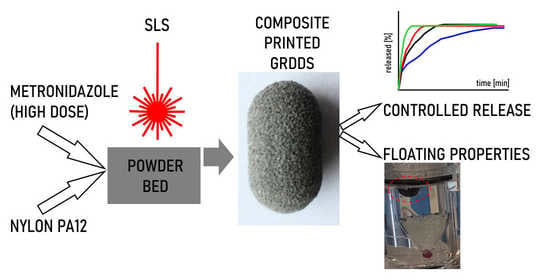
Development of Composite, Reinforced, Highly Drug-Loaded Pharmaceutical Printlets Manufactured by Selective Laser Sintering—In Search of Relevant Excipients for Pharmaceutical 3D Printing
Abstract
:1. Introduction
2. Materials and Methods
2.1. 3D Printing
2.2. Scanning Electron Microscopy (SEM)
2.3. Differential Scanning Calorimetry (DSC)
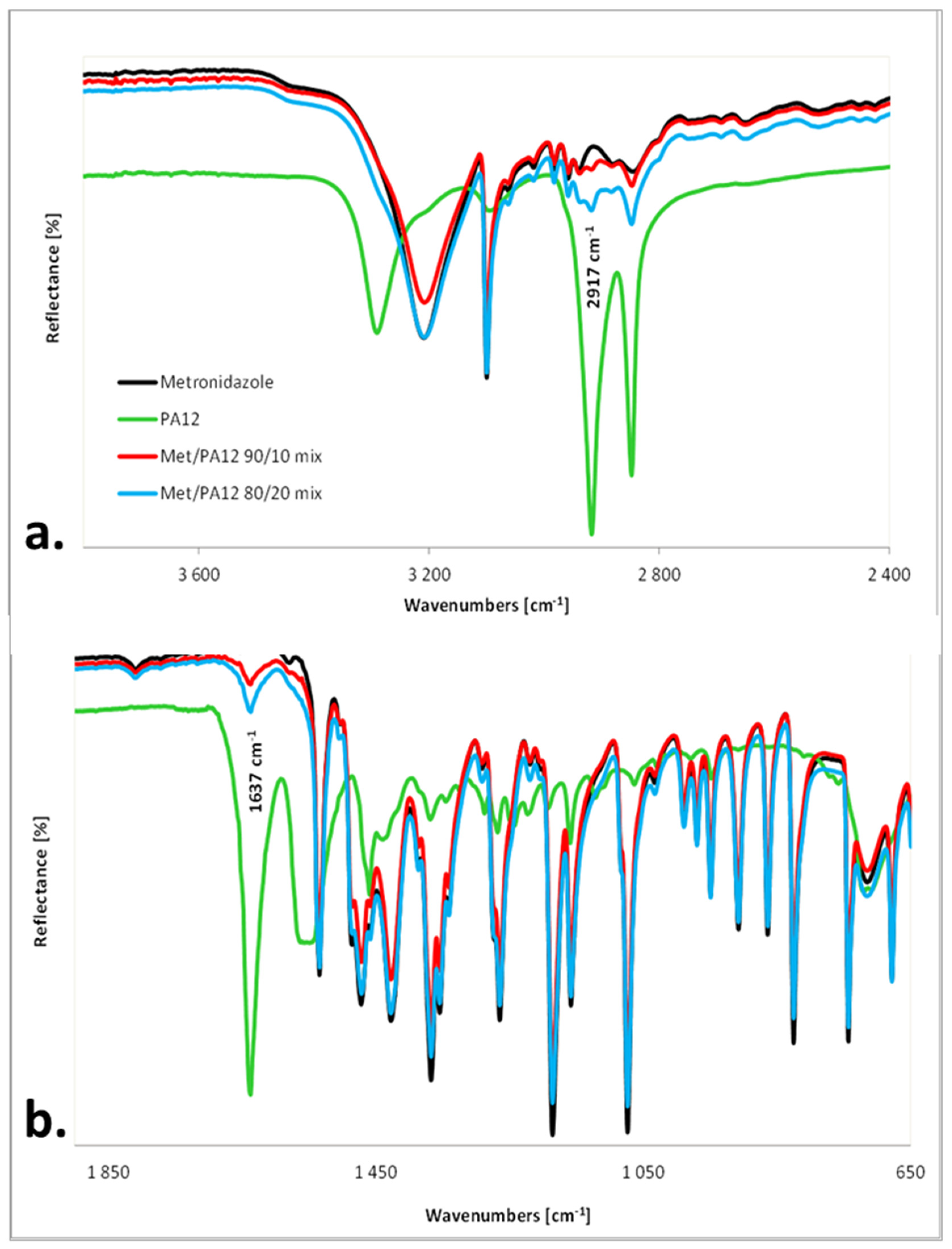
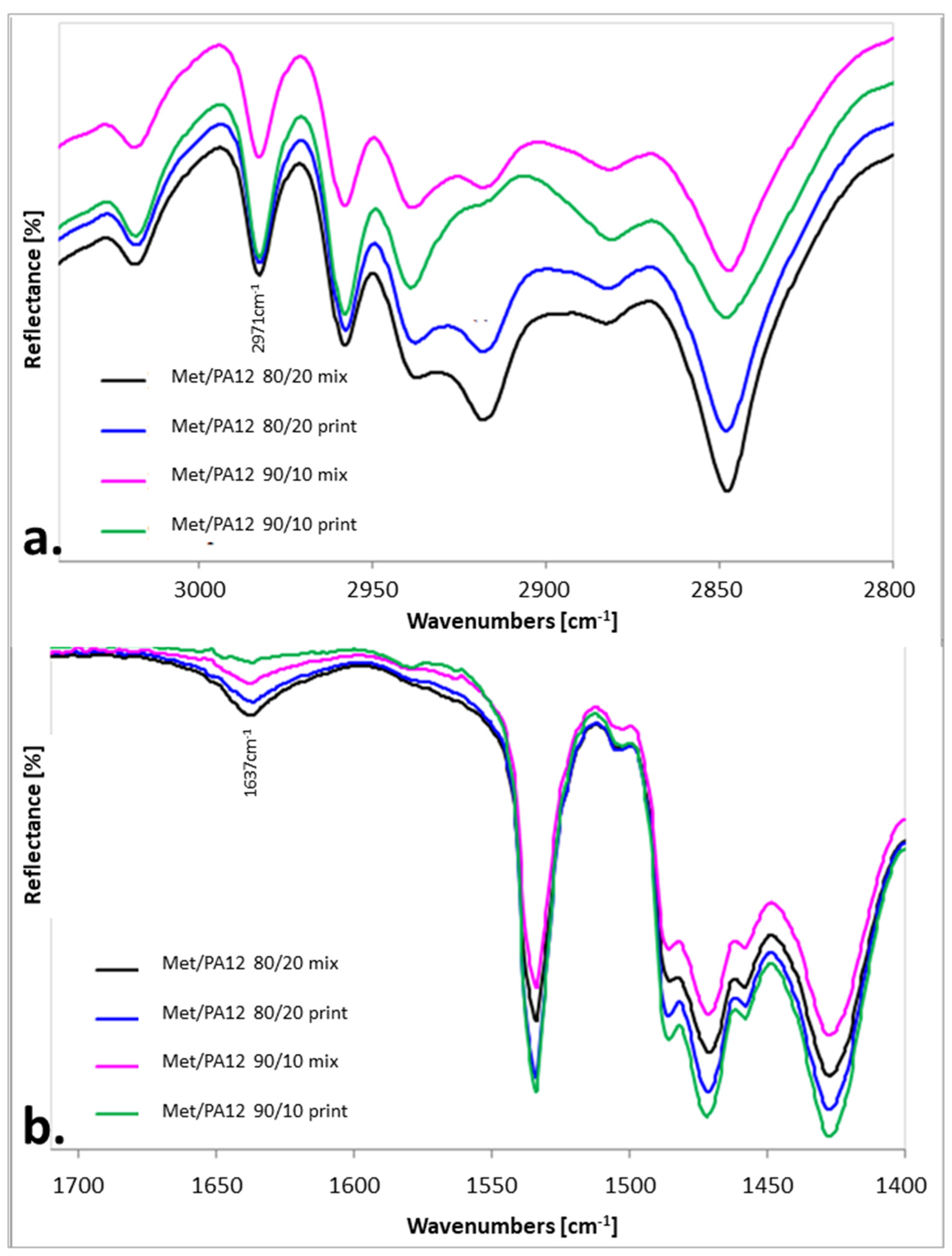
2.4. IR Measurements
2.5. Mechanical Parameters of the Printlets and Apparent Density of Printlets
2.6. Drug Release Studies and Buoyancy Observation
3. Results and Discussion
3.1. The Characteristics of the Printlets
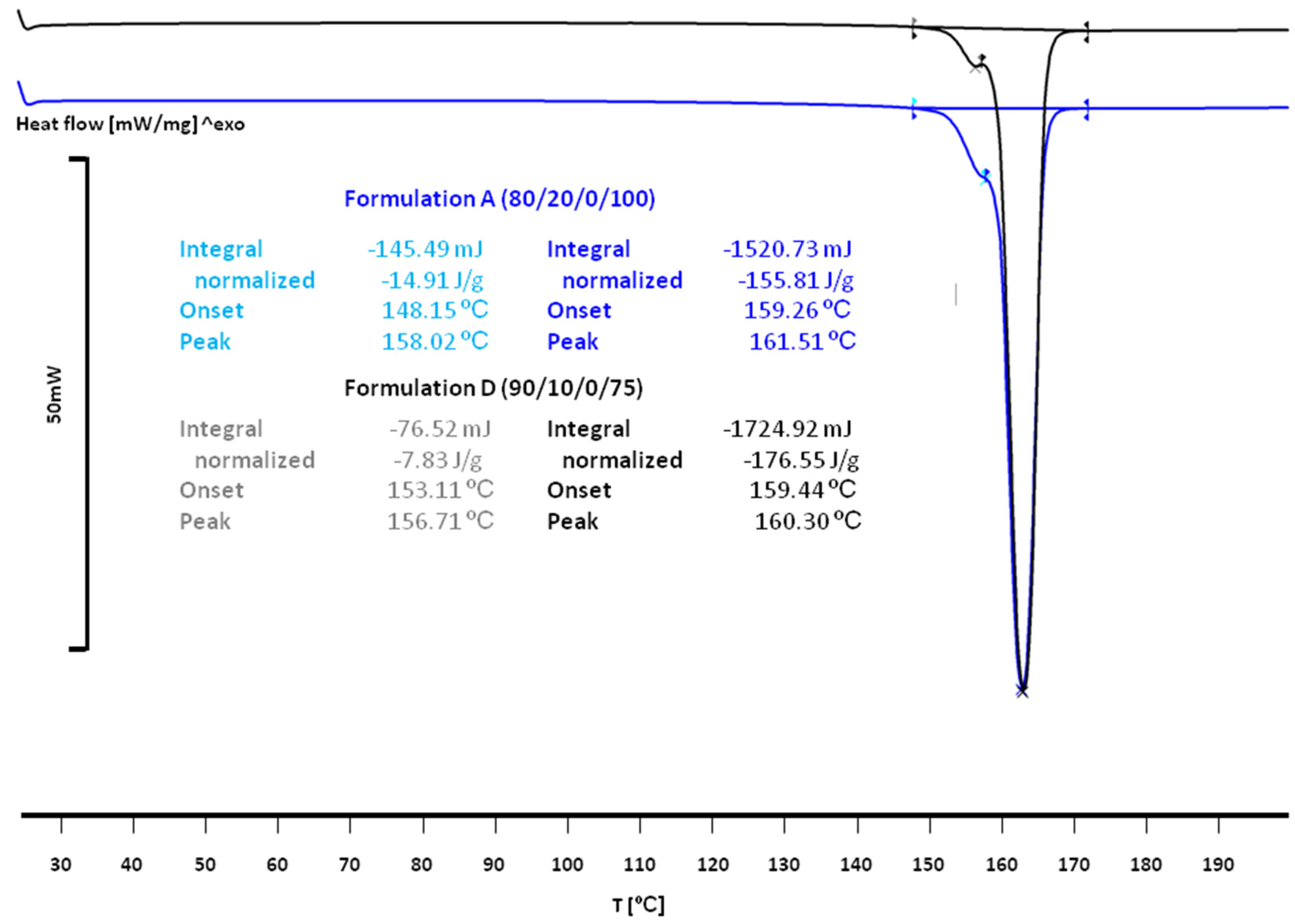
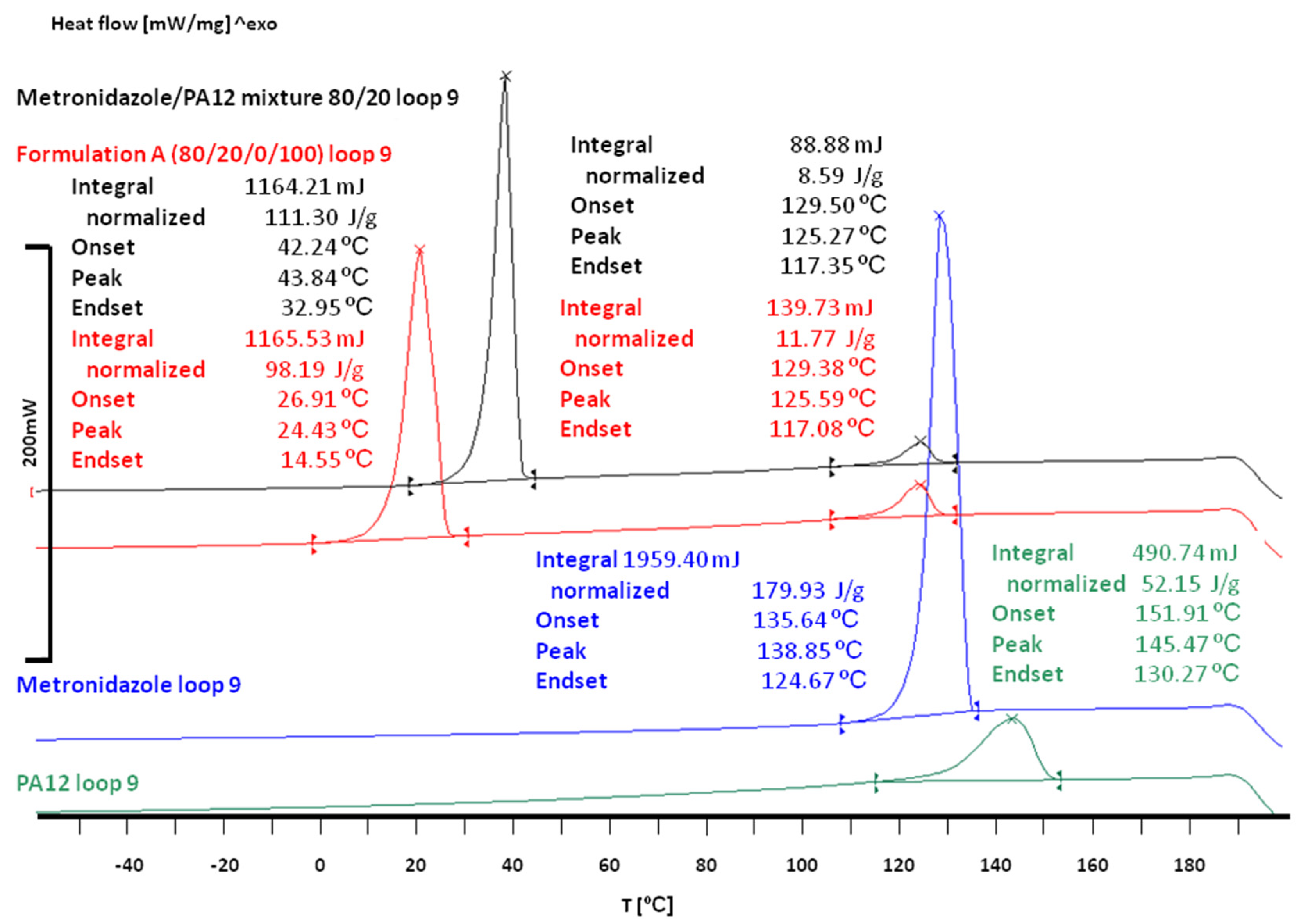
3.2. The Effect of Sintering on the Phase Transitions of Printlet Components
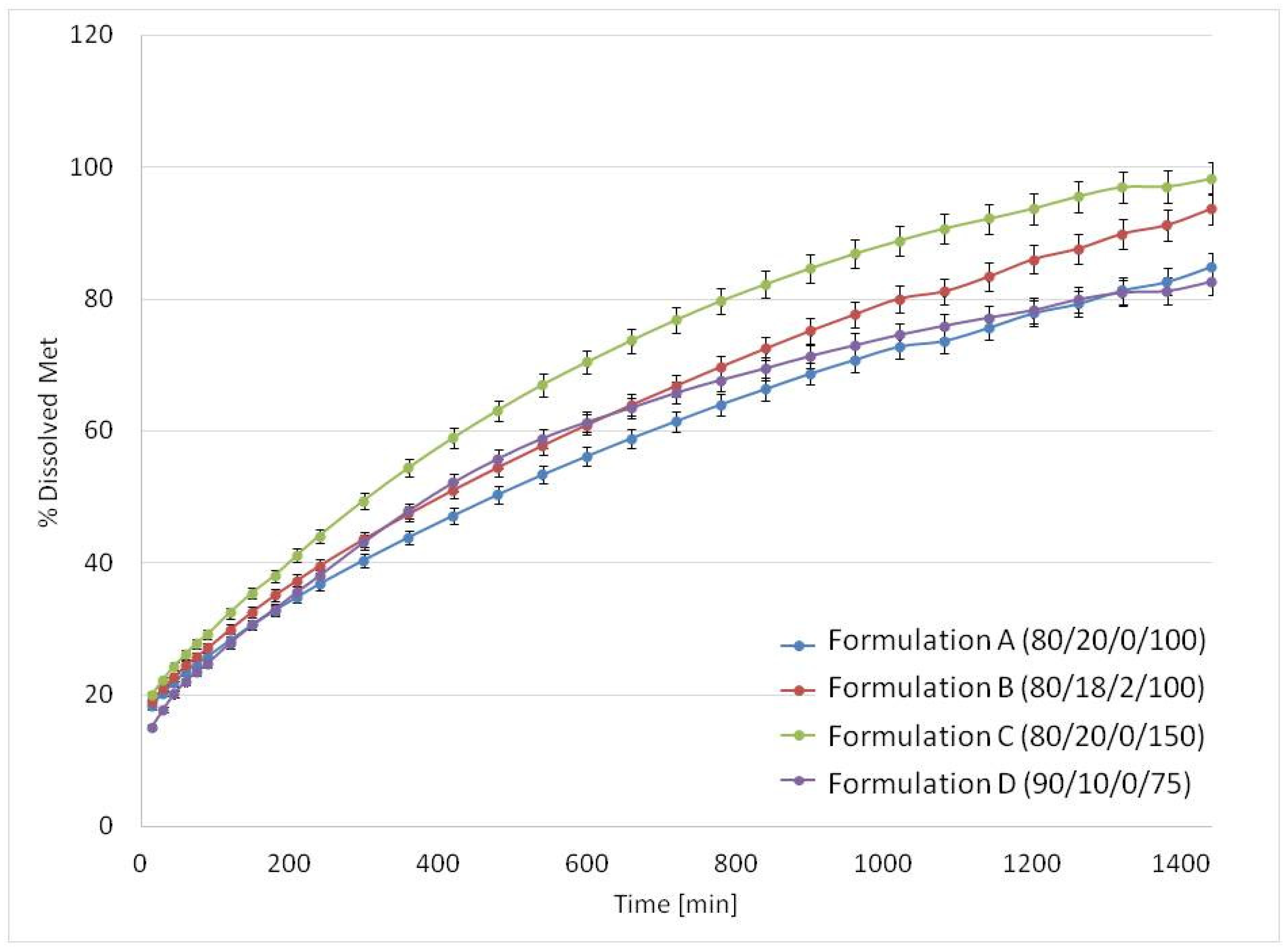
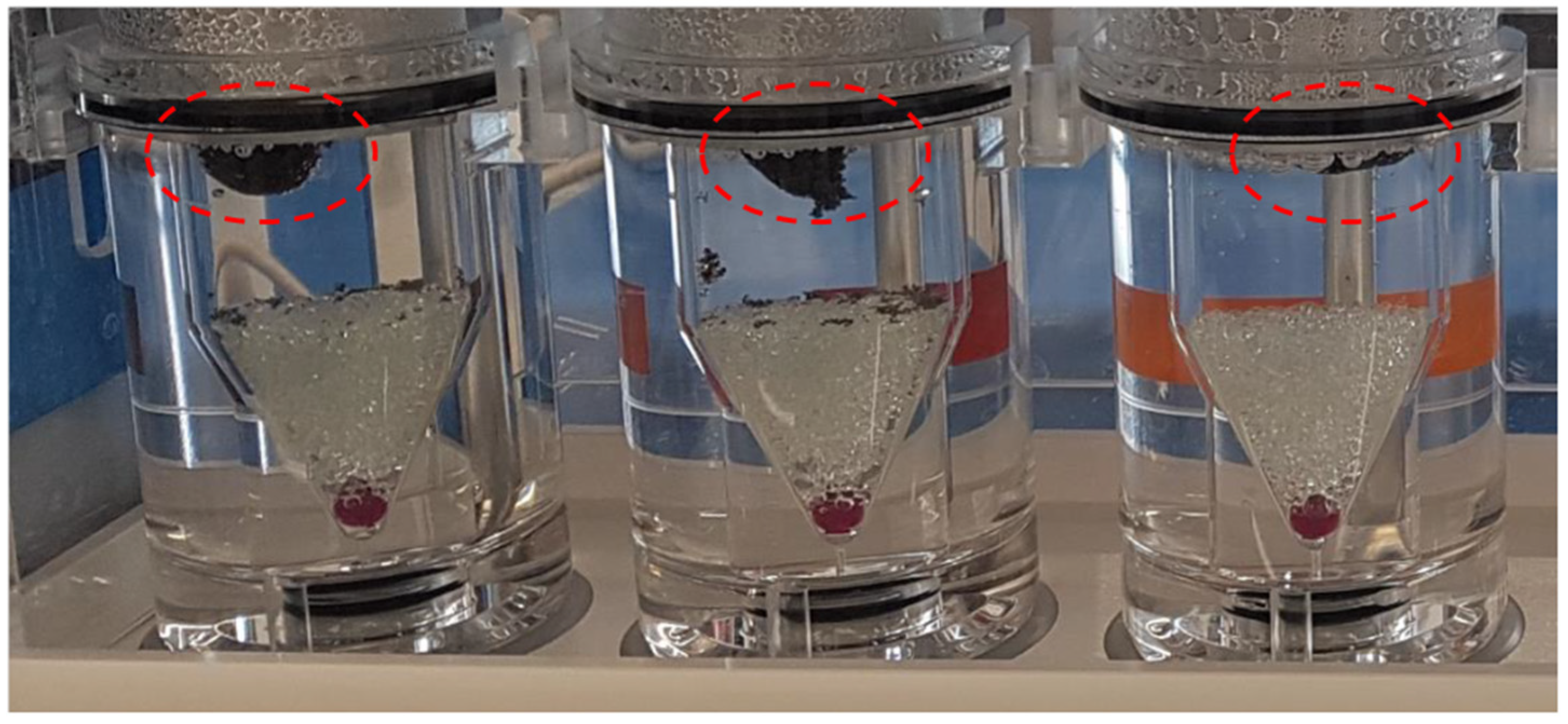
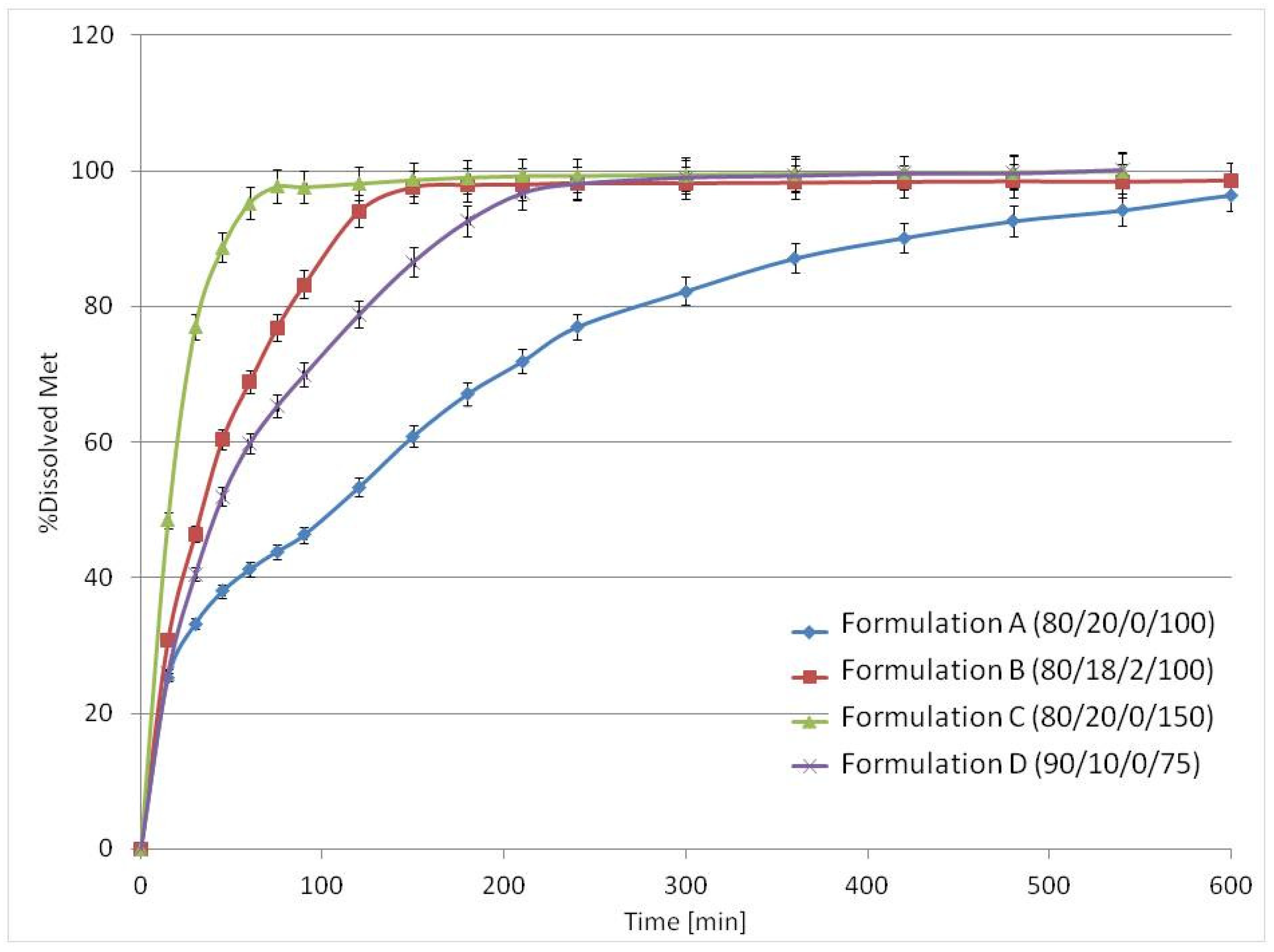
3.3. Drug Release Studies and Floating Properties
3.4. General Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Souza, M.P.C.; de Camargo, B.A.F.; Spósito, L.; Fortunato, G.C.; Carvalho, G.C.; Marena, G.D.; Meneguin, A.B.; Bauab, T.M.; Chorilli, M. Highlighting the use of micro and nanoparticles based-drug delivery systems for the treatment of Helicobacter pylori infections. Crit. Rev. Microbiol. 2021, 47, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, A.K.; Cotter, T.G.; Oxentenko, A.S. Helicobacter pylori. Mayo Clin. Proc. 2017, 92, 599–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- T3DB Version 2.0. Available online: http://www.t3db.ca/toxins/T3D4703 (accessed on 12 December 2021).
- Li, J.; Hao, X.; Wang, C.; Liu, H.; Liu, L.; He, X.; Sun, C. Improving the Solubility, Dissolution, and Bioavailability of Metronidazole via Cocrystallization with Ethyl Gallate. Pharmaceutics 2021, 13, 546. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Qaqish, R.; Patel, V.; Amiji, M. Evaluation of the Factors Influencing Stomach-specific Delivery of Antibacterial Agents for Helicobacter pylori Infection. J. Pharm. Pharmacol. 1999, 51, 667–672. [Google Scholar] [CrossRef]
- Lopes, C.M.; Bettencourt, C.; Rossi, A.; Buttini, F.; Barata, P. Overview on gastroretentive drug delivery systems for improving drug bioavailability. Int. J. Pharm. 2016, 510, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Namdev, A.; Jain, D. Floating Drug Delivery Systems: An Emerging Trend for the Treatment of Peptic Ulcer. Curr. Drug Deliv. 2019, 16, 874–886. [Google Scholar] [CrossRef]
- Wallis, M.; Al-Dulimi, Z.; Tan, D.K.; Maniruzzaman, M.; Nokhodchi, A. 3D printing for enhanced drug delivery: Current state-of-the-art and challenges. Drug Dev. Ind. Pharm. 2020, 46, 1385–1401. [Google Scholar] [CrossRef] [PubMed]
- Jamróz, W.; Szafraniec-Szczęsny, J.; Kurek, M.; Jachowicz, R. 3D Printing in Pharmaceutical and Medical Applications-Recent Achievements and Challenges. Pharm. Res. 2018, 35, 176. [Google Scholar] [CrossRef] [Green Version]
- Beg, S.; Almalki, W.H.; Malik, A.; Farhan, M.; Aatif, M.; Rahman, Z.; Alruwaili, N.K.; Alrobaian, M.; Tarique, M.; Rahman, M. 3D printing for drug delivery and biomedical applications. Drug Discov. Today 2020, 25, 1668–1681. [Google Scholar] [CrossRef]
- Elkasabgy, N.A.; Mahmoud, A.A.; Maged, A. 3D printing: An appealing route for customized drug delivery systems. Int. J. Pharm. 2020, 588, 119732. [Google Scholar] [CrossRef]
- Pandey, M.; Choudhury, H.; Fern, J.L.C.; Kee, A.T.K.; Kou, J.; Jing, J.L.J.; Her, H.C.; Yong, H.S.; Ming, H.C.; Bhattamisra, S.K.; et al. 3D printing for oral drug delivery: A new tool to customize drug delivery. Drug Deliv. Transl. Res. 2020, 10, 986–1001. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.; Aghda, N.H.; Pillai, A.R.; Thakkar, R.; Nokhodchi, A.; Maniruzzaman, M. Emerging 3D printing technologies for drug delivery devices: Current status and future perspective. Adv. Drug Deliv. Rev. 2021, 174, 294–316. [Google Scholar] [CrossRef]
- Govender, R.; Kissi, E.O.; Larsson, A.; Tho, I. Polymers in pharmaceutical additive manufacturing: A balancing act between printability and product performance. Adv. Drug Deliv. Rev. 2021, 177, 113923. [Google Scholar] [CrossRef] [PubMed]
- Charoenying, T.; Patrojanasophon, P.; Ngawhirunpat, T.; Rojanarata, T.; Akkaramongkolporn, P.; Opanasopit, P. Three-dimensional (3D)-printed devices composed of hydrophilic cap and hydrophobic body for improving buoyancy and gastric retention of domperidone tablets. Eur. J. Pharm. Sci. 2020, 155, 105555. [Google Scholar] [CrossRef] [PubMed]
- Falcone, G.; Saviano, M.; Aquino, R.P.; Del Gaudio, P.; Russo, P. Coaxial semi-solid extrusion and ionotropic alginate gelation: A successful duo for personalized floating formulations via 3D printing. Carbohydr. Polym. 2021, 260, 117791. [Google Scholar] [CrossRef]
- Li, P.; Zhang, S.; Sun, W.; Cui, M.; Wen, H.; Li, Q.; Pan, W.; Yang, X. Flexibility of 3D Extruded Printing for a Novel Controlled-Release Puerarin Gastric Floating Tablet: Design of Internal Structure. AAPS PharmSciTech 2019, 20, 236. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guan, X.; Cui, M.; Zhu, Z.; Chen, K.; Wen, H.; Jia, D.; Hou, J.; Xu, W.; Yang, X.; et al. Preparation and investigation of novel gastro-floating tablets with 3D extrusion-based printing. Int. J. Pharm. 2018, 535, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.M.; Weon, K.-Y.; Shin, B.S.; Shin, S. 3D-Printed Gastroretentive Sustained Release Drug Delivery System by Applying Design of Experiment Approach. Molecules 2020, 25, 2330. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Yin, H.; Yu, X.; Xie, C.; Jiang, H.; Jin, Y.; Sheng, F. Combination of 3D printing technologies and compressed tablets for preparation of riboflavin floating tablet-in-device (TiD) systems. Int. J. Pharm. 2018, 549, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Kim, T.H.; Jeong, S.W.; Chung, S.E.; Lee, D.Y.; Kim, D.-H.; Shin, B.S. Development of a gastroretentive delivery system for acyclovir by 3D printing technology and its in vivo pharmacokinetic evaluation in Beagle dogs. PLoS ONE 2019, 14, e0216875. [Google Scholar] [CrossRef] [PubMed]
- Dumpa, N.R.; Bandari, S.; Repka, M.A. Novel Gastroretentive Floating Pulsatile Drug Delivery System Produced via Hot-Melt Extrusion and Fused Deposition Modeling 3D Printing. Pharmaceutics 2020, 12, 52. [Google Scholar] [CrossRef] [Green Version]
- Chai, X.; Chai, H.; Wang, X.; Yang, J.; Li, J.; Zhao, Y.; Cai, W.; Tao, T.; Xiang, X. Fused Deposition Modeling (FDM) 3D Printed Tablets for Intragastric Floating Delivery of Domperidone. Sci. Rep. 2017, 7, 2829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Xu, X.-Y.; Li, R.; Zang, G.-A.; Zhang, Y.; Wang, M.-R.; Xiong, M.-F.; Xu, J.-R.; Wang, T.; Fu, H.; et al. Preparation and In vitro Evaluation of FDM 3D-Printed Ellipsoid-Shaped Gastric Floating Tablets with Low Infill Percentages. AAPS PharmSciTech 2019, 21, 6. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Luo, H.; Huang, S.; Liu, J.; Lin, M.; Yang, F.; Ban, J.; Huang, Z.; Lu, Z.; Xie, Q.; et al. Preparation of High-Drug-Loaded Clarithromycin Gastric-Floating Sustained-Release Tablets Using 3D Printing. AAPS PharmSciTech 2021, 22, 131. [Google Scholar] [CrossRef]
- Charoo, N.A.; Ali, S.F.B.; Mohamed, E.M.; Kuttolamadom, M.A.; Ozkan, T.; Khan, M.A.; Rahman, Z. Selective laser sintering 3D printing—an overview of the technology and pharmaceutical applications. Drug Dev. Ind. Pharm. 2020, 46, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.; Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D printing: Principles and pharmaceutical applications of selective laser sintering. Int. J. Pharm. 2020, 586, 119594. [Google Scholar] [CrossRef]
- Awad, A.; Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. Advances in powder bed fusion 3D printing in drug delivery and healthcare. Adv. Drug Deliv. Rev. 2021, 174, 406–424. [Google Scholar] [CrossRef]
- Gueche, Y.; Sanchez-Ballester, N.; Cailleaux, S.; Bataille, B.; Soulairol, I. Selective Laser Sintering (SLS), a New Chapter in the Production of Solid Oral Forms (SOFs) by 3D Printing. Pharmaceutics 2021, 13, 1212. [Google Scholar] [CrossRef] [PubMed]
- Allahham, N.; Fina, F.; Marcuta, C.; Kraschew, L.; Mohr, W.; Gaisford, S.; Basit, A.W.; Goyanes, A. Selective Laser Sintering 3D Printing of Orally Disintegrating Printlets Containing Ondansetron. Pharmaceutics 2020, 12, 110. [Google Scholar] [CrossRef] [Green Version]
- Awad, A.; Yao, A.; Trenfield, S.J.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D Printed Tablets (Printlets) with Braille and Moon Patterns for Visually Impaired Patients. Pharmaceutics 2020, 12, 172. [Google Scholar] [CrossRef] [Green Version]
- Fina, F.; Madla, C.M.; Goyanes, A.; Zhang, J.; Gaisford, S.; Basit, A.W. Fabricating 3D printed orally disintegrating printlets using selective laser sintering. Int. J. Pharm. 2018, 541, 101–107. [Google Scholar] [CrossRef]
- Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. Selective laser sintering (SLS) 3D printing of medicines. Int. J. Pharm. 2017, 529, 285–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fina, F.; Goyanes, A.; Madla, C.M.; Awad, A.; Trenfield, S.J.; Kuek, J.M.; Patel, P.; Gaisford, S.; Basit, A.W. 3D printing of drug-loaded gyroid lattices using selective laser sintering. Int. J. Pharm. 2018, 547, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.; Fina, F.; Trenfield, S.J.; Patel, P.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D Printed Pellets (Miniprintlets): A Novel, Multi-Drug, Controlled Release Platform Technology. Pharmaceutics 2019, 11, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulinowski, P.; Malczewski, P.; Pesta, E.; Łaszcz, M.; Mendyk, A.; Polak, S.; Dorożyński, P. Selective laser sintering (SLS) technique for pharmaceutical applications—Development of high dose controlled release printlets. Addit. Manuf. 2021, 38, 101761. [Google Scholar] [CrossRef]
- Schmid, M.; Amado, A.; Wegener, K. Materials perspective of polymers for additive manufacturing with selective laser sintering. J. Mater. Res. 2014, 29, 1824–1832. [Google Scholar] [CrossRef] [Green Version]
- Shakiba, M.; Ghomi, E.R.; Khosravi, F.; Jouybar, S.; Bigham, A.; Zare, M.; Abdouss, M.; Moaref, R.; Ramakrishna, S. Nylon—A material introduction and overview for biomedical applications. Polym. Adv. Technol. 2021, 32, 3368–3383. [Google Scholar] [CrossRef]
- Adeleke, O.A. In vitro characterization of a synthetic polyamide-based erodible compact disc for extended drug release. SN Appl. Sci. 2020, 2, 2152. [Google Scholar] [CrossRef]
- Kolawole, O.A.; Pillay, V.; Choonara, Y.E.; du Toit, L.; Ndesendo, V.M. The influence of polyamide 6, 10 synthesis variables on the physicochemical characteristics and drug release kinetics from a monolithic tablet matrix. Pharm. Dev. Technol 2009, 15, 595–612. [Google Scholar] [CrossRef]
- Leong, K.F.; Phua, K.K.S.; Chua, C.K.; Du, Z.H.; Teo, K.O.M. Fabrication of porous polymeric matrix drug delivery devices using the selective laser sintering technique. J. Eng. Med. 2001, 215, 191–192. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Liu, W.; Ma, L.; He, S.; Liu, H.; Zhang, Z.; Sun, A.; Huang, M.; Zhu, C. Crystal transition and thermal behavior of Nylon 12. e-Polymers 2020, 20, 346–352. [Google Scholar] [CrossRef]
- Ramesh, C. Crystalline Transitions in Nylon 12. Macromolecules 1999, 32, 5704–5706. [Google Scholar] [CrossRef]
- Dadbakhsh, S.; Verbelen, L.; Verkinderen, O.; Strobbe, D.; Van Puyvelde, P.; Kruth, J.-P. Effect of PA12 powder reuse on coalescence behaviour and microstructure of SLS parts. Eur. Polym. J. 2017, 92, 250–262. [Google Scholar] [CrossRef]
- Martynková, G.S.; Slíva, A.; Kratošová, G.; Barabaszová, K.; Študentová, S.; Klusák, J.; Brožová, S.; Dokoupil, T.; Holešová, S. Polyamide 12 Materials Study of Morpho-Structural Changes during Laser Sintering of 3D Printing. Polymers 2021, 13, 810. [Google Scholar] [CrossRef] [PubMed]
- Agafonova, E.V.; Moshchenskiy, Y.V.; Tkachenko, M. DSC study and calculation of metronidazole and clarithromycin thermodynamic melting parameters for individual substances and for eutectic mixture. Thermochim. Acta 2014, 580, 1–6. [Google Scholar] [CrossRef]
- Ali, H.R.H.; Ali, R.; Batakoushy, H.A.; Derayea, S.M. Solid-State FTIR Spectroscopic Study of Two Binary Mixtures: Cefepime-Metronidazole and Cefoperazone-Sulbactam. J. Spectrosc. 2017, 2017, 5673214. [Google Scholar] [CrossRef]
- Pezzini, B.R.; Issa, M.G.; Duque, M.D.; Ferraz, H.G. Applications of USP apparatus 3 in assessing the in vitro release of solid oral dosage forms. Braz. J. Pharm. Sci. 2015, 51, 265–272. [Google Scholar] [CrossRef] [Green Version]
- Fukui, S.; Yano, H.; Yada, S.; Mikkaichi, T.; Minami, H. Design and evaluation of an extended-release matrix tablet formulation; the combination of hypromellose acetate succinate and hydroxypropylcellulose. Asian J. Pharm. Sci. 2016, 12, 149–156. [Google Scholar] [CrossRef]
- Low, K.; Leong, K.; Chua, C.; Du, Z.; Cheah, C. Characterization of SLS parts for drug delivery devices. Rapid Prototyp. J. 2001, 7, 262–268. [Google Scholar] [CrossRef]
- Cheah, C.M.; Leong, K.F.; Chua, C.K.; Low, K.H.; Quek, H.S. Characterization of microfeatures in selective laser sintered drug delivery devices. J. Eng. Med. 2002, 216, 369–383. [Google Scholar] [CrossRef]
- Leong, K.F.; Chua, C.K.; Gui, W.S. Verani Building Porous Biopolymeric Microstructures for Controlled Drug Delivery Devices Using Selective Laser Sintering. Int. J. Adv. Manuf. Technol. 2006, 31, 483–489. [Google Scholar] [CrossRef]
- Leong, K.F.; Wiria, F.E.; Chua, C.K.; Li, S.H. Characterization of a poly-epsilon-caprolactone polymeric drug delivery device built by selective laser sintering. Bio-Med. Mater. Eng. 2007, 17, 147–157. [Google Scholar]
- Salmoria, G.V.; Klauss, P.; Zepon, K.M.; Kanis, L.A. The effects of laser energy density and particle size in the selective laser sintering of polycaprolactone/progesterone specimens: Morphology and drug release. Int. J. Adv. Manuf. Technol. 2012, 66, 1113–1118. [Google Scholar] [CrossRef]
- Salmoria, G.V.; Klauss, P.; Kanis, L.A. Laser Printing of PCL/Progesterone Tablets for Drug Delivery Applications in Hormone Cancer Therapy. Lasers Manuf. Mater. Process. 2017, 4, 108–120. [Google Scholar] [CrossRef]
- Thakkar, R.; Davis, D.A.; Williams, R.O.; Maniruzzaman, M. Selective Laser Sintering of a Photosensitive Drug: Impact of Processing and Formulation Parameters on Degradation, Solid State, and Quality of 3D-Printed Dosage Forms. Mol. Pharm. 2021, 18, 3894–3908. [Google Scholar] [CrossRef]
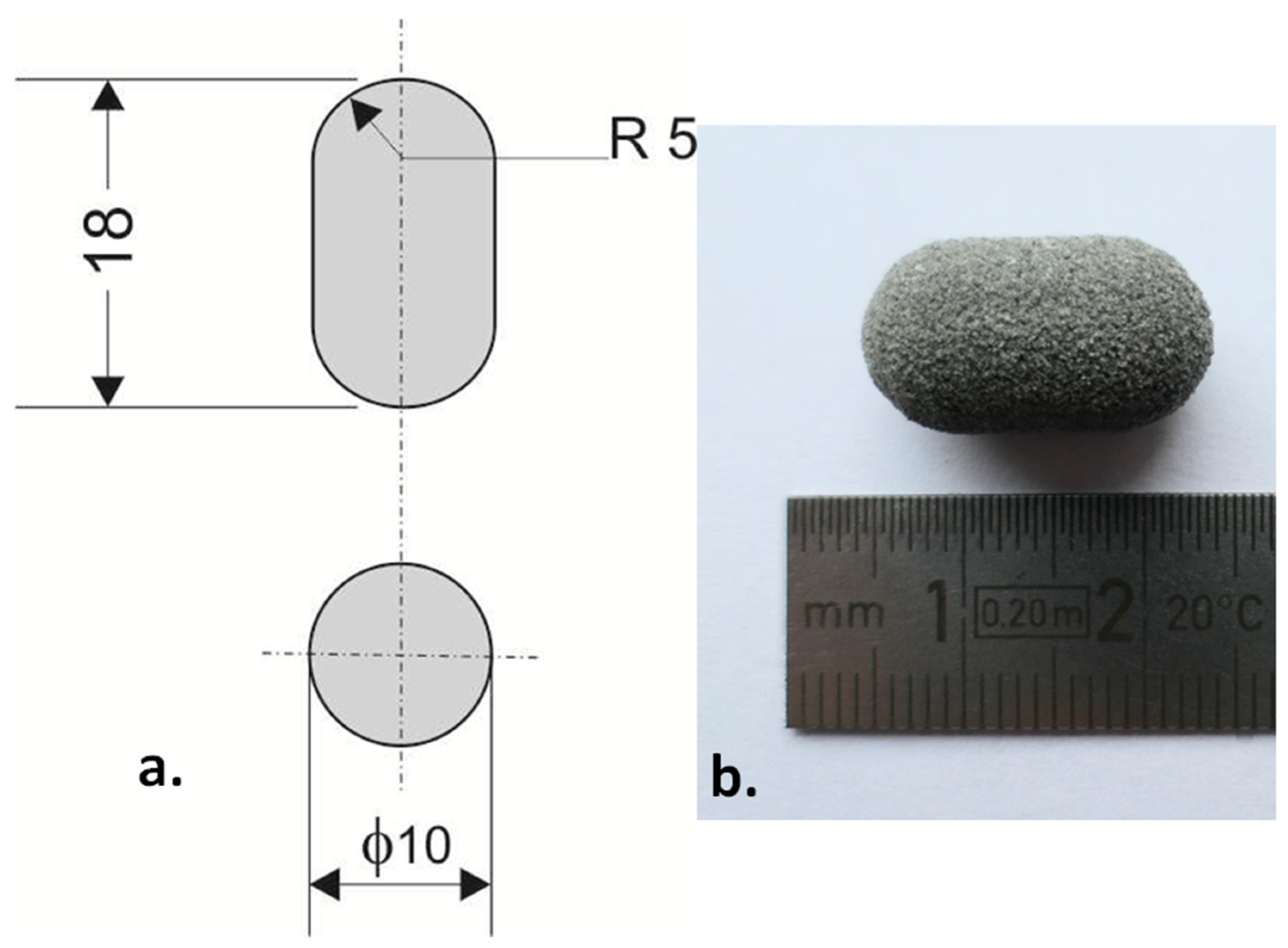


| Metronidazole | PA12 | Sodium Chloride | Laser Speed (mm/s) | ||||
|---|---|---|---|---|---|---|---|
| (% v/v) | (% w/w) | (% v/v) | (% w/w) | (% v/v) | (% w/w) | ||
| Formulation A (80/20/0/100) | 80 | 84.1 | 20 | 15.9 | 0 | 0 | 100 |
| Formulation B (80/18/2/100) | 80 | 81.7 | 18 | 13.9 | 2 | 4.4 | 100 |
| Formulation C (80/20/0/150) | 80 | 84.1 | 20 | 15.9 | 0 | 0 | 150 |
| Formulation D (90/10/0/75) | 90 | 92.2 | 10 | 7.8 | 0 | 0 | 75 |
| Formulation A (80/20/0/100) | Formulation B (80/18/2/100) | Formulation C (80/20/0/150) | Formulation D (90/10/0/75) | |
|---|---|---|---|---|
| Mean apparent density (g/cm3) | 0.76 | 0.73 | 0.62 | 0.82 |
| SD (n = 10) | 0.02 | 0.02 | 0.01 | 0.02 |
| Buoyancy lag time (min) | 0 | 0 | 10 | 0 |
| Total floating time (min) | 30 | 60 | 600 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulinowski, P.; Malczewski, P.; Łaszcz, M.; Baran, E.; Milanowski, B.; Kuprianowicz, M.; Dorożyński, P. Development of Composite, Reinforced, Highly Drug-Loaded Pharmaceutical Printlets Manufactured by Selective Laser Sintering—In Search of Relevant Excipients for Pharmaceutical 3D Printing. Materials 2022, 15, 2142. https://doi.org/10.3390/ma15062142
Kulinowski P, Malczewski P, Łaszcz M, Baran E, Milanowski B, Kuprianowicz M, Dorożyński P. Development of Composite, Reinforced, Highly Drug-Loaded Pharmaceutical Printlets Manufactured by Selective Laser Sintering—In Search of Relevant Excipients for Pharmaceutical 3D Printing. Materials. 2022; 15(6):2142. https://doi.org/10.3390/ma15062142
Chicago/Turabian StyleKulinowski, Piotr, Piotr Malczewski, Marta Łaszcz, Ewelina Baran, Bartłomiej Milanowski, Mateusz Kuprianowicz, and Przemysław Dorożyński. 2022. "Development of Composite, Reinforced, Highly Drug-Loaded Pharmaceutical Printlets Manufactured by Selective Laser Sintering—In Search of Relevant Excipients for Pharmaceutical 3D Printing" Materials 15, no. 6: 2142. https://doi.org/10.3390/ma15062142
APA StyleKulinowski, P., Malczewski, P., Łaszcz, M., Baran, E., Milanowski, B., Kuprianowicz, M., & Dorożyński, P. (2022). Development of Composite, Reinforced, Highly Drug-Loaded Pharmaceutical Printlets Manufactured by Selective Laser Sintering—In Search of Relevant Excipients for Pharmaceutical 3D Printing. Materials, 15(6), 2142. https://doi.org/10.3390/ma15062142