Effectiveness of Ternary Blend Incorporating Rice Husk Ash, Silica Fume, and Cement in Preparing ASR Resilient Concrete
Abstract
:1. Introduction
2. Materials and Mixture Proportions
2.1. Materials
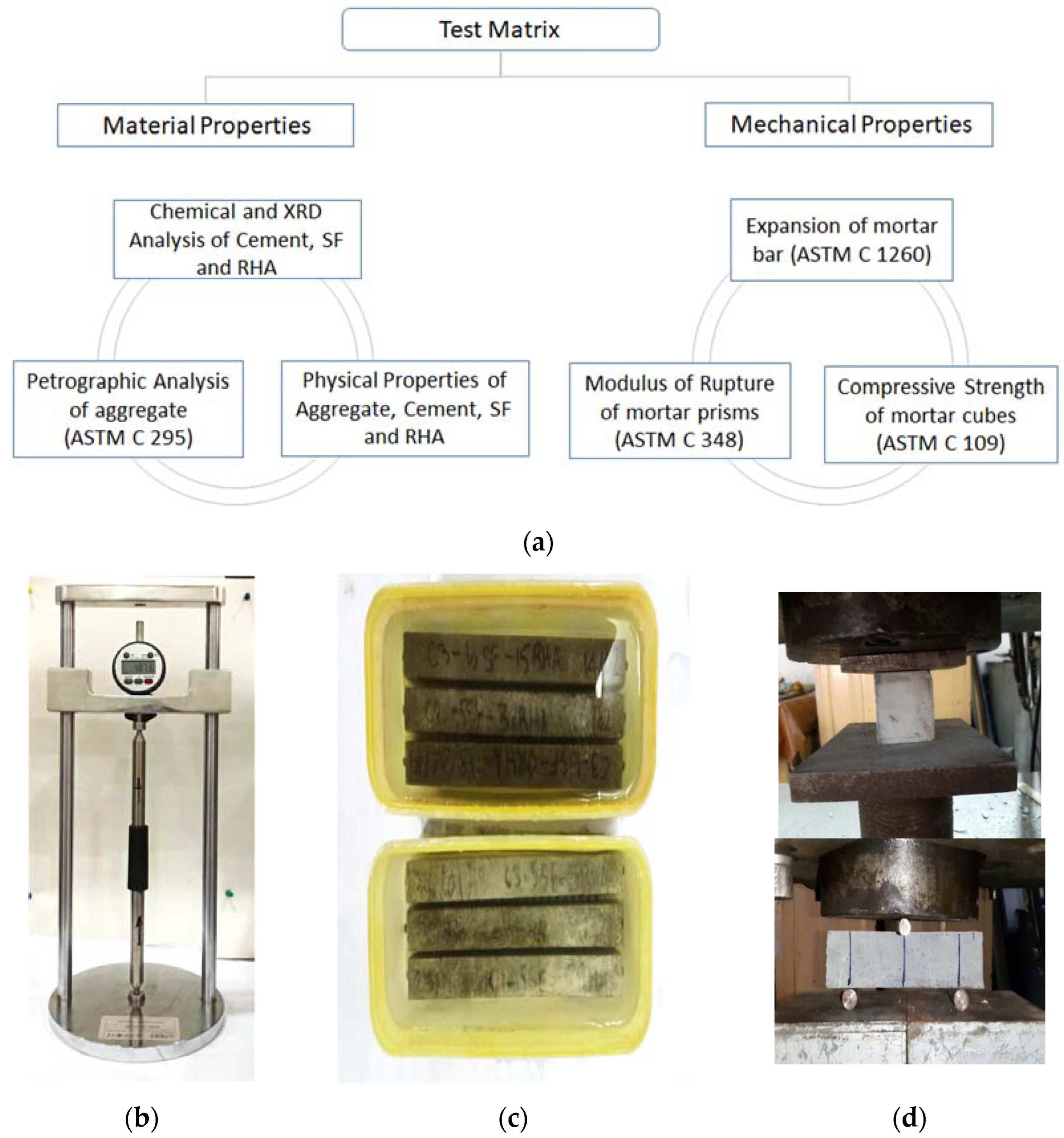
2.2. Tests on Raw Materials
2.3. Fresh Properties of Mixtures Incorporating RHA and SF
2.4. Cube and Prism Specimens
2.5. Mortar Bar Specimens
3. Results and Discussion
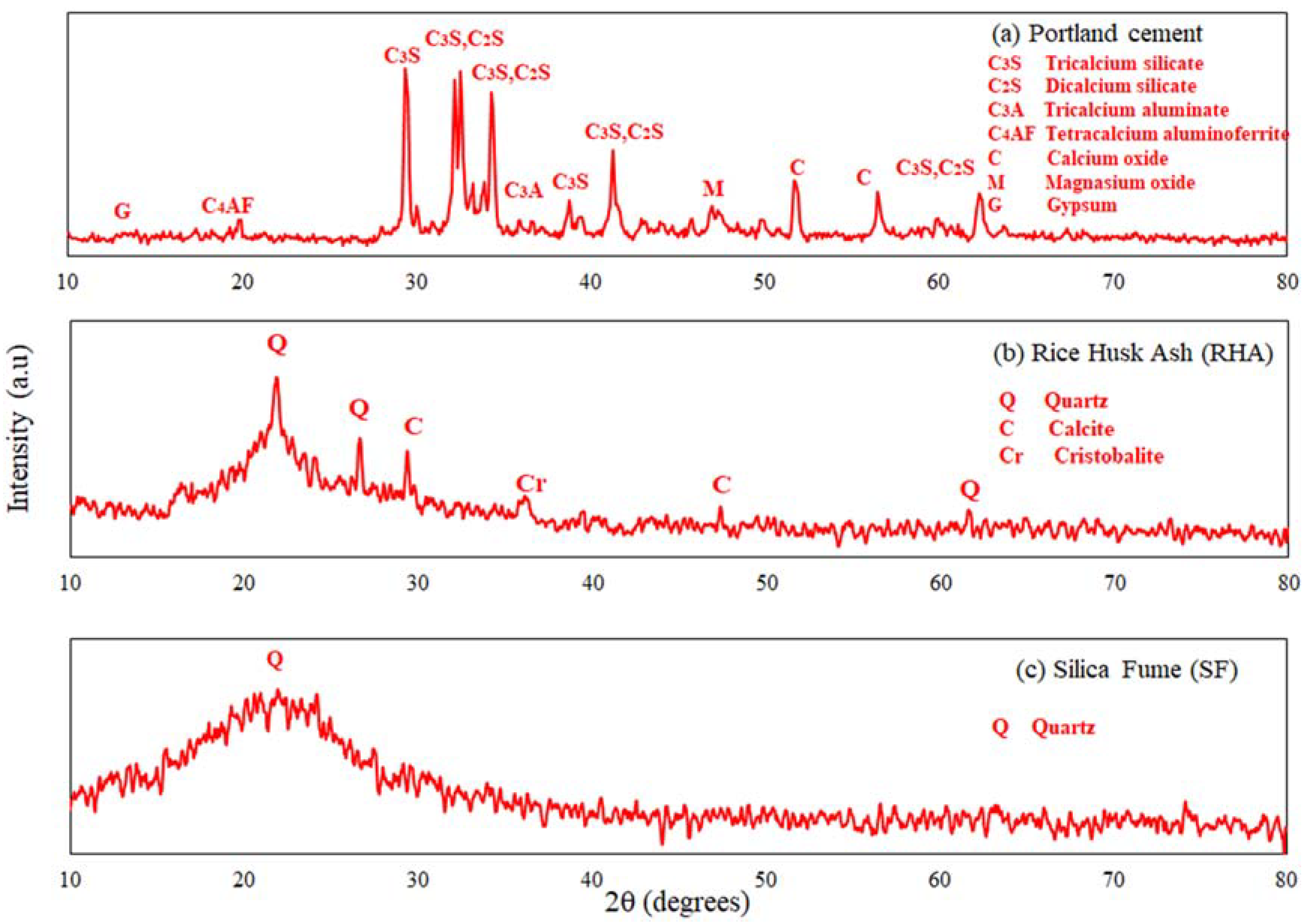
3.1. Material Characterization
3.2. Fresh Properties of Mixtures Incorporating SF and RHA
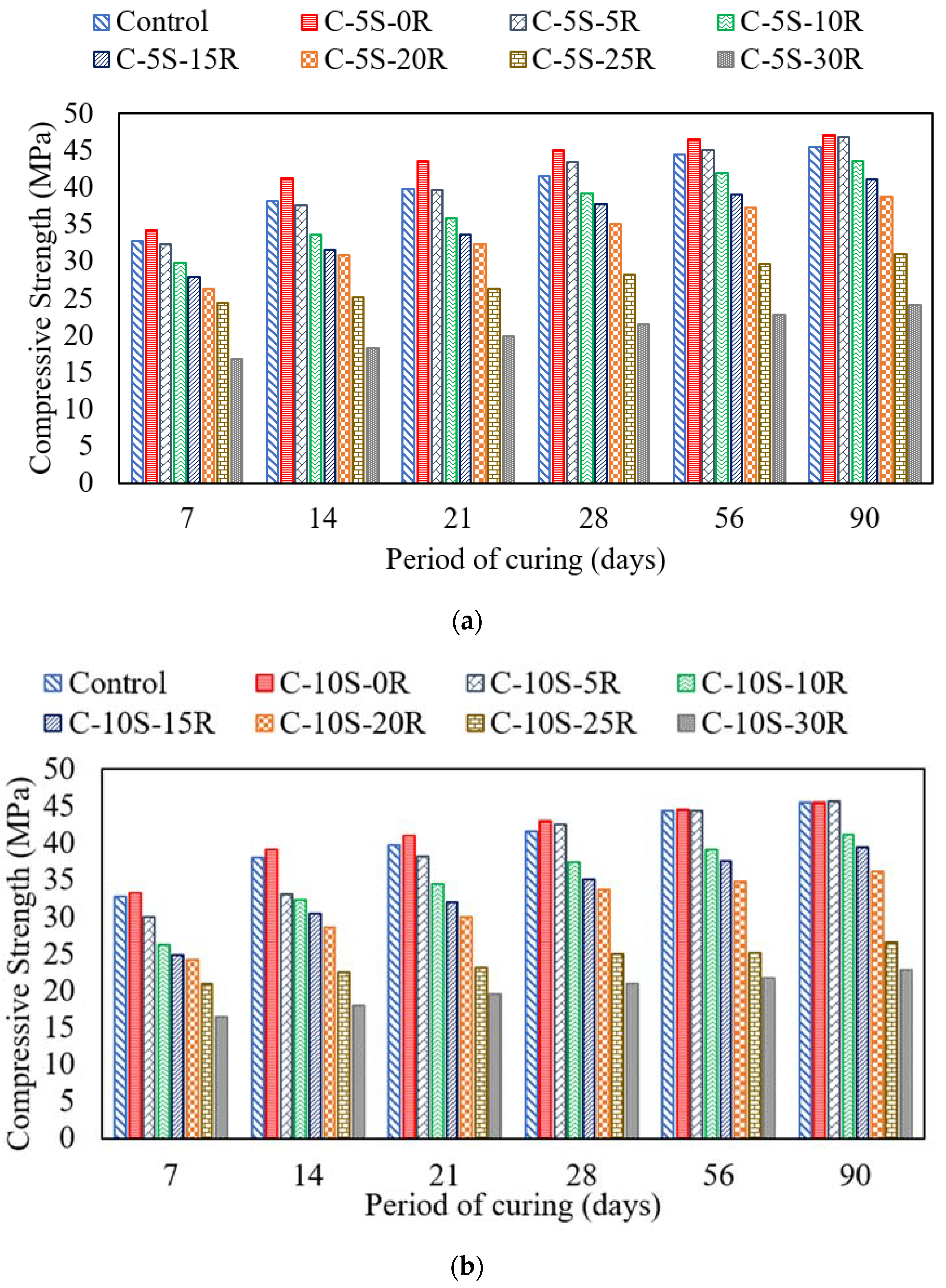
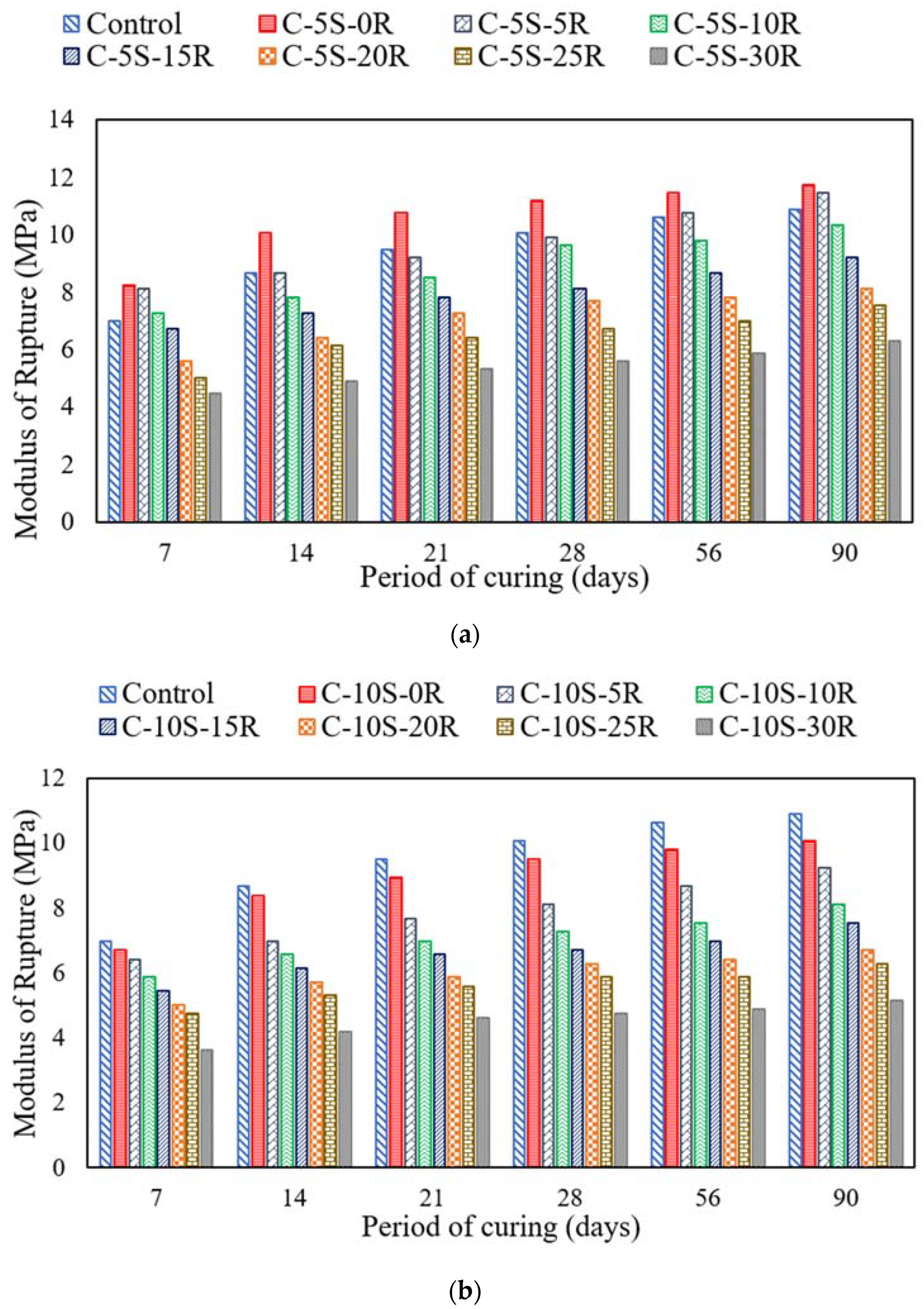
3.3. Effect of SF and RHA on Compressive and Flexural Strength
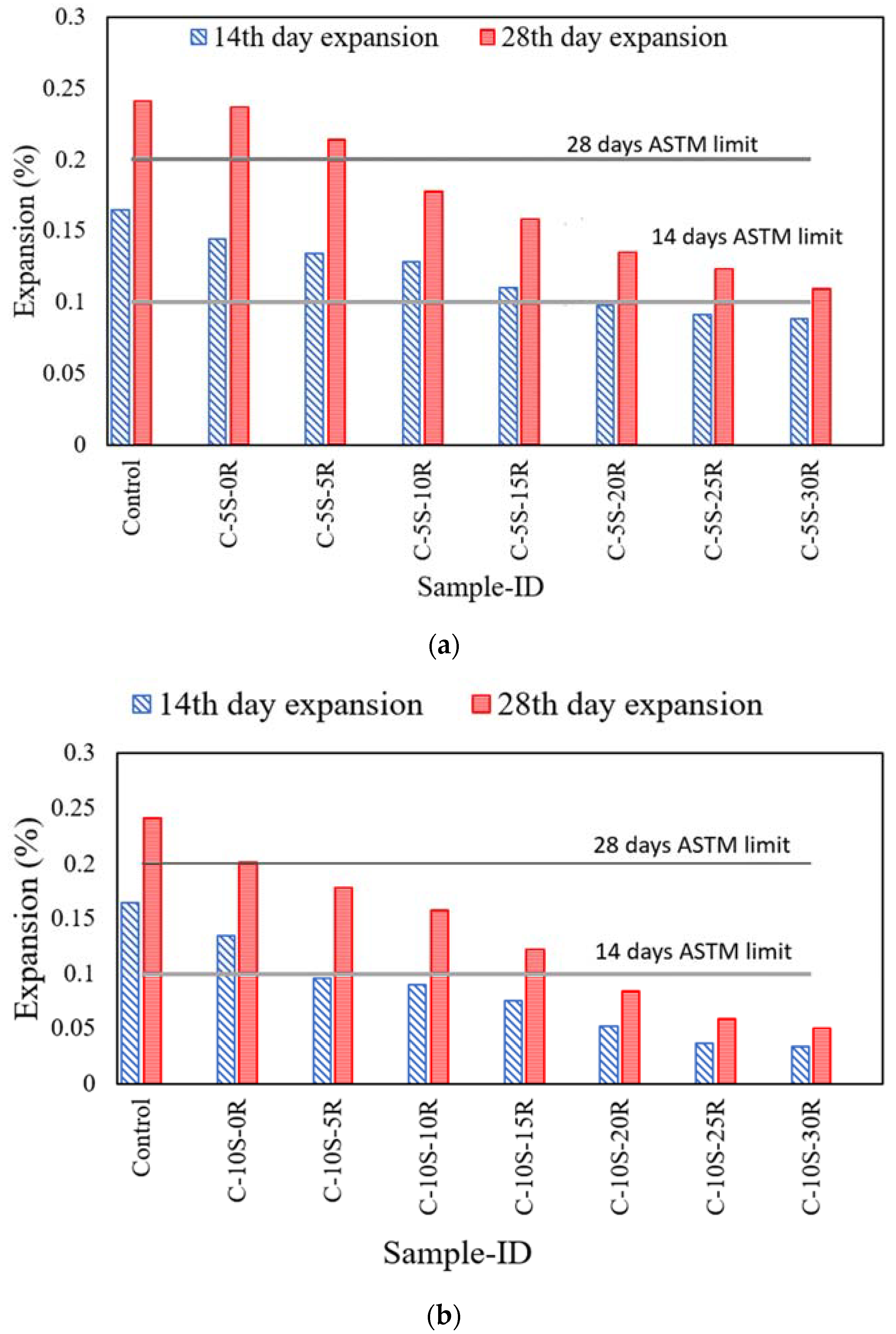
3.4. Expansion due to Alkali-Silica Reaction in Concrete
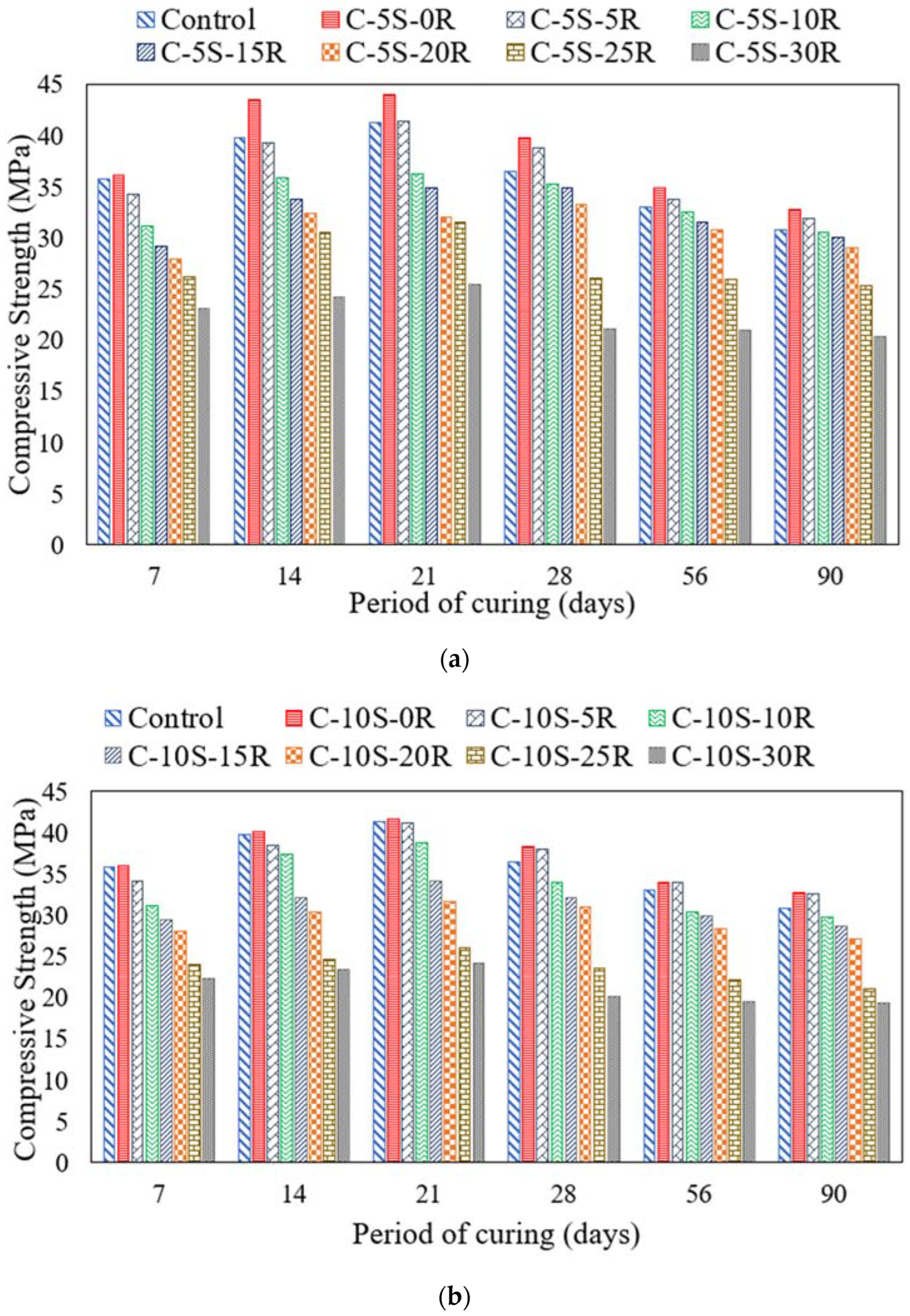
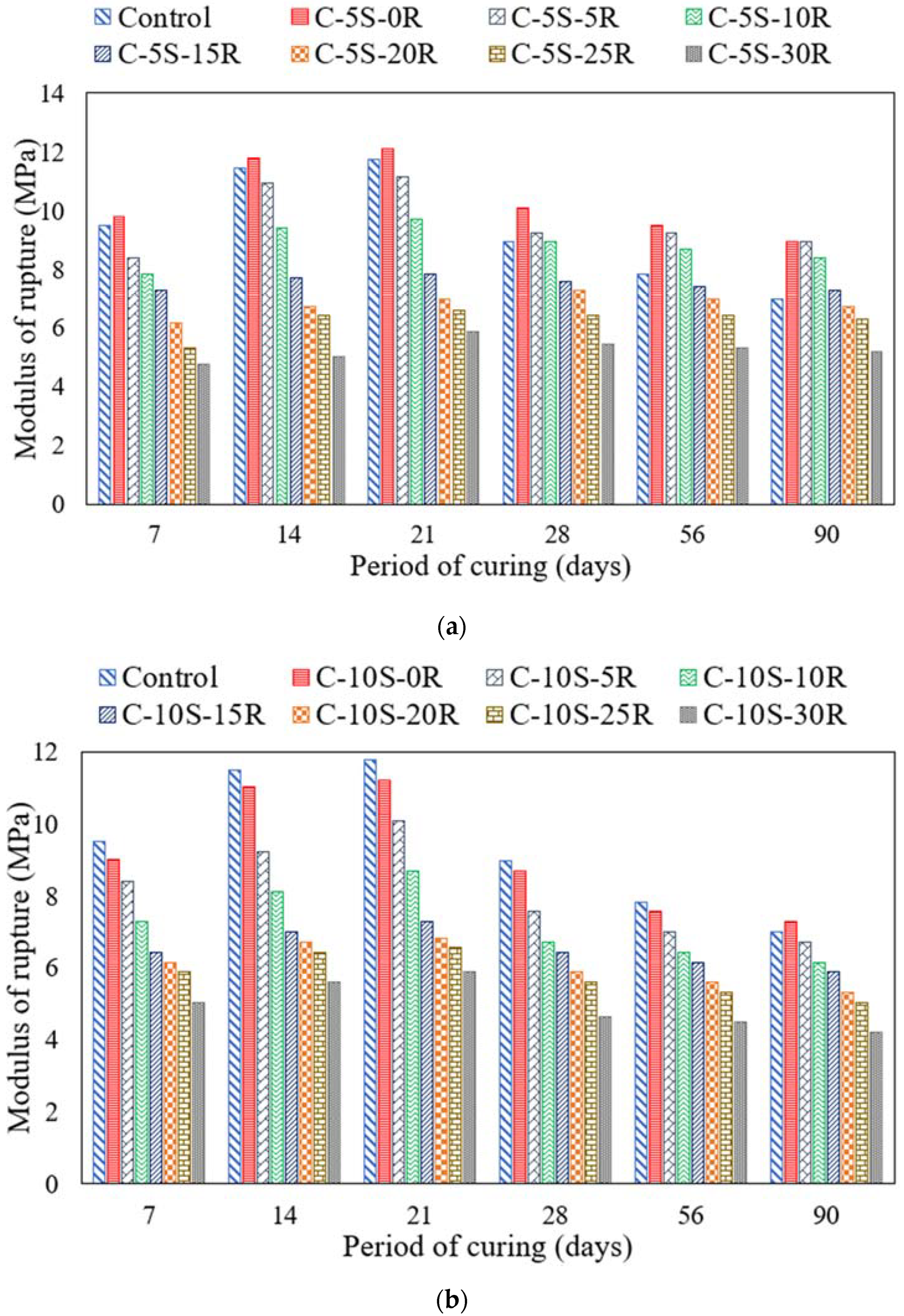
3.5. Effect of ASR on Compressive and Flexural Strengths
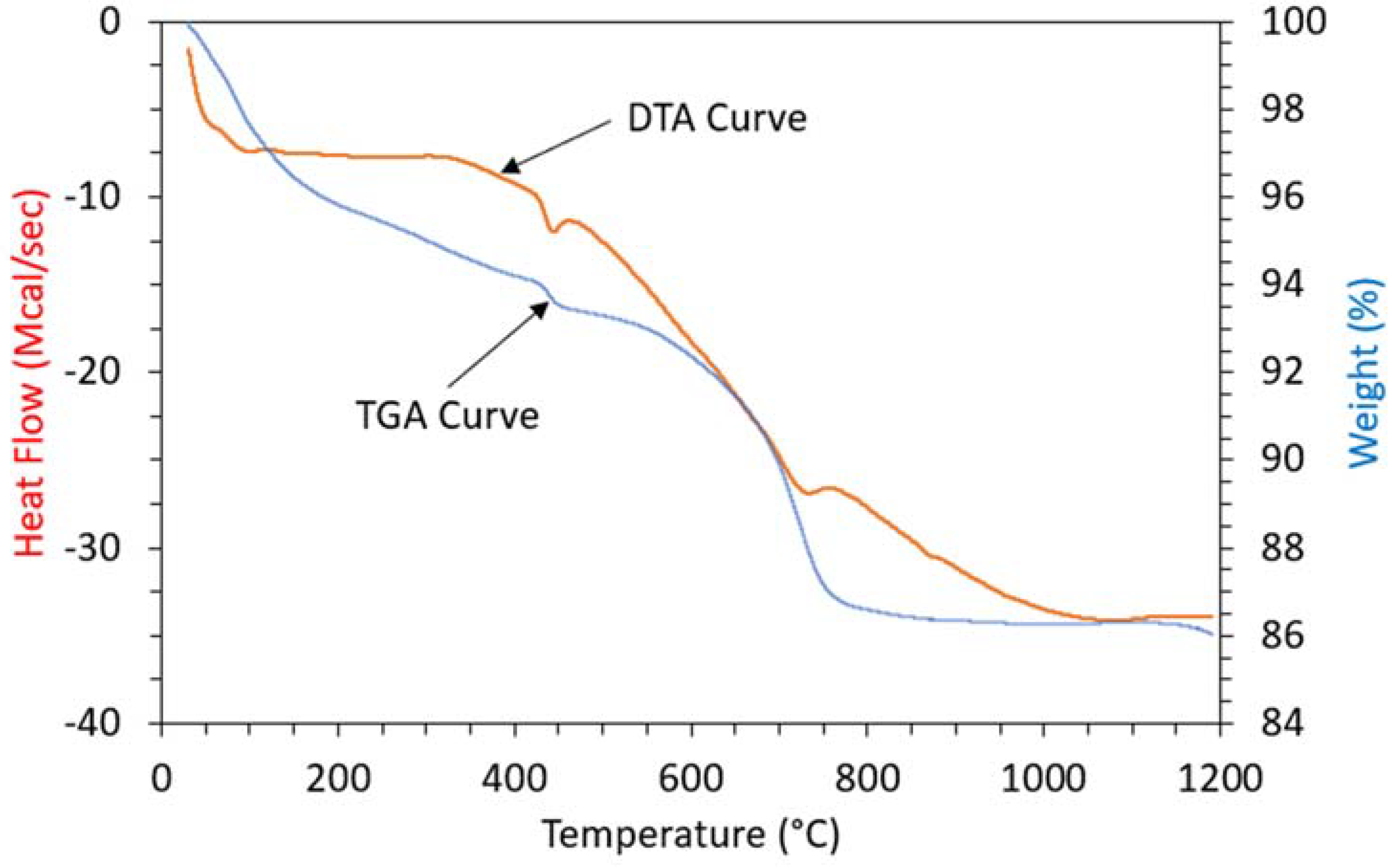
3.6. Thermal Analysis
4. Conclusions
- The incorporation of waste ashes (SF and RHA) reduces the flow of concrete mixtures due to the porous nature and increased surface area.
- The incorporation of 5% or 10% SF along with 5% RHA exhibited improved compressive strength at 28 days of curing age. However, after increasing the waste ash content, the compressive strength was reduced. Similarly, this behavior was also observed in the case of flexural strength.
- A decrease in the expansion of the mortar bar was noticed with an increase in the SF and RHA replacement due to pozzolanic reaction and dilution effect. The expansion of the mortar bar incorporating 5% SF and 20% RHA was 0.10% and 0.13% at 14 days and 28 days, respectively, which were less than the limit specified by ASTM C 1260. Moreover, the expansion of the mortar bar when incorporating 10% SF and 5% RHA was 0.096 and 0.178% at 14 and 28 days, respectively.
5. Future Recommendations
- A durability test should be conducted and the sulfate attack and chloride penetration of concrete incorporating SF and RHA should be studied.
- A more detailed study on effect of SF and RHA on the alkalinity of mortar may be studied by alkali leaching.
- The effects of using ternary blends of other pozzolanic materials should be studied.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, T.; Burley, E.; Rigden, S.; Abu-Tair, A.I. The effect of alkali reactivity on the mechanical properties of concrete. Constr. Build. Mater. 2003, 17, 123–144. [Google Scholar] [CrossRef]
- Miyagawa, T. Fracture of reinforcing steels in concrete damaged by ASR. Constr. Build. Mater. 2012, 39, 105–112. [Google Scholar] [CrossRef]
- Abbas, S.; Arshad, U.; Abbass, W.; Nehdi, M.; Ahmed, A. Recycling Untreated Coal Bottom Ash with Added Value for Mitigating Alkali–Silica Reaction in Concrete: A Sustainable Approach. Sustainability 2020, 12, 10631. [Google Scholar] [CrossRef]
- Marzouk, H.; Langdon, S. The effect of alkali-aggregate reactivity on the mechanical properties of high and normal strength concrete. Cem. Concr. Compos. 2003, 25, 549–556. [Google Scholar] [CrossRef]
- Ono, K. Damaged concrete structures in Japan due to alkali silica reaction. Int. J. Cem. Compos. Light. Concr. 1988, 10, 247–257. [Google Scholar] [CrossRef]
- Stark, D. Handbook for the Identification of Alkali-Silica Reactivity in Highway Structures; SHRP-C/FR-91-101; TRB National Research Council: Washington, DC, USA, 1991. [Google Scholar]
- Thomas, M.D.; Folliard, K.J.; Fournier, B.; Rivard, P.; Drimalas, T.; Garber, S.I. Methods for Evaluating and Treating ASR-Affected Structures: Results of Field Application and Demonstration Projects: Volume II—Details of Field Applications and Analysis; Federal Highway Administration: Washington, DC, USA, 2013. [Google Scholar] [CrossRef]
- Owsiak, Z.; Zapała-Sławeta, J.; Czapik, P. Diagnosis of concrete structures distress due to alkali-aggregate reaction. Bull. Pol. Acad. Sci. Tech. Sci. 2015, 63, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Jensen, V. Alkali–silica reaction damage to Elgeseter Bridge, Trondheim, Norway: A review of construction, research and repair up to 2003. Mater. Charact. 2004, 53, 155–170. [Google Scholar] [CrossRef]
- Munir, M.J.; Qazi, A.U.; Kazmi, S.M.; Khitab, A.; Ashiq, S.Z.; Ahmed, I. A literature review on alkali silica reactivity of concrete in Pakistan. Pak. J. Sci. 2016, 68, 53–62. [Google Scholar]
- Ferdous, W.; Ngo, T.D.; Nguyen, K.T.; Ghazlan, A.; Mendis, P.; Manalo, A. Effect of fire-retardant ceram powder on the properties of phenolic-based GFRP composites. Compos. Part B Eng. 2018, 155, 414–424. [Google Scholar] [CrossRef]
- Siddika, A.; Hajimohammadi, A.; Mamun, M.A.A.; Alyousef, R.; Ferdous, W. Waste Glass in Cement and Geopolymer Concretes: A Review on Durability and Challenges. Polymers 2021, 13, 2071. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M. The effect of supplementary cementing materials on alkali-silica reaction: A review. Cem. Concr. Res. 2011, 41, 1224–1231. [Google Scholar] [CrossRef]
- Carles-Gibergues, A.; Cyr, M.; Moisson, M.; Ringot, E. A simple way to mitigate alkali-silica reaction. Mater. Struct. 2007, 41, 73–83. [Google Scholar] [CrossRef]
- Monteiro, P.; Wang, K.; Sposito, G.; Santos, M.; de Andrade, W. Influence of mineral admixtures on the alkali-aggregate reaction. Cem. Concr. Res. 1997, 27, 1899–1909. [Google Scholar] [CrossRef]
- Panesar, D.K. Supplementary cementing materials. In Developments in the Formulation and Reinforcement of Concrete; Woodhead Publishing: Sawston, UK, 2019. [Google Scholar]
- ACI 234R-06; 234R-06 Guide for the Use of Silica Fume in Concrete ACI 234R-06. American Concrete Institute: Farmington Hills, MI, USA, 2006.
- Aquino, W.; Lange, D.; Olek, J. The influence of metakaolin and silica fume on the chemistry of alkali–silica reaction products. Cem. Concr. Compos. 2001, 23, 485–493. [Google Scholar] [CrossRef]
- Boddy, A.; Hooton, R.; Thomas, M. The effect of product form of silica fume on its ability to control alkali–silica reaction. Cem. Concr. Res. 2000, 30, 1139–1150. [Google Scholar] [CrossRef]
- Kumar, S.; Sangwan, P.; Dhankhar, R.M.; Bidra, S. Utilization of Rice Husk and Their Ash: A Review. J. Chem. Environ. Sci. 2013, 1, 126–129. [Google Scholar]
- ASTM Standard C 1260; Standard Test Method for Potential Alkali Reactivity of Aggregates (Mortar-Bar Method). ASTM International: West Conshohocken, PA, USA, 2014. [CrossRef]
- ASTM C151; Standard Test Method for Autoclave Expansion of Hydraulic Cement. ASTM International: West Conshohocken, PA, USA, 2015.
- ASTM C188; ASTM C188-16, Standard Test Method for Density of Hydraulic Cement. ASTM International: West Conshohocken, PA, USA, 2016.
- ASTM C184; Standard Test Method for Fineness of Hydraulic Cement by Air-Permeability. ASTM International: West Conshohocken, PA, USA, 2000.
- ASTM 295; Standard Guide for Petrographic Examination of Aggregates for Concrete. ASTM: West Conshohocken, PA, USA, 2015. [CrossRef]
- ASTM-C127-15.2; Standard Test Method for Relative Density (Specific Gravity) and Absorption of Coarse Aggregate. ASTM Subcommittee C09.20. ASTM International: West Conshohocken, PA, USA, 2015; p. 5. [CrossRef]
- Astm:C29/C29M-09; Standard Test Method for Bulk Density (‘Unit Weight’) and Voids in Aggregate. ASTM International: West Conshohocken, PA, USA, 2009. [CrossRef]
- ASTM C535; Standard Test Method for Resistance to Degradation of Large-Size Coarse Aggregate by Abrasion and Impact in the Los Angeles Machine. ASTM International: West Conshohocken, PA, USA, 2010. [CrossRef]
- BS-812-110; Aggregate Crushing Value. British Standards Institution: London, UK, 1990.
- BS-812-112; Testing Aggregates—Methods for Determination of Aggregate Crushing Value (ACV). British Standards Institution: London, UK, 1990.
- ASTM C187; Standard Test Method for Amount of Water Required for Normal Consistency of Hydraulic Cement Paste. ASTM International: West Conshohocken, PA, USA, 2016.
- ASTM C191; Standard Test Methods for Time of Setting of Hydraulic Cement by Vicat Needle. ASTM International: West Conshohocken, PA, USA, 2018. [CrossRef]
- ASTM C1437; Standard Test Method for Flow of Hydraulic Cement Mortar. ASTM International: West Conshohocken, PA, USA, 2016. [CrossRef]
- ASTM Committee C09.26; ASTM C1260-14 Standard Test Method for Potential Alkali Reactivity of Aggregates (Mortar-Bar Method). Annu. B. ASTM Stand. Vol. 04.02. ASTM: West Conshohocken, PA, USA, 2014. [CrossRef]
- ASTM C109; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50-mm] Cube Specimens). ASTM: West Conshohocken, PA, USA, 2010. [CrossRef]
- ASTM C348; Standard Test Method for Flexural Strength of Hydraulic-Cement Mortars. ASTM: West Conshohocken, PA, USA, 2020. [CrossRef]
- ASTM C305; Standard Practice for Mechanical Mixing of Hydraulic Cement Pastes and Mortars. ASTM: West Conshohocken, PA, USA, 2020.
- ASTM C490; Standard Practice for Use of Apparatus for the Determination of Length Change of Hardened Cement Paste, Mortar, and Concrete. ASTM: West Conshohocken, PA, USA, 2019, 2019. [CrossRef]
- ASTM C618-17a; Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete. Annu. B. ASTM Stand. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM C114; Standard Test Methods for Chemical Analysis of Hydraulic Cement. ASTM International: West Conshohocken, PA, USA, 2020. [CrossRef]
- Abbas, S.; Kazmi, S.M.; Munir, M.J. Potential of rice husk ash for mitigating the alkali-silica reaction in mortar bars incorporating reactive aggregates. Constr. Build. Mater. 2017, 132, 61–70. [Google Scholar] [CrossRef]
- Pradhan, D.; Dutta, D. Influence of Silica Fume on Normal Concrete. Int. J. Eng. Res. Appl. 2013, 3, 79–82. [Google Scholar]
- Kontoleontos, F.; Tsakiridis, P.; Marinos, A.; Katsiotis, N.; Kaloidas, V.; Katsioti, M. Dry-grinded ultrafine cements hydration. physicochemical and microstructural characterization. Mater. Res. 2013, 16, 404–416. [Google Scholar] [CrossRef]
- Mor, S.; Chhoden, K.; Ravindra, K. Application of agro-waste rice husk ash for the removal of phosphate from the wastewater. J. Clean. Prod. 2016, 129, 673–680. [Google Scholar] [CrossRef]
- Soliman, N.; Tagnit-Hamou, A. Partial substitution of silica fume with fine glass powder in UHPC: Filling the micro gap. Constr. Build. Mater. 2017, 139, 374–383. [Google Scholar] [CrossRef]
- ASTM Standard C33; Standard Specification for Concrete Aggregates. ASTM International: West Conshohocken, PA, USA, 2003. [CrossRef]
- Chusilp, N.; Jaturapitakkul, C.; Kiattikomol, K. Effects of LOI of ground bagasse ash on the compressive strength and sulfate resistance of mortars. Constr. Build. Mater. 2009, 23, 3523–3531. [Google Scholar] [CrossRef]
- Shearer, C.R.; Kurtis, K.E. Use of Biomass and Co-Fired Fly Ash in Concrete. ACI Mater. J. 2015, 112, 209–218. [Google Scholar] [CrossRef]
- Arif, E.; Clark, M.W.; Lake, N. Sugar cane bagasse ash from a high efficiency co-generation boiler: Applications in cement and mortar production. Constr. Build. Mater. 2016, 128, 287–297. [Google Scholar] [CrossRef]
- Medina, C.; del Bosque, I.S.; Frías, M.; de Rojas, M.S. Design and characterisation of ternary cements containing rice husk ash and fly ash. Constr. Build. Mater. 2018, 187, 65–76. [Google Scholar] [CrossRef]
- Hossain, T.; Sarker, S.K.; Basak, B.C. Utilization potential of rice husk ash as a construction material in rural areas. J. Civ. Eng. 2011, 39, 175–188. [Google Scholar]
- Vishnumaya, L.; Ambi, R. Early age properties of silica fume modified cement mortar with M sand as fine aggregate. Int. J. Eng. Res. Appl. 2014, 78–81. Available online: https://www.ijera.com/special_issue/Trace/IJERA%20206.pdf (accessed on 14 February 2022).
- Park, K.-B.; Kwon, S.-J.; Wang, X.-Y. Analysis of the effects of rice husk ash on the hydration of cementitious materials. Constr. Build. Mater. 2016, 105, 196–205. [Google Scholar] [CrossRef]
- Mosaberpanah, M.A.; Umar, S.A. Utilizing Rice Husk Ash as Supplement to Cementitious Materials on Performance of Ultra High Performance Concrete—A review. Mater. Today Sustain. 2020, 7, 100030. [Google Scholar] [CrossRef]
- Salim, P.M.; Prasad, B.S. An investigation on the influence of silica fume on the properties of mineral admixture concrete. Int. J. Civ. Eng. Technol. 2018, 9, 1217–1227. [Google Scholar]
- Younes, M.; Abdel-Rahman, H.; Khattab, M.M. Utilization of rice husk ash and waste glass in the production of ternary blended cement mortar composites. J. Build. Eng. 2018, 20, 42–50. [Google Scholar] [CrossRef]
- Ahsan, M.B.; Hossain, Z. Supplemental use of rice husk ash (RHA) as a cementitious material in concrete industry. Constr. Build. Mater. 2018, 178, 1–9. [Google Scholar] [CrossRef]
- Gowda, T.S.; Ranganath, R. Utilization of Coal Fly ash and ultra fine additives in producing HPC: Influence on early strength, flexural parameters and microstructure. Mater. Today Proc. 2020, 32, 677–685. [Google Scholar] [CrossRef]
- Saha, A.K.; Sarker, P.K. Potential alkali silica reaction expansion mitigation of ferronickel slag aggregate by fly ash. Struct. Concr. 2018, 19, 1376–1386. [Google Scholar] [CrossRef] [Green Version]
- Afshinnia, K.; Rangaraju, P.R. Influence of fineness of ground recycled glass on mitigation of alkali–silica reaction in mortars. Constr. Build. Mater. 2015, 81, 257–267. [Google Scholar] [CrossRef]
- Turk, K.; Kina, C.; Bagdiken, M. Use of binary and ternary cementitious blends of F-Class fly-ash and limestone powder to mitigate alkali-silica reaction risk. Constr. Build. Mater. 2017, 151, 422–427. [Google Scholar] [CrossRef]
- Nixon, P.J.; Sims, I. RILEM Recommendations for the Prevention of Damage by Alkali-Aggregate Reactions in New Concrete Structures: State-of-the-Art Report of the RILEM Technical Committee 219-ACS; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Abbas, S.; Ahmed, A.; Nehdi, M.L.; Saeed, D.; Abbass, W.; Amin, F. Eco-Friendly Mitigation of Alkali-Silica Reaction in Concrete Using Waste-Marble Powder. J. Mater. Civ. Eng. 2020, 32, 04020270. [Google Scholar] [CrossRef]
- Abbas, S.; Sharif, A.; Ahmed, A.; Abbass, W.; Shaukat, S. Prospective of sugarcane bagasse ash for controlling the alkali-silica reaction in concrete incorporating reactive aggregates. Struct. Concr. 2019, 21, 781–793. [Google Scholar] [CrossRef]
- Yazıcı, H. The effect of steel micro-fibers on ASR expansion and mechanical properties of mortars. Constr. Build. Mater. 2012, 30, 607–615. [Google Scholar] [CrossRef]
- Shehata, M.H.; Thomas, M.D.A. Use of ternary blends containing silica fume and fly ash to suppress expansion due to alkali–silica reaction in concrete. Cem. Concr. Res. 2002, 32, 341–349. [Google Scholar] [CrossRef]
- Nagrockienė, D.; Rutkauskas, A. The effect of fly ash additive on the resistance of concrete to alkali silica reaction. Constr. Build. Mater. 2019, 201, 599–609. [Google Scholar] [CrossRef]
- Moropoulou, A.; Bakolas, A.; Aggelakopoulou, E. Evaluation of pozzolanic activity of natural and artificial pozzolans by thermal analysis. Thermochim. Acta 2004, 420, 135–140. [Google Scholar] [CrossRef]
- Almeida, A.E.F.D.S.; Sichieri, E.P. Thermogravimetric analyses and mineralogical study of polymer modified mortar with silica fume. Mater. Res. 2006, 9, 321–326. [Google Scholar] [CrossRef] [Green Version]
- Munir, M.J.; Kazmi, S.M.; Wu, Y.-F. Efficiency of waste marble powder in controlling alkali–silica reaction of concrete: A sustainable approach. Constr. Build. Mater. 2017, 154, 590–599. [Google Scholar] [CrossRef]
- Kandasamy, S.; Shehata, M.H. The capacity of ternary blends containing slag and high-calcium fly ash to mitigate alkali silica reaction. Cem. Concr. Compos. 2014, 49, 92–99. [Google Scholar] [CrossRef]
- Lei, J.; Law, W.; Yang, E.H. Effect of calcium hydroxide on the alkali-silica reaction of alkali-activated slag mortars activated by sodium hydroxide. Constr. Build. Mater. 2021, 272. [Google Scholar] [CrossRef]
- Bleszynski, R.F.; Thomas, M.D. Microstructural Studies of Alkali-Silica Reaction in Fly Ash Concrete Immersed in Alkaline Solutions. Adv. Cem. Based Mater. 1998, 7, 66–78. [Google Scholar] [CrossRef]

| Sample | Cement | Silica Fume | Rice Husk Ash |
|---|---|---|---|
| % | % | % | |
| Control | 100 | 0 | 0 |
| C-5S-0R | 95 | 5 | 0 |
| C-5S-5R | 90 | 5 | 5 |
| C-5S-10R | 85 | 5 | 10 |
| C-5S-15R | 80 | 5 | 15 |
| C-5S-20R | 75 | 5 | 20 |
| C-5S-25R | 70 | 5 | 25 |
| C-5S-30R | 65 | 5 | 30 |
| C-10S-0R | 90 | 10 | 0 |
| C-10S-5R | 85 | 10 | 5 |
| C-10S-10R | 80 | 10 | 10 |
| C-10S-15R | 75 | 10 | 15 |
| C-10S-20R | 70 | 10 | 20 |
| C-10S-25R | 65 | 10 | 25 |
| C-10S-30R | 60 | 10 | 30 |
| Oxides | Cement | SF | RHA |
|---|---|---|---|
| CaO | 59.62 | 1.23 | 0.65 |
| MgO | 3.85 | 0.89 | 1.43 |
| SiO2 | 21.73 | 90.13 | 88.51 |
| Al2O3 | 4.87 | 0.42 | 0.21 |
| Fe2O3 | 2.12 | 0.515 | 0.61 |
| SO3 | 1.65 | 1.23 | 0.40 |
| K2O | 0.67 | 1.51 | 1.89 |
| Na2O | 0.21 | 0.512 | 0.18 |
| L. O. I | 2.61 | 2.08 | 2.89 |
| Property | Standard | Cement | SF | RHA |
|---|---|---|---|---|
| Specific Gravity | ASTM C188 | 3.12 | 2.25 | 2.09 |
| Unit Weight (Kg/m3) | ASTM C29 | 1428 | 580.4 | 554.8 |
| Fineness (passing No. 200) | ASTM C184 | 96.5% | 100% | 100% |
| Autoclave expansion | ASTM C151 | 0.11% | - | - |
| Property | Standard | Value |
|---|---|---|
| Impact Value | BS-812 | 22% |
| Crushing Value | BS-812 | 26% |
| Abrasion Test | ASTM C 535 | 28% |
| Specific Gravity | ASTM C 127 | 2.58 |
| Water Absorption (%) | ASTM C 127 | 0.96 |
| Bulk Density (Kg/m3) | ASTM C 29 | 1393 |
| Voids Content (%) | ASTM C 29 | 36.75% |
| Sr. | Sample | Cement | SF | RHA | Aggregates | Water | Flow Dia |
|---|---|---|---|---|---|---|---|
| No | - | (Grams) | (Grams) | (Grams) | (Grams) | (Grams) | (mm) |
| 1 | C | 220 | 0 | 0 | 495 | 103.4 | 115 |
| 2 | C-5S-0R | 209 | 11 | 0 | 495 | 103.4 | 114 |
| 3 | C-5S-5R | 198 | 11 | 11 | 495 | 103.4 | 113 |
| 4 | C-5S-10R | 187 | 11 | 22 | 495 | 103.4 | 112 |
| 5 | C-5S-15R | 176 | 11 | 33 | 495 | 103.4 | 109 |
| 6 | C-5S-20R | 165 | 11 | 44 | 495 | 103.4 | 106 |
| 7 | C-5S-25R | 154 | 11 | 55 | 495 | 103.4 | 104 |
| 8 | C-5S-30R | 143 | 11 | 66 | 495 | 103.4 | 102 |
| 9 | C-10S-0R | 198 | 22 | 0 | 495 | 103.4 | 113 |
| 10 | C-10S-5R | 187 | 22 | 11 | 495 | 103.4 | 112 |
| 11 | C-10S-10R | 176 | 22 | 22 | 495 | 103.4 | 111 |
| 12 | C-10S-15R | 165 | 22 | 33 | 495 | 103.4 | 107 |
| 13 | C-10S-20R | 154 | 22 | 44 | 495 | 103.4 | 104 |
| 14 | C-10S-25R | 143 | 22 | 55 | 495 | 103.4 | 103 |
| 15 | C-10S-30R | 132 | 22 | 66 | 495 | 103.4 | 101 |
| Sr. No | Sample | Normal Consistency | Initial Setting Time | Final Setting Time |
|---|---|---|---|---|
| - | - | (%) | (Minutes) | (Minutes) |
| 1 | C | 24.03 | 128 | 203 |
| 2 | C-5S-0R | 28.8 | 85 | 215 |
| 3 | C-5S-10R | 32.7 | 104 | 228 |
| 4 | C-5S-20R | 36.8 | 124 | 243 |
| 5 | C-5S-30R | 40.7 | 147 | 265 |
| 6 | C-10S-0R | 30.3 | 69 | 211 |
| 7 | C-10S-10R | 33.4 | 90 | 225 |
| 8 | C-10S-20R | 37.7 | 108 | 239 |
| 9 | C-10S-30R | 43 | 132 | 258 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, A.; Ameer, S.; Abbas, S.; Abbass, W.; Razzaq, A.; Mohamed, A.M.; Mohamed, A. Effectiveness of Ternary Blend Incorporating Rice Husk Ash, Silica Fume, and Cement in Preparing ASR Resilient Concrete. Materials 2022, 15, 2125. https://doi.org/10.3390/ma15062125
Ahmed A, Ameer S, Abbas S, Abbass W, Razzaq A, Mohamed AM, Mohamed A. Effectiveness of Ternary Blend Incorporating Rice Husk Ash, Silica Fume, and Cement in Preparing ASR Resilient Concrete. Materials. 2022; 15(6):2125. https://doi.org/10.3390/ma15062125
Chicago/Turabian StyleAhmed, Ali, Shoaib Ameer, Safeer Abbas, Wasim Abbass, Afia Razzaq, Abdeliazim Mustafa Mohamed, and Abdullah Mohamed. 2022. "Effectiveness of Ternary Blend Incorporating Rice Husk Ash, Silica Fume, and Cement in Preparing ASR Resilient Concrete" Materials 15, no. 6: 2125. https://doi.org/10.3390/ma15062125
APA StyleAhmed, A., Ameer, S., Abbas, S., Abbass, W., Razzaq, A., Mohamed, A. M., & Mohamed, A. (2022). Effectiveness of Ternary Blend Incorporating Rice Husk Ash, Silica Fume, and Cement in Preparing ASR Resilient Concrete. Materials, 15(6), 2125. https://doi.org/10.3390/ma15062125







