Effect of Annealing Temperature on the Interfacial Microstructure and Bonding Strength of Cu/Al Clad Sheets with a Stainless Steel Interlayer
Abstract
:1. Introduction
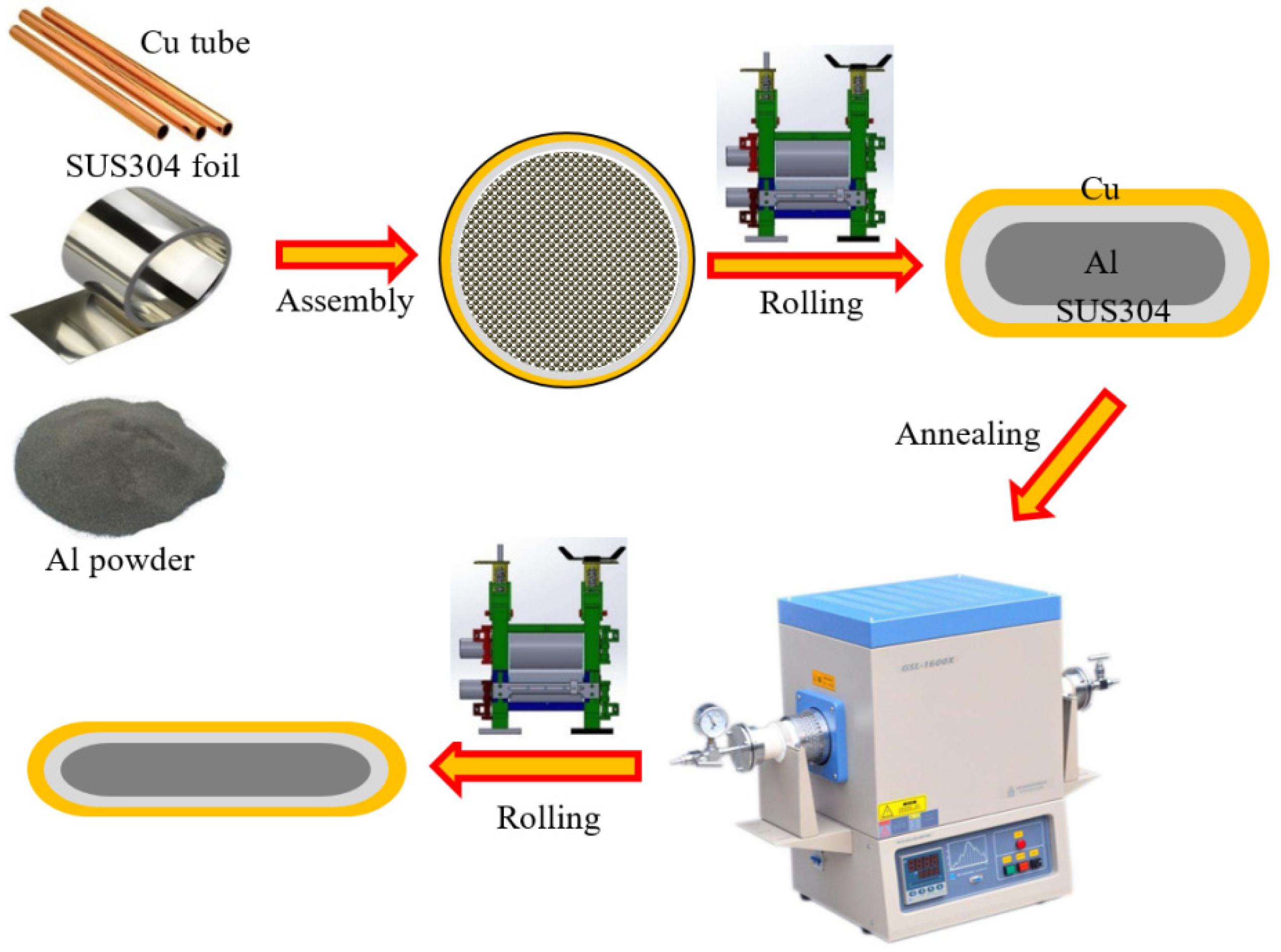
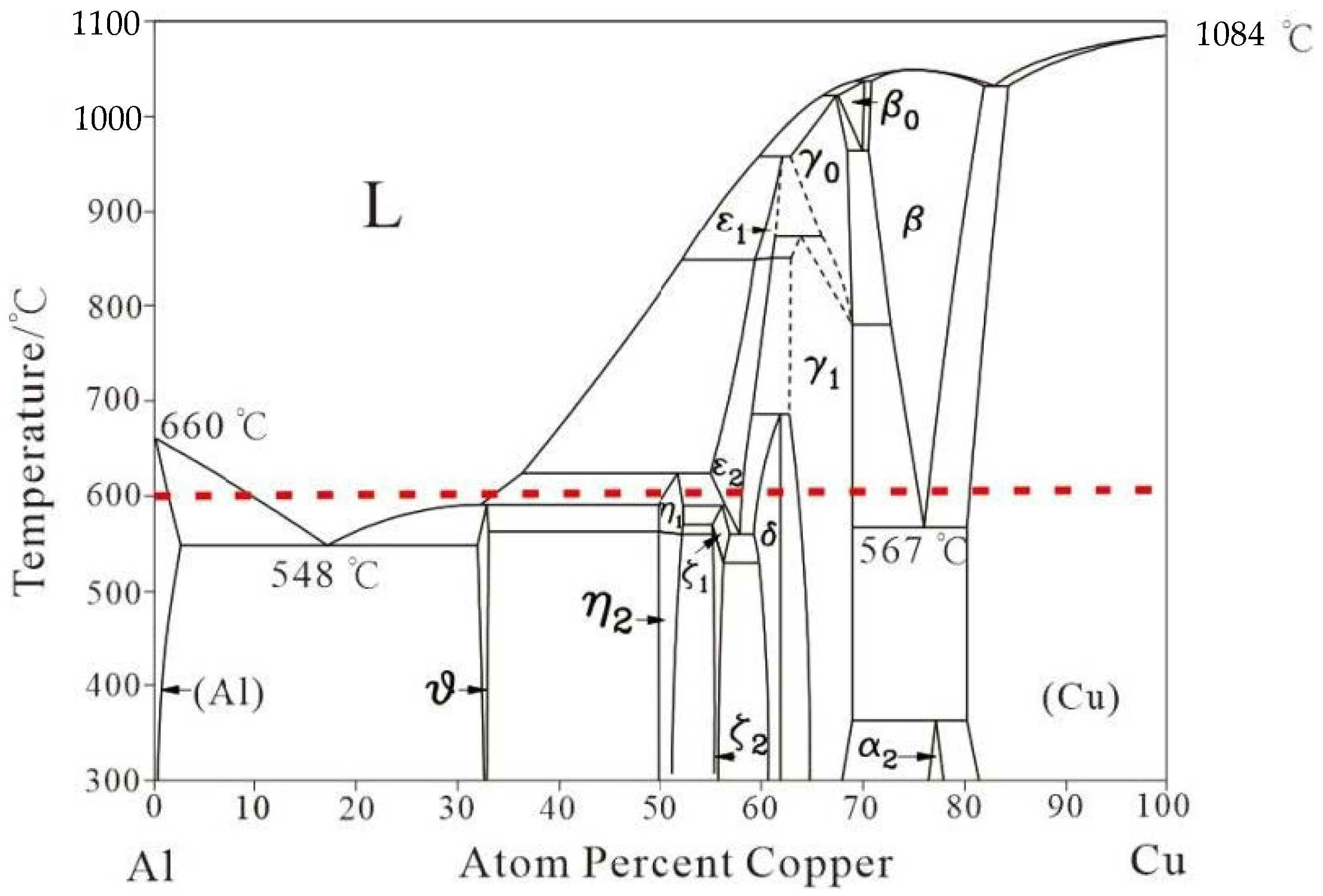
2. Materials and Methods
3. Results and Discussion
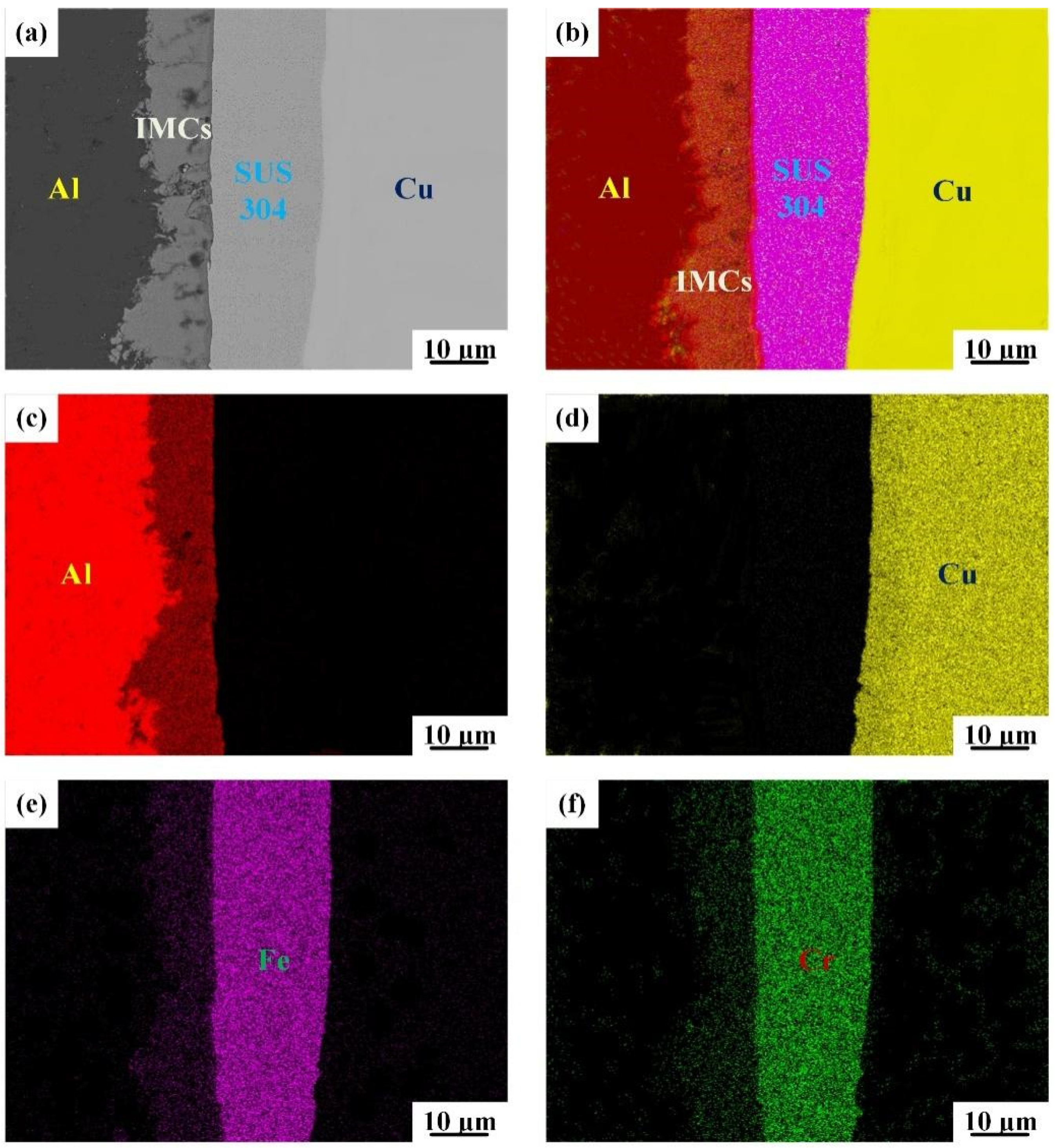
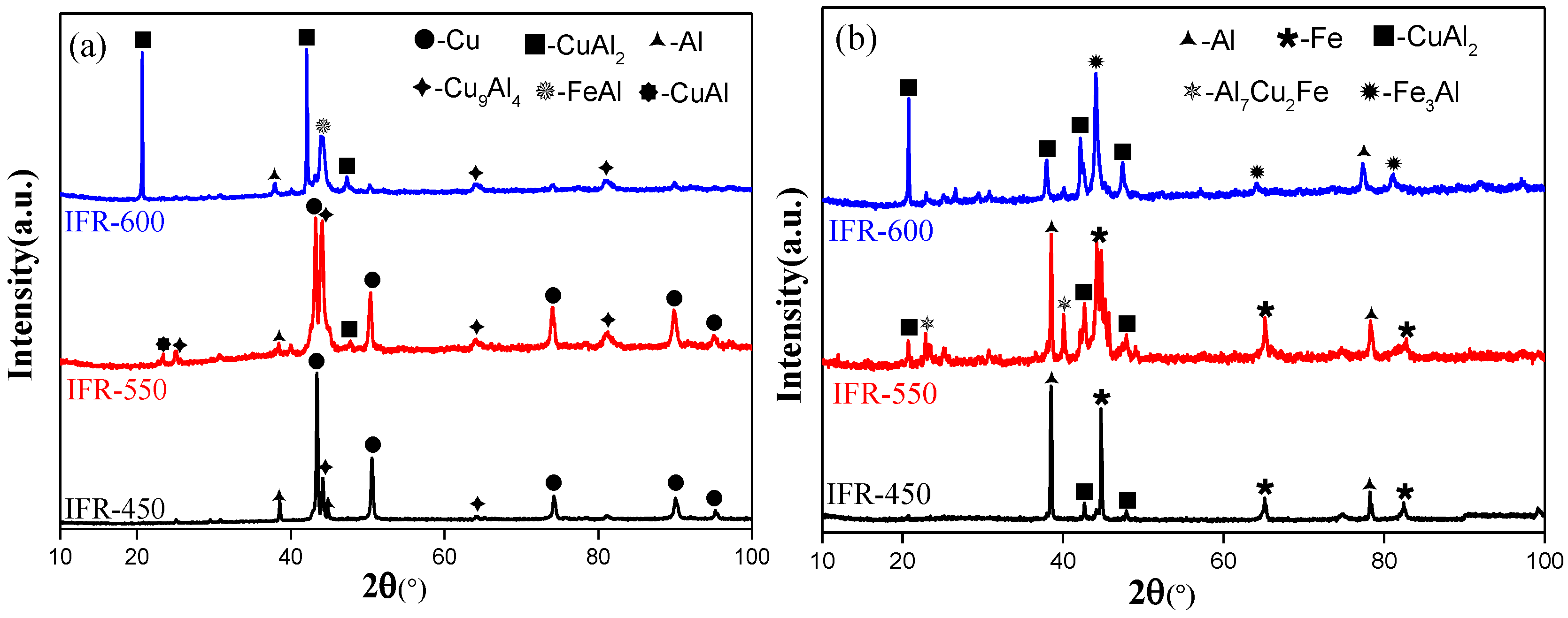
3.1. Interfacial Microstructure
3.2. Elemental Diffusion across the Bonding Interface
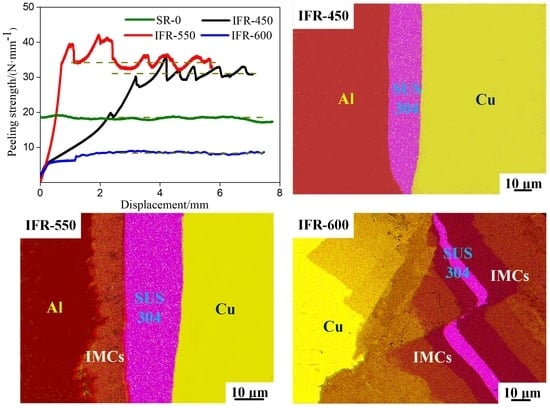
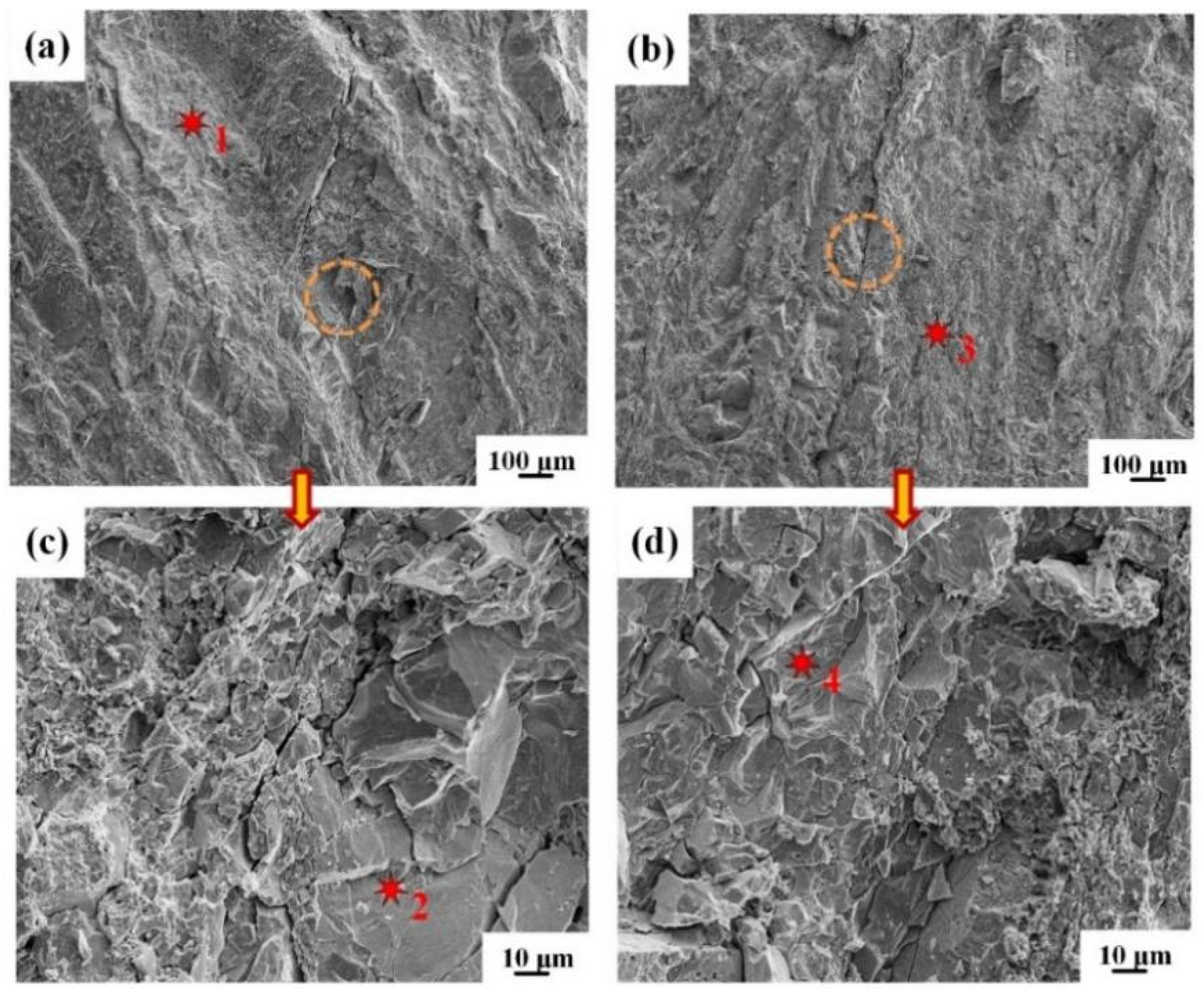
3.3. Peeling Surface of the Clad Sheets
3.4. Peeling Strength of the Clad Sheets
4. Discussions
5. Conclusions
- (1)
- The interfacial atomic diffusion is significantly enhanced by increasing the intermediate annealing temperature. Nevertheless, after a high-temperature annealing treatment (IFR-600), a liquid phase is formed at the bonding interface and the clear Cu/SUS304/Al interface in IFR-450 and IFR-550 is replaced by the chaotic composite interfaces.
- (2)
- For IFR-450 and IFR-550, the interfacial crack is propagated along the Cu/SUS304 interface, Cu-Al IMCs layer, and Al matrix. Compared with IFR-450, the interfacial bonding strength for IFR-550 is improved by 11%, from 30.9 N/mm to 34.3 N/mm, which is proven by the obvious wrinkles on the surface of SUS304 fragments and the formation of a new Al7Cu2Fe phase.
- (3)
- As the intermediate annealing temperature is increased to 600 °C (IFR-600), the propagation path of interfacial crack is changed into the Cu-Al IMCs layer and Fe-Al IMCs layer. The clad sheets broke completely in the unduly thick IMCs layer, resulting in the sharp decrease of interfacial bonding strength, only 8.3 N/mm.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, M.J.; Lee, K.S.; Han, S.H.; Hong, S.I. Interface strengthening of a roll-bonded two-ply Al/Cu sheet by short annealing. Mater. Charact. 2021, 174, 111021. [Google Scholar] [CrossRef]
- Sheng, L.Y.; Yang, F.; Xi, T.F.; Lai, C.; Ye, H.Q. Influence of heat treatment on interface of Cu/Al bimetal composite fabricated by cold rolling. Compos. Part B 2011, 42, 1468–1473. [Google Scholar] [CrossRef]
- Huang, H.G.; Dong, Y.K.; Yan, M.; Du, F.S. Evolution of bonding interface in solid–liquid cast-rolling bonding of Cu/Al clad strip. Trans. Nonferrous Met. Soc. 2017, 27, 1019–1025. [Google Scholar] [CrossRef]
- Elango, E.; Saravanan, S.; Raghukandan, K. Experimental and numerical studies on aluminum-stainless steel explosive cladding. J. Cent. South Univ. 2020, 27, 1742–1753. [Google Scholar] [CrossRef]
- Alikhani, A.; Beygi, R.; Zarezadeh-Mehrizi, M.; Nematzadeh, F.; Galvao, I. Effect of Mg and Si on intermetallic formation and fracture behavior of pure aluminum-galvanized carbon-steel joints made by weld-brazing. J. Cent. South Univ. 2021, 28, 3626–3638. [Google Scholar] [CrossRef]
- Gao, H.T.; Liu, X.H.; Qi, J.L.; Ai, Z.R.; Liu, L.Z. Microstructure and mechanical properties of Cu/Al/Cu clad strip processed by the powder-in-tube method. J. Mater. Process. Technol. 2018, 251, 1–11. [Google Scholar] [CrossRef]
- Simsir, M.; Kumruoğlu, L.C.; Özer, A. An investigation into stainless-steel/structural-alloy-steel bimetal produced by shell mould casting. Mater. Des. 2009, 30, 264–270. [Google Scholar] [CrossRef]
- Lee, K.S.; Yoon, D.H.; Kim, H.K.; Kwon, Y.N.; Lee, Y.S. Effect of annealing on the interface microstructure and mechanical properties of a STS–Al–Mg 3-ply clad sheet. Mater. Sci. Eng. A 2012, 556, 319–330. [Google Scholar] [CrossRef]
- Lee, S.; Son, I.S.; Lee, J.K.; Lee, J.S.; Kim, Y.B.; Lee, G.A.; Lee, S.P.; Cho, Y.R.; Bae, D.S. Effect of aging treatment on bonding interface properties of hot-pressed Cu/Al clad material. Int. J. Precis. Eng. Manuf. 2015, 16, 525–530. [Google Scholar] [CrossRef]
- Gao, K.; Song, S.J.; Li, S.M.; Fu, H.Z. Characterization of microstructures and growth orientation deviating of Al2Cu phase dendrite at different directional solidification rates. J. Alloys Compd. 2016, 660, 73–79. [Google Scholar] [CrossRef]
- Pelzer, R.; Nelhiebel, M.; Zink, R.; Woehlert, S.; Lassnig, A.; Khatibi, G. High temperature storage reliability investigation of the Al–Cu wire bond interface. Microelectron. Reliab. 2012, 52, 1966–1970. [Google Scholar] [CrossRef]
- Lee, W.B.; Bang, K.S.; Jung, S.B. Effects of intermetallic compound on the electrical and mechanical properties of friction welded Cu/Al bimetallic joints during annealing. J. Alloys Compd. 2005, 390, 212–219. [Google Scholar] [CrossRef]
- Li, H.Y.; Chen, W.G.; Dong, L.L.; Shi, Y.G.; Liu, J.; Fu, Y.Q. Interfacial bonding mechanism and annealing effect on Cu-Al joint produced by solid-liquid compound casting. J. Mater. Process. Technol. 2018, 252, 795–803. [Google Scholar] [CrossRef]
- Mao, Z.P.; Xie, J.P.; Wang, A.Q.; Wang, W.Y.; Ma, D.Q.; Liu, P. Effects of annealing temperature on the interfacial microstructure and bonding strength of Cu/Al clad sheets produced by twin-roll casting and rolling. J. Mater. Process. Technol. 2020, 285, 116804. [Google Scholar] [CrossRef]
- Gao, H.T.; Wang, L.; Liu, S.L.; Li, J.; Kong, C.; Yu, H.L. Effects of a stainless steel interlayer on the interfacial microstructure and bonding strength of Cu/Al clad sheets prepared via the powder-in-tube method. J. Mater. Res. Technol. 2021, 15, 3514–3524. [Google Scholar] [CrossRef]
- Chen, G.; Xu, G.M. Interfacial reaction in twin-roll cast AA1100/409L clad sheet during different sequence of cold rolling and annealing. Met. Mater. Int. 2021, 27, 3013–3025. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Y.Z.; Wang, L.; Kong, C.; Pesin, A.; Zhilyaev, A.P.; Yu, H.L. Fabrication and characterization of high bonding strength Al/Ti/Al-laminated composites via cryorolling. Acta Metall. Sin. 2020, 33, 871–880. [Google Scholar] [CrossRef]
- Hasanniah, A.; Movahedi, M. Welding of Al-Mg aluminum alloy to aluminum clad steel sheet using pulsed gas tungsten arc process. J. Manuf. Process. 2018, 31, 494–501. [Google Scholar] [CrossRef]
- Jannot, Y.; Bal, H.M.; Degiovanni, A.; Moyne, C. Influence of heat transfer on the estimation of water vapor diffusion coefficient in transient regime. Int. J. Heat Mass Transf. 2021, 177, 121558. [Google Scholar] [CrossRef]
- Ai-Ghamdi, K.A.; Hussain, G. Parameter-formability relationship in ISF of tri-layered Cu-Steel-Cu composite sheet metal: Response surface and microscopic analyses. Int. J. Precis. Eng. Manuf. 2016, 17, 1633–1642. [Google Scholar] [CrossRef]
- Gao, H.T.; Li, J.; Lei, G.; Song, L.L.; Kong, C.; Yu, H.L. High strength and thermal stability of multilayered Cu/Al composites fabricated through accumulative roll bonding and cryorolling. Metall. Mater. Trans. A 2022. [Google Scholar] [CrossRef]
- Sahar, R.; Ali, A.; Stanislav, J.; Alireza, G.K.; Fredrick, M.; Carlos, L.; Dinara, S.; Sławomir, K.; Reza, S.; Miroslaw, B.; et al. Effect of annealing on the micromorphology and corrosion properties of Ti/SS thin films. Superlattice Microstruct. 2020, 146, 106681. [Google Scholar]
- Peng, X.K.; Wuhrer, R.; Heness, G.; Yeung, W.Y. Rolling strain effects on the interlaminar properties of roll bonded copper/aluminium metal laminates. J. Mater. Sci. 2000, 35, 4357–4363. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chen, H.L.; Hwang, W.S. Influence of interfacial structure development on the fracture mechanism and bond strength of aluminum/copper bimetal plate. Mater. Trans. 2006, 47, 1232–1239. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.Q.; Liu, X.H.; Yan, S.; Yu, Q.B. Experimental study on bending capacity of copper/steel/copper cold rolled composite strip. J. Northeast Univ. (Nat. Sci.) 2019, 40, 647–652. [Google Scholar]
- Yu, Q.B.; Liu, X.H.; Sun, Y.; Qi, J.L. Deformation-induced reaction diffusion of Cu/Al multilayered composite by rolling at room temperature. Sci. Sin. Technol. 2016, 46, 1166–1174. [Google Scholar]
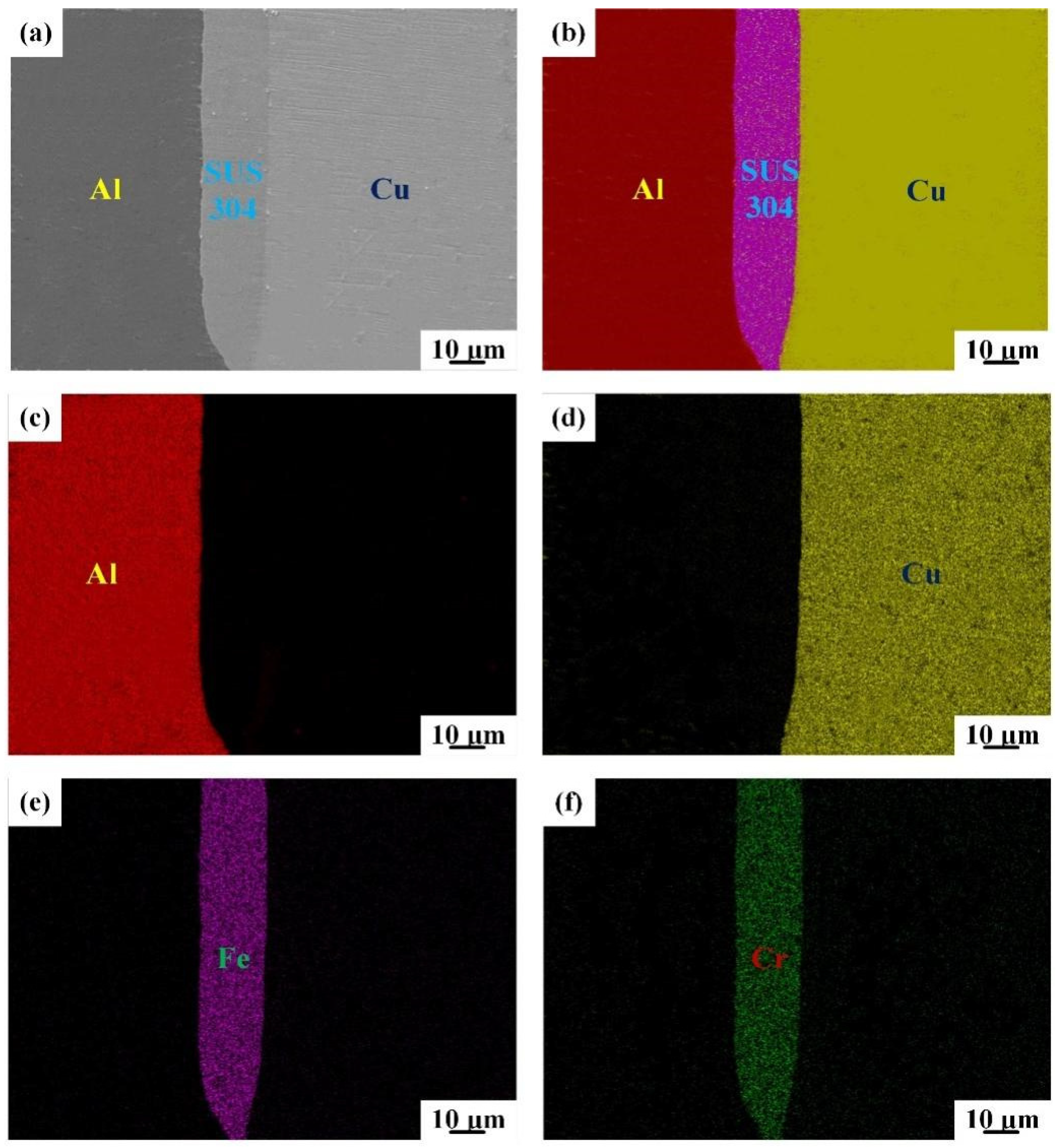



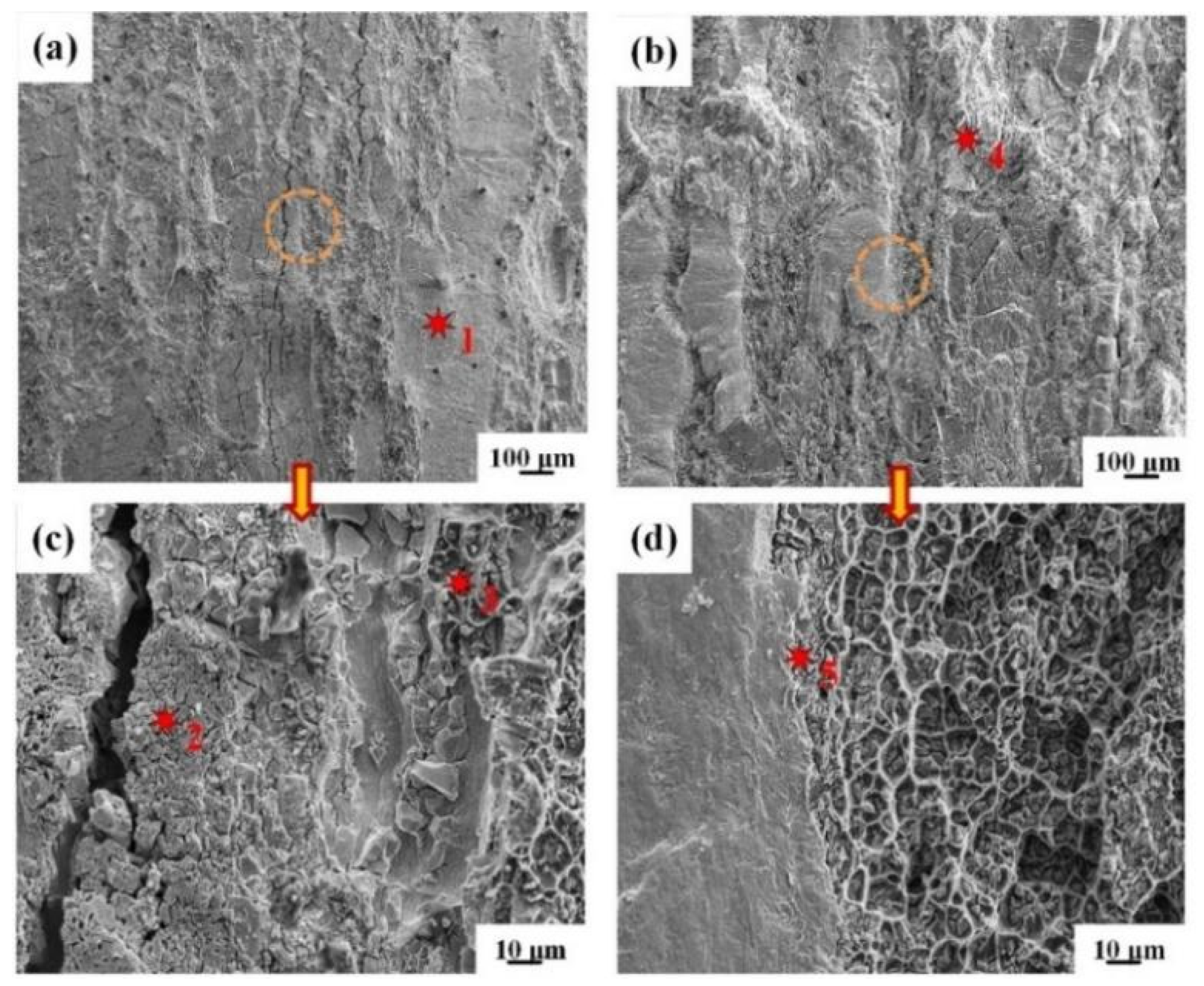


| Points | Cu | Al | Fe | Cr | Ni | Phase |
|---|---|---|---|---|---|---|
| 1 | 97.3 | 2.7 | - | - | - | Cu |
| 2 | - | - | 78.6 | 16.1 | 5.3 | SUS304 |
| 3 | 1.6 | 98.4 | - | - | - | Al |
| 4 | 67.2 | 32.8 | - | - | - | Cu9Al4 |
| Points | Cu | Al | Fe | Cr | Ni | Phase |
|---|---|---|---|---|---|---|
| 1 | 92.6 | 7.4 | - | - | - | Cu |
| 2 | 63.7 | 36.3 | - | - | - | Cu9Al4 |
| 3 | 3.2 | 96.8 | - | - | - | Al |
| 4 | 72.5 | 27.5 | - | - | - | Cu9Al4 |
| 5 | 68.2 | 19.3 | 9.1 | 2.5 | 0.9 | Al7Cu2Fe |
| Points | Cu | Al | Fe | Cr | Ni | Phase |
|---|---|---|---|---|---|---|
| 1 | 26.8 | 73.2 | - | - | - | CuAl2 |
| 2 | 5.2 | 45.6 | 43.2 | 4.3 | 1.7 | FeAl |
| 3 | 39.5 | 60.5 | - | - | - | CuAl2 |
| 4 | 4.3 | 18.7 | 59.6 | 13.8 | 3.6 | Fe3Al |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, H.; Gu, H.; Wang, S.; Xuan, Y.; Yu, H. Effect of Annealing Temperature on the Interfacial Microstructure and Bonding Strength of Cu/Al Clad Sheets with a Stainless Steel Interlayer. Materials 2022, 15, 2119. https://doi.org/10.3390/ma15062119
Gao H, Gu H, Wang S, Xuan Y, Yu H. Effect of Annealing Temperature on the Interfacial Microstructure and Bonding Strength of Cu/Al Clad Sheets with a Stainless Steel Interlayer. Materials. 2022; 15(6):2119. https://doi.org/10.3390/ma15062119
Chicago/Turabian StyleGao, Haitao, Hao Gu, Sai Wang, Yanni Xuan, and Hailiang Yu. 2022. "Effect of Annealing Temperature on the Interfacial Microstructure and Bonding Strength of Cu/Al Clad Sheets with a Stainless Steel Interlayer" Materials 15, no. 6: 2119. https://doi.org/10.3390/ma15062119
APA StyleGao, H., Gu, H., Wang, S., Xuan, Y., & Yu, H. (2022). Effect of Annealing Temperature on the Interfacial Microstructure and Bonding Strength of Cu/Al Clad Sheets with a Stainless Steel Interlayer. Materials, 15(6), 2119. https://doi.org/10.3390/ma15062119