Synthesis of Si-Modified Pseudo-Boehmite@kaolin Composite and Its Application as a Novel Matrix Material for FCC Catalyst
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
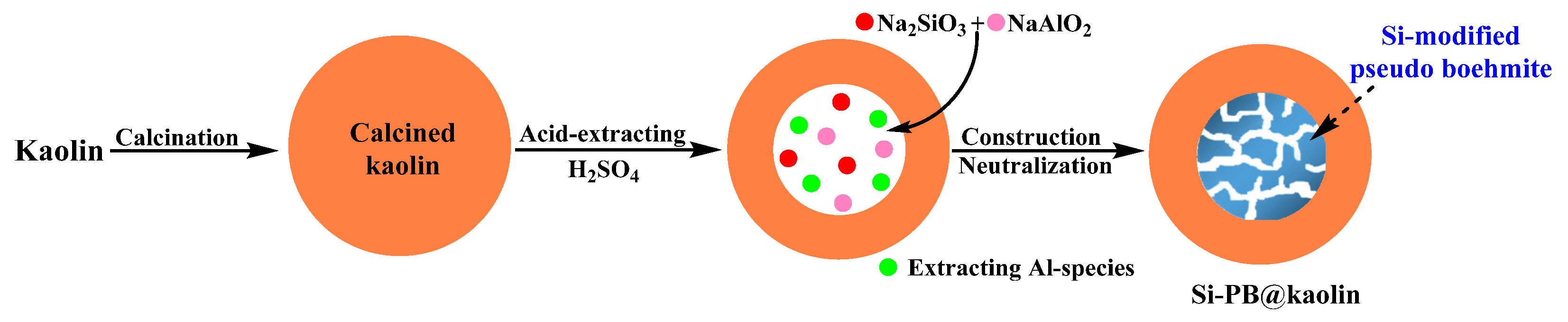
2.2. Synthesis of Si-PB@kaolin Composite
2.3. Preparation of FCC Catalysts
2.4. Characterizations and Evaluations
3. Results
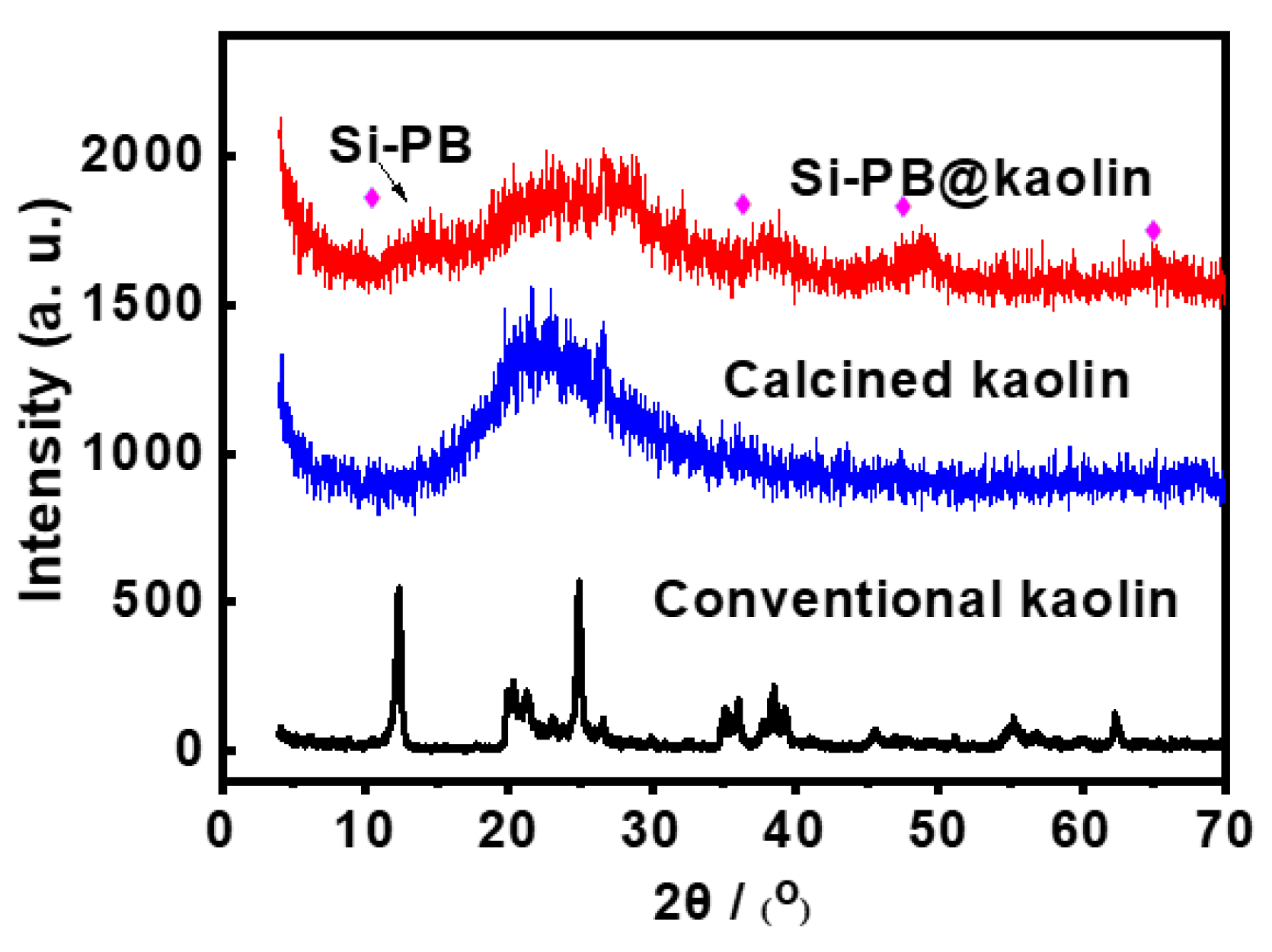
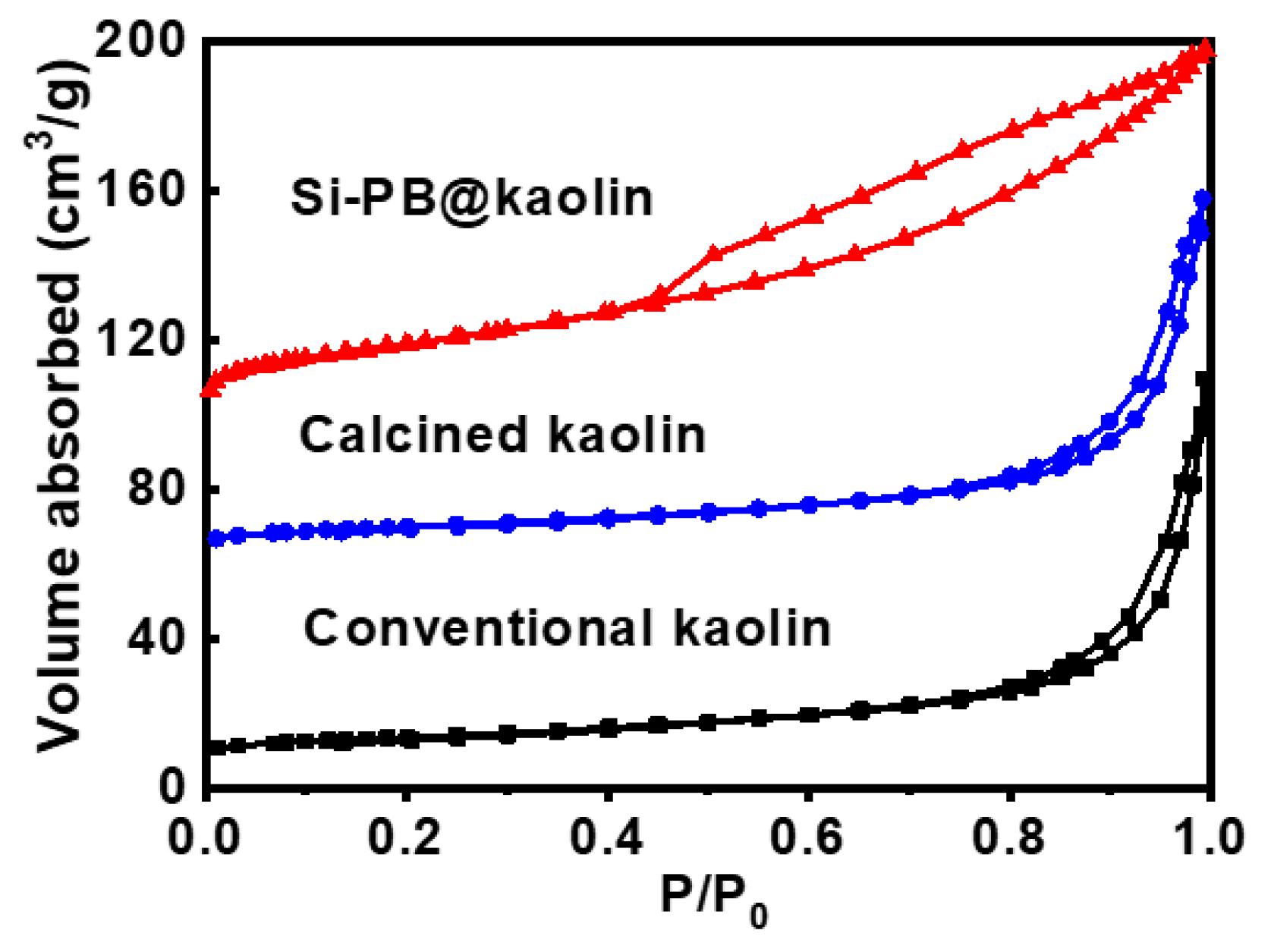
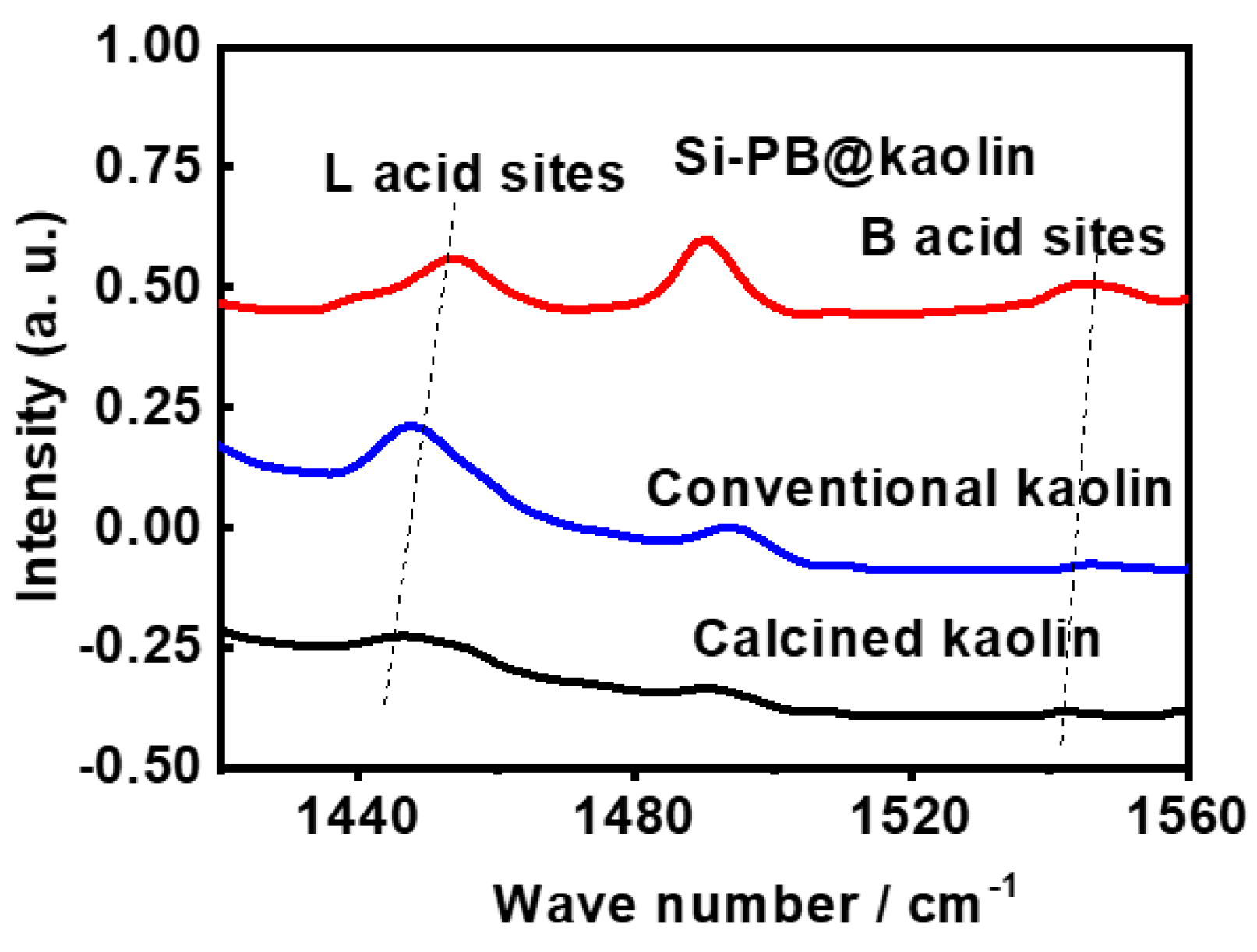
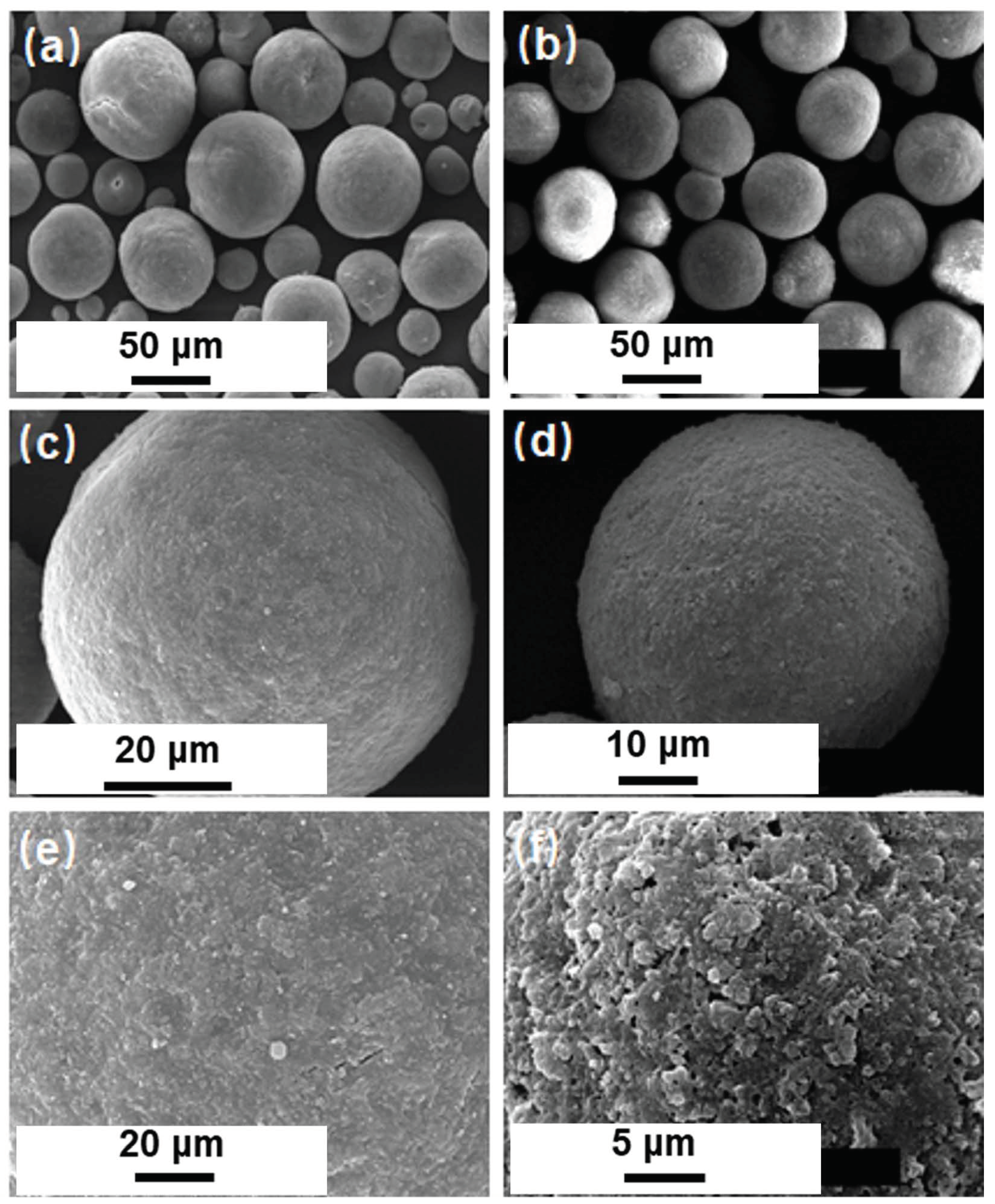
3.1. Characterization Results
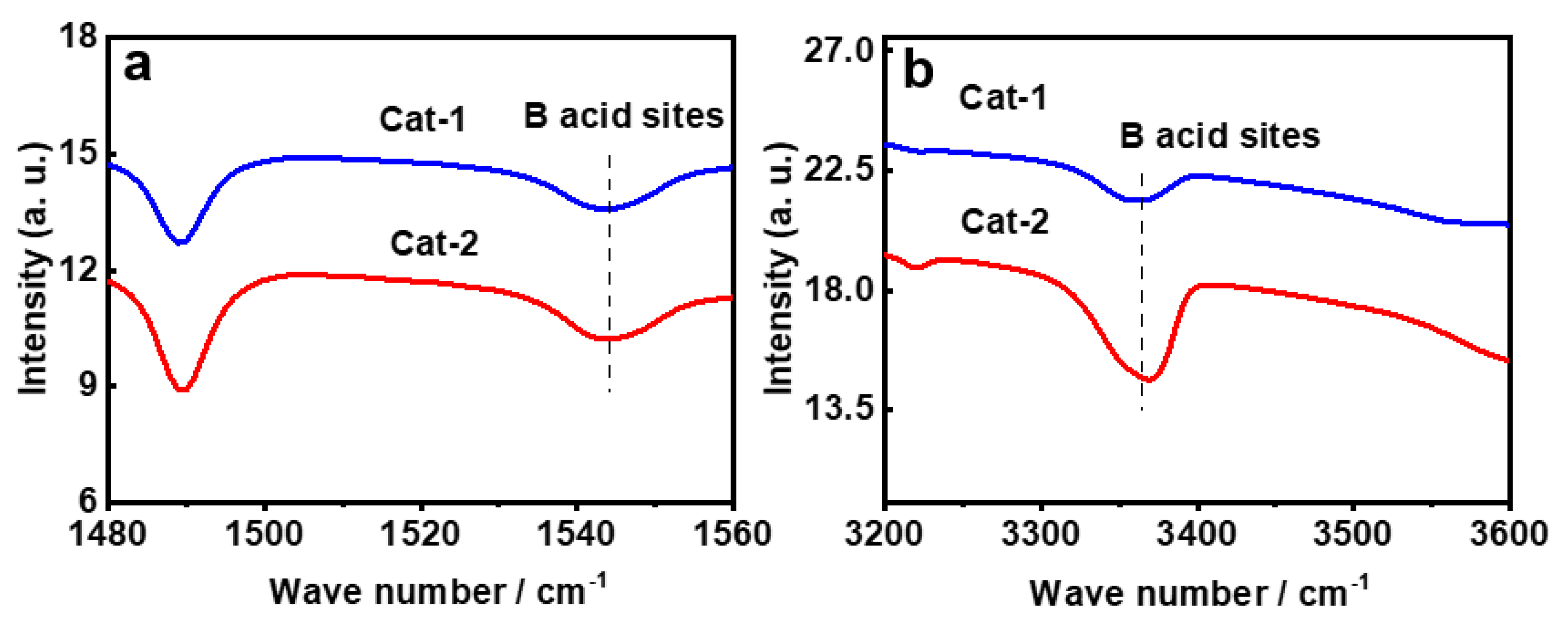
3.2. Characterizations for Prepared FCC Catalysts
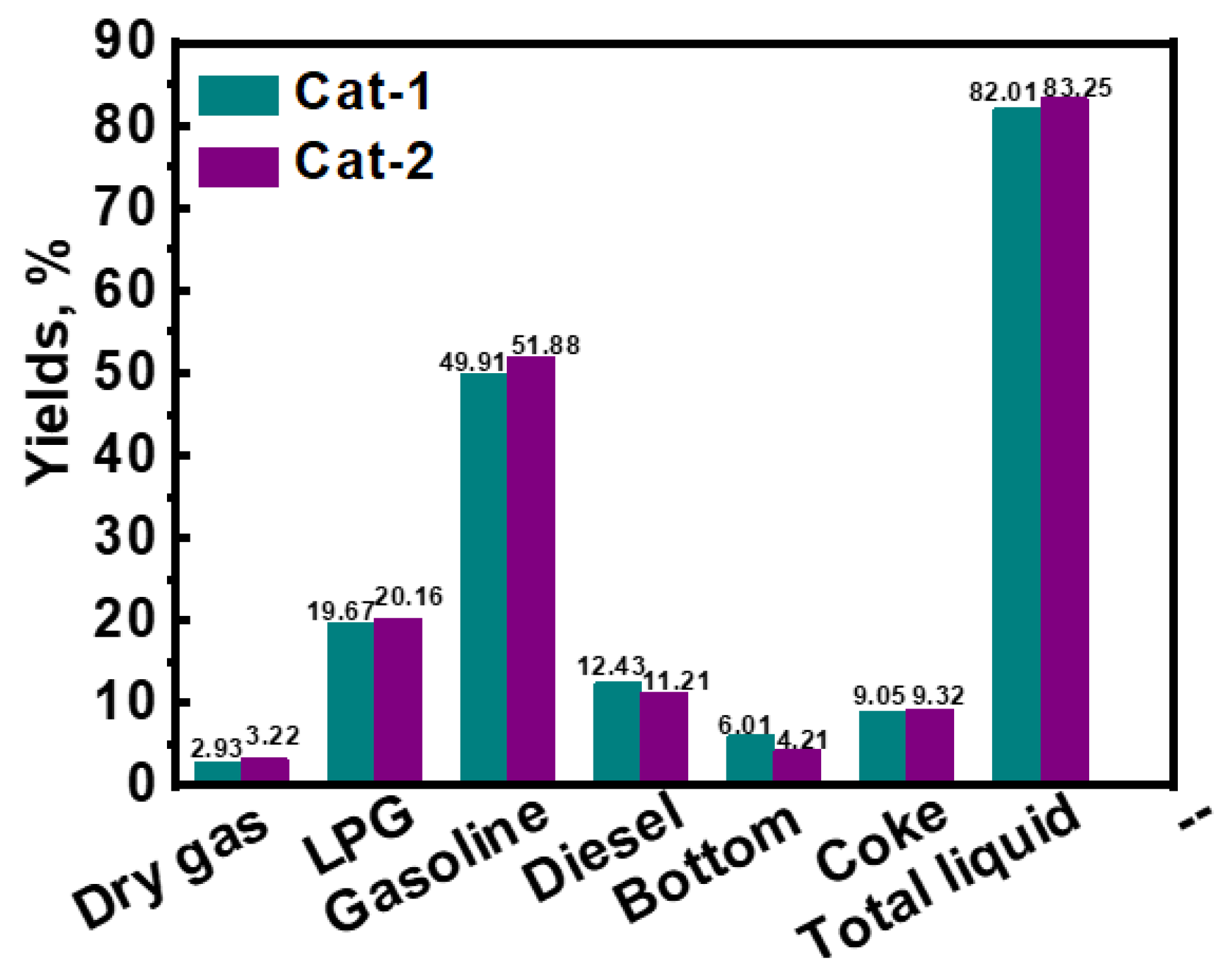
3.3. Evaluation Results for Heavy Oil Catalytic Cracking
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.-M. Recent advances in FCC technology. Powder Technol. 2006, 163, 2–8. [Google Scholar] [CrossRef]
- Rodríguez, E.; Izaddoust, S.; Valecillos, J.; Bilbao, J.; Arandes, J.M.; Castaño, P.; Epelde, E.; Elordi, G. Lessening coke formation and boosting gasoline yield by incorporating scrap tire pyrolysis oil in the cracking conditions of an FCC unit. Energy Convers. Manag. 2020, 224, 113327–113339. [Google Scholar] [CrossRef]
- Rodríguez, E.; Palos, R.; Gutiérrez, A.; Arandes, J.M.; Bilbao, J. Scrap tires pyrolysis oil as a co-feeding stream on the catalytic cracking of vacuum gasoil under fluid catalytic cracking conditions. Waste Manag. 2020, 105, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Travert, A.; Schiller, R.; Ziebarth, M.S.; Wormsbecher, R.; Cheng, W.-C. Effect of Ionic Radius of Rare Earth on USY Zeolite in Fluid Catalytic Cracking: Fundamentals and Commercial Application. Top. Catal. 2018, 58, 334–342. [Google Scholar] [CrossRef]
- Vogt, E.T.C.; Weckhuysen, B.M. Fluid catalytic cracking: Recent developments on the grand old lady of zeolite catalysis. Chem. Rev. 2015, 44, 7342–7370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, K.; Valla, J.; Garcia-Martinez, J. Realizing the Commercial Potential of Hierarchical Zeolites: New Opportunities in Catalytic Cracking. ChemCatChem 2013, 6, 46–66. [Google Scholar] [CrossRef]
- Du, X.; Gao, X.; Zhang, H.; Li, X.; Liu, P. Effect of cation location on the hydrothermal stability of rare earth-exchanged Y zeolites. Catal. Commun. 2013, 35, 17–22. [Google Scholar] [CrossRef]
- Mi, S.; Wei, T.; Sun, J.; Liu, P.; Li, X.; Zheng, Q.; Gong, K.; Liu, X.; Gao, X.; Wang, B.; et al. Catalytic function of boron to creating interconnected mesoporosity in microporous Y zeolites and its high performance in hydrocarbon cracking. J. Catal. 2017, 347, 116–126. [Google Scholar] [CrossRef]
- Ishihara, A.; Wakamatsu, T.; Nasu, H.; Hashimoto, T. Preparation of amorphous silica-alumina using polyethylene glycol and its role for matrix in catalytic cracking of n-dodecane. Appl. Catal. A Gen. 2014, 478, 58–65. [Google Scholar] [CrossRef]
- Ishihara, A.; Negura, H.; Hashimoto, T.; Nasu, H. Catalytic properties of amorphous silica-alumina prepared using malic acid as a matrix in catalytic cracking of n-dodecane. Appl. Catal. A Gen. 2010, 388, 68–76. [Google Scholar] [CrossRef]
- Ishihara, A.; Nakajima, K.; Hirado, M.; Hashimoto, T.; Nasu, H. Novel method for generating large mesopores in an amor-phous silica–alumina by controlling the pore size with the gel skeletal reinforcement and its catalytic cracking properties as a catalyst matrix. Chem. Lett. 2011, 40, 558–560. [Google Scholar] [CrossRef]
- Ishihara, A.; Kimura, K.; Owaki, A.; Inui, K.; Hashimoto, T.; Nasu, H. Catalytic cracking of VGO by hierarchical ZSM-5 zeolite containing mesoporous silica–aluminas using a Curie point pyrolyzer. Catal. Commun. 2012, 28, 163–167. [Google Scholar] [CrossRef]
- Ishihara, A.; Inui, K.; Hashimoto, T.; Nasu, H. Preparation of hierarchical β and Y zeolite-containing mesoporous silica–aluminas and their properties for catalytic cracking of n-dodecane. J. Catal. 2012, 295, 81–90. [Google Scholar] [CrossRef]
- Ravichyandran, J.; Sivasankar, B. Properties and catalytic activity of acid-modified montmorillonite and vermiculite. Clays Clay Miner. 1997, 45, 854–858. [Google Scholar] [CrossRef]
- Rong, T.-J.; Xiao, J.-K. The catalytic cracking activity of the kaolin-group minerals. Chem. Lett. 2002, 57, 297–301. [Google Scholar] [CrossRef]
- He, M.-Y. The development of catalytic cracking catalysts: Acidic property related catalytic performance. Catal. Today 2002, 73, 49–55. [Google Scholar] [CrossRef]
- Liu, H.; Ma, J.; Gao, X. Synthesis, characterization and evaluation of a novel resid FCC catalyst based on in situ synthesis on kaolin microspheres. Catal. Lett. 2006, 110, 229–234. [Google Scholar] [CrossRef]
- Qi, Y.P.; Chen, S.L.; Dong, P.; Xu, K.; Shen, B. Novel macroporous residua FCC catalysts. J. Fuel Chem. Technol. 2006, 34, 685–690. [Google Scholar]
- Rao, W.; Lv, G.; Liao, L. Modification of kaolinite and its effect on the catalytic cracking performance of fluid catalytic cracking. J. Am. Ceram. Soc. 2019, 6, 848–854. [Google Scholar]
- Lenarda, M.; Storaro, L.; Talon, A.; Moretti, E.; Riello, P. Solid acid catalysts from clays: Preparation of mesoporous catalysts by chemical activation of metakaolin under acid conditions. J. Colloid Interface Sci. 2007, 311, 537–543. [Google Scholar] [CrossRef]
- Perissinotto, M.; Lenarda, M.; Storaro, L.; Ganzerla, R. Solid acid catalysts from clays: Acid leached metakaolin as isopropanol dehydration and 1-butene isomerization catalyst. J. Mol. Catal. A Chem. 1997, 121, 103–109. [Google Scholar] [CrossRef]
- Lussier, R.J. A novel clay-based catalytic material?preparation and properties. J. Catal. 1991, 129, 225–237. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, Y.; Han, B.; Xu, B.; Liu, X.; Yan, Z. Effects of synthetic conditions on the textural structure of pseudo-boehmite. J. Colloid Interface Sci. 2016, 469, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Murrell, L.L. Catalysts Comprising Silica Supported on a Boehmite-Like Surface, Their Preparation and Use. U.S. Patent No. 4,708,945, 13 April 1987. [Google Scholar]
- Zheng, J.; Luo, Y.; Mu, X.; Shu, X. Effect of silica modification on the structure and cracking properties of industurial alumina materials. Acta Pet. Sin. (Pet. Process. Sect.) 2012, 26, 846–851. [Google Scholar]
- Hou, X.; Wang, X.; Xu, G.; Zhang, S.; Zheng, J.; Zhou, K. Effects of acidity and pore size of silica alumina materials on contact cracking of vacuum residues. Acta Pet. Sin. (Pet. Process. Sect.) 2019, 35, 1045–1051. [Google Scholar]
- Liu, H.; Zhao, H.; Gao, X.; Ma, J. A novel FCC catalyst synthesized via in situ overgrowth of NaY zeolite on kaolin microspheres for maximizing propylene yield. Catal. Today 2007, 125, 163–168. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, H.; Lin, J.; Ji, Y. Surface modification of calcined kaolin with toluene diisocyanate based on high energy ball milling. Appl. Surf. Sci. 2013, 284, 214–221. [Google Scholar] [CrossRef]
- Toledo-Chávez, G.; Paniagua-Rodríguez, J.-C.; Zárate-Medina, J.; Maya-Yescas, R. Reactions analysis during the synthesis of pseudo-boehmite as precursor of gamma-alumina. Catal. Today 2016, 271, 207–212. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution. Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.Y.; Huo, Q.S.; Feng, J.L.; Chmelka, B.F.; Stucky, G.D. Nonionic Triblock and Star Diblock Copolymer and Oligomeric Surfactant Syntheses of Highly Ordered, Hydrothermally Stable, Mesoporous Silica Structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Thomas, O.; Guillaume, C.; Houalla, M. Quantitative IR characterization of the acidity of various oxide catalysts. Microporous Mesoporous Mater. 2005, 82, 99–104. [Google Scholar]
- Xue, Z.; Zhang, T.; Ma, J.; Miao, H.; Fan, W.; Zhang, Y.; Li, R. Accessibility and catalysis of acidic sites in hierarchical ZSM-5 prepared by silanization. Microporous Mesoporous Mater. 2012, 151, 271–276. [Google Scholar] [CrossRef]
- Yuan, C.; Tan, Z.; Pan, Z.; Zhang, H.; Gao, X. Research on catalytic cracking performance improvement of waste FCC catalyst by magnesium modification. China Pet. Process. Petrochem. 2018, 20, 48–55. [Google Scholar]
- Hosseinpour, N.; Mortazavi, A.; Bazyari, A.; Khodadadi, A.A. Synergetic effects of Y-zeolite and amorphous silica-alumina as main FCC catalyst components on triisopropylbenzene cracking and coke formation. Fuel Process. Technol. 2009, 90, 171–179. [Google Scholar] [CrossRef]
- Thibault-Starzyk, F.; Stan, I.; Abelló, S.; Bonilla, A.; Thomas, K.; Fernandez, C.; Gilson, J.-P.; Pérez-Ramírez, J. Quantification of enhanced acid site accessibility in hierarchical zeolites—The accessibility index. J. Catal. 2009, 264, 11–14. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, Z.; Zhang, H.; Tan, Z.; Pan, Z.; Gao, X. Preparation of core-shell composite of Y@mesoporous alumina and its application in heavy oil cracking. China Pet. Process. Petrochem. 2016, 18, 29–35. [Google Scholar]



| Items | Value |
|---|---|
| Molecular weight, g/mol | 473 |
| Viscosity (50 °C), mm2/s | 17.27 |
| Carbon residue, wt% | 4.17 |
| Density (70 °C), g/mL | 0.87 |
| Metals, μg/g | |
| Fe | 5.16 |
| Ni | 6.52 |
| Ca | 6.94 |
| Cu | 0.27 |
| V | 9.59 |
| Pb | 0.03 |
| Na | 200 |
| Hydrocarbons, wt% | |
| Saturate | 63 |
| Aromatic | 33.6 |
| Resin | 3.4 |
| Elementals, wt% | |
| C | 84.97 |
| H | 12.64 |
| N | 0.11 |
| S | 0.52 |
| Samples | Surface Area, m2/g | Pore Volume, cm3/g | L Acid Sites, μmol/g | B Acid Sites, μmol/g | Total Acid Sites, μmol/g |
|---|---|---|---|---|---|
| Conventional kaolin | 23 | 0.10 | 26.8 | – | 26.8 |
| Calcined kaolin | 9 | 0.04 | 4.6 | – | 4.6 |
| Si-PB@kaolin | 106 | 0.28 | 86.4 | 11.3 | 97.7 |
| FCC Catalysts | Surface Area, m2/g | Pore Volume, cm3/g | Total Acid Sites, μmol/g | B/L | MAT, % | ||||
|---|---|---|---|---|---|---|---|---|---|
| Smicro | Smeso | Total | Vmicro | Vmeso | Total | ||||
| Cat-1 | 164.9 | 36.3 | 201.2 | 0.19 | 0.06 | 0.25 | 264 | 1.17 | 63 |
| Cat-2 | 161.6 | 65.9 | 227.5 | 0.20 | 0.13 | 0.33 | 348 | 1.56 | 67 |
| Items | Cat-1 | Cat-2 |
|---|---|---|
| Paraffins, % | 40.58 | 37.66 |
| Olefins, % | 11.86 | 12.19 |
| Naphthenes, % | 10.01 | 10.86 |
| Aromatics, % | 37.55 | 39.29 |
| RON | 91.1 | 91.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, C.; Li, Z.; Zhou, L.; Ju, G. Synthesis of Si-Modified Pseudo-Boehmite@kaolin Composite and Its Application as a Novel Matrix Material for FCC Catalyst. Materials 2022, 15, 2169. https://doi.org/10.3390/ma15062169
Yuan C, Li Z, Zhou L, Ju G. Synthesis of Si-Modified Pseudo-Boehmite@kaolin Composite and Its Application as a Novel Matrix Material for FCC Catalyst. Materials. 2022; 15(6):2169. https://doi.org/10.3390/ma15062169
Chicago/Turabian StyleYuan, Chengyuan, Zhongfu Li, Lei Zhou, and Guannan Ju. 2022. "Synthesis of Si-Modified Pseudo-Boehmite@kaolin Composite and Its Application as a Novel Matrix Material for FCC Catalyst" Materials 15, no. 6: 2169. https://doi.org/10.3390/ma15062169






