Adsorption of Inositol Phosphate on Hydroxyapatite Powder with High Specific Surface Area
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Hydroxyapatite Powder with High Specific Surface Area
2.2. HApHS Powder Characterization
2.3. Surface Modification of HApHS Powder with IP6
2.4. Adsorption Isotherm of IP6
3. Results and Discussion
3.1. Synthesis of HApHS Powders
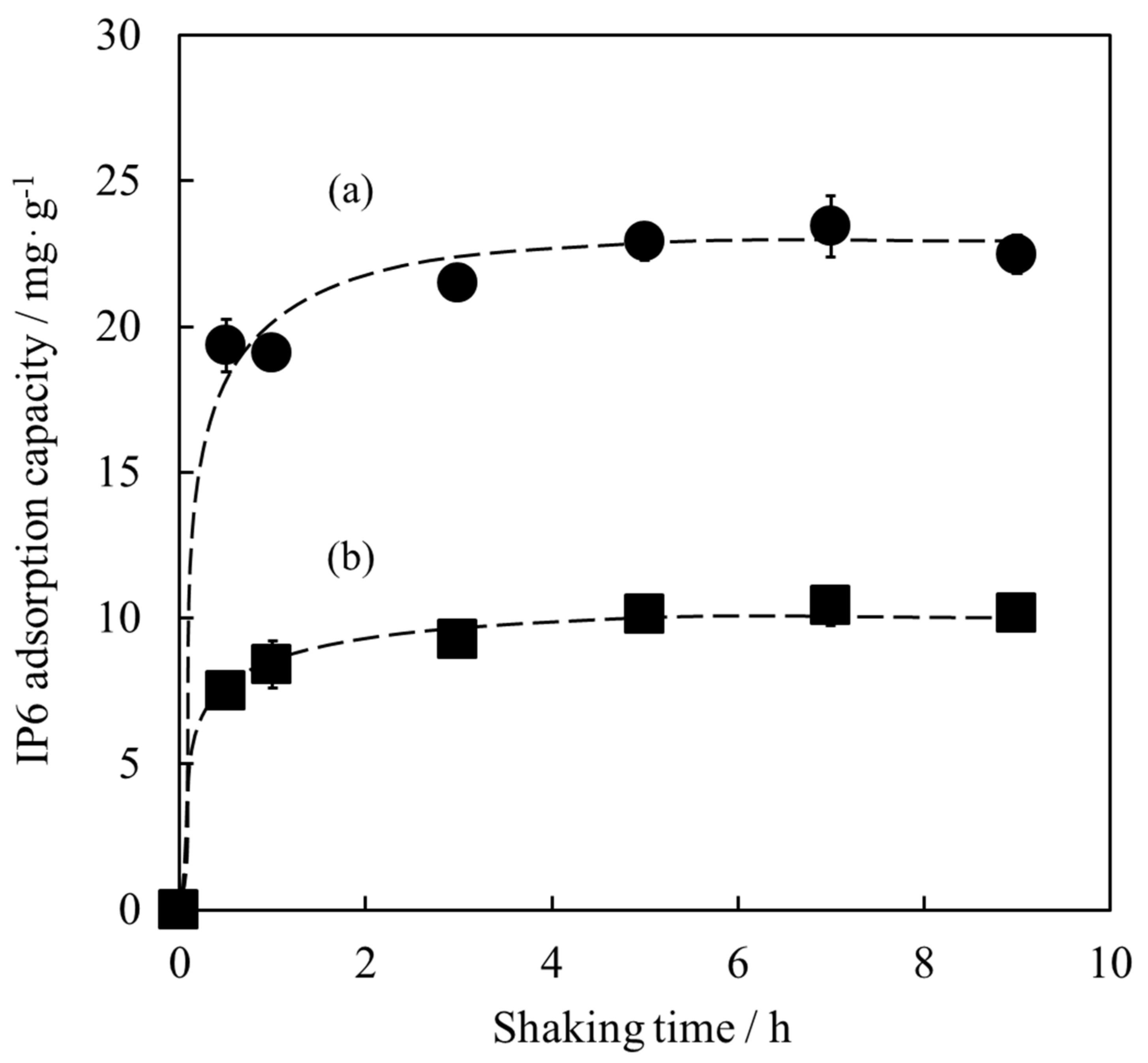
3.2. Adsorption of IP6 for Determination of Shaking Time
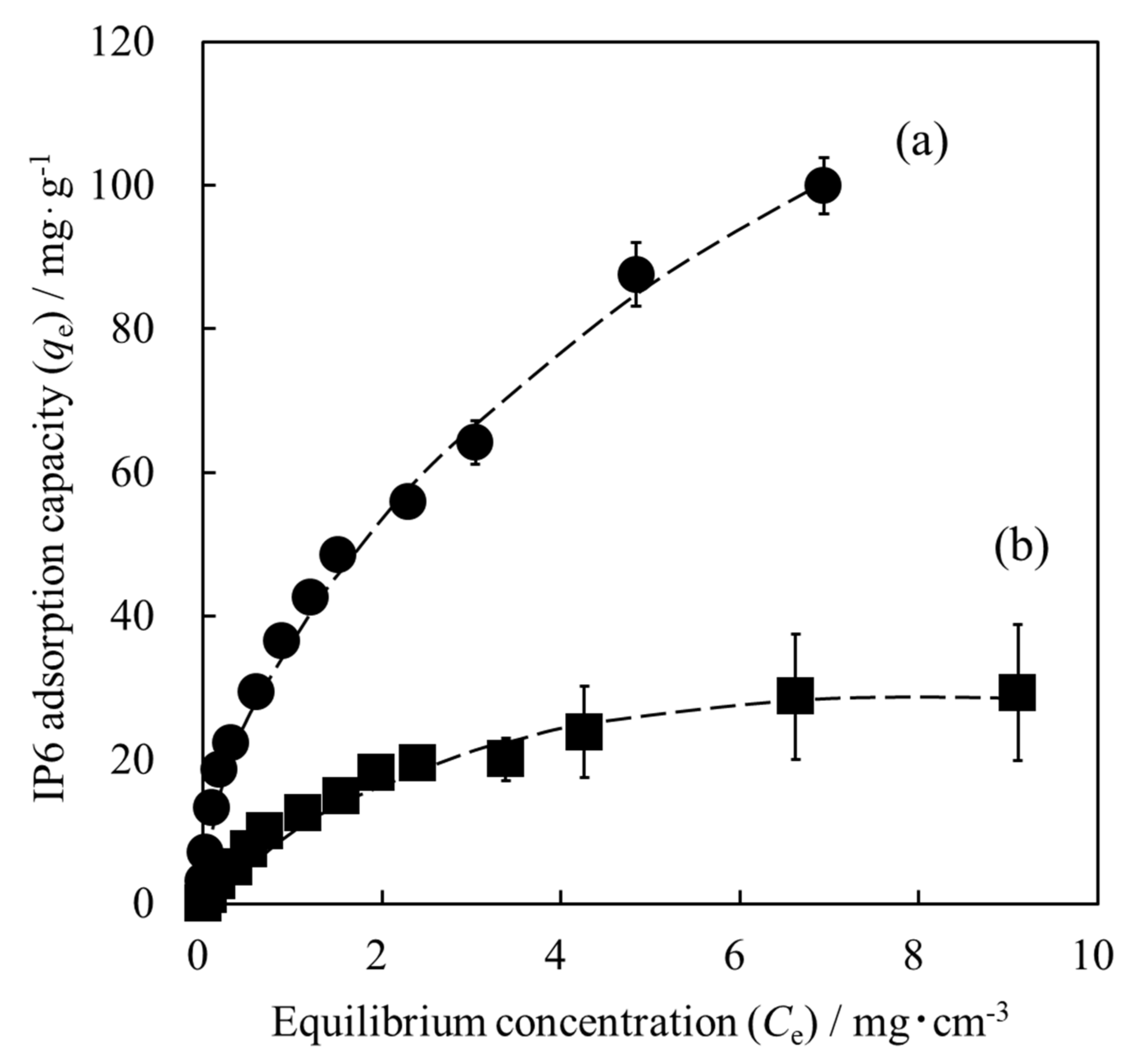
3.3. Adsorption Isotherm of IP6
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wen, S.; Liu, X.; Ding, J.; Liu, Y.; Lan, Z.; Zhang, Z.; Chen, G. Hydrothermal Synthesis of Hydroxyapatite Coating on the Surface of Medical Magnesium Alloy and Its Corrosion Resistance. Prog. Nat. Sci. Mater. Int. 2021, 31, 324–333. [Google Scholar] [CrossRef]
- Xu, N.; Ye, X.; Wei, D.; Zhong, J.; Chen, Y.; Xu, G.; He, D. 3D Artificial Bones for Bone Repair Prepared by Computed Tomography-Guided Fused Deposition Modeling for Bone Repair. ACS Appl. Mater. Interfaces 2014, 6, 14952–14963. [Google Scholar] [CrossRef]
- Oonishi, H.; Hench, L.L.; Wilson, J.; Sugihara, F.; Tsuji, E.; Kushitani, S.; Iwaki, H. Comparative Bone Growth Behavior in Granules of Bioceramic Materials of Various Sizes. J. Biomed. Mater. Res. 1999, 44, 31–43. [Google Scholar] [CrossRef]
- Tanaka, K.; Tsuchiya, A.; Ogino, Y.; Koyano, K.; Ishikawa, K. Fabrication and Histological Evaluation of a Fully Interconnected Porous CO3Ap Block Formed by Hydrate Expansion of CaO Granules. ACS Appl. Bio Mater. 2020, 3, 8872–8878. [Google Scholar] [CrossRef]
- Le Gars Santoni, B.; Niggli, L.; Dolder, S.; Loeffel, O.; Sblendorio, G.A.; Heuberger, R.; Maazouz, Y.; Stähli, C.; Döbelin, N.; Bowen, P.; et al. Effect of Minor Amounts of β-Calcium Pyrophosphate and Hydroxyapatite on the Physico-Chemical Properties and Osteoclastic Resorption of β-Tricalcium Phosphate Cylinders. Bioact. Mater. 2022, 10, 222–235. [Google Scholar] [CrossRef]
- Bu, S.; Yan, S.; Wang, R.; Xia, P.; Zhang, K.; Li, G.; Yin, J. In Situ Precipitation of Cluster and Acicular Hydroxyapatite onto Porous Poly(γ-benzyl-l-glutamate) Microcarriers for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2020, 12, 12468–12477. [Google Scholar] [CrossRef]
- Lewis, G. Properties of Acrylic Bone Cement: State of the Art Review. J. Biomed. Mater. Res. 1997, 38, 155–182. [Google Scholar] [CrossRef]
- He, Z.; Zhai, Q.; Hu, M.; Cao, C.; Wang, J.; Yang, H.; Li, B. Bone Cements for Percutaneous Vertebroplasty and Balloon Kyphoplasty: Current Status and Future Developments. J. Orthop. Transl. 2015, 3, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Bohner, M.; Gbureck, U.; Barralet, J.E. Technological Issues for the Development of More Efficient Calcium Phosphate Bone Cements: A Critical Assessment. Biomaterials 2005, 26, 6423–6429. [Google Scholar] [CrossRef]
- Montufar, E.B.; Maazouz, Y.; Ginebra, M.P. Relevance of the Setting Reaction to the Injectability of Tricalcium Phosphate Pastes. Acta Biomater. 2013, 9, 6188–6198. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, K.; Takagi, S.; Chow, L.C.; Ishikawa, Y. Properties and Mechanism of Fast-Setting Calcium Phosphate Cements. J. Mater. Sci. Mater. Med. 1995, 6, 528–533. [Google Scholar] [CrossRef]
- Gbureck, U.; Barralet, J.E.; Spatz, K.; Grover, L.M.; Thull, R. Ionic Modification of Calcium Phosphate Cement Viscosity. Part 1: Hypodermic Injection and Strength Improvement of Apatite Cement. Biomaterials 2004, 25, 2187–2195. [Google Scholar] [CrossRef]
- O’Neill, R.; McCarthy, H.O.; Montufar, E.B.; Ginebra, M.P.; Wilson, D.I.; Lennon, A.; Dunne, N. Critical Review: Injectability of Calcium Phosphate Pastes and Cements. Acta Biomater. 2017, 50, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Browm, W.E.; Chow, L.C. Dental Restoration Cement Pastes. U.S. Patent 4518430, 21 May 1985. [Google Scholar]
- Miyamoto, Y.; Ishikawa, K.; Takechi, M.; Toh, T.; Yuasa, T.; Nagayama, M.; Suzuki, K. Histological and Compositional Evaluations of Three Types of Calcium Phosphate Cements When Implanted in Subcutaneous Tissue Immediately after Mixing. J. Biomed. Mater. Res. 1999, 48, 36–42. [Google Scholar] [CrossRef]
- Horiguchi, Y.; Yoshikawa, A.; Oribe, K.; Aizawa, M. Fabrication of Chelate-Setting Hydroxyapatite Cements from Four Kinds of Commercially-Available Powder with Various Shape and Crystallinity and Their Mechanical Property. J. Ceram. Soc. Jpn. 2008, 116, 50–55. [Google Scholar] [CrossRef] [Green Version]
- Ando, A.; Kamikura, M.; Takeoka, Y.; Rikukawa, M.; Nakano, K.; Nagaya, M.; Nagashima, H.; Aizawa, M. Bioresorbable Porous β-Tricalcium Phosphate Chelate-Setting Cements with Poly(Lactic-co-Glycolic Acid) Particles as Pore-Forming Agent: Fabrication, Material Properties, Cytotoxicity, and In Vivo Evaluation. Sci. Technol. Adv. Mater. 2021, 22, 511–521. [Google Scholar] [CrossRef]
- Nassar, M.; Nassar, R.; Maki, H.; Al-Yagoob, A.; Hachim, M.; Senok, A.; Williams, D.; Hiraishi, N. Phytic Acid: Properties and Potential Applications in Dentistry. Front. Mater. 2021, 8, 638909. [Google Scholar] [CrossRef]
- Park, H.R.; Ahn, H.J.; Kim, J.H.; Yook, H.S.; Kim, S.; Lee, C.H.; Byun, M.W. Effects of Irradiated Phytic Acid on Antioxidation and Color Stability in Meat Models. J. Agric. Food Chem. 2004, 52, 2572–2576. [Google Scholar] [CrossRef]
- Hiasa, Y.; Kitahori, Y.; Morimoto, J.; Konishi, N.; Nakaoka, S.; Nishioka, H. Carcinogenicity Study in Rats of Phytic Acid ‘Daiichi’, a Natural Food Additive. Food Chem. Toxicol. 1992, 30, 117–125. [Google Scholar] [CrossRef]
- Oh, B.C.; Kim, M.H.; Yun, B.S.; Choi, W.C.; Park, S.C.; Bae, S.C.; Oh, T.K. Ca2+-Inositol Phosphate Chelation Mediates the Substrate Specificity of β-Propeller Phytase. Biochemistry 2006, 45, 9531–9539. [Google Scholar] [CrossRef]
- Minamisawa, H.; Umegaki, T.; Kojima, Y. Synthesis of Ultrafine Hydroxyapatite in a Ca(OH)2-H3PO4-H2O Reaction System Using Ultrasound Irradiation. Phosphorus Res. Bull. 2019, 35, 42–47. [Google Scholar] [CrossRef] [Green Version]
- Minamisawa, H.; Nomura, K.; Onda, A.; Umegaki, T.; Kojima, Y. Properties of Fine Sr2+-Substituted Hydroxyapatite Synthesized Using Ultrasonication. IOP Conf. Ser. Mater. Sci. Eng. 2020, 839, 012016. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Konishi, T.; Mizumoto, M.; Honda, M.; Zhuang, Z.; Aizawa, M. Fabrication of Chelate-setting Cements from Hydroxyapatite Powders Surface-modified with Various Sodium Inositol Hexaphosphate Concentrations and their mechanical Properties. Procedia Eng. 2012, 36, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Konishi, T.; Mizumoto, M.; Honda, M.; Aizawa, M. Adsorption Behavior of Sodium Inositol Hexaphosphate on the Surface of Hydroxyapatite. Key Eng. Mater. 2012, 529–530, 161–166. [Google Scholar] [CrossRef]
- Arakawa, M. Measurement of Powder Characteristics (IV). J. Soc. Mater. Sci. Jpn. 1970, 19, 685–691. [Google Scholar] [CrossRef]
- Correa-Piña, B.A.; Gomez-Vazquez, O.M.; Londoño-Restrepo, S.M.; Zubieta-Otero, L.F.; Millan-Malo, B.M.; Rodriguez-García, M.E. Synthesis and Characterization of Nano-Hydroxyapatite Added with Magnesium Obtained by Wet Chemical Precipitation. Prog. Nat. Sci. Mater. Int. 2021, 31, 575–582. [Google Scholar] [CrossRef]
- Castillo-Paz, A.M.; Londoño-Restrepo, S.M.; Tirado-Mejía, L.; Mondragón, M.A.; Rodríguez-García, M.E. Nano to Micro Size Transition of Hydroxyapatite in Porcine Bone during Heat Treatment with Low Heating Rates. Prog. Nat. Sci. Mater. Int. 2020, 30, 494–501. [Google Scholar] [CrossRef]
- Koutsopoulos, S. Synthesis and Characterization of Hydroxyapatite Crystals: A Review Study on the Analytical Methods. J. Biomed. Mater. Res. 2002, 62, 600–612. [Google Scholar] [CrossRef]
- Nadal, M.; Trombe, J.C.; Bonel, G.; Montel, G. Étude par spectrométrie d’absorption dans l’infrarouge de quelques substitutions dans les apatites carbonatées. J. Chim. Phys. 1970, 67, 1161–1167. [Google Scholar] [CrossRef]
- Mobasherpour, I.; Salahi, E.; Pazouki, M. Comparative of the Removal of Pb2+, Cd2+ and Ni2+ by Nano Crystallite Hydroxyapatite from Aqueous Solutions: Adsorption Isotherm Study. Arab. J. Chem. 2012, 5, 439–446. [Google Scholar] [CrossRef]
- Meski, S.; Ziani, S.; Khireddine, H. Removal of Lead Ions by Hydroxyapatite Prepared from the Egg Shell. J. Chem. Eng. Data 2010, 55, 3923–3928. [Google Scholar] [CrossRef]
- Yuan, N.; Gong, X.R.; Han, B.H. Hydrophobic Fluorous Metal-Organic Framework Nanoadsorbent for Removal of Hazardous Wastes from Water. ACS Appl. Nano Mater. 2021, 4, 1576–1585. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Misra, D.N. Adsorption on Hydroxyapatite: Role of Hydrogen Bonding and Interphase Coupling. Langmuir 1988, 4, 953–958. [Google Scholar] [CrossRef]
- Zhang, Q.; Dan, S.; Du, K. Fabrication and Characterization of Magnetic Hydroxyapatite Entrapped Agarose Composite Beads with High Adsorption Capacity for Heavy Metal Removal. Ind. Eng. Chem. Res. 2017, 56, 8705–8712. [Google Scholar] [CrossRef]
- Li, X.; Zhai, Q. Nano Mesocellular Foam Silica (MCFs): An Effective Adsorbent for Removing Ni2+ from Aqueous Solution. Water Sci. Eng. 2019, 12, 298–306. [Google Scholar] [CrossRef]
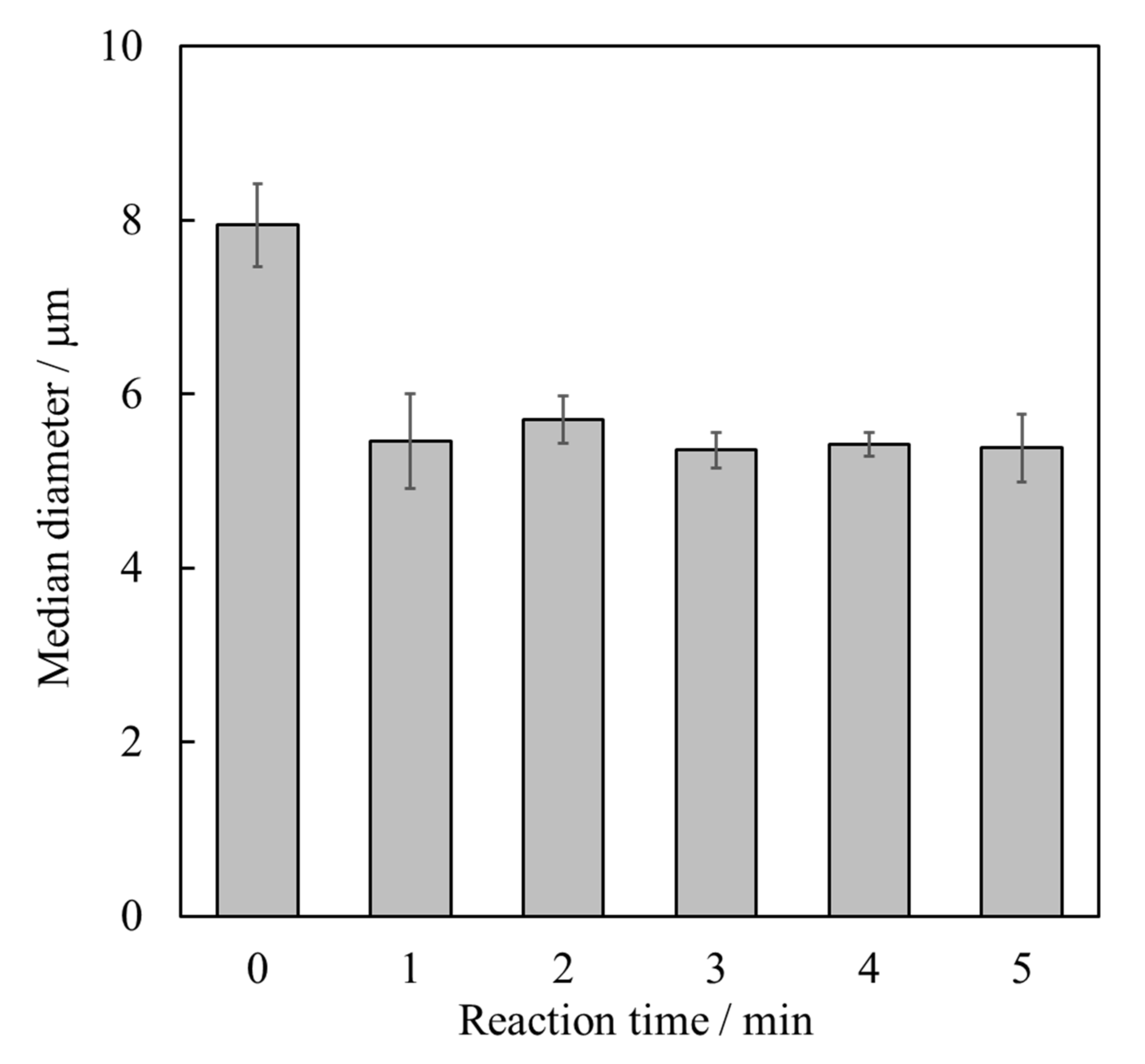

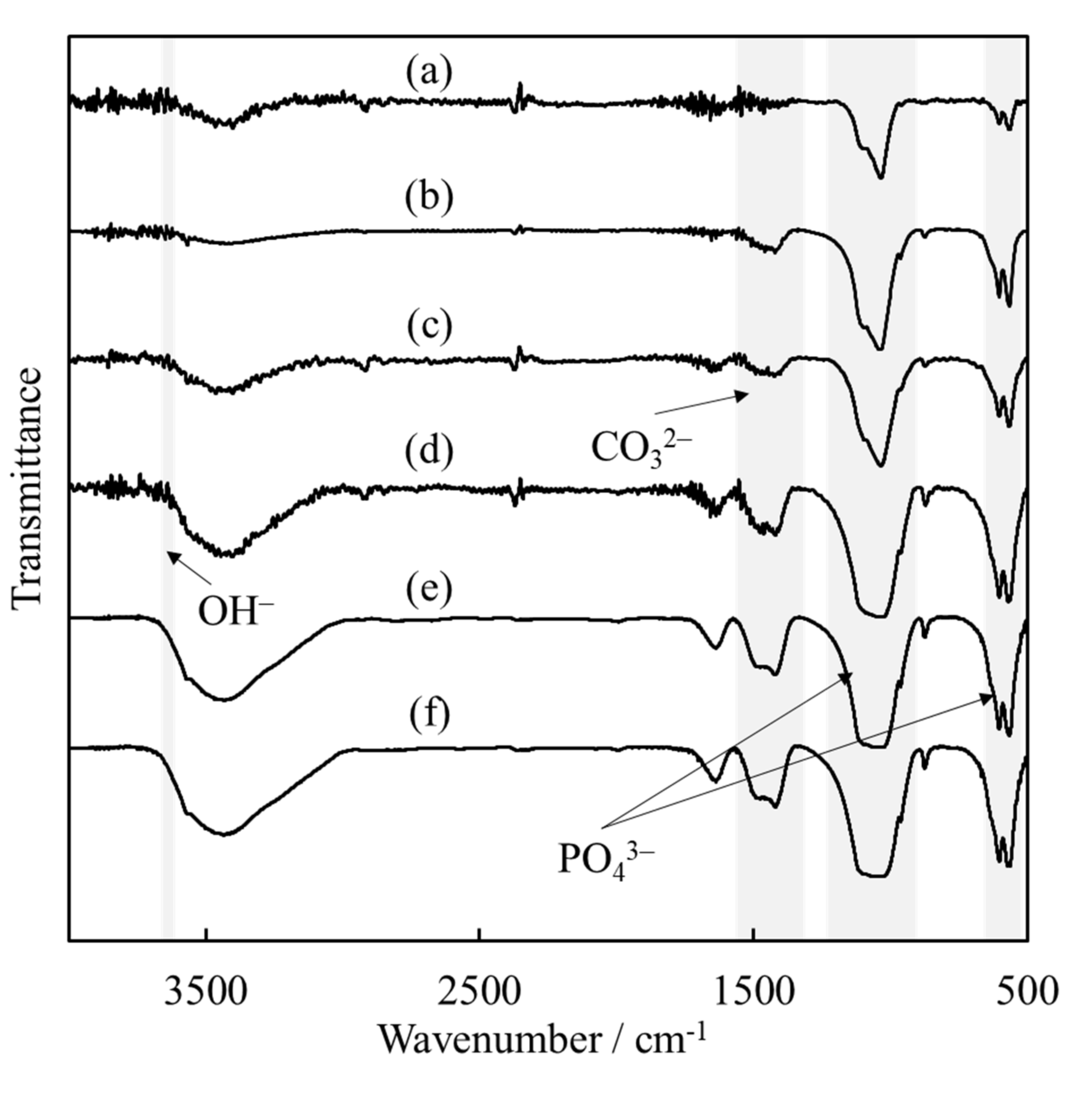

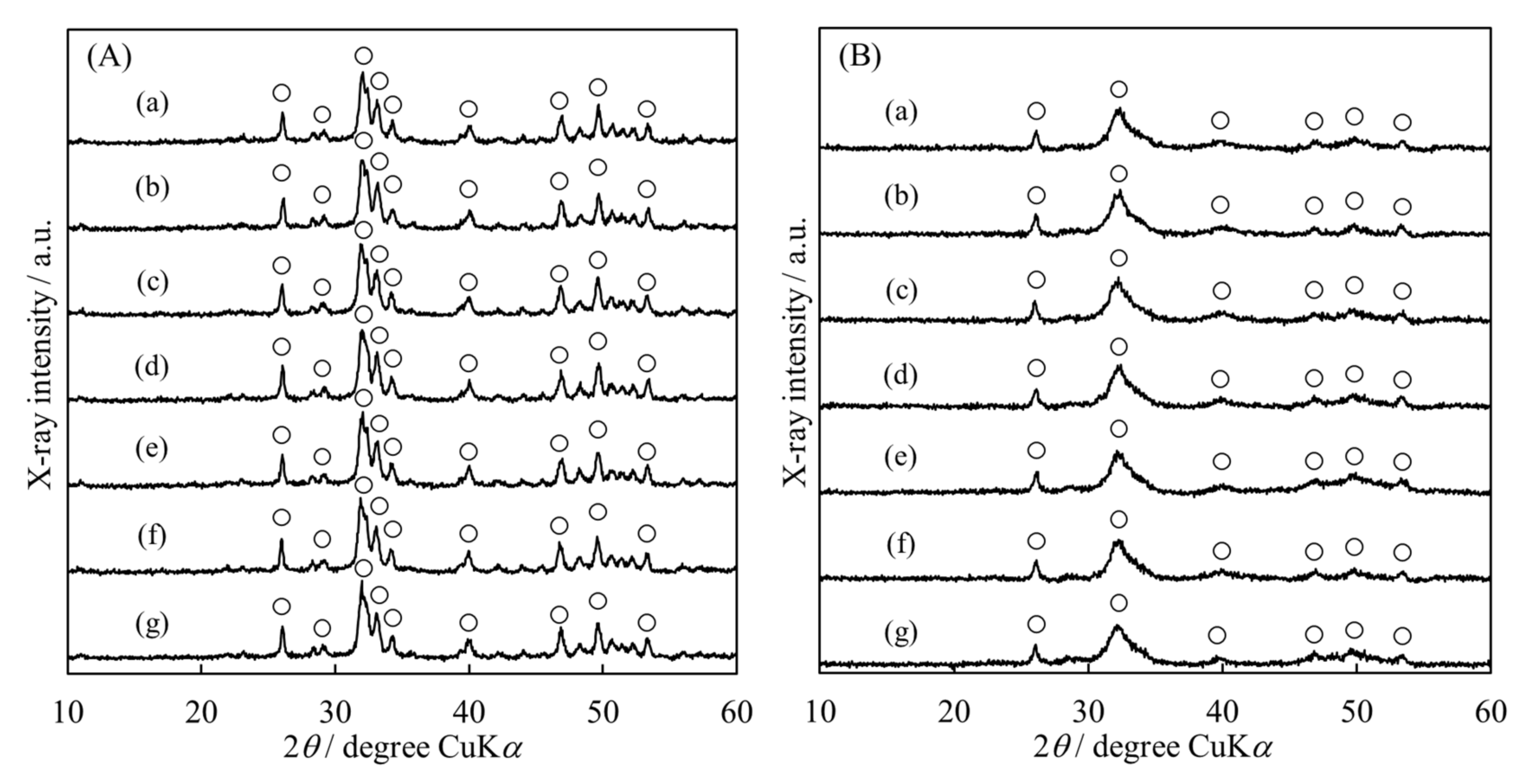
| HApHS(X) X/min | Specific Surface Area /m2/g | Ca/P Molar Ratio /- |
|---|---|---|
| 0 | 147 ± 18 | 1.63 ± 0.02 |
| 1 | 214 ± 7 | 1.71 ± 0.03 |
| 2 | 211 ± 13 | 1.70 ± 0.03 |
| 3 | 223 ± 17 | 1.71 ± 0.01 |
| 4 | 204 ± 9 | 1.69 ± 0.04 |
| 5 | 216 ± 13 | 1.73 ± 0.08 |
| Sample | Specific Surface Area /m2/g | Langmuir Model | Freundlich Model | |||
|---|---|---|---|---|---|---|
| KL | R2 | KF | n | R2 | ||
| HApHS | 208 | 0.8173 | 0.9163 | 39.02 | 2.039 | 0.9978 |
| HAp | 62.9 | 0.5809 | 0.9943 | 9.977 | 1.572 | 0.9581 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minamisawa, H.; Kojima, Y.; Aizawa, M. Adsorption of Inositol Phosphate on Hydroxyapatite Powder with High Specific Surface Area. Materials 2022, 15, 2176. https://doi.org/10.3390/ma15062176
Minamisawa H, Kojima Y, Aizawa M. Adsorption of Inositol Phosphate on Hydroxyapatite Powder with High Specific Surface Area. Materials. 2022; 15(6):2176. https://doi.org/10.3390/ma15062176
Chicago/Turabian StyleMinamisawa, Hirogo, Yoshiyuki Kojima, and Mamoru Aizawa. 2022. "Adsorption of Inositol Phosphate on Hydroxyapatite Powder with High Specific Surface Area" Materials 15, no. 6: 2176. https://doi.org/10.3390/ma15062176
APA StyleMinamisawa, H., Kojima, Y., & Aizawa, M. (2022). Adsorption of Inositol Phosphate on Hydroxyapatite Powder with High Specific Surface Area. Materials, 15(6), 2176. https://doi.org/10.3390/ma15062176






