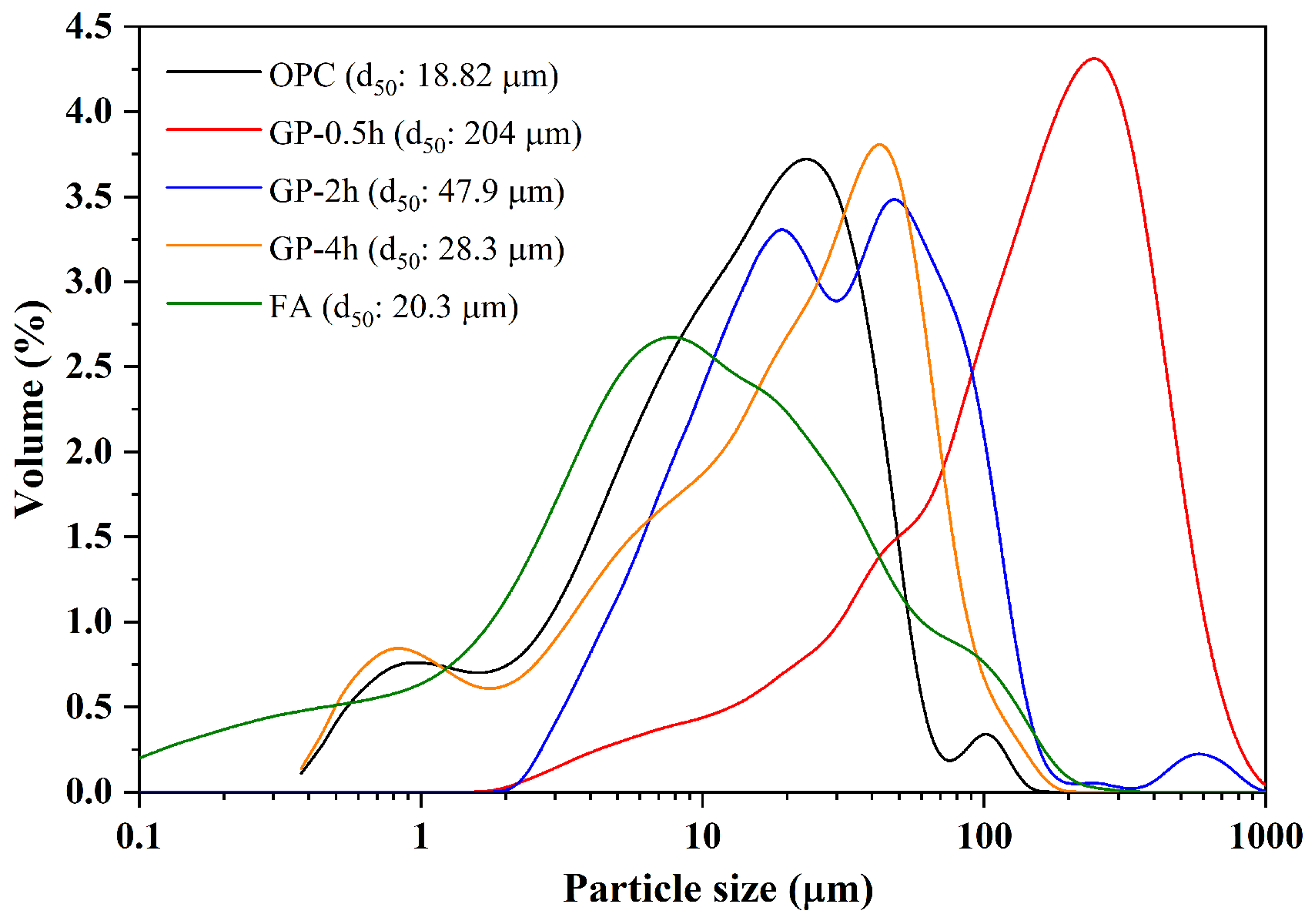
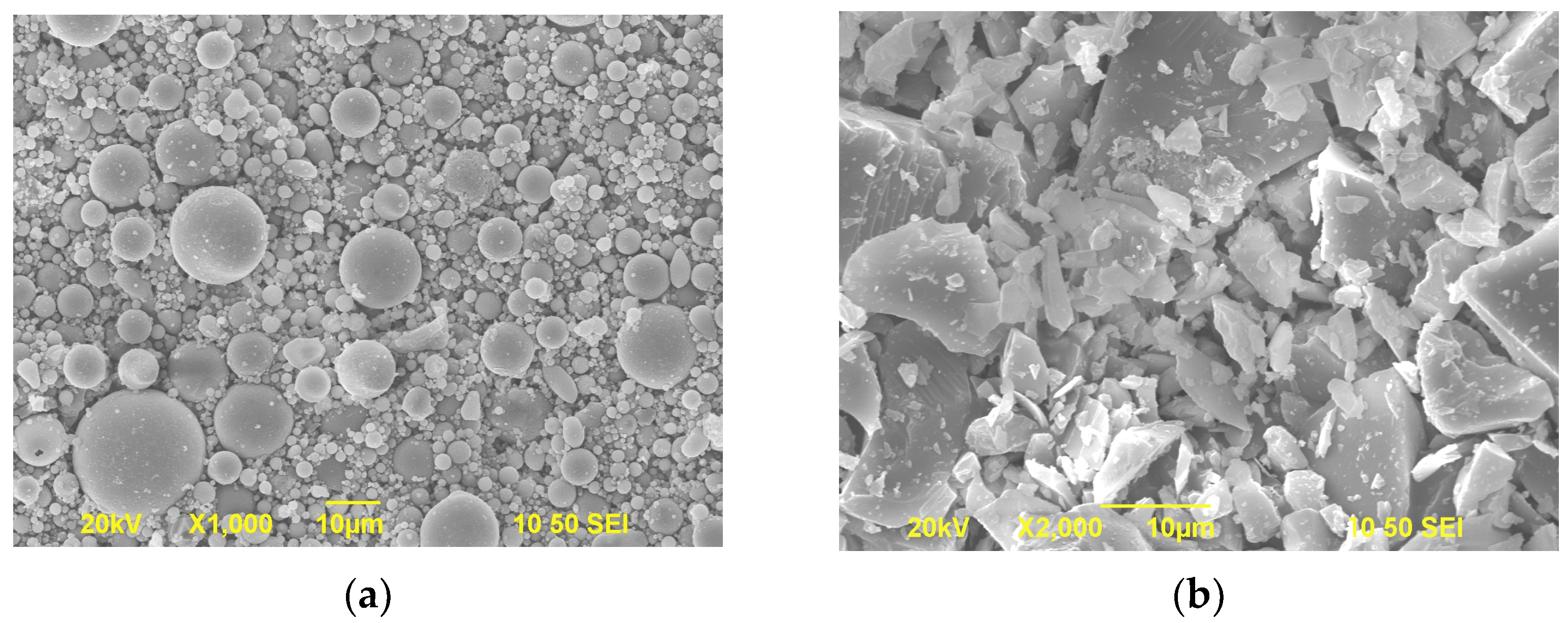
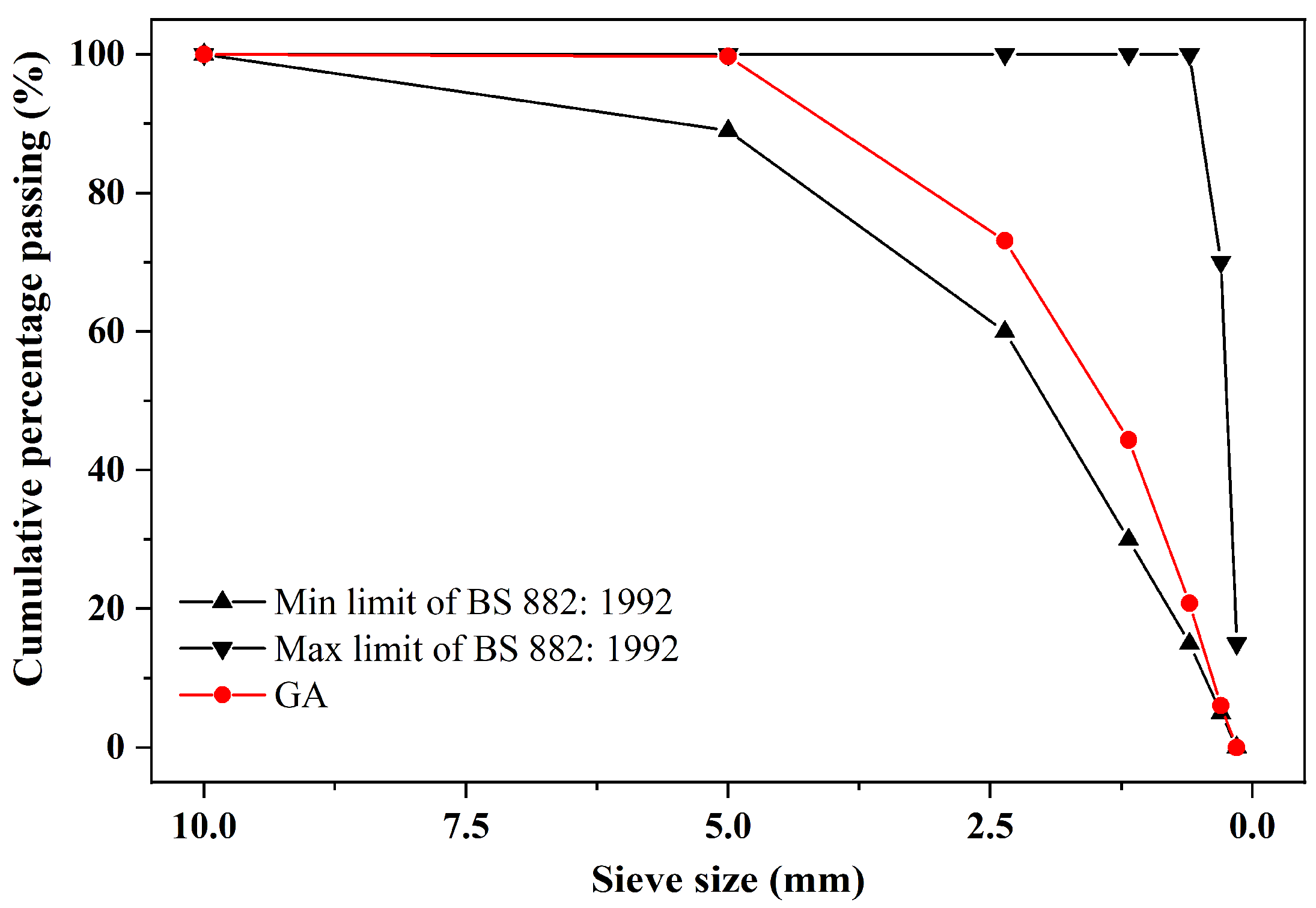
2.1. Raw Materials
2.2. Test Methods
2.2.1. Sample Preparation
2.2.2. Volume Stability Test
2.2.3. Compressive Strength Test
2.2.4. Scanning Electron Microscope (SEM) Test
3. Results and Discussion
3.1. Feasibility of the Water Curing at 80 °C on GA Mortars
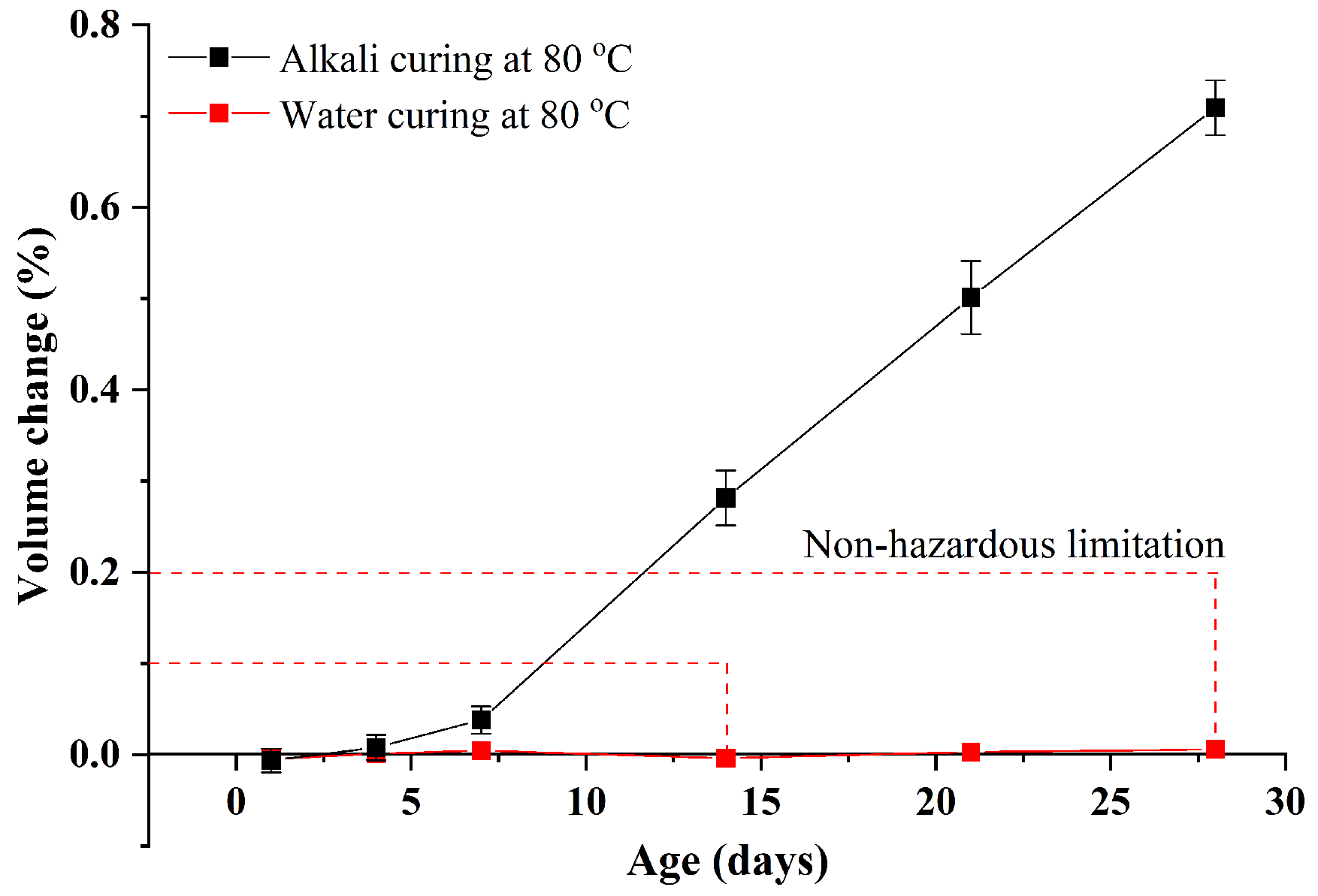
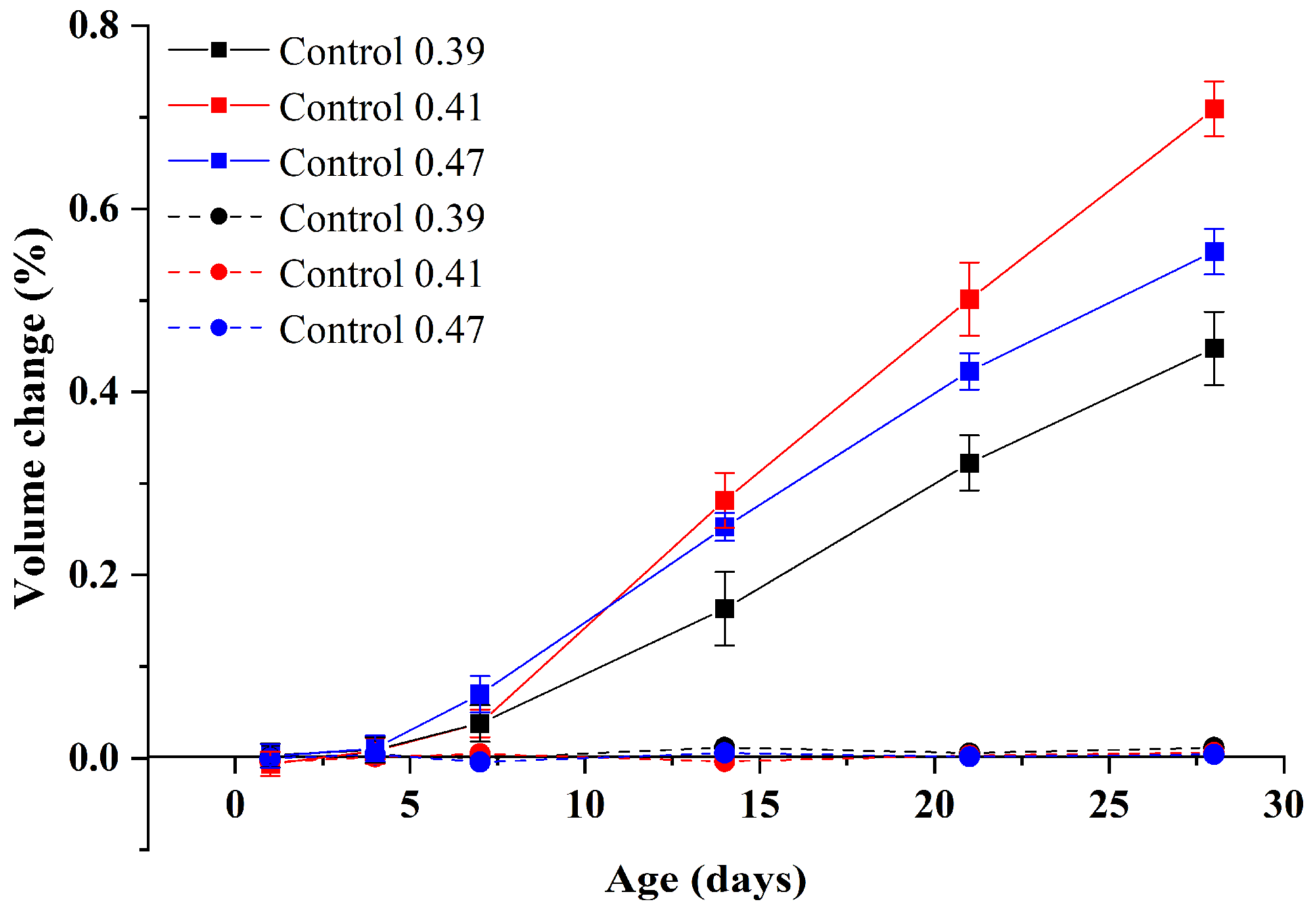
3.1.1. Volume Change Analysis
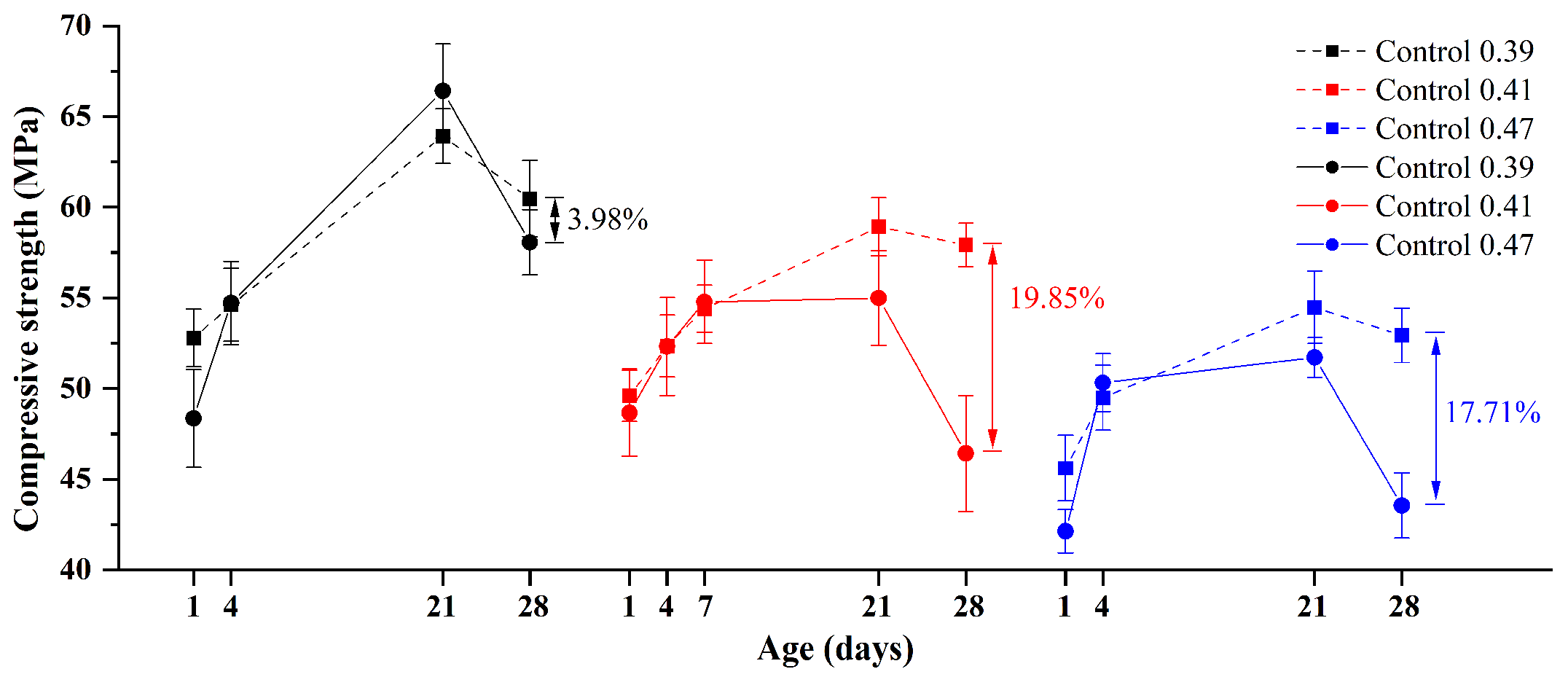
3.1.2. Compressive Strength Analysis
3.1.3. Effect of w/b Ratios
3.2. Effects of GP Addition and Fineness on GA Mortars under 80 °C Curing
3.2.1. Volume Change Analysis
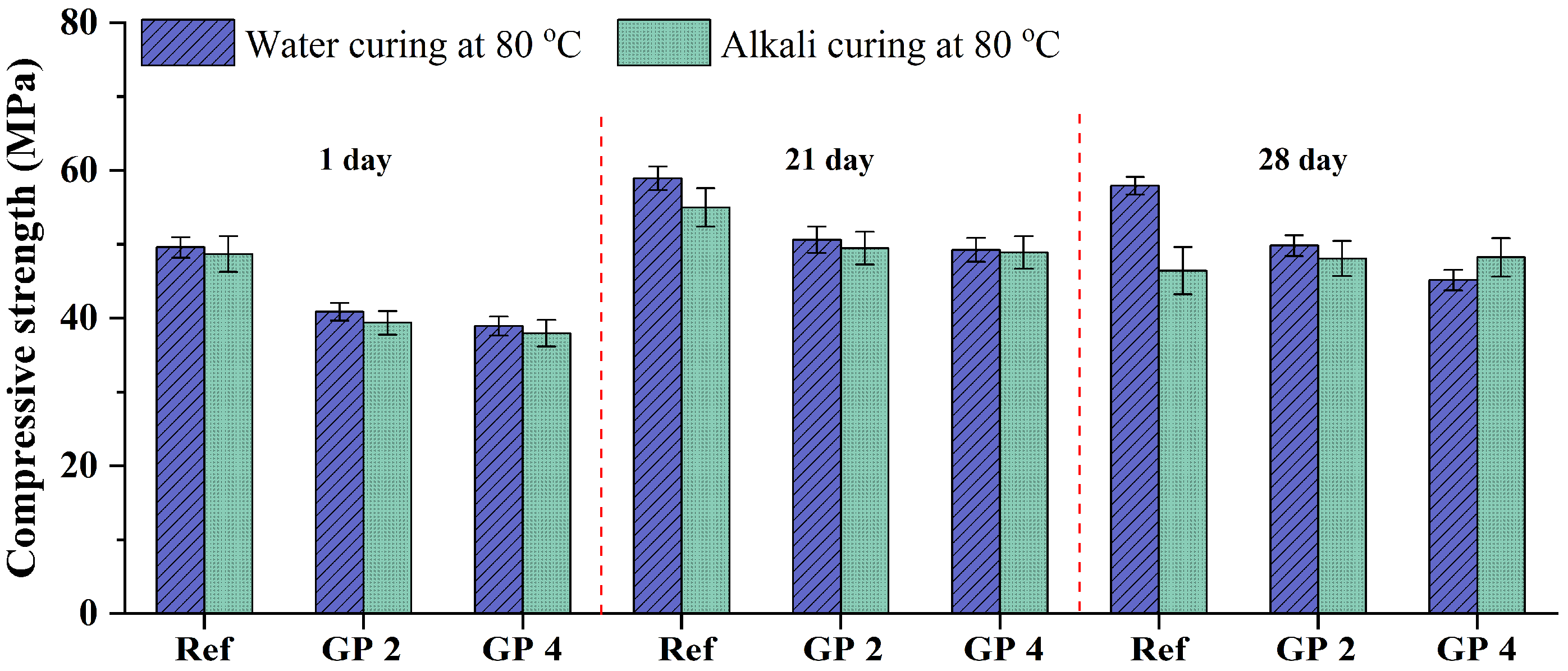
3.2.2. Compressive Strength Analysis
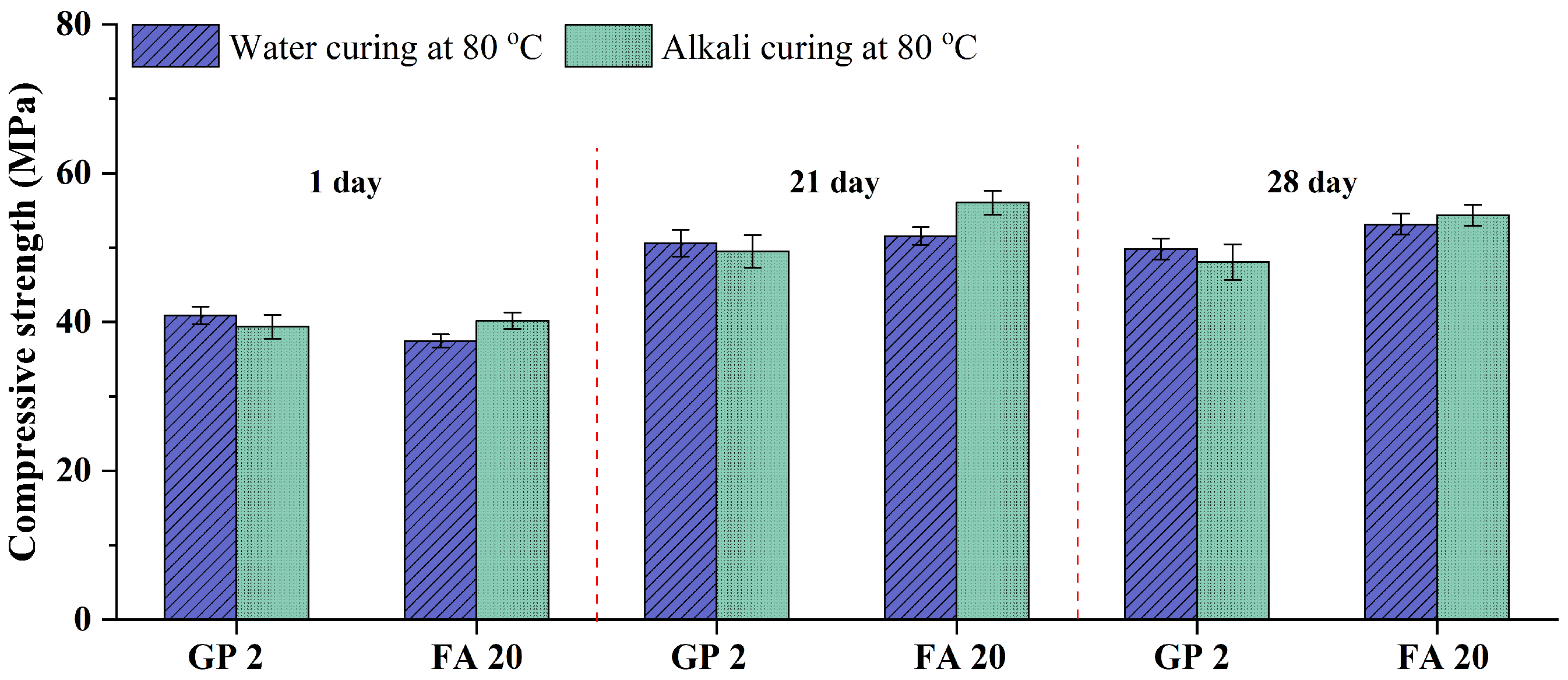
3.3. A Comparison of GP and FA
4. Conclusions
- (1)
- Compared with alkali curing at 80 °C, the volume change of GA mortars under water curing at 80 °C is negligible. On the other hand, the compressive strength of GA mortars after the 1-day water curing at 80 °C is comparable with that after the 28-day water curing at 20 °C. Therefore, the 1-day water curing at 80 °C can be used as an accelerated curing method for GA mortars. In addition, when the w/b ratio increases from 0.39 to 0.47, the ASR expansion of GA mortars increases and then decreases.
- (2)
- With the addition of 20 wt.% GP having the mean diameter of 47.9 and 28.3 μm, the ASR expansion of GA mortars can be effectively mitigated. Furthermore, under water curing at 80 °C, the addition of GP (47.9 μm) can always obtain the higher compressive strength. It is attributed to the fact that the addition of GP (28.3 μm) cannot endow mortars with a denser particle packing state because it has a similar particle size to OPC. Therefore, the GP (47.9 μm) is suggested as an alternative material to mitigate the ASR expansion of GA mortars.
- (3)
- The addition of FA and GP (47.9 μm) obtains the same effects on the ASR expansion of GA mortars. Moreover, after the 1-day water curing at 80 °C, the addition of GP (47.9 μm) can gain the higher compressive strength. Although the high-temperature curing and the alkaline environment are beneficial to the FA hydration, the addition of GP (47.9 μm) is still a better choice when applying the 1-day water curing at 80 °C as the accelerated curing method.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Z.; Poon, C.S.; Li, J.S.; Xue, Q. Utilization of glass cullet to enhance the performance of recycled aggregate unbound sub-base. J. Clean. Prod. 2021, 288, 125083. [Google Scholar] [CrossRef]
- He, P.P.; Zhang, B.Y.; Lu, J.X.; Poon, C.S. ASR expansion of alkali-activated cement glass aggregate mortars. Constr. Build. Mater. 2020, 261, 119925. [Google Scholar] [CrossRef]
- Zhao, H.; Poon, C.S. Recycle of large amount cathode ray tube funnel glass sand to mortar with supplementary cementitious materials. Constr. Build. Mater. 2021, 308, 124953. [Google Scholar] [CrossRef]
- Thornton, E.B.; Sallenger, A.; Sesto, J.C.; Egley, L.; McGee, T.; Parsons, R. Sand mining impacts on long-term dune erosion in southern Monterey Bay. Mar. Geol. 2006, 229, 45–58. [Google Scholar] [CrossRef]
- Sun, L.F.; Zhu, X.J.; Kim, M.; Zi, G. Alkali-silica reaction and strength of concrete with pretreated glass particles as fine aggregates. Constr. Build. Mater. 2021, 271, 121809. [Google Scholar] [CrossRef]
- Khan, M.N.N.; Sarker, P.K. Effect of waste glass fine aggregate on the strength, durability and high temperature resistance of alkali-activated fly ash and GGBFS blended mortar. Constr. Build. Mater. 2020, 263, 120177. [Google Scholar] [CrossRef]
- Flores-Ales, V.; Martin-del-Rio, J.J.; Alducin-Ochoa, J.M.; Torres-Gonzalez, M. Rehydration on high temperature-mortars based on recycled glass as aggregate. J. Clean. Prod. 2020, 275, 124139. [Google Scholar] [CrossRef]
- Shi, Z.G.; Shi, C.J.; Wan, S.; Li, N.; Zhang, Z.H. Effect of of alkali dosage and silicate modulus on carbonation of alkali-activated slag mortars. Cem. Concr. Res. 2018, 113, 55–64. [Google Scholar] [CrossRef]
- Shi, Z.G.; Shi, C.J.; Wan, S.; Zhang, Z.H. Effects of alkali dosage and silicate modulus on alkali-silica reaction in alkali-activated slag mortars. Cem. Concr. Res. 2018, 111, 104–115. [Google Scholar] [CrossRef]
- Dong, W.K.; Li, W.G.; Tao, Z. A comprehensive review on performance of cementitious and geopolymeric concretes with recycled waste glass as powder, sand or cullet. Resour. Conserv. Recy. 2021, 172, 105664. [Google Scholar] [CrossRef]
- Saha, A.K.; Khan, M.N.N.; Sarker, P.K.; Shaikh, F.A.; Pramanik, A. The ASR mechanism of reactive aggregates in concrete and its mitigation by fly ash: A critical review. Constr. Build. Mater. 2018, 171, 743–758. [Google Scholar] [CrossRef]
- Mohammadi, A.; Ghiasvand, E.; Nili, M. Relation between mechanical properties of concrete and alkali-silica reaction (ASR); a review. Constr. Build. Mater. 2020, 258, 119567. [Google Scholar] [CrossRef]
- Hay, R.; Ostertag, C.P. New insights into the role of fly ash in mitigating alkali-silica reaction (ASR) in concrete. Cem. Concr. Res. 2021, 144, 106440. [Google Scholar] [CrossRef]
- Khalifa, A.Z.; Cizer, O.; Pontikes, Y.; Heath, A.; Patureau, P.; Bernal, S.A.; Marsh, A.T.M. Advances in alkali-activation of clay minerals. Cem. Concr. Res. 2020, 132, 106050. [Google Scholar] [CrossRef]
- Afshinnia, K.; Rangaraju, P.R. Impact of combined use of ground glass powder and crushed glass aggregate on selected properties of Portland cement concrete. Constr. Build. Mater. 2016, 117, 263–272. [Google Scholar] [CrossRef]
- Liu, S.H.; Wang, S.; Tang, W.; Hu, N.N.; Wei, J.P. Inhibitory Effect of Waste Glass Powder on ASR Expansion Induced by Waste Glass Aggregate. Materials 2015, 8, 6849–6862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Idir, R.; Cyr, M.; Tagnit-Hamou, A. Use of fine glass as ASR inhibitor in glass aggregate mortars. Constr. Build. Mater. 2010, 24, 1309–1312. [Google Scholar] [CrossRef]
- Lu, J.X.; Duan, Z.H.; Poon, C.S. Combined use of waste glass powder and cullet in architectural mortar. Cem. Concr. Comp. 2017, 82, 34–44. [Google Scholar] [CrossRef]
- Lu, J.X.; Duan, Z.H.; Poon, C.S. Fresh properties of cement pastes or mortars incorporating waste glass powder and cullet. Constr. Build. Mater. 2017, 131, 793–799. [Google Scholar] [CrossRef]
- Lu, J.X.; Zhan, B.J.; Duan, Z.H.; Poon, C.S. Using glass powder to improve the durability of architectural mortar prepared with glass aggregates. Mater. Des. 2017, 135, 102–111. [Google Scholar] [CrossRef]
- Lu, J.X.; Zhan, B.J.; Duan, Z.H.; Poon, C.S. Improving the performance of architectural mortar containing 100% recycled glass aggregates by using SCMs. Constr. Build. Mater. 2017, 153, 975–985. [Google Scholar] [CrossRef]
- Robayo, R.A.; Mulford, A.; Munera, J.; de Gutierrez, R.M. Alternative cements based on alkali-activated red clay brick waste. Constr. Build. Mater. 2016, 128, 163–169. [Google Scholar] [CrossRef]
- Silva, G.; Castaneda, D.; Kim, S.; Castaneda, A.; Bertolotti, B.; Ortega-San-Martin, L.; Nakamatsu, J.; Aguilar, R. Analysis of the production conditions of geopolymer matrices from natural pozzolana and fired clay brick wastes. Constr. Build. Mater. 2019, 215, 633–643. [Google Scholar] [CrossRef]
- Huo, W.W.; Zhu, Z.D.; Chen, W.; Zhang, J.; Kang, Z.A.Z.A.; Pu, S.Y.; Wan, Y. Effect of synthesis parameters on the development of unconfined compressive strength of recycled waste concrete powder-based geopolymers. Constr. Build. Mater. 2021, 292, 123264. [Google Scholar] [CrossRef]
- Elkhadiri, I.; Palacios, M.; Puertas, F. Effect of curing temperature on cement hydration. Ceram.-Silik. 2009, 53, 65–75. [Google Scholar]
- Shi, Z.; Park, S.; Lothenbach, B.; Leemann, A. Formation of shlykovite and ASR-P1 in concrete under accelerated alkali-silica reaction at 60 and 80 °C. Cem. Concr. Res. 2020, 137, 106213. [Google Scholar] [CrossRef]
- Zhang, F.Y.; Yao, X.; Yang, T.; Gao, X.; Geng, C.Z.; Jiang, T. Rheology and alkali-silica reaction of alkali-activated slag mortars modified by fly ash microsphere: A comparative analysis to OPC mortars. Mater. Res. Express 2021, 8, 065501. [Google Scholar] [CrossRef]
- Sajedi, F. Effect of curing regime and temperature on the compressive strength of cement-slag mortars. Constr. Build. Mater. 2012, 36, 549–556. [Google Scholar] [CrossRef]
- Sajedi, F.; Razak, H.A.; Bin Mahmud, H.; Shafigh, P. Relationships between compressive strength of cement-slag mortars under air and water curing regimes. Constr. Build. Mater. 2012, 31, 188–196. [Google Scholar] [CrossRef] [Green Version]
- Komnitsas, K.; Zaharaki, D.; Vlachou, A.; Bartzas, G.; Galetakis, M. Effect of synthesis parameters on the quality of construction and demolition wastes (CDW) geopolymers. Adv. Powder Technol. 2015, 26, 368–376. [Google Scholar] [CrossRef] [Green Version]
- Mo, B.H.; He, Z.; Cui, X.M.; He, Y.; Gong, S.Y. Effect of curing temperature on geopolymerization of metakaolin-based geopolymers. Appl. Clay Sci. 2014, 99, 144–148. [Google Scholar] [CrossRef]
- Hu, J.; Ge, Z.; Wang, K.J. Influence of cement fineness and water-to-cement ratio on mortar early-age heat of hydration and set times. Constr. Build. Mater. 2014, 50, 657–663. [Google Scholar] [CrossRef]
- Allahverdi, A.; Mehrpour, K.; Kani, E.N. Investigating the possibility of utilizing pumice-type natural pozzonal in production of geopolymer cement. Ceram.-Silik. 2008, 52, 16–23. [Google Scholar]
- Komnitsas, K.; Zaharaki, D.; Perdikatsis, V. Geopolymerisation of low calcium ferronickel slags. J. Mater. Sci. 2007, 42, 3073–3082. [Google Scholar] [CrossRef]
- Nixon, P.J.; Page, C.L.; Bollinghaus, R.; Canham, I. The Effect of a Pfa with a High Total Alkali Content on Pore Solution Composition and Alkali Silica Reaction. Mag. Concr. Res. 1986, 38, 30–35. [Google Scholar] [CrossRef]
- Shafaatian, S.M.H.; Akhavan, A.; Maraghechi, H.; Rajabipour, F. How does fly ash mitigate alkali-silica reaction (ASR) in accelerated mortar bar test (ASTM C1567)? Cem. Concr. Comp. 2013, 37, 143–153. [Google Scholar] [CrossRef]
- Monteiro, P.J.M.; Wang, K.; Sposito, G.; dos Santos, M.C.; de Andrade, W.P. Influence of mineral admixtures on the alkali-aggregate reaction. Cem. Concr. Res. 1997, 27, 1899–1909. [Google Scholar] [CrossRef]
- Xu, G.J.Z.; Watt, D.F.; Hudec, P.P. Effectiveness of Mineral Admixtures in Reducing Asr Expansion. Cem. Concr. Res. 1995, 25, 1225–1236. [Google Scholar] [CrossRef]
- Raju, A.S.; Anand, K.B.; Rakesh, P. Partial replacement of Ordinary Portland cement by LCD glass powder in concrete. Mater. Today-Proc. 2021, 46, 5131–5137. [Google Scholar] [CrossRef]
- Vayghan, A.G.; Rajabipour, F.; Rosenberger, J.L. Composition-rheology relationships in alkali-silica reaction gels and the impact on the gel’s deleterious behavior. Cem. Concr. Res. 2016, 83, 45–56. [Google Scholar] [CrossRef] [Green Version]
- Long, W.J.; Lin, C.; Ye, T.H.; Dong, B.Q.; Xing, F. Stabilization/solidification of hazardous lead glass by geopolymers. Constr. Build. Mater. 2021, 294, 123574. [Google Scholar] [CrossRef]
- Ichikawa, T. Alkali-silica reaction, pessimum effects and pozzolanic effect. Cem. Concr. Res. 2009, 39, 716–726. [Google Scholar] [CrossRef]
- Afshinnia, K.; Rangaraju, P.R. Influence of fineness of ground recycled glass on mitigation of alkali-silica reaction in mortars. Constr. Build. Mater. 2015, 81, 257–267. [Google Scholar] [CrossRef]
- Hwang, K.; Noguchi, T.; Tomosawa, F. Prediction model of compressive strength development of fly-ash concrete. Cem. Concr. Res. 2004, 34, 2269–2276. [Google Scholar] [CrossRef]
- Yang, J.; Hu, H.C.; He, X.Y.; Su, Y.; Wang, Y.B.; Tan, H.B.; Pan, H. Effect of steam curing on compressive strength and microstructure of high volume ultrafine fly ash cement mortar. Constr. Build. Mater. 2021, 266, 120894. [Google Scholar] [CrossRef]
- Vasquez, A.; Cardenas, V.; Robayo, R.A.; de Gutierrez, R.M. Geopolymer based on concrete demolition waste. Adv. Powder Technol. 2016, 27, 1173–1179. [Google Scholar] [CrossRef]
- Rakhimova, N.R.; Rakhimov, R.Z. Alkali-activated cements and mortars based on blast furnace slag and red clay brick waste. Mater. Des. 2015, 85, 324–331. [Google Scholar] [CrossRef]
- Long, W.J.; Li, H.D.; Ma, H.Y.; Fang, Y.; Xing, F. Green alkali-activated mortar: Sustainable use of discarded cathode-ray tube glass powder as precursor. J. Clean. Prod. 2019, 229, 1082–1092. [Google Scholar] [CrossRef]






| Notation | OPC | GP | FA | GA | Water | Consistency (mm) | |
|---|---|---|---|---|---|---|---|
| Series 1 | Control 0.41 | 1 | – | – | 2 | 0.41 | 97 ± 2 |
| GP 0.5/GP 2/GP 4 | 0.8 | 0.2 | – | 2 | 0.41 | 101 ± 2/95 ± 1/93 ± 2 | |
| FA 20 | 0.8 | – | 0.2 | 2 | 0.41 | 104 ± 3 | |
| Series 2 | Control 0.41 | 1 | – | – | 2 | 0.41 | 97 ± 2 |
| Control 0.39 | 1 | – | – | 2 | 0.39 | 93 ± 1 | |
| Control 0.47 | 1 | – | – | 2 | 0.47 | 107 ± 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, T.; Lu, J.; Duan, Z.; Li, L.; Zhu, D. Accelerated Curing for Glass-Based Mortars Using Water at 80 °C. Materials 2022, 15, 2109. https://doi.org/10.3390/ma15062109
Ye T, Lu J, Duan Z, Li L, Zhu D. Accelerated Curing for Glass-Based Mortars Using Water at 80 °C. Materials. 2022; 15(6):2109. https://doi.org/10.3390/ma15062109
Chicago/Turabian StyleYe, Taohua, Jianxin Lu, Zhenhua Duan, Lei Li, and Dayu Zhu. 2022. "Accelerated Curing for Glass-Based Mortars Using Water at 80 °C" Materials 15, no. 6: 2109. https://doi.org/10.3390/ma15062109





