Plasmon Tuning of Liquid Gallium Nanoparticles through Surface Anodization
Abstract
:1. Introduction
2. Materials and Methods
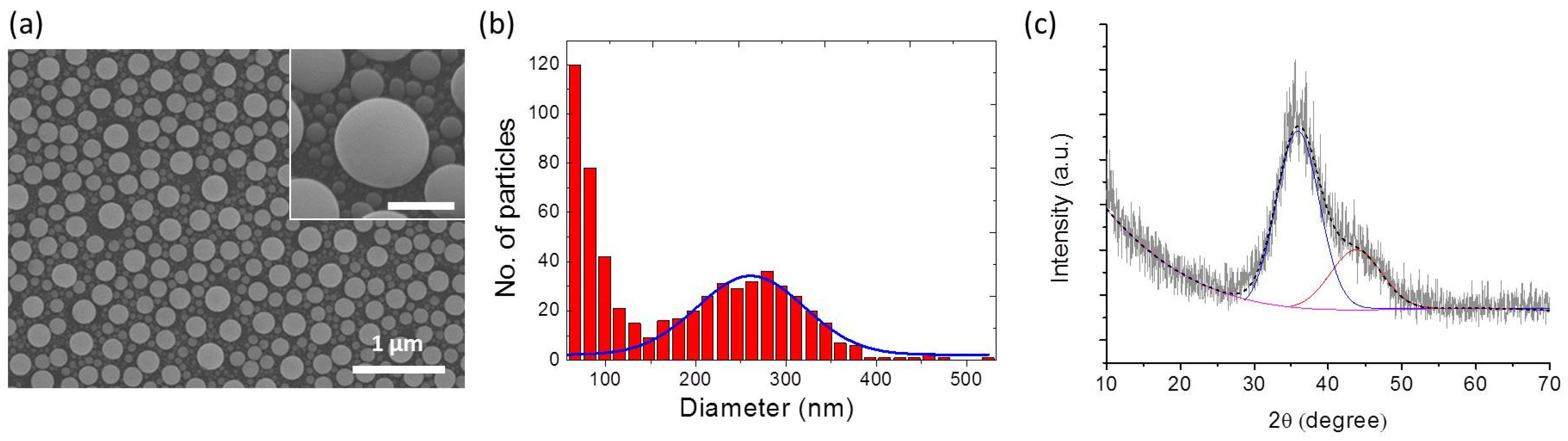
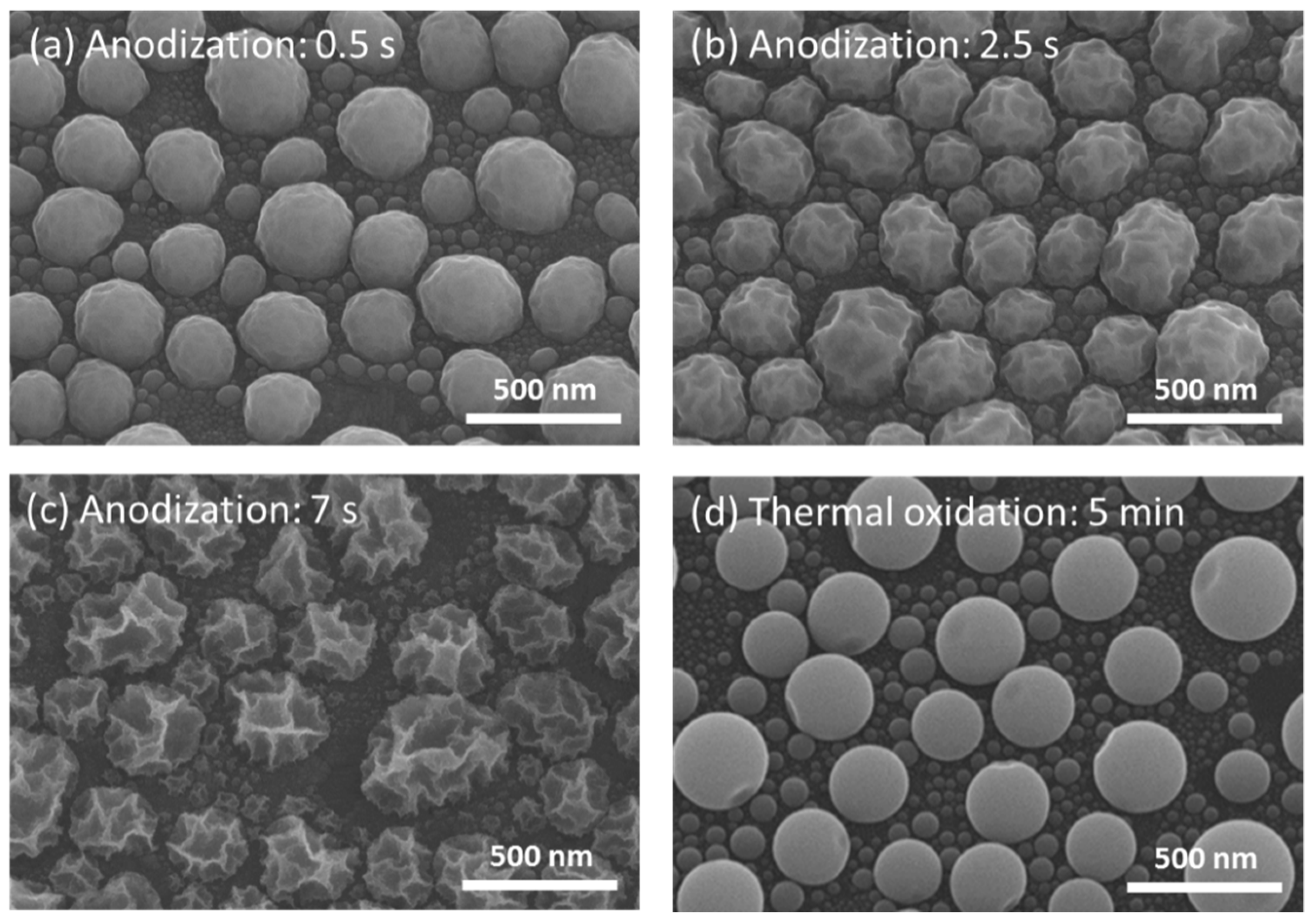
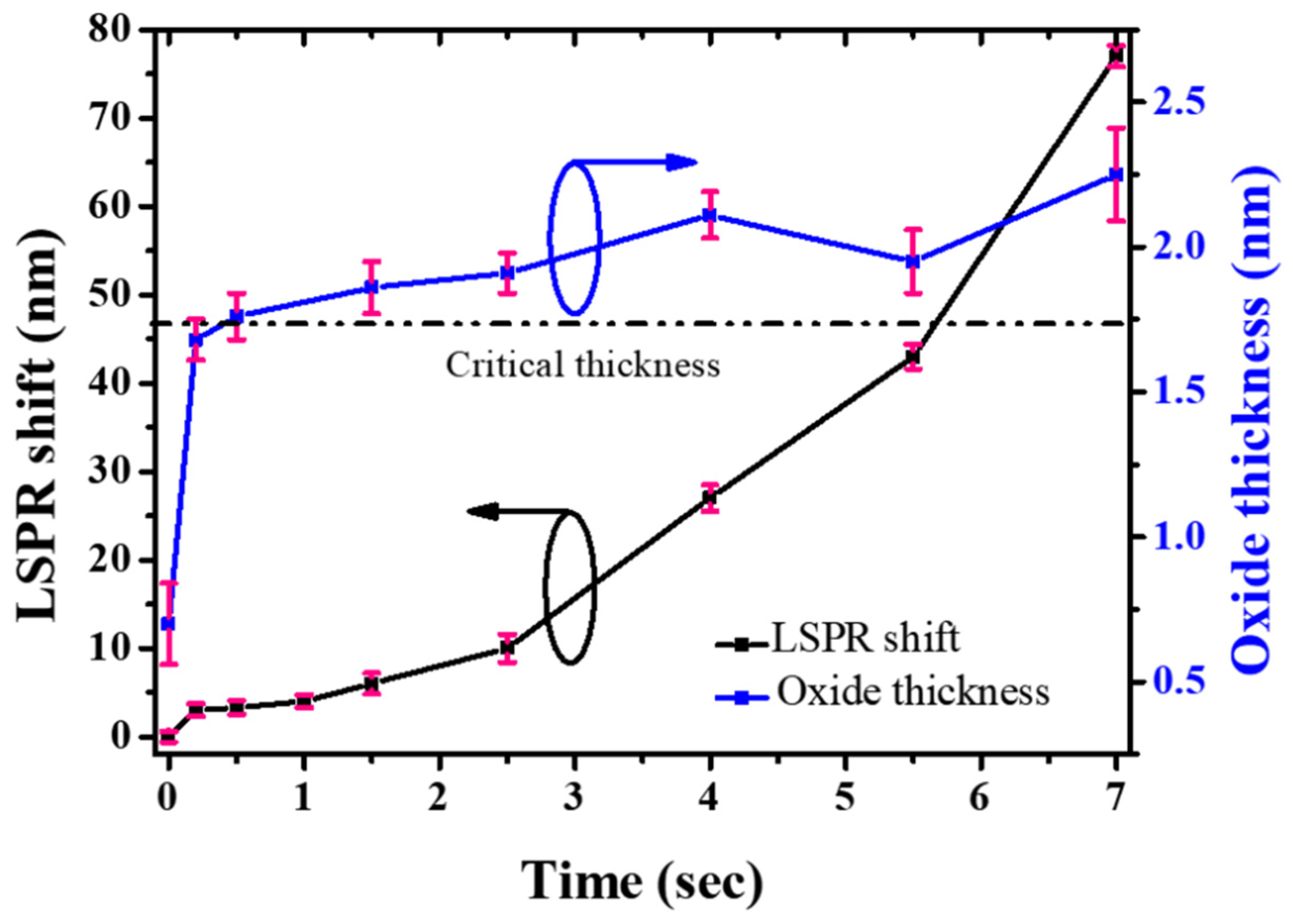
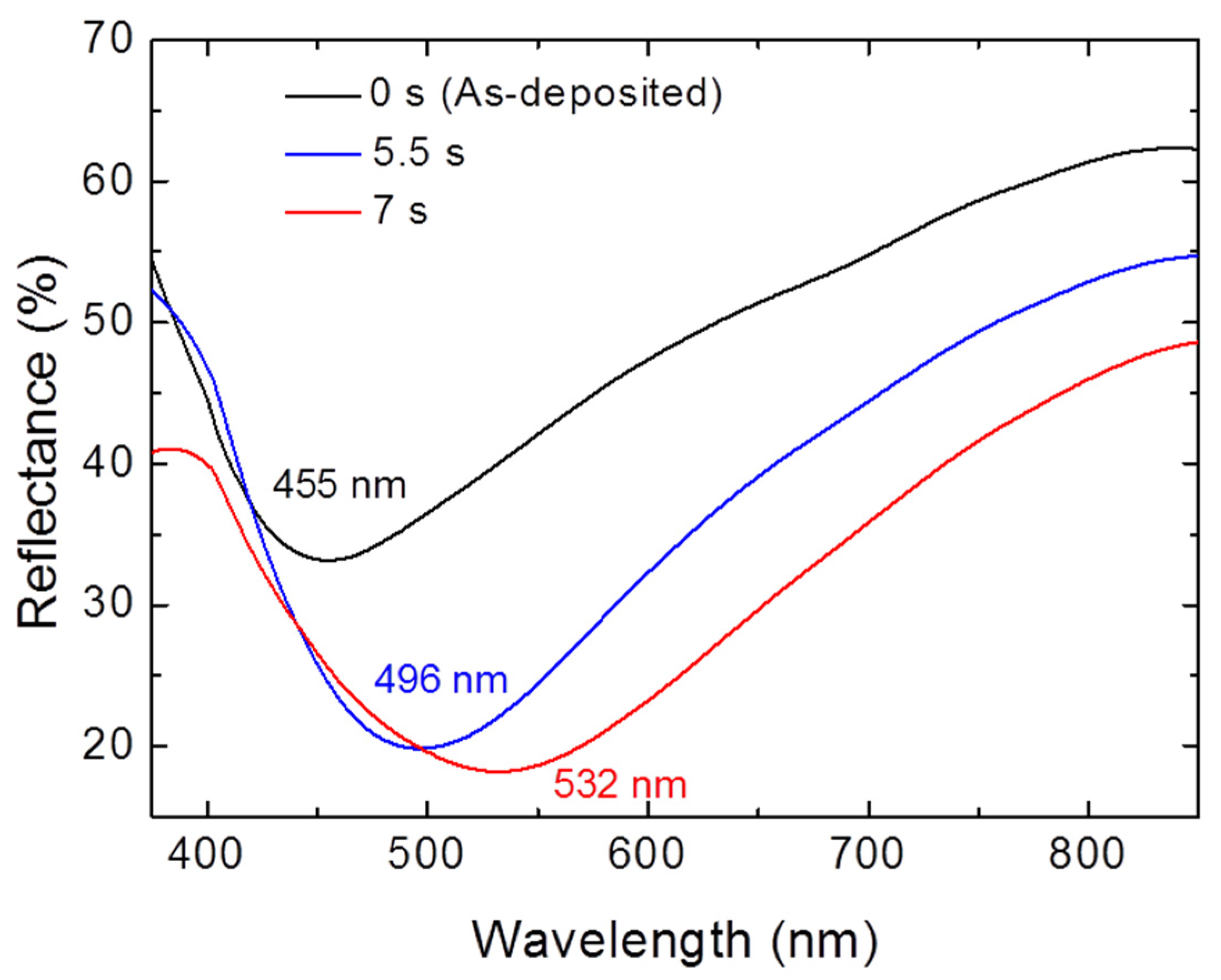
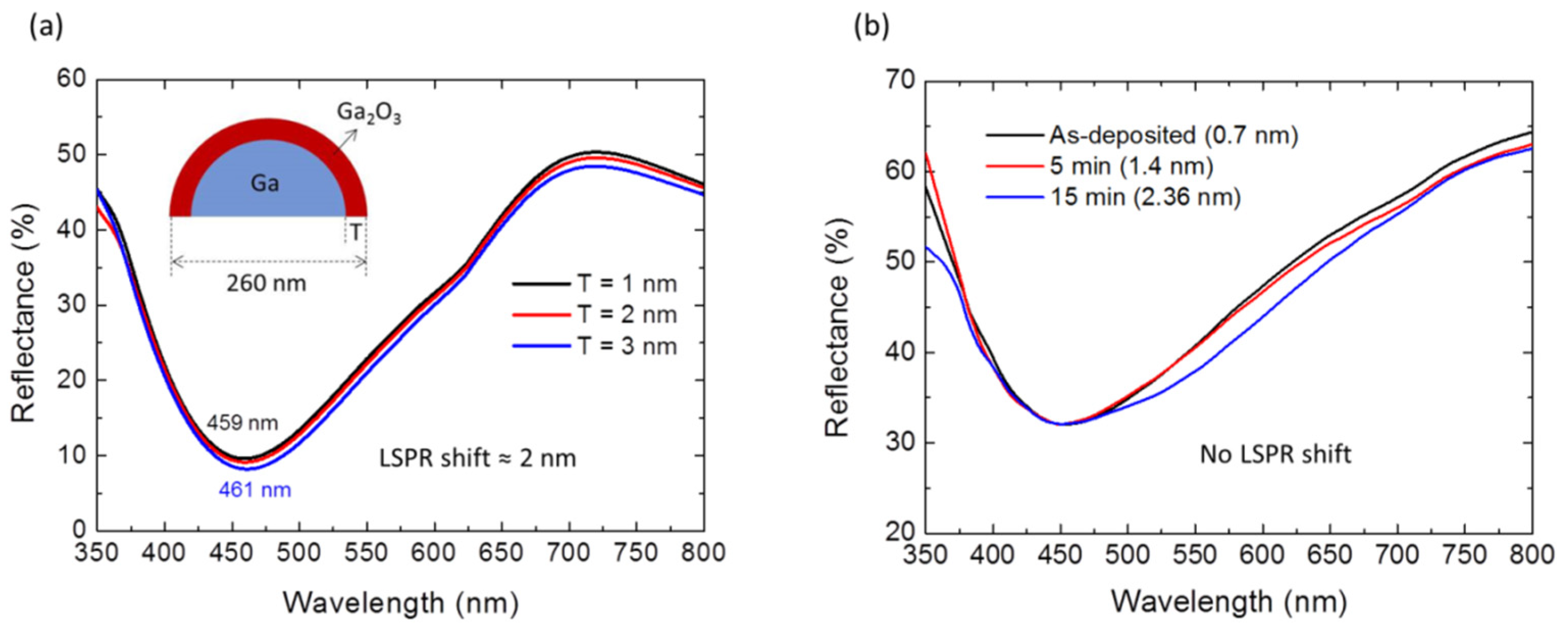
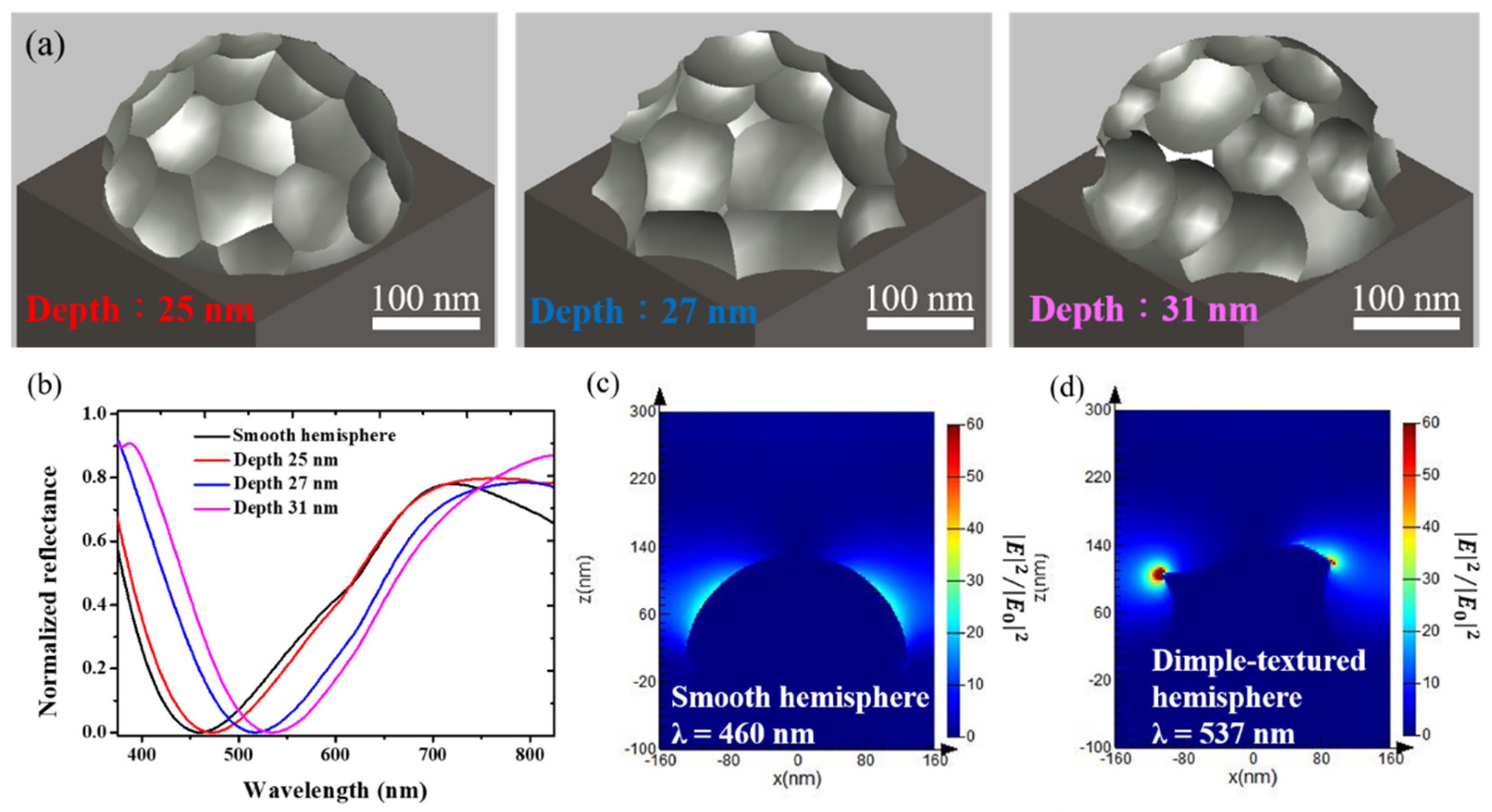
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Butun, S.; Tongay, S.; Aydin, K. Enhanced Light Emission from Large-Area Monolayer MoS2 Using Plasmonic Nanodisc Arrays. Nano Lett. 2015, 15, 2700–2704. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Shi, L.; Schmidt, M.S.; Boisen, A.; Hansen, O.; Zi, J.; Xiao, S.; Mortensen, N.A. Enhanced Light–Matter Interactions in Graphene-Covered Gold Nanovoid Arrays. Nano Lett. 2013, 13, 4690–4696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baranov, D.; Wersäll, M.; Cuadra, J.; Antosiewicz, T.J.; Shegai, T. Novel Nanostructures and Materials for Strong Light–Matter Interactions. Acs Photonics 2018, 5, 24–42. [Google Scholar] [CrossRef]
- Schuller, J.A.; Barnard, E.; Cai, W.; Jun, Y.C.; White, J.S.; Brongersma, M.L. Plasmonics for extreme light concentration and manipulation. Nat. Mater. 2010, 9, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Quidant, R.; Girard, C. Surface-plasmon-based optical manipulation. Laser Photonics Rev. 2008, 2, 47–57. [Google Scholar] [CrossRef]
- Webb, J.A.; Bardhan, R. Emerging advances in nanomedicine with engineered gold nanostructures. Nanoscale 2014, 6, 2502–2530. [Google Scholar] [CrossRef] [PubMed]
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shan, N.C.; Zhao, J.; Van Duyne, R.P. Biosensing with plasmonic nanosensors. Nat. Mater. 2008, 7, 442–453. [Google Scholar] [CrossRef]
- Atwater, H.; Polman, A. Erratum: Plasmonics for improved photovoltaic devices. Nat. Mater. 2010, 9, 865. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Libisch, F.; Large, N.; Neumann, O.; Brown, L.V.; Cheng, J.; Lassiter, J.B.; Carter, E.A.; Nordlander, P.; Halas, N.J. Hot Electrons Do the Impossible: Plasmon-Induced Dissociation of H2 on Au. Nano Lett. 2012, 13, 240–247. [Google Scholar] [CrossRef]
- Zijlstra, P.; Chon, J.; Gu, M. Five-Dimensional Optical Recording Mediated by Surface Plasmons in Gold Nanorods. Nature 2009, 459, 410–413. [Google Scholar] [CrossRef]
- Garcia, M.A. Surface plasmons in metallic nanoparticles: Fundamentals and applications. J. Phys. D Appl. Phys. 2011, 44, 283001. [Google Scholar] [CrossRef]
- Hunderi, O.; Ryberg, R. Band structure and optical properties of gallium. J. Phys. F Met. Phys. 1974, 4, 2084–2095. [Google Scholar] [CrossRef]
- Sanz, J.M.; Ortiz, D.; de la Osa, R.A.; González, F.; Brown, A.S.; Losurdo, M.; Everitt, H.O.; Moreno, F. UV Plasmonic Behavior of Various Metal Nanoparticles in the Near- and Far-Field Regimes: Geometry and Substrate Effects. J. Phys. Chem. C 2013, 117, 19606–19615. [Google Scholar] [CrossRef]
- Wu, P.C.; Khoury, C.G.; Kim, T.-H.; Yang, Y.; Losurdo, M.; Bianco, G.V.; Vo-Dinh, T.; Brown, A.S.; Everitt, H.O. Demonstration of Surface-Enhanced Raman Scattering by Tunable, Plasmonic Gallium Nanoparticles. J. Am. Chem. Soc. 2009, 131, 12032–12033. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Callahan, J.M.; Kim, T.-H.; Brown, A.S.; Everitt, H.O. Ultraviolet Nanoplasmonics: A Demonstration of Surface-Enhanced Raman Spectroscopy, Fluorescence, and Photodegradation Using Gallium Nanoparticles. Nano Lett. 2013, 13, 2837–2841. [Google Scholar] [CrossRef]
- Soares, B.F.; Jonsson, F.; Zheludev, N.I. All-Optical Phase-Change Memory in a Single Gallium Nanoparticle. Phys. Rev. Lett. 2007, 98, 153905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krasavin, A.V.; Zheludev, N. Active plasmonics: Controlling signals in Au/Ga waveguide using nanoscale structural transformations. Appl. Phys. Lett. 2004, 84, 1416–1418. [Google Scholar] [CrossRef] [Green Version]
- Marín, A.G.; Hernández, M.J.; Ruiz, E.; Abad, J.M.; Lorenzo, E.; Piqueras, J.; Pau, J.L. Immunosensing platform based on gallium nanoparticle arrays on silicon substrates. Biosens. Bioelectron. 2015, 74, 1069–1075. [Google Scholar] [CrossRef] [Green Version]
- Marín, A.G.; García-Mendiola, T.; Bernabeu, C.N.; Hernández, M.J.; Piqueras, J.; Pau, J.L.; Pariente, F.; Lorenzo, E. Gallium plasmonic nanoparticles for label-free DNA and single nucleotide polymorphism sensing. Nanoscale 2016, 8, 9842–9851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujiwara, H.; Suzuki, T.; Pin, C.; Sasaki, K. Localized ZnO Growth on a Gold Nanoantenna by Plasmon-Assisted Hydrothermal Synthesis. Nano Lett. 2020, 20, 389–394. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Chen, H.J.; Kou, X.S.; Yang, Z.; Ni, W.H.; Wang, J.F. Shape- and Size-Dependent Refractive Index Sensitivity of Gold Nanoparticles. Langmuir 2008, 24, 5233–5237. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Li, G.; Tang, Z. Self-assembly of noble metal nanocrystals: Fabrication, optical property, and application. Nano Today 2012, 7, 564–585. [Google Scholar] [CrossRef]
- Haynes, C.L.; McFarland, A.D.; Zhao, L.; Van Duyne, R.P.; Schatz, G.C.; Gunnarsson, L.; Prikulis, J.; Kasemo, B.; Käll, M. Nanoparticle Optics: The Importance of Radiative Dipole Coupling in Two-Dimensional Nanoparticle Arrays. J. Phys. Chem. B 2003, 107, 7337–7342. [Google Scholar] [CrossRef]
- Hulteen, J.C.; Van Duyne, R.P. Nanosphere lithography: A materials general fabrication process for periodic particle array surfaces. J. Vac. Sci. Technol. A Vac. Surf. Film. 1995, 13, 1553–1558. [Google Scholar] [CrossRef]
- Zheng, Y.B.; Jensen, L.; Yan, W.; Walker, T.R.; Juluri, B.K.; Jensen, L.; Huang, T.J. Chemically Tuning the Localized Surface Plasmon Resonances of Gold Nanostructure Arrays. J. Phys. Chem. C 2009, 113, 7019–7024. [Google Scholar] [CrossRef]
- Bao, Y.; Zhao, B.; Tang, X.; Hou, D.; Cai, J.; Tang, S.; Liu, J.; Wang, F.; Cui, T. Tuning surface plasmon resonance by the plastic deformation of Au nanoparticles within a diamond anvil cell. Appl. Phys. Lett. 2015, 107, 201909. [Google Scholar] [CrossRef]
- Ameer, F.S.; Varahagiri, S.; Benza, D.W.; Willett, D.R.; Wen, Y.; Wang, F.; Chumanov, G.; Anker, J.N. Tuning Localized Surface Plasmon Resonance Wavelengths of Silver Nanoparticles by Mechanical Deformation. J. Phys. Chem. C 2016, 120, 20886–20895. [Google Scholar] [CrossRef] [Green Version]
- Hida, M.; Sakakibara, A.; Kamiyabu, H. Surface Tension and Supercooling Phenomenon of Liquid Ga. J. Jpn. Inst. Met. 1989, 53, 1263–1267. [Google Scholar] [CrossRef] [Green Version]
- Ghigna, P.; Spinolo, G.; Parravicini, G.B.; Stella, A.; Migliori, A.A.; Kofman, R. Metallic versus Covalent Bonding: Ga Nanoparticles as a Case Study. J. Am. Chem. Soc. 2007, 129, 8026–8033. [Google Scholar] [CrossRef]
- Knight, M.W.; Coenen, T.; Yang, Y.; Brenny, B.J.M.; Losurdo, M.; Brown, A.S.; Everitt, H.O.; Polman, A. Gallium Plasmonics: Deep Subwavelength Spectroscopic Imaging of Single and Interacting Gallium Nanoparticles. ACS Nano 2015, 9, 2049–2060. [Google Scholar] [CrossRef] [PubMed]
- Rebien, M.; Henrion, W.; Hong, M.; Mannaerts, J.P.; Fleischer, M. Optical properties of gallium oxide thin films. Appl. Phys. Lett. 2002, 81, 250–252. [Google Scholar] [CrossRef] [Green Version]
- Palik, E.D. Handbook of Optical Constants of Solids; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Spinelli, P.; Verschuuren, M.; Polman, A. Broadband omnidirectional antireflection coating based on subwavelength surface Mie resonators. Nat. Commun. 2012, 3, 692. [Google Scholar] [CrossRef] [Green Version]
- Bosio, L.; Windsor, C.G. Observation of a Metastability Limit in Liquid Gallium. Phys. Rev. Lett. 1975, 35, 1652–1655. [Google Scholar] [CrossRef]
- Yarema, M.; Wörle, M.; Rossell, M.D.; Erni, R.; Caputo, R.; Protesescu, L.; Kravchyk, K.V.; Dirin, D.N.; Lienau, K.; von Rohr, F.; et al. Monodisperse Colloidal Gallium Nanoparticles: Synthesis, Low Temperature Crystallization, Surface Plasmon Resonance and Li-Ion Storage. J. Am. Chem. Soc. 2014, 136, 12422. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.T.; Jung, Y.S.; Kim, T.; Kim, C.H.; Kim, J.H.; Kwon, J.Y.; Oh, S.M. Liquid Gallium Electrode Confined in Porous Carbon Matrix as Anode for Lithium Secondary Batteries. Electrochem. Solid-State Lett. 2008, 11, A21–A24. [Google Scholar] [CrossRef]
- Catalán-Gómez, S.; Redondo-Cubero, A.; Palomares, F.J.; Nucciarelli, F.; Pau, J.L. Tunable plasmonic resonance of gallium nanoparticles by thermal oxidation at low temperatures. Nanotechnology 2017, 28, 405705. [Google Scholar] [CrossRef]
- Zhang, F.; Sun, Y.; Wang, X.D.; Cao, Q.P.; Jiang, J.Z.; Wang, C.Z.; Ho, K.M. Structural connection between gallium crystals and near-Tm liquids under ambient pressure. Scr. Mater. 2018, 143, 86–89. [Google Scholar] [CrossRef]
- Palomares, F.J.; Alonso, M.; Jiménez, I.; Avila, J.; Sacedon, J.L.; Soria, F. Electron-beam-induced reactions at O2/GaAs(100) interfaces. Surf. Sci. 2001, 482–485, 121–127. [Google Scholar] [CrossRef]
- Gocalinska, A.; Rubini, S.; Pelucchi, E. Native oxides formation and surface wettability of epitaxial III–V materials: The case of InP and GaAs. Appl. Surf. Sci. 2016, 383, 19–27. [Google Scholar] [CrossRef]
- Gómez, S.C.; Redondo-Cubero, A.; Palomares, F.J.; Vázquez, L.; Nogales, E.; Nucciarelli, F.; Mendez, B.; Gordillo, N.; Pau, J.L. Size-selective breaking of the core–shell structure of gallium nanoparticles. Nanotechnology 2018, 29, 355707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surdu-Bob, C.; Saied, S.; Sullivan, J. An X-ray photoelectron spectroscopy study of the oxides of GaAs. Appl. Surf. Sci. 2001, 183, 126–136. [Google Scholar] [CrossRef]
- Greenwood, N.N. The Chemistry of Gallium. In Advances in Inorganic Chemistry and Radiochemistry; Academic Press: Cambridge, MA, USA, 1963; Volume 5, pp. 91–134. [Google Scholar]
- Shard, A.G. A Straightforward Method For Interpreting XPS Data From Core–Shell Nanoparticles. J. Phys. Chem. C 2012, 116, 16806–16813. [Google Scholar] [CrossRef]
- Di Cicco, A. Phase Transitions in Confined Gallium Droplets. Phys. Rev. Lett. 1998, 81, 2942–2945. [Google Scholar] [CrossRef]
- Eaker, C.B.; Hight, D.C.; O’Regan, J.D.; Dickey, M.D.; Daniels, K.E. Oxidation-Mediated Fingering in Liquid Metals. Phys. Rev. Lett. 2017, 119, 174502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Çapraz, Ö.Ö.; Shrotriya, P.; Hebert, K.R. Measurement of Stress Changes during Growth and Dissolution of Anodic Oxide Films on Aluminum. J. Electrochem. Soc. 2014, 161, D256–D262. [Google Scholar] [CrossRef] [Green Version]
- Losurdo, M.; Suvorova, A.; Rubanov, S.; Hingerl, K.; Brown, A.S. Thermally stable coexistence of liquid and solid phases in gallium nanoparticles. Nat. Mater. 2016, 15, 995–1002. [Google Scholar] [CrossRef]
- Li, R.; Li, L.; Yu, T.; Wang, L.; Chen, J.; Wang, Y.; Cai, Z.; Chen, J.; Rivers, M.L.; Liu, H. Study of Liquid Gallium as a Function of Pressure and Temperature Using Synchrotron X-ray Microtomography and X-ray Diffraction. Appl. Phys. Lett. 2014, 105, 41906. [Google Scholar] [CrossRef]
- Gibson, K.D.; Scheraga, H.A. Volume of the intersection of three spheres of unequal size: A simplified formula. J. Phys. Chem. 1987, 91, 4121–4122. [Google Scholar] [CrossRef]
- Hao, F.; Nehl, C.L.; Hafner, J.H.; Nordlander, P. Plasmon Resonances of a Gold Nanostar. Nano Lett. 2007, 7, 729–732. [Google Scholar] [CrossRef]
- Kumar, P.S.; Pastoriza-Santos, I.; Rodríguez-González, B.; Garcia de Abajo, F.J.; Liz-Marzán, L.M. High-yield synthesis and optical response of gold nanostars. Nanotechnology 2008, 19, 15606. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Khoury, C.G.; Hwang, H.; Wilson, C.M.; Grant, G.A.; Vo-Dinh, T. Gold nanostars: Surfactant-free synthesis, 3D modelling, and two-photon photoluminescence imaging. Nanotechnology 2012, 23, 75102. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-Y.; Chien, C.-Y.; Wang, C.-M.; Lin, R.-S.; Chen, I.-C. Plasmon Tuning of Liquid Gallium Nanoparticles through Surface Anodization. Materials 2022, 15, 2145. https://doi.org/10.3390/ma15062145
Chen C-Y, Chien C-Y, Wang C-M, Lin R-S, Chen I-C. Plasmon Tuning of Liquid Gallium Nanoparticles through Surface Anodization. Materials. 2022; 15(6):2145. https://doi.org/10.3390/ma15062145
Chicago/Turabian StyleChen, Chih-Yao, Ching-Yun Chien, Chih-Ming Wang, Rong-Sheng Lin, and I-Chen Chen. 2022. "Plasmon Tuning of Liquid Gallium Nanoparticles through Surface Anodization" Materials 15, no. 6: 2145. https://doi.org/10.3390/ma15062145





