Evaluation of the Effect of Selected Physiological Fluid Contaminants on the Mechanical Properties of Selected Medium-Viscosity PMMA Bone Cements
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparations
2.2. Mechanical Testing
2.3. Statistical Analysis
3. Results and Discussion
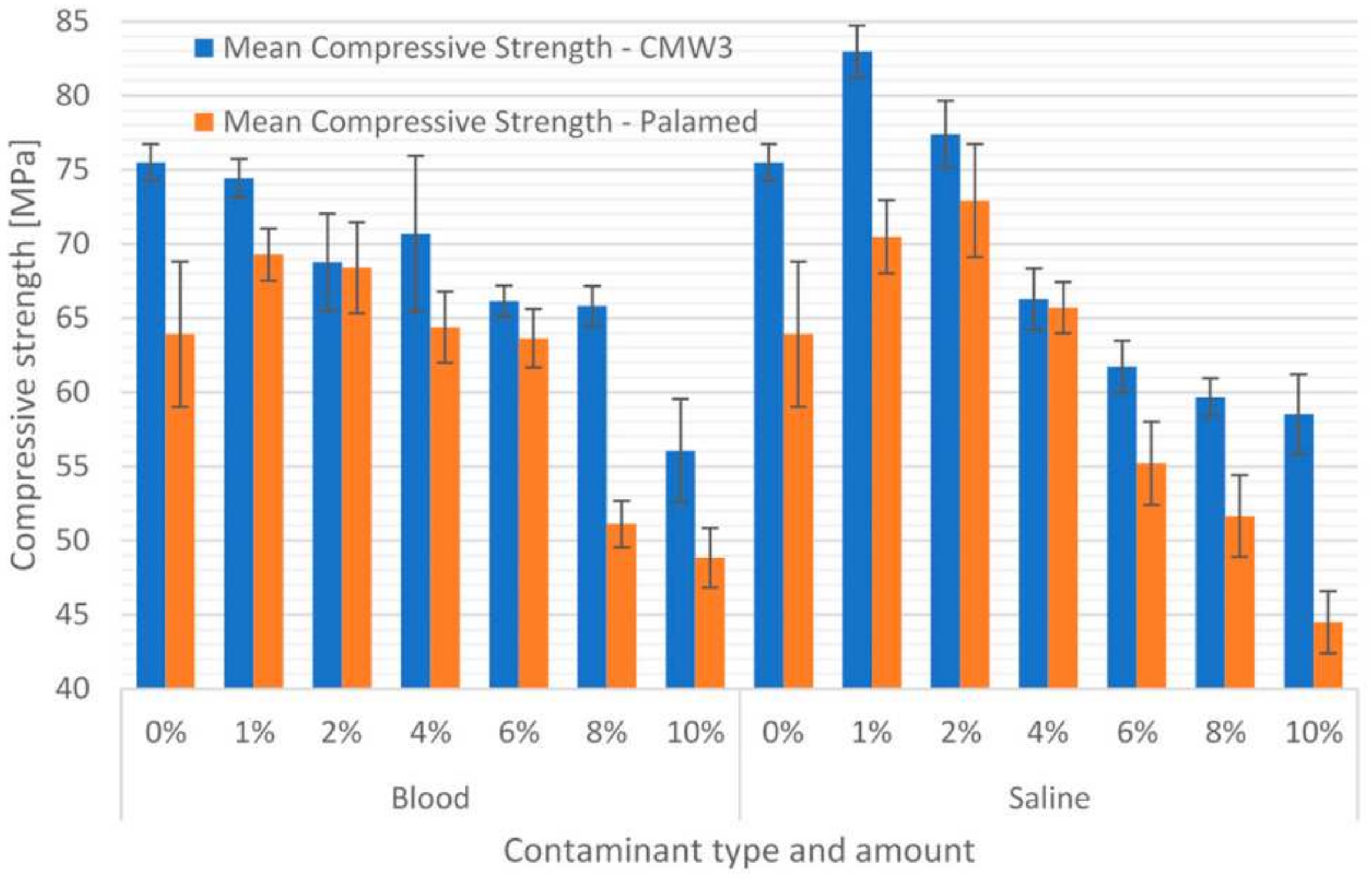
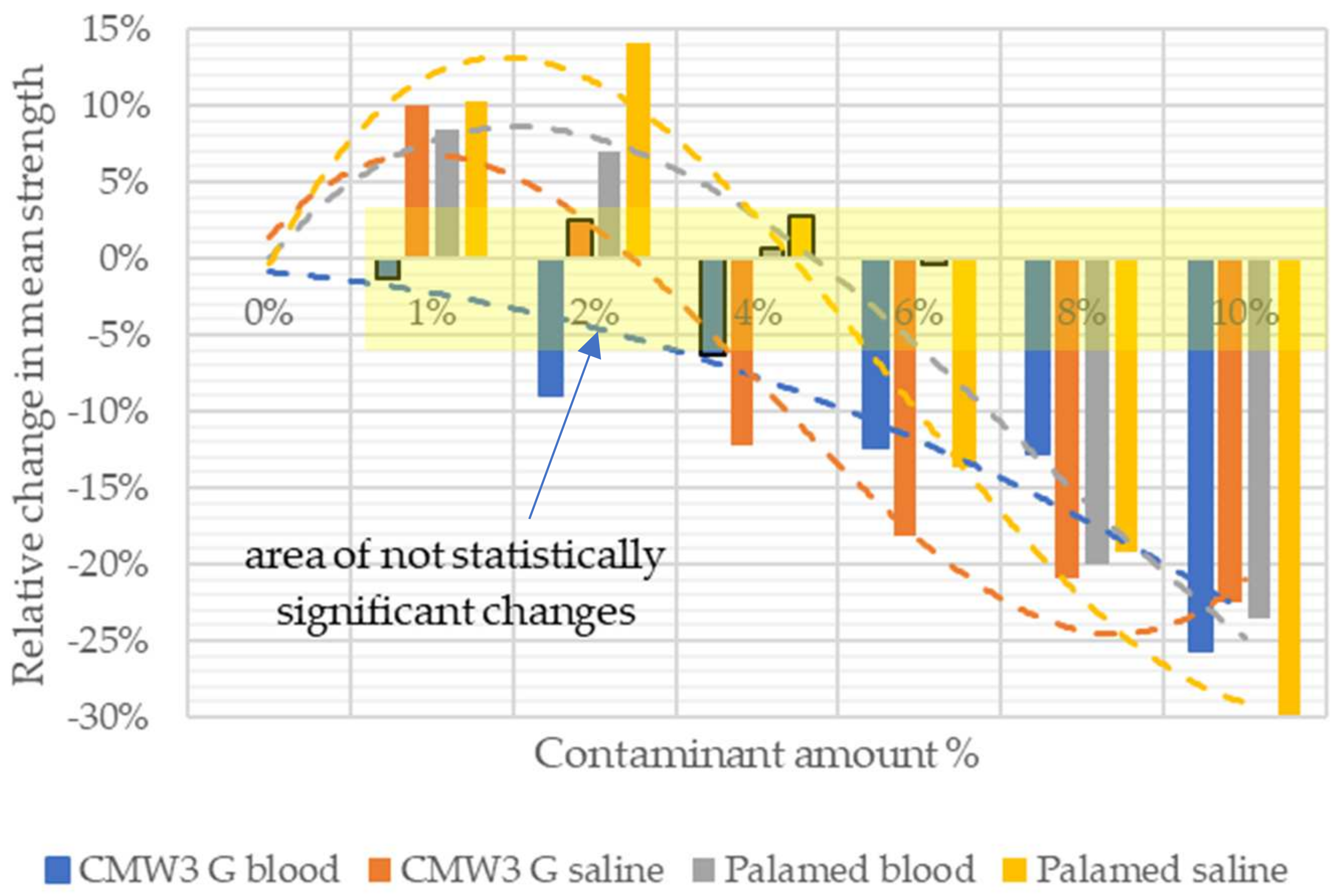
3.1. Compressive Strength
3.2. Compressive Modulus of Elasticity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cameron, K.; Hsiao, M.; Owens, B.; Burks, R.; Svoboda, S.J. Incidence of Physician-Diagnosed Osteoarthritis among Active Duty United States Military Service Members. Arthr. Rheumatol. 2011, 63, 2974–2982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dulay, G.S.; Cooper, C.; Dennison, E.M. Knee Pain, Knee Injury, Knee Osteoarthritis & Work. Best Pract. Res. Clin. Rheumatol. 2015, 29, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Krakowski, P.; Karpiński, R.; Maciejewski, R.; Jonak, J.; Jurkiewicz, A. Short-Term Effects of Arthroscopic Microfracturation of Knee Chondral Defects in Osteoarthritis. Appl. Sci. 2020, 10, 8312. [Google Scholar] [CrossRef]
- Krakowski, P.; Nogalski, A.; Jurkiewicz, A.; Karpiński, R.; Maciejewski, R.; Jonak, J. Comparison of Diagnostic Accuracy of Physical Examination and MRI in the Most Common Knee Injuries. Appl. Sci. 2019, 9, 4102. [Google Scholar] [CrossRef] [Green Version]
- Widmyer, M.R.; Utturkar, G.M.; Leddy, H.A.; Coleman, J.L.; Spritzer, C.E.; Moorman, C.T.; DeFrate, L.E.; Guilak, F. High Body Mass Index Is Associated with Increased Diurnal Strains in the Articular Cartilage of the Knee. Arthritis Rheum. 2013, 65, 2615–2622. [Google Scholar] [CrossRef] [Green Version]
- Martin-Rodriguez, E.; Guillen-Grima, F.; Martí, A.; Brugos-Larumbe, A. Comorbidity Associated with Obesity in a Large Population: The APNA Study. Obes. Res. Clin. Pract. 2015, 9, 435–447. [Google Scholar] [CrossRef]
- Thijssen, E.; van Caam, A.; van der Kraan, P.M. Obesity and Osteoarthritis, More than Just Wear and Tear: Pivotal Roles for Inflamed Adipose Tissue and Dyslipidaemia in Obesity-Induced Osteoarthritis. Rheumatology 2015, 54, 588–600. [Google Scholar] [CrossRef] [Green Version]
- Furman, B.D.; Olson, S.A.; Guilak, F. The Development of Posttraumatic Arthritis after Articular Fracture. J. Orthop. Trauma 2006, 20, 719–725. [Google Scholar] [CrossRef]
- Friel, N.A.; Chu, C.R. The Role of ACL Injury in the Development of Posttraumatic Knee Osteoarthritis. Clin. Sports Med. 2013, 32, 1–12. [Google Scholar] [CrossRef]
- Krakowski, P.; Karpiński, R.; Jojczuk, M.; Nogalska, A.; Jonak, J. Knee MRI Underestimates the Grade of Cartilage Lesions. Appl. Sci. 2021, 11, 1552. [Google Scholar] [CrossRef]
- Cibere, J.; Sayre, E.C.; Guermazi, A.; Nicolaou, S.; Kopec, J.A.; Esdaile, J.M.; Thorne, A.; Singer, J.; Wong, H. Natural History of Cartilage Damage and Osteoarthritis Progression on Magnetic Resonance Imaging in a Population-Based Cohort with Knee Pain. Osteoarthr. Cartil. 2011, 19, 683–688. [Google Scholar] [CrossRef] [Green Version]
- Shiers, L.G. Arthroplasty of the Knee; Preliminary Report of New Method. J. Bone Jt. Surg. Br. 1954, 36-B, 553–560. [Google Scholar] [CrossRef] [Green Version]
- Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR). Hip, Knee & Shoulder Arthroplasty: 2020 Annual Report; AOANJRR: Adelaide, Australia, 2020. [Google Scholar]
- Singh, J.A.; Yu, S.; Chen, L.; Cleveland, J.D. Rates of Total Joint Replacement in the United States: Future Projections to 2020–2040 Using the National Inpatient Sample. J. Rheumatol. 2019, 46, 1134–1140. [Google Scholar] [CrossRef]
- Learmonth, I.D.; Young, C.; Rorabeck, C. The Operation of the Century: Total Hip Replacement. Lancet 2007, 370, 1508–1519. [Google Scholar] [CrossRef]
- Serbetci, K.; Korkusuz, F.; Hasirci, N. Thermal and Mechanical Properties of Hydroxyapatite Impregnated Acrylic Bone Cements. Polym. Test. 2004, 23, 145–155. [Google Scholar] [CrossRef]
- Rodriguez, L.; Chari, J.; Aghyarian, S.; Gindri, I.; Kosmopoulos, V.; Rodrigues, D. Preparation and Characterization of Injectable Brushite Filled-Poly (Methyl Methacrylate) Bone Cement. Materials 2014, 7, 6779–6795. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.-J.; Xu, J.; Qiu, Z.-Y.; Ma, X.-L.; Zhang, Z.-Q.; Tan, X.-X.; Cui, Y.; Cui, F.-Z. Mechanical Properties and Cytocompatibility Improvement of Vertebroplasty PMMA Bone Cements by Incorporating Mineralized Collagen. Materials 2015, 8, 2616–2634. [Google Scholar] [CrossRef]
- Lewis, G. Injectable Bone Cements for Use in Vertebroplasty and Kyphoplasty: State-of-the-Art Review. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 76B, 456–468. [Google Scholar] [CrossRef]
- Liu, H.; Liu, B.; Gao, C.; Meng, B.; Yang, H.; Yu, H.; Yang, L. Injectable, Biomechanically Robust, Biodegradable and Osseointegrative Bone Cement for Percutaneous Kyphoplasty and Vertebroplasty. Int. Orthop. 2018, 42, 125–132. [Google Scholar] [CrossRef]
- Robertsson, O.; Lidgren, L.; Sundberg, M.; W-Dahl, A. The Swedish Knee Arthroplasty Register Annual Report 2020; Media-Tryck: Lund, Sweden, 2020; ISBN 978-91-88017-32-1. [Google Scholar]
- Barnett, A.J.; Toms, A.D. Revision Total Hip and Knee Replacement. Clin. Geriatr. Med. 2012, 28, 431–446. [Google Scholar] [CrossRef]
- Lee, A.J.C. The Time-Dependent Properties of Polymethylmethacrylate Bone Cement: The Interaction of Shape of Femoral Stems, Surface Finish and Bone Cement. In Interfaces in Total Hip Arthroplasty; Learmonth, I.D., Ed.; Springer: London, UK, 2000; pp. 11–19. ISBN 978-1-4471-1150-4. [Google Scholar]
- Wekwejt, M.; Moritz, N.; Świeczko-Żurek, B.; Pałubicka, A. Biomechanical Testing of Bioactive Bone Cements—A Comparison of the Impact of Modifiers: Antibiotics and Nanometals. Polym. Test. 2018, 70, 234–243. [Google Scholar] [CrossRef]
- Wekwejt, M.; Michalska-Sionkowska, M.; Bartmański, M.; Nadolska, M.; Łukowicz, K.; Pałubicka, A.; Osyczka, A.M.; Zieliński, A. Influence of Several Biodegradable Components Added to Pure and Nanosilver-Doped PMMA Bone Cements on Its Biological and Mechanical Properties. Mater. Sci. Eng. C 2020, 117, 111286. [Google Scholar] [CrossRef]
- Matuszewski, Ł.; Olchowik, G.; Mazurkiewicz, T.; Kowalczyk, B.; Zdrojewska, A.; Matuszewska, A.; Ciszewski, A.; Gospodarek, M.; Morawik, I. Biomechanical Parameters of the BP-Enriched Bone Cement. Eur. J. Orthop. Surg. Traumatol. 2014, 24, 435–441. [Google Scholar] [CrossRef] [Green Version]
- Callaghan, J.J.; Rosenberg, A.G.; Rubash, H.E. The Adult Hip; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; Volume 1, ISBN 0-7817-5092-X. [Google Scholar]
- Montaño, C.J.; Campos, T.P.R.; Lemos, B.R.S.; Yoshida, M.I.; Almeida, N.G.S.; Aguilar, M.T.P.; Lima, C.V. Effects of Hydroxyapatite on PMMA-HAp Cement for Biomedical Applications. Biomed. Mater. Eng. 2020, 31, 191–201. [Google Scholar] [CrossRef]
- de Souza Leão, R.; de Moraes SL, D.; de Luna Gomes, J.M.; Lemos CA, A.; da Silva Casado, B.G.; do Egito Vasconcelos, B.C.; Pellizzer, E.P. Influence of Addition of Zirconia on PMMA: A Systematic Review. Mater. Sci. Eng. C 2020, 106, 110292. [Google Scholar] [CrossRef]
- Prakash, J.; Prema, D.; Venkataprasanna, K.S.; Balagangadharan, K.; Selvamurugan, N.; Venkatasubbu, G.D. Nanocomposite Chitosan Film Containing Graphene Oxide/Hydroxyapatite/Gold for Bone Tissue Engineering. Int. J. Biol. Macromol. 2020, 154, 62–71. [Google Scholar] [CrossRef]
- Céspedes-Valenzuela, D.N.; Sánchez-Rentería, S.; Cifuentes, J.; Gantiva-Diaz, M.; Serna, J.A.; Reyes, L.H.; Ostos, C.; Cifuentes-De la Portilla, C.; Muñoz-Camargo, C.; Cruz, J.C. Preparation and Characterization of an Injectable and Photo-Responsive Chitosan Methacrylate/Graphene Oxide Hydrogel: Potential Applications in Bone Tissue Adhesion and Repair. Polymers 2021, 14, 126. [Google Scholar] [CrossRef]
- Zapata, M.E.V.; Tovar, C.D.G.; Hernandez, J.H.M. The Role of Chitosan and Graphene Oxide in Bioactive and Antibacterial Properties of Acrylic Bone Cements. Biomolecules 2020, 10, 1616. [Google Scholar] [CrossRef]
- Tan, J.; Koh, B.T.; Ramruttun, A.; Wang, W. Compression and Flexural Strength of Bone Cement Mixed with Blood. J. Orthop. Surg. 2016, 24, 240–244. [Google Scholar] [CrossRef] [Green Version]
- Karpiński, R.; Szabelski, J.; Maksymiuk, J. Effect of Physiological Fluids Contamination on Selected Mechanical Properties of Acrylate Bone Cement. Materials 2019, 12, 3963. [Google Scholar] [CrossRef] [Green Version]
- Szabelski, J.; Karpiński, R.; Krakowski, P.; Jonak, J. The Impact of Contaminating Poly (Methyl Methacrylate) (PMMA) Bone Cements on Their Compressive Strength. Materials 2021, 14, 2555. [Google Scholar] [CrossRef] [PubMed]
- Machrowska, A.; Szabelski, J.; Karpiński, R.; Krakowski, P.; Jonak, J.; Jonak, K. Use of Deep Learning Networks and Statistical Modeling to Predict Changes in Mechanical Parameters of Contaminated Bone Cements. Materials 2020, 13, 5419. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; O’Donnell, J.; Cochrane, N.; Ryan, S.; Belay, E.; Myntti, M.; Seyler, T. Effect of Commonly Used Lavage Solutions on the Polymerization of Bone Cement. Orthop. Traumatol. Surg. Res. 2022, 103243. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.; Pruitt, L.; Ries, M.; Gundiah, N. Fracture and Fatigue Properties of Acrylic Bone Cement: The Effects of Mixing Method, Sterilization Treatment, and Molecular Weight. J. Arthroplast. 2000, 15, 1028–1035. [Google Scholar] [CrossRef]
- Lewis, G. Effect of Mixing Method and Storage Temperature of Cement Constituents on the Fatigue and Porosity of Acrylic Bone Cement. J. Biomed. Mater. Res. 1999, 48, 143–149. [Google Scholar] [CrossRef]
- Wang, J.-S.; Breusch, S.J. Mixing: Choice of Mixing System. In The Well-Cemented Total Hip Arthroplasty—Theory and Practice; Springer: Heidelberg, Germany, 2005; p. 113. [Google Scholar]
- Boehm, A.; Meininger, S.; Tesch, A.; Gbureck, U.; Müller, F. The Mechanical Properties of Biocompatible Apatite Bone Cement Reinforced with Chemically Activated Carbon Fibers. Materials 2018, 11, 192. [Google Scholar] [CrossRef] [Green Version]
- Kotha, S.; Li, C.; Schmid, S.; Mason, J. Reinforcement of Bone Cement Using Zirconia Fibers with and without Acrylic Coating. J. Biomed. Mater. Res. A 2009, 88A, 898–906. [Google Scholar] [CrossRef]
- Knoell, A.; Maxwell, H.; Bechtol, C. Graphite Fiber Reinforced Bone Cement: An Experimental Feasibility Investigation. Ann. Biomed. Eng. 1975, 3, 225–229. [Google Scholar] [CrossRef]
- Tavakoli, M.; Bakhtiari, S.S.E.; Karbasi, S. Incorporation of Chitosan/Graphene Oxide Nanocomposite in to the PMMA Bone Cement: Physical, Mechanical and Biological Evaluation. Int. J. Biol. Macromol. 2020, 149, 783–793. [Google Scholar] [CrossRef]
- Paz, E.; Ballesteros, Y.; Forriol, F.; Dunne, N.J.; del Real, J.C. Graphene and Graphene Oxide Functionalisation with Silanes for Advanced Dispersion and Reinforcement of PMMA-Based Bone Cements. Mater. Sci. Eng. C 2019, 104, 109946. [Google Scholar] [CrossRef]
- Paz, E.; Forriol, F.; del Real, J.C.; Dunne, N. Graphene Oxide versus Graphene for Optimisation of PMMA Bone Cement for Orthopaedic Applications. Mater. Sci. Eng. C 2017, 77, 1003–1011. [Google Scholar] [CrossRef]
- Paz, E.; Ballesteros, Y.; Abenojar, J.; del Real, J.C.; Dunne, N.J. Graphene Oxide and Graphene Reinforced PMMA Bone Cements: Evaluation of Thermal Properties and Biocompatibility. Materials 2019, 12, 3146. [Google Scholar] [CrossRef] [Green Version]
- Wekwejt, M.; Chen, S.; Kaczmarek-Szczepańska, B.; Nadolska, M.; Łukowicz, K.; Pałubicka, A.; Michno, A.; Osyczka, A.M.; Michálek, M.; Zieliński, A. Nanosilver-Loaded PMMA Bone Cement Doped with Different Bioactive Glasses—Evaluation of Cytocompatibility, Antibacterial Activity, and Mechanical Properties. Biomater. Sci. 2021, 9, 3112–3126. [Google Scholar] [CrossRef]
- Wekwejt, M.; Michno, A.; Truchan, K.; Pałubicka, A.; Świeczko-Żurek, B.; Osyczka, A.M.; Zieliński, A. Antibacterial Activity and Cytocompatibility of Bone Cement Enriched with Antibiotic, Nanosilver, and Nanocopper for Bone Regeneration. Nanomaterials 2019, 9, 1114. [Google Scholar] [CrossRef] [Green Version]
- Rahighi, R.; Panahi, M.; Akhavan, O.; Mansoorianfar, M. Pressure-Engineered Electrophoretic Deposition for Gentamicin Loading within Osteoblast-Specific Cellulose Nanofiber Scaffolds. Mater. Chem. Phys. 2021, 272, 125018. [Google Scholar] [CrossRef]
- Mansoorianfar, M.; Shahin, K.; Mirström, M.M.; Li, D. Cellulose-Reinforced Bioglass Composite as Flexible Bioactive Bandage to Enhance Bone Healing. Ceram. Int. 2021, 47, 416–423. [Google Scholar] [CrossRef]
- Letchmanan, K.; Shen, S.-C.; Ng, W.K.; Kingshuk, P.; Shi, Z.; Wang, W.; Tan, R.B.H. Mechanical Properties and Antibiotic Release Characteristics of Poly(Methyl Methacrylate)-Based Bone Cement Formulated with Mesoporous Silica Nanoparticles. J. Mech. Behav. Biomed. Mater. 2017, 72, 163–170. [Google Scholar] [CrossRef]
- Slane, J.; Vivanco, J.; Ebenstein, D.; Squire, M.; Ploeg, H.-L. Multiscale Characterization of Acrylic Bone Cement Modified with Functionalized Mesoporous Silica Nanoparticles. J. Mech. Behav. Biomed. Mater. 2014, 37, 141–152. [Google Scholar] [CrossRef]
- He, X.; Qu, Y.; Peng, J.; Peng, T.; Qian, Z. A Novel Botryoidal Aramid Fiber Reinforcement of a PMMA Resin for a Restorative Biomaterial. Biomater. Sci. 2017, 5, 808–816. [Google Scholar] [CrossRef]
- Xu, H.H.K.; Quinn, J.B.; Takagi, S.; Chow, L.C.; Eichmiller, F.C. Strong and Macroporous Calcium Phosphate Cement: Effects of Porosity and Fiber Reinforcement on Mechanical Properties. J. Biomed. Mater. Res. 2001, 57, 457–466. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Mayer, J.; Wintermantel, E.; Leong, K.W. Biomedical Applications of Polymer-Composite Materials: A Review. Compos. Sci. Technol. 2001, 61, 1189–1224. [Google Scholar] [CrossRef]
- Khaled, S.M.Z.; Charpentier, P.A.; Rizkalla, A.S. Physical and Mechanical Properties of PMMA Bone Cement Reinforced with Nano-Sized Titania Fibers. J. Biomater. Appl. 2011, 25, 515–537. [Google Scholar] [CrossRef]
- Kotha, S.P.; Li, C.; McGinn, P.; Schmid, S.R.; Mason, J.J. Improved Mechanical Properties of Acrylic Bone Cement with Short Titanium Fiber Reinforcement. J. Mater. Sci. Mater. Med. 2006, 17, 743–748. [Google Scholar] [CrossRef]
- Yang, J.-M.; Huang, P.-Y.; Yang, M.-C. The Effect of Ultra-High Molecular Weight Polyethylene Fiber on the Mechanical Properties of Acrylic Bone Cement. J. Polym. Res. 1997, 4, 41–46. [Google Scholar] [CrossRef]
- Yang, J.-M.; Li, H.-M.; Yang, M.-C.; Shih, C.-H. Characterization of Acrylic Bone Cement Using Dynamic Mechanical Analysis. J. Biomed. Mater. Res. 1999, 48, 52–60. [Google Scholar] [CrossRef]
- Shirdar, M.R.; Taheri, M.M.; Qi, M.-L.; Gohari, S.; Farajpour, N.; Narayanan, S.; Foroozan, T.; Sharifi-Asl, S.; Shahbazian-Yassar, R.; Shokuhfar, T. Optimization of the Mechanical Properties and the Cytocompatibility for the PMMA Nanocomposites Reinforced with the Hydroxyapatite Nanofibers and the Magnesium Phosphate Nanosheets. Materials 2021, 14, 5893. [Google Scholar] [CrossRef]
- Phakatkar, A.H.; Shirdar, M.R.; Qi, M.; Taheri, M.M.; Narayanan, S.; Foroozan, T.; Sharifi-Asl, S.; Huang, Z.; Agrawal, M.; Lu, Y.; et al. Novel PMMA Bone Cement Nanocomposites Containing Magnesium Phosphate Nanosheets and Hydroxyapatite Nanofibers. Mater. Sci. Eng. C 2020, 109, 110497. [Google Scholar] [CrossRef]
- Karpiński, R.; Szabelski, J.; Maksymiuk, J. Seasoning Polymethyl Methacrylate (PMMA) Bone Cements with Incorrect Mix Ratio. Materials 2019, 12, 3073. [Google Scholar] [CrossRef] [Green Version]
- Ayre, W.N.; Denyer, S.P.; Evans, S.L. Ageing and Moisture Uptake in Polymethyl Methacrylate (PMMA) Bone Cements. J. Mech. Behav. Biomed. Mater. 2014, 32, 76–88. [Google Scholar] [CrossRef] [Green Version]
- Gbureck, U.; Grübel, S.; Thull, R.; Barralet, J.E. Modified PMMA Cements for a Hydrolysis Resistant Metal–Polymer Interface in Orthopaedic Applications. Acta Biomater. 2005, 1, 671–676. [Google Scholar] [CrossRef]
- Tham, W.L.; Chow, W.S.; Mohd Ishak, Z.A. Simulated Body Fluid and Water Absorption Effects on Poly(Methyl Methacrylate)/Hydroxyapatite Denture Base Composites. Express Polym. Lett. 2010, 4, 517–528. [Google Scholar] [CrossRef]
- Machrowska, A.; Karpiński, R.; Jonak, J.; Szabelski, J.; Krakowski, P. Numerical Prediction of the Component-Ratio-Dependent Compressive Strength of Bone Cement. Appl. Comput. Sci. 2020, 87–101. [Google Scholar] [CrossRef]
- Karpinski, R.; Szabelski, J.; Maksymiuk, J. Analysis of the Properties of Bone Cement with Respect to Its Manufacturing and Typical Service Lifetime Conditions. MATEC Web Conf. 2018, 244, 01004. [Google Scholar] [CrossRef]
- Harper, E.J.; Bonfield, W. Tensile Characteristics of Ten Commercial Acrylic Bone Cements. J. Biomed. Mater. Res. 2000, 53, 605–616. [Google Scholar] [CrossRef]
- Kühn, K.-D. Bone Cements up to Date Comparison of Physical and Chemical Properties of Commercial Materials; with 132 Tables; Springer: Berlin, Germany, 2000; ISBN 978-3-540-67207-4. [Google Scholar]
- Sophie, H.; Yuhan, C.; Clemens, K.; Klaus-Dieter, K. Properties of Orthopaedic Cements Biomechanically Little Affected by Exceptional Use of Liquid Antibiotics. Orthop. Surg. 2021, 13, 2153–2162. [Google Scholar] [CrossRef]
- ISO 5833:2002; Implants for Surgery—Acrylic Resin Cements. ISO: Geneva, Switzerland, 2002.
- Rabiej, M. Analizy Statystyczne z Programami Statistica i Excel; Wydawnictwo Helion: Gliwice, Poland, 2018; ISBN 978-83-283-3922-4. [Google Scholar]
- Mccabe, J.F.; Walls, A. Applied Dental Materials; Wiley-Blackwell: Oxford, UK, 2013; ISBN 978-1-118-69712-2. [Google Scholar]
- Zafar, M.S. Prosthodontic Applications of Polymethyl Methacrylate (PMMA): An Update. Polymers 2020, 12, 2299. [Google Scholar] [CrossRef]
- Umeda, T.; Itatani, K.; Endo, H.; Takeuchi, H.; Mizutani, K.; Musha, Y. Effect of Blood Addition on the Biocompatibility of Calcium Phosphate Paste. J. Eur. Ceram. Soc. 2006, 26, 525–531. [Google Scholar] [CrossRef]
- Kiyasu, K.; Takemasa, R.; Ikeuchi, M.; Tani, T. Differential Blood Contamination Levels and Powder–Liquid Ratios Can Affect the Compressive Strength of Calcium Phosphate Cement (CPC): A Study Using a Transpedicular Vertebroplasty Model. Eur. Spine J. 2013, 22, 1643–1649. [Google Scholar] [CrossRef] [Green Version]
- Lodoso-Torrecilla, I.; van den Beucken, J.J.J.P.; Jansen, J.A. Calcium Phosphate Cements: Optimization toward Biodegradability. Acta Biomater. 2021, 119, 1–12. [Google Scholar] [CrossRef]
- Karpiński, R.; Szabelski, J.; Krakowski, P.; Jonak, J. Effect of Physiological Saline Solution Contamination on Selected Mechanical Properties of Seasoned Acrylic Bone Cements of Medium and High Viscosity. Materials 2021, 14, 110. [Google Scholar] [CrossRef]
- Miettinen, V.M.; Vallittu, P.K. Water Sorption and Solubility of Glass Fiber-Reinforced Denture Polymethyl Methacrylate Resin. J. Prosthet. Dent. 1997, 77, 531–534. [Google Scholar] [CrossRef]
- Stich, T.; Alagboso, F.; Křenek, T.; Kovářík, T.; Alt, V.; Docheva, D. Implant-Bone-Interface: Reviewing the Impact of Titanium Surface Modifications on Osteogenic Processes In Vitro and In Vivo. Bioeng. Transl. Med. 2022, 7, e10239. [Google Scholar] [CrossRef]
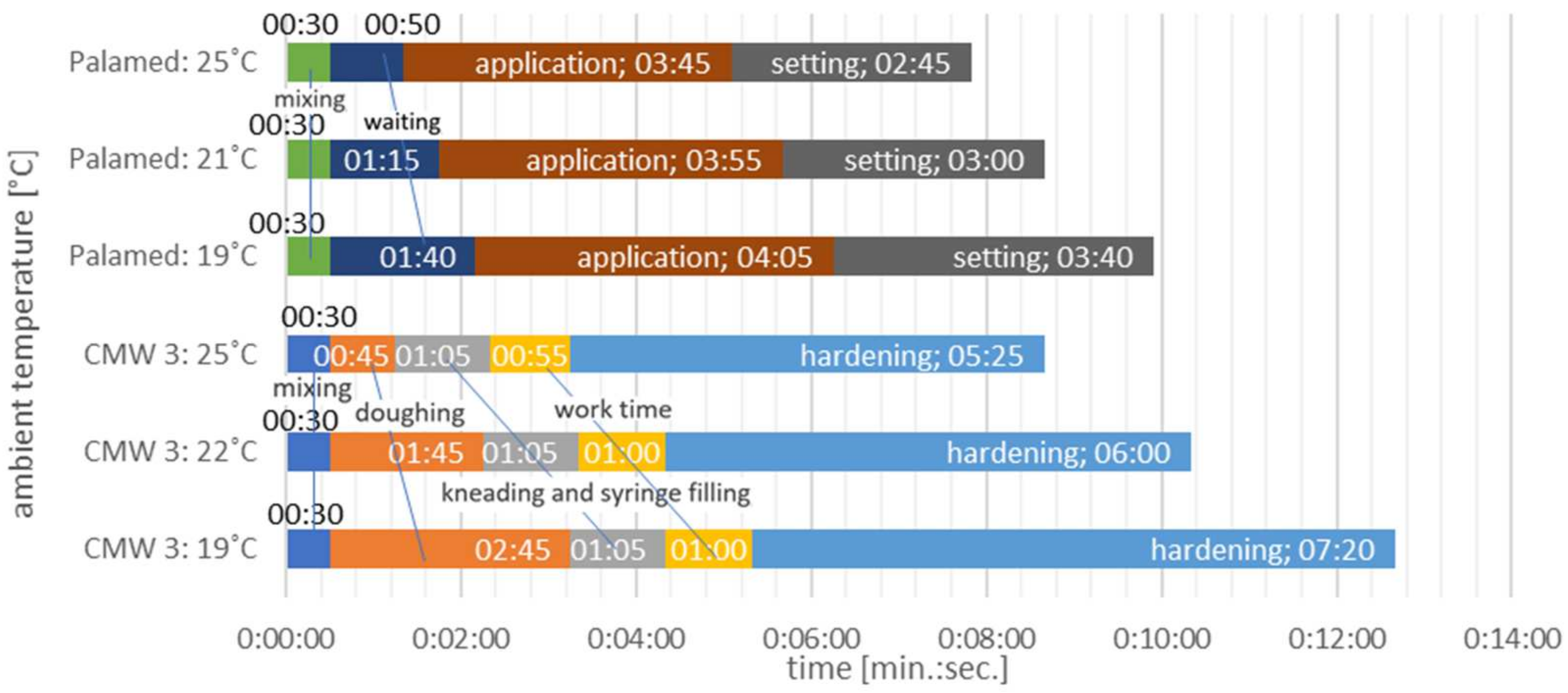




| DePuy CMW3 GENTAMICIN | Heraeus Palamed | |
|---|---|---|
| Powder | ||
| polymethyl methacrylate (PMMA) | ||
| initiator | benzoyl peroxide | |
| radiopaque agents | barium sulphate | zirconium dioxide |
| colorant | E141 (chlorophyllin) | |
| antibiotic | gentamicin sulphate | |
| Liquid | ||
| methyl methacrylate (MMA) | ||
| accelerator | N,N-dimethyl-ptoluidine (DMPT) | |
| stabilizer | hydroquinone | |
| colorant | E141 (chlorophyllin) | |
| Blood | Saline | ||||
|---|---|---|---|---|---|
| Contamination Amount | Mean Compressive Strength (MPa) | SD (MPa) | Mean Compressive Strength (MPa) | SD (MPa) | |
| CMW3 Gentamicin | 0% | 75.47 | 1.26 | 75.47 | 1.26 |
| 1% | 74.46 | 1.28 | 82.98 | 1.76 | |
| 2% | 68.77 | 3.27 | 77.39 | 2.26 | |
| 4% | 70.70 | 5.25 | 66.27 | 2.08 | |
| 6% | 66.14 | 1.05 | 61.73 | 1.74 | |
| 8% | 65.82 | 1.35 | 59.67 | 1.27 | |
| 10% | 56.07 | 3.48 | 58.52 | 2.70 | |
| Palamed | 0% | 63.92 | 4.89 | 63.92 | 4.89 |
| 1% | 69.28 | 1.75 | 70.48 | 2.46 | |
| 2% | 68.40 | 3.06 | 72.91 | 3.81 | |
| 4% | 64.39 | 2.41 | 65.71 | 1.72 | |
| 6% | 63.64 | 1.96 | 55.21 | 2.79 | |
| 8% | 51.11 | 1.58 | 51.65 | 2.76 | |
| 10% | 48.84 | 2.00 | 44.50 | 2.09 | |
| Blood | Saline | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean Compressive Strength (MPa) | 1 | 2 | 3 | 4 | Mean Compressive Strength (MPa) | 1 | 2 | 3 | 4 | 5 | ||||
| CMW3 Gentamicin | 0% | 75.47 | X | CMW3 Gentamicin | 0% | 75.47 | X | |||||||
| 1% | 74.46 | X | 1% | 82.98 | X | |||||||||
| 2% | 68.77 | X | 2% | 77.39 | X | |||||||||
| 4% | 70.70 | X | X | 4% | 66.27 | X | ||||||||
| 6% | 66.14 | X | 6% | 61.73 | X | |||||||||
| 8% | 65.82 | X | 8% | 59.67 | X | |||||||||
| 10% | 56.07 | X | 10% | 58.52 | X | |||||||||
| Palamed | 0% | 63.92 | X | X | Palamed | 0% | 63.92 | X | ||||||
| 1% | 69.28 | X | 1% | 70.48 | X | X | ||||||||
| 2% | 68.40 | X | X | 2% | 72.91 | X | ||||||||
| 4% | 64.39 | X | X | 4% | 65.71 | X | X | |||||||
| 6% | 63.64 | X | 6% | 55.21 | X | |||||||||
| 8% | 51.11 | X | 8% | 51.65 | X | |||||||||
| 10% | 48.84 | X | 10% | 44.85 | X | |||||||||
| Contaminant | Amount of Admixture/ Mean Compressive Strength | |||||||
|---|---|---|---|---|---|---|---|---|
| CMW3 Gentamicin | Saline | 0% | 1% | 2% | 4% | 6% | 8% | 10% |
| 75.474 | 82.979 | 77.392 | 66.272 | 61.731 | 59.673 | 58.523 | ||
| 0% | 0.00 | 0.62 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| 1% | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| 2% | 0.62 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| 4% | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| 6% | 0.00 | 0.00 | 0.00 | 0.00 | 0.54 | 0.09 | ||
| 8% | 0.00 | 0.00 | 0.00 | 0.00 | 0.54 | 0.95 | ||
| 10% | 0.00 | 0.00 | 0.00 | 0.00 | 0.09 | 0.95 | ||
| Blood | 0% | 1% | 2% | 4% | 6% | 8% | 10% | |
| 75.474 | 56.074 | 68.766 | 70.698 | 66.141 | 65.816 | 56.074 | ||
| 0% | 1.00 | 0.01 | 0.08 | 0.00 | 0.00 | 0.00 | ||
| 1% | 1.00 | 0.04 | 0.26 | 0.00 | 0.00 | 0.00 | ||
| 2% | 0.01 | 0.04 | 0.93 | 0.75 | 0.64 | 0.00 | ||
| 4% | 0.08 | 0.26 | 0.93 | 0.10 | 0.07 | 0.00 | ||
| 6% | 0.00 | 0.00 | 0.75 | 0.10 | 1.00 | 0.00 | ||
| 8% | 0.00 | 0.00 | 0.64 | 0.07 | 1.00 | 0.00 | ||
| 10% | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| Palamed | Saline | 0% | 1% | 2% | 4% | 6% | 8% | 10% |
| 63.921 | 70.482 | 72.914 | 65.706 | 55.207 | 51.655 | 44.853 | ||
| 0% | 0.01 | 0.00 | 0.94 | 0.00 | 0.00 | 0.00 | ||
| 1% | 0.01 | 0.78 | 0.09 | 0.00 | 0.00 | 0.00 | ||
| 2% | 0.00 | 0.78 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| 4% | 0.94 | 0.09 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| 6% | 0.00 | 0.00 | 0.00 | 0.00 | 0.37 | 0.00 | ||
| 8% | 0.00 | 0.00 | 0.00 | 0.00 | 0.37 | 0.00 | ||
| 10% | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| Blood | 0% | 1% | 2% | 4% | 6% | 8% | 10% | |
| 63.921 | 69.281 | 68.397 | 64.387 | 63.636 | 51.114 | 48.844 | ||
| 0% | 0.00 | 0.05 | 1.00 | 1.00 | 0.00 | 0.00 | ||
| 1% | 0.00 | 1.00 | 0.01 | 0.01 | 0.00 | 0.00 | ||
| 2% | 0.05 | 1.00 | 0.11 | 0.03 | 0.00 | 0.00 | ||
| 4% | 1.00 | 0.01 | 0.11 | 1.00 | 0.00 | 0.00 | ||
| 6% | 1.00 | 0.01 | 0.03 | 1.00 | 0.00 | 0.00 | ||
| 8% | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.71 | ||
| 10% | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.71 | ||
| Blood | Saline | ||||
|---|---|---|---|---|---|
| Contamination Amount | Mean Compressive Modulus (MPa) | SD (MPa) | Mean Compressive Modulus (MPa) | SD (MPa) | |
| CMW3 Gentamicin | 0% | 985.63 | 65.46 | 985.63 | 65.46 |
| 1% | 938.92 | 44.44 | 2010.83 | 54.60 | |
| 2% | 921.44 | 59.03 | 1788.51 | 78.18 | |
| 4% | 895.77 | 49.50 | 1539.44 | 171.37 | |
| 6% | 848.44 | 49.57 | 1461.00 | 122.83 | |
| 8% | 862.71 | 37.28 | 1320.94 | 106.20 | |
| 10% | 794.87 | 76.02 | 1296.67 | 136.53 | |
| Palamed | 0% | 1179.82 | 237.02 | 1179.82 | 237.02 |
| 1% | 1456.79 | 143.96 | 1381.56 | 210.58 | |
| 2% | 1328.59 | 238.62 | 1410.47 | 242.67 | |
| 4% | 1129.35 | 185.60 | 1255.44 | 153.08 | |
| 6% | 1122.43 | 103.54 | 1017.85 | 160.87 | |
| 8% | 989.53 | 93.83 | 1086.30 | 92.30 | |
| 10% | 1029.29 | 139.58 | 823.40 | 135.13 | |
| Blood | Saline | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean Compressive Modulus (MPa) | 1 | 2 | 3 | Mean Compressive Modulus (MPa) | 1 | 2 | 3 | 4 | 5 | ||||
| CMW3 Gentamicin | 0% | 985.63 | X | CMW3 Gentamicin | 0% | 985.63 | X | ||||||
| 1% | 938.92 | X | X | 1% | 2010.83 | X | |||||||
| 2% | 921.44 | X | X | 2% | 1788.51 | X | |||||||
| 4% | 895.77 | X | X | X | 4% | 1539.44 | X | ||||||
| 6% | 848.44 | X | X | 6% | 1461.00 | X | X | ||||||
| 8% | 862.71 | X | X | 8% | 1320.94 | X | |||||||
| 10% | 794.87 | X | 10% | 1296.67 | X | ||||||||
| Palamed | 0% | 1179.82 | X | X | Palamed | 0% | 1179.82 | X | X | X | |||
| 1% | 1456.79 | X | 1% | 1381.56 | X | X | |||||||
| 2% | 1328.59 | X | X | 2% | 1410.47 | X | |||||||
| 4% | 1129.35 | X | X | 4% | 1255.45 | X | X | X | |||||
| 6% | 1122.43 | X | X | 6% | 1017.85 | X | X | ||||||
| 8% | 989.53 | X | 8% | 1086.30 | X | X | X | ||||||
| 10% | 1029.29 | X | 10% | 823.40 | X | ||||||||
| Contaminant | Amount of Admixture/ Mean Compressive Strength | |||||||
|---|---|---|---|---|---|---|---|---|
| CMW3 Gentamicin | Saline | 0% | 1% | 2% | 4% | 6% | 8% | 10% |
| 985.63 | 2010.8 | 1788.5 | 1539.4 | 1461.0 | 1320.9 | 1296.7 | ||
| 0% | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| 1% | 0.00 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | ||
| 2% | 0.00 | 0.02 | 0.01 | 0.00 | 0.00 | 0.00 | ||
| 4% | 0.00 | 0.00 | 0.01 | 0.88 | 0.03 | 0.01 | ||
| 6% | 0.00 | 0.00 | 0.00 | 0.88 | 0.33 | 0.17 | ||
| 8% | 0.00 | 0.00 | 0.00 | 0.03 | 0.33 | 1.00 | ||
| 10% | 0.00 | 0.00 | 0.00 | 0.01 | 0.17 | 1.00 | ||
| Blood | 0% | 1% | 2% | 4% | 6% | 8% | 10% | |
| 985.63 | 938.92 | 921.44 | 895.77 | 848.44 | 862.71 | 794.87 | ||
| 0% | 0.76 | 0.53 | 0.10 | 0.00 | 0.01 | 0.00 | ||
| 1% | 0.76 | 1.00 | 0.82 | 0.10 | 0.23 | 0.00 | ||
| 2% | 0.53 | 1.00 | 0.99 | 0.38 | 0.63 | 0.01 | ||
| 4% | 0.10 | 0.82 | 0.99 | 0.75 | 0.94 | 0.09 | ||
| 6% | 0.00 | 0.10 | 0.38 | 0.75 | 1.00 | 0.72 | ||
| 8% | 0.01 | 0.23 | 0.63 | 0.94 | 1.00 | 0.46 | ||
| 10% | 0.00 | 0.00 | 0.01 | 0.09 | 0.72 | 0.46 | ||
| Palamed | Saline | 0% | 1% | 2% | 4% | 6% | 8% | 10% |
| 1179.8 | 1381.6 | 1410.5 | 1255.4 | 1017.8 | 1086.3 | 823.40 | ||
| 0% | 0.41 | 0.26 | 0.99 | 0.66 | 0.96 | 0.03 | ||
| 1% | 0.41 | 1.00 | 0.86 | 0.01 | 0.07 | 0.00 | ||
| 2% | 0.26 | 1.00 | 0.71 | 0.01 | 0.03 | 0.00 | ||
| 4% | 0.99 | 0.86 | 0.71 | 0.23 | 0.62 | 0.00 | ||
| 6% | 0.66 | 0.01 | 0.01 | 0.23 | 0.99 | 0.55 | ||
| 8% | 0.96 | 0.07 | 0.03 | 0.62 | 0.99 | 0.20 | ||
| 10% | 0.03 | 0.00 | 0.00 | 0.00 | 0.55 | 0.20 | ||
| Blood | 0% | 1% | 2% | 4% | 6% | 8% | 10% | |
| 1179.8 | 1456.8 | 1328.6 | 1129.4 | 1122.4 | 989.53 | 1029.3 | ||
| 0% | 0.04 | 0.68 | 1.00 | 1.00 | 0.39 | 0.66 | ||
| 1% | 0.04 | 0.80 | 0.00 | 0.01 | 0.00 | 0.00 | ||
| 2% | 0.68 | 0.80 | 0.34 | 0.30 | 0.01 | 0.03 | ||
| 4% | 1.00 | 0.00 | 0.34 | 1.00 | 0.73 | 0.93 | ||
| 6% | 1.00 | 0.01 | 0.30 | 1.00 | 0.78 | 0.95 | ||
| 8% | 0.39 | 0.00 | 0.01 | 0.73 | 0.78 | 1.00 | ||
| 10% | 0.66 | 0.00 | 0.03 | 0.93 | 0.95 | 1.00 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karpiński, R.; Szabelski, J.; Krakowski, P.; Jojczuk, M.; Jonak, J.; Nogalski, A. Evaluation of the Effect of Selected Physiological Fluid Contaminants on the Mechanical Properties of Selected Medium-Viscosity PMMA Bone Cements. Materials 2022, 15, 2197. https://doi.org/10.3390/ma15062197
Karpiński R, Szabelski J, Krakowski P, Jojczuk M, Jonak J, Nogalski A. Evaluation of the Effect of Selected Physiological Fluid Contaminants on the Mechanical Properties of Selected Medium-Viscosity PMMA Bone Cements. Materials. 2022; 15(6):2197. https://doi.org/10.3390/ma15062197
Chicago/Turabian StyleKarpiński, Robert, Jakub Szabelski, Przemysław Krakowski, Mariusz Jojczuk, Józef Jonak, and Adam Nogalski. 2022. "Evaluation of the Effect of Selected Physiological Fluid Contaminants on the Mechanical Properties of Selected Medium-Viscosity PMMA Bone Cements" Materials 15, no. 6: 2197. https://doi.org/10.3390/ma15062197
APA StyleKarpiński, R., Szabelski, J., Krakowski, P., Jojczuk, M., Jonak, J., & Nogalski, A. (2022). Evaluation of the Effect of Selected Physiological Fluid Contaminants on the Mechanical Properties of Selected Medium-Viscosity PMMA Bone Cements. Materials, 15(6), 2197. https://doi.org/10.3390/ma15062197







