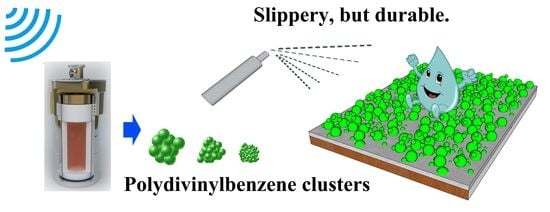
Large-Scale Fabrication of Graded Convex Structure for Superhydrophobic Coating Inspired by Nature
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials Synthesis
2.1.1. Fabrication of Polydivinylbenzene Nanoparticles
2.1.2. Fabrication of Superhydrophobic Coating
2.2. Measurements and Characterizations
2.3. Stability Testing of the Superhydrophobic Coatings
2.4. Oil−Water Separation Experiment
3. Results and Discussion
3.1. Chemical Analysis, Morphology and Wettability
3.2. Durability and Chemical Stability of the Superhydrophobic Coating
3.3. Self-Cleaning Capability of the Superhydrophobic Coating
3.4. Oil−Water Separation Capability of the Superhydrophobic Coating
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nosonovsky, M.; Bhushan, B. Biologically inspired surfaces: Broadening the scope of roughness. Adv. Funct. Mater. 2008, 6, 843–855. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, W. Biomimic from the superhydrophobic plant leaves in nature: Binary structure and unitary structure. Plant Sci. 2007, 6, 1103–1112. [Google Scholar] [CrossRef]
- Otten, A.; Herminghaus, S. How plants keep dry: A physicist’s point of view. Langmuir 2004, 6, 2405–2408. [Google Scholar] [CrossRef] [PubMed]
- Neinhuis, C.; Barthlott, W. Characterization and distribution of water-repellent, self-cleaning plant surfaces. Ann. Bot. 1997, 6, 667–677. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Jiang, L. Water-repellent legs of water striders. Nature 2004, 7013, 36. [Google Scholar] [CrossRef]
- Feng, X.; Jiang, L. Design and creation of superwetting/antiwetting surfaces. Adv. Mater. 2006, 23, 3063–3078. [Google Scholar] [CrossRef]
- Sun, T.; Feng, L.; Gao, X.; Jiang, L. Bioinspired surfaces with special wettability. Acc. Chem. Res. 2005, 8, 644–652. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, F.; Niu, J.; Jiang, Y.; Wang, Z. Superhydrophobic surfaces: From structural control to functional application. J. Mater. Chem. 2008, 6, 621–633. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Yang, X.B.; Yan, L.L.; Bai, Y.P.; Li, S.W.; Sorokin, P.; Shao, L. Biomimetic nanoparticle-engineered superwettablemembranes for efficient oil/water separation. J. Membr. Sci. 2021, 618, 118525. [Google Scholar] [CrossRef]
- Huang, G.; Lai, B.W.; Xu, H.D.; Jin, Y.K.; Huo, L.; Li, Z.R.; Deng, Y.L. Fabrication of a superhydrophobic fabric with a uniformhierarchical structure via a bottom-blown stirring method for highlyefficient oil-water separation. Sep. Purif. Technol. 2021, 258, 118063. [Google Scholar] [CrossRef]
- Gao, Y.N.; Wang, Y.; Yue, T.N.; Weng, Y.X.; Wang, M. Multifunctional cotton non-woven fabrics coated with silver nano-particles and polymers for antibacterial, superhydrophobic and highperformance microwave shielding. J. Colloid Interface Sci. 2021, 582, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Otero-Fernández, A.; Diaz, P.; Otero, J.A.; Ibanez, R.; Maroto-Valiente, A.; Palacio, L.; Pradanos, P.; Carmona, F.J.; Hernandez, A. Morphological, chemical and electrical characterization of a family of commercial nanofiltration polyvinyl alcohol coated polypiperazinea-mide membranes. Eur. Polym. J. 2020, 126, 109544. [Google Scholar] [CrossRef]
- Nine, M.J.; Tung, T.T.; Alotaibi, F.; Tran, D.N.H.; Losic, D. Facile adhesion-tuning of superhydrophobic surfaces between “lotus”and “petal” effect and their influence on icing and deicing properties. ACS Appl. Mater. Interfaces 2017, 9, 8393–8402. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.Y.; Zhang, X.M.; Liu, Z.Y.; Huo, K.F.; Chu, P.K.; Zhai, J.; Jiang, L. Regulating water adhesion on superhydrophobicTiO2nanotube arrays. Adv. Funct. Mater. 2014, 24, 6381–6388. [Google Scholar] [CrossRef]
- Zhou, C.L.; Chen, Z.D.; Yang, H.; Hou, K.; Zeng, X.J.; Zheng, Y.F.; Cheng, J. Nature-inspired strategy toward superhydrophobicfabrics for versatile oil/water separation. ACS Appl. Mater. Interfaces 2017, 9, 9184–9194. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.L.; Cheng, H.F.; Fane, A.G.; Wang, R.; Zhang, H. Recent development of advanced materials with special wettability forselective oil/water separation. Small 2016, 12, 2186–2202. [Google Scholar] [CrossRef]
- Gao, X.; Zhou, J.Y.; Du, R.; Xie, Z.Q.; Deng, S.B.; Liu, R.; Liu, Z.F.; Zhang, J. Robust superhydrophobic foam: A graphdiyne-based hierarchical architecture for oil/water separation. Adv. Mater. 2016, 28, 168–173. [Google Scholar] [CrossRef]
- Ren, Z.; Gang, W.; Guo, Z. Biomimetic high-intensity superhydrophobic metal rubber with anti-corrosion property for industrial oil-water separation. New J. Chem. 2018, 4, 1894–1899. [Google Scholar] [CrossRef]
- Zha, J.; Ala, S.S.; Peyroux, J.; Batisse, N.; Claves, D.; Dubois, M.; Kharitonov, A.P.; Monier, G.; Darmanin, T.; Guittard, F. Superhydrophobicity of polymer films via fluorine atoms covalent attachment and surface nano-texturing. J. Fluor. Chem. 2017, 200, 123–132. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Zhang, Q. Superhydrophobic SiO2 micro/nanofibrous membranes with porous surface prepared by freeze electrospinning for oil adsorption. Colloids Surf. A 2019, 568, 356–361. [Google Scholar] [CrossRef]
- Ma, W.; Guo, Z.; Zhao, J.; Yu, Q.; Wang, F.; Han, J.; Pan, H.; Yao, J.; Zhang, Q.; Samal, S.K. Polyimide/cellulose acetate core/shell electrospun fibrous membranes for oil-water separation. Sep. Purif. Technol. 2017, 177, 71–85. [Google Scholar] [CrossRef] [Green Version]
- Yuan, D.; Zhang, T.; Guo, Q.; Qiu, F.; Yang, D.; Ou, Z. Recyclable biomass carbon@SiO2@MnO2 aerogel with hierarchical architectures for fast and selective oil-water separation. Chem. Eng. J. 2018, 351, 622–630. [Google Scholar] [CrossRef]
- Hajar, M.; Lawrence, W.; Nicola, H. Novel multifunctional polymethylsilsesquioxane–silk fibroin aerogel hybrids for environmental and thermal insulation applications. J. Mater. Chem. A 2018, 6, 12598–12612. [Google Scholar] [CrossRef] [Green Version]
- Bu, Y.; Huang, J.; Zhang, S.; Wang, Y.; Gu, S.; Cao, G.; Yang, H.; Ye, D.; Zhou, Y.; Xu, W. Robust superhydrophobic surface by nature-inspired polyphenol chemistry for effective oil-water separation. Appl. Surf. Sci. 2018, 440, 535–546. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, C.; Wu, Z.; Wang, Y.; Du, X.; Kong, W.; Pan, D.; Guan, G.; Hao, X. A novel 3D porous modified material with cage-like structure: Fabrication and its demulsification effect for efficient oil/water separation. J. Mater. Chem. A 2017, 12, 5895–5904. [Google Scholar] [CrossRef]
- Chen, J.; Yuan, L.; Shi, C.; Wu, C.; Fan, Q.H. Nature-Inspired Hierarchical Protrusion Structure Construction for Washable and Wear-Resistant Superhydrophobic Textiles with Self-Cleaning Ability. ACS Appl. Mater. Interfaces 2021, 13, 18142–18151. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Lim, Y.T.; Jang, W.; Koo, H.Y.; Choi, W.S. Polydopamine-mediated all-in-one device with superhydrophilicity and superhydrophobicity for one-step oil/water separation and pollutant purification. Polymer 2016, 107, 1–11. [Google Scholar] [CrossRef]
- Huang, J.; Wang, S.; Shaoyi, L. Facile Preparation of a robust and durable superhydrophobic coating using biodegradable lignin-coated cellulose nanocrystal particles. Materials 2017, 9, 1080. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.F.; Zheng, Y.M.; Zou, S.W.; Chen, J.P. Characterization of copper adsorption onto an alginate encapsulated magnetic sorbent by a combined FT-IR, XPS and mathematical modeling study. Environ. Sci. Technol. 2008, 7, 2551. [Google Scholar] [CrossRef]
- Guo, Z.; Sun, Y. Recent advances of bioinspired functional materials with specific wettability: From nature and beyond nature. Nanoscale Horiz. 2018, 4, 52–76. [Google Scholar] [CrossRef]
- Wang, S.; Li, D.; Zhou, Y.; Jiang, L. Hierarchical Ti3C2Tx MXene/Ni chain/ZnO array hybrid nanostructures on cotton fabric for durable self-cleaning and enhanced microwave absorption. ACS Nano 2020, 14, 8634–8645. [Google Scholar] [CrossRef] [PubMed]














Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Huang, J.-T. Large-Scale Fabrication of Graded Convex Structure for Superhydrophobic Coating Inspired by Nature. Materials 2022, 15, 2179. https://doi.org/10.3390/ma15062179
Wang Y, Huang J-T. Large-Scale Fabrication of Graded Convex Structure for Superhydrophobic Coating Inspired by Nature. Materials. 2022; 15(6):2179. https://doi.org/10.3390/ma15062179
Chicago/Turabian StyleWang, Yu, and Jin-Tian Huang. 2022. "Large-Scale Fabrication of Graded Convex Structure for Superhydrophobic Coating Inspired by Nature" Materials 15, no. 6: 2179. https://doi.org/10.3390/ma15062179