pH and Redox Dual-Responsive Mesoporous Silica Nanoparticle as Nanovehicle for Improving Fungicidal Efficiency
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of BMMs
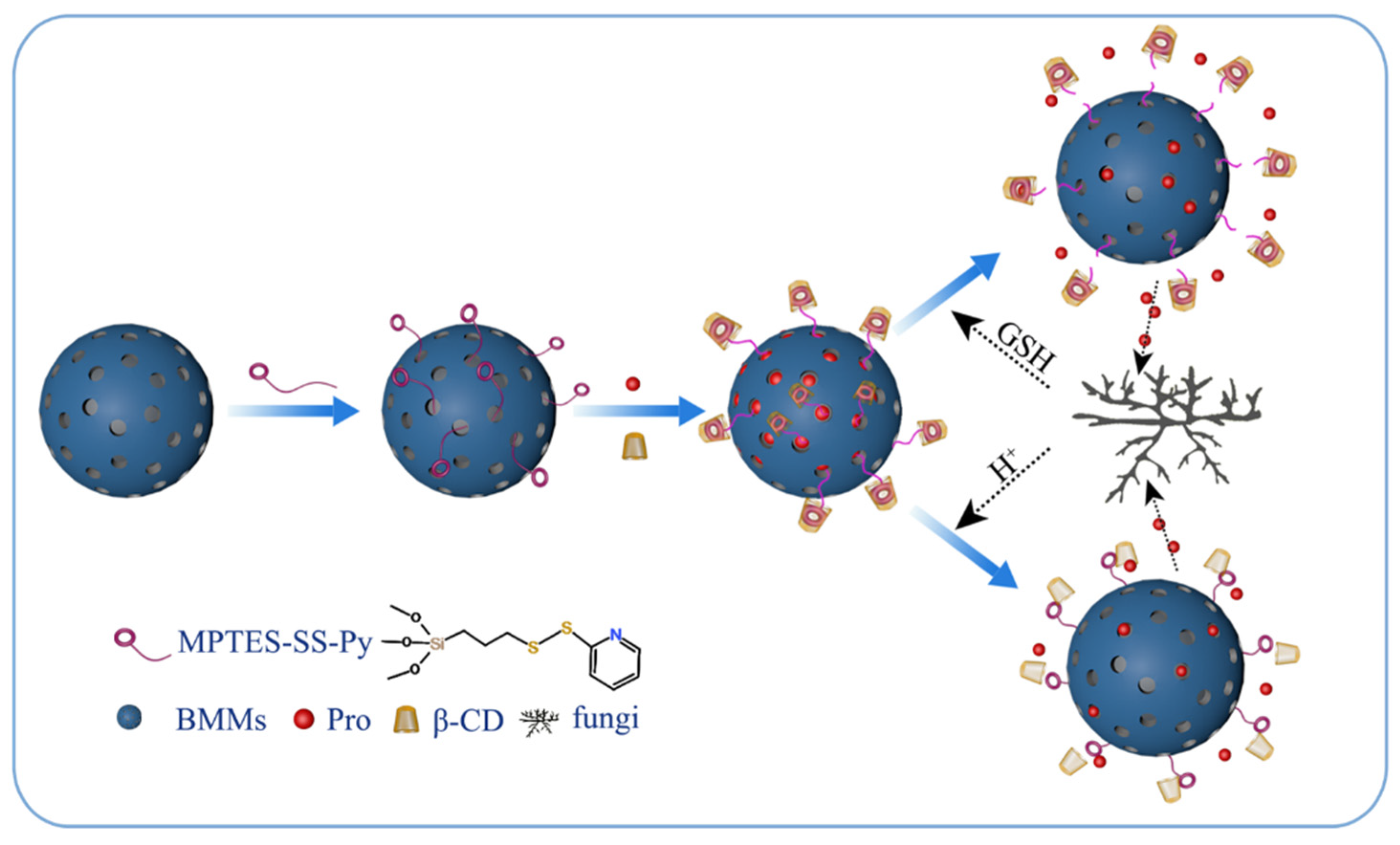
2.3. Preparation of BMMs-SS-Py NPs
2.4. Preparation of Pro@BMMs-SS-Py NPs
2.5. Preparation of Pro@BMMs-SS-Py/β-CD NPs
2.6. Characterization of Pro@BMMs-SS-Py/β-CD NPs
2.7. Pro Measurement
2.8. pH/Redox Dual-Responsive Release
2.9. Stability Study
2.10. Foliage Adhesion Test
2.11. Bioefficacy Test
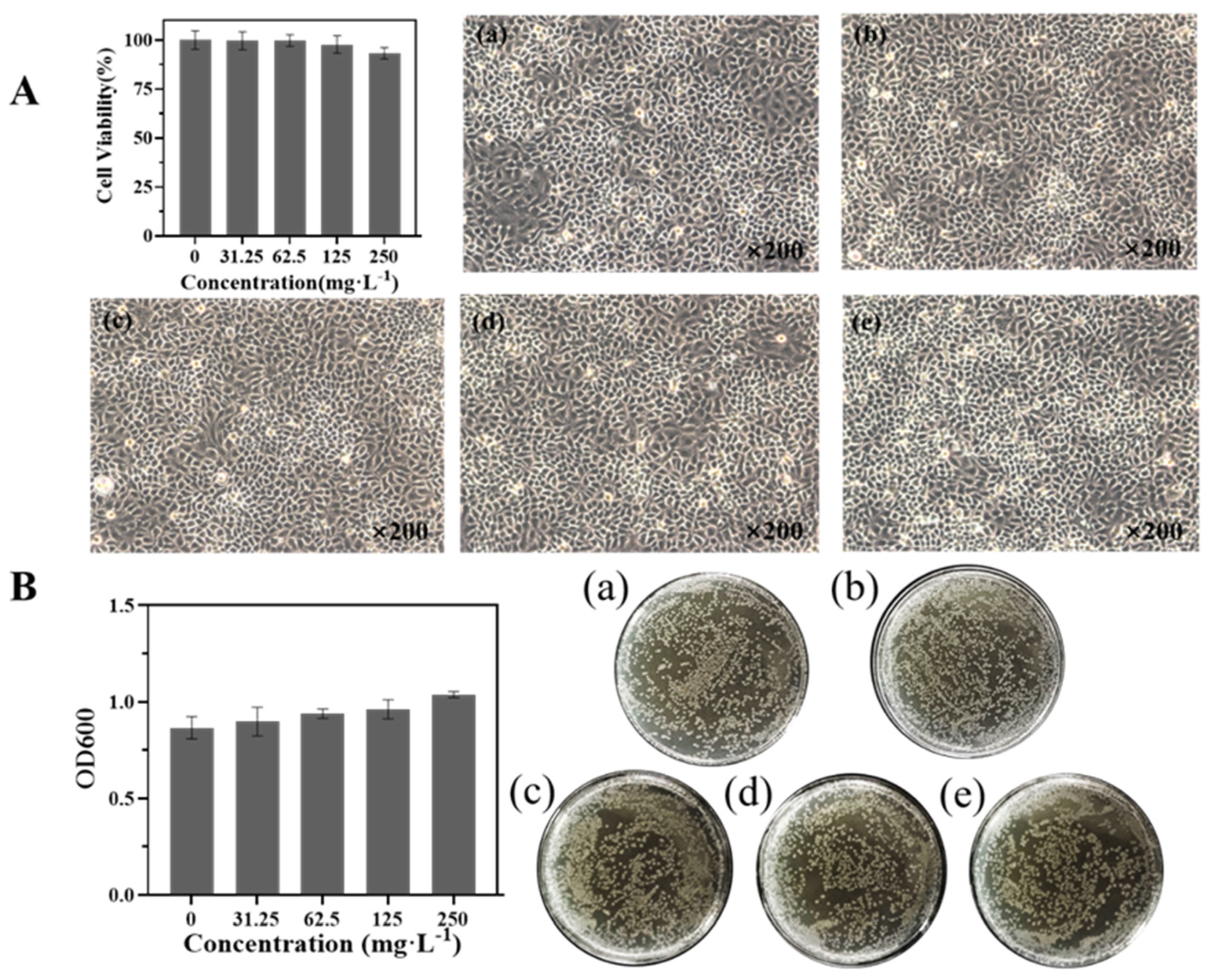
2.12. Biosafety Evaluation
2.13. Data Analysis
3. Results and Discussion
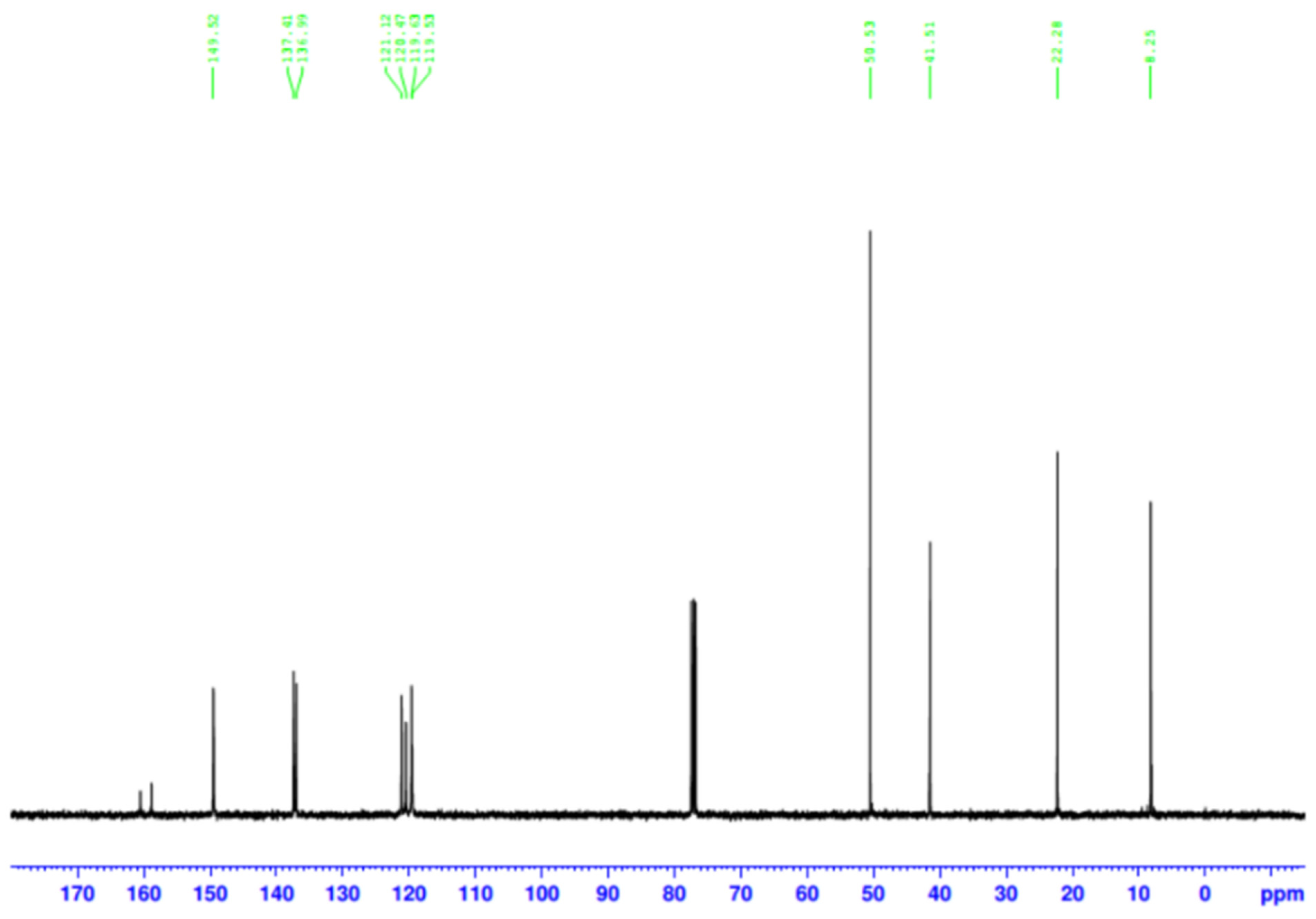
3.1. Characterization and Interaction Analysis
3.2. Foliage Adhesion
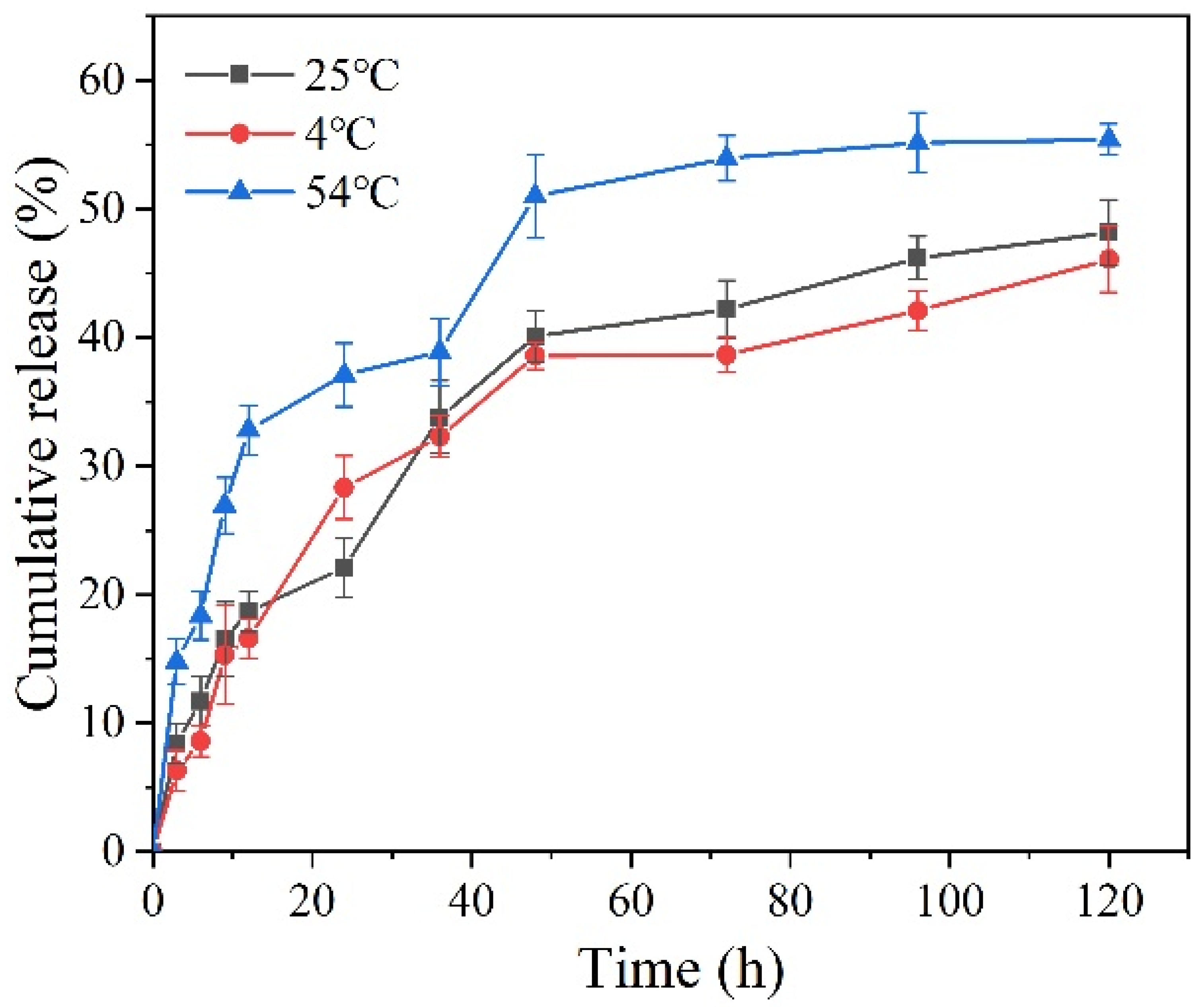
3.3. Stability Study
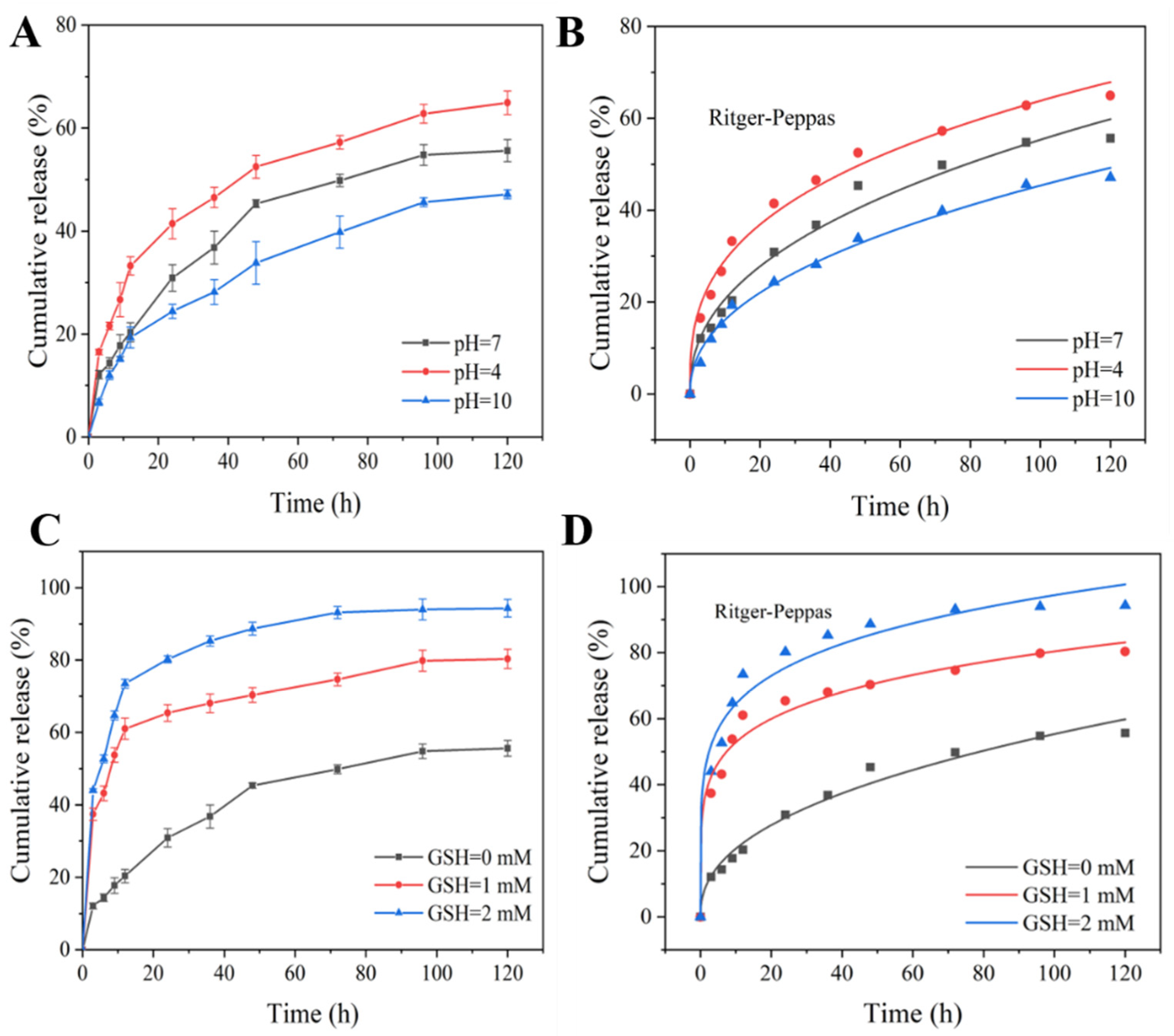
3.4. pH/Redox Dual-Responsive Release Behavior
3.5. Release-Kinetics Analysis
3.6. Bioactivity Test
3.7. Biosafety Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
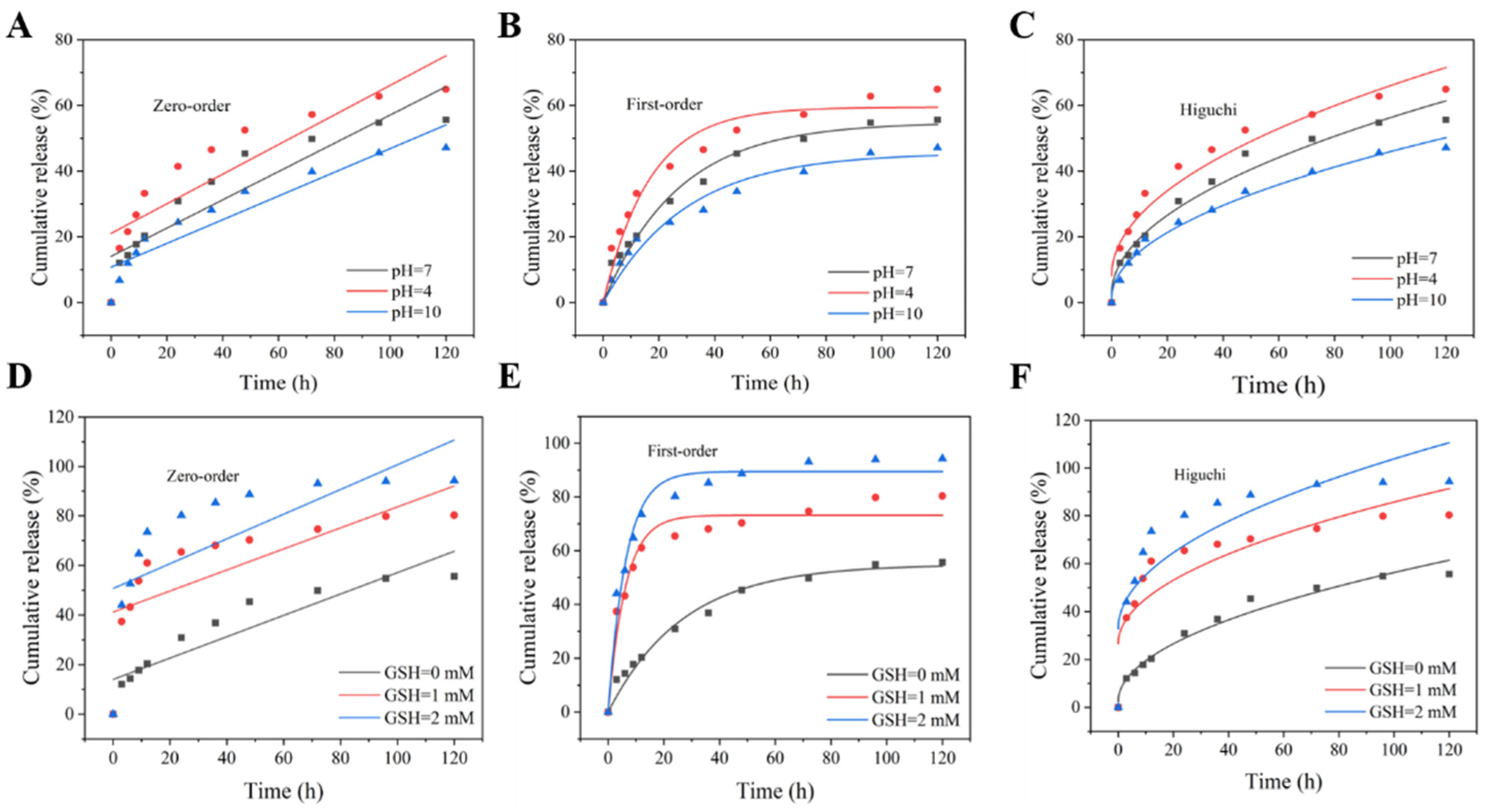
| pH | Fitting Methods | Kinetic Equation | Determiation Coefficient, R2 |
|---|---|---|---|
| 4 | Zero-order fitting | 0.7863 | |
| First-order fitting | 0.9585 | ||
| Higuchi fitting | 0.9537 | ||
| Ritger–Peppas fitting | 0.9891 | ||
| 7 | Zero-order fitting | 0.8501 | |
| First-order fitting | 0.9787 | ||
| Higuchi fitting | 0.9766 | ||
| Ritger–Peppas fitting | 0.9836 | ||
| 10 | Zero-order fitting | 0.8805 | |
| First-order fitting | 0.9670 | ||
| Higuchi fitting | 0.9888 | ||
| Ritger–Peppas fitting | 0.9924 |
| GSH | Fitting Methods | Kinetic Equation | Determination Coefficient, R2 |
|---|---|---|---|
| 0 | Zero-order fitting | 0.8501 | |
| First-order fitting | 0.9787 | ||
| Higuchi fitting | 0.9766 | ||
| Ritger–Peppas fitting | 0.9836 | ||
| 1 | Zero-order fitting | 0.5394 | |
| First-order fitting | 0.9520 | ||
| Higuchi fitting | 0.7704 | ||
| Ritger-Peppas fitting | 0.9792 | ||
| 2 | Zero-order fitting | 0.7098 | |
| First-order fitting | 0.9698 | ||
| Higuchi fitting | 0.7481 | ||
| Ritger–Peppas fitting | 0.9699 |
References
- Huang, B.; Chen, F.; Shen, Y.; Qian, K.; Wang, Y.; Sun, C.; Zhao, X.; Cui, B.; Gao, F.; Zeng, Z.; et al. Advances in Targeted Pesticides with Environmentally Responsive Controlled Release by Nanotechnology. Nanomaterials 2018, 8, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.; Dhiman, N.; Kar, A.K.; Singh, D.; Purohit, M.P.; Ghosh, D.; Patnaik, S. Advances in controlled release pesticide formulations: Prospects to safer integrated pest management and sustainable agriculture. J. Hazard. Mater. 2020, 385, 121525. [Google Scholar] [CrossRef] [PubMed]
- Kookana, R.S.; Boxall, A.B.; Reeves, P.T.; Ashauer, R.; Beulke, S.; Chaudhry, Q.; Cornelis, G.; Fernandes, T.F.; Gan, J.; Kah, M.; et al. Nanopesticides: Guiding principles for regulatory evaluation of environmental risks. J. Agric. Food Chem. 2014, 62, 4227–4240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Nehra, M.; Dilbaghi, N.; Marrazza, G.; Hassan, A.A.; Kim, K.H. Nano-based smart pesticide formulations: Emerging opportunities for agriculture. J. Control Release 2019, 294, 131–153. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Chen, W.; Shen, Y.; Chen, Q.; Yang, J.; Zhang, M.; Yang, W.; Yuan, S. Fabrication of abamectin-loaded mesoporous silica nanoparticles by emulsion-solvent evaporation to improve photolysis stability and extend insecticidal activity. Nanotechnology 2020, 31, 345705. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.P.; Zhang, B.H.; Wang, J. Multiple Roles of Mesoporous Silica in Safe Pesticide Application by Nanotechnology: A Review. J. Agric. Food Chem. 2021, 69, 6735–6754. [Google Scholar] [CrossRef] [PubMed]
- Worrall, E.; Hamid, A.; Mody, K.; Mitter, N.; Pappu, H. Nanotechnology for Plant Disease Management. Agronomy 2018, 8, 285. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Zhang, Y.; He, S.; Xiao, Y.; Qin, X.; Zhang, Y.; Li, D.; Ma, H.; You, H.; Li, J. Fabrication of a hollow mesoporous silica hybrid to improve the targeting of a pesticide. Chem. Eng. J. 2019, 364, 361–369. [Google Scholar] [CrossRef]
- Li, W.; Wang, Q.; Zhang, F.; Shang, H.; Bai, S.; Sun, J. pH-sensitive thiamethoxam nanoparticles based on bimodal mesoporous silica for improving insecticidal efficiency. R. Soc. Open Sci. 2021, 8, 201967. [Google Scholar] [CrossRef]
- Ma, X.; Xiang, S.; Xie, H.; He, L.; Sun, X.; Zhang, Y.; Huang, J. Fabrication of pH-Sensitive Tetramycin Releasing Gel and Its Antibacterial Bioactivity against Ralstonia solanacearum. Molecules 2019, 24, 3606. [Google Scholar] [CrossRef] [Green Version]
- Chi, Y.; Zhang, G.; Xiang, Y.; Cai, D.; Wu, Z. Fabrication of a Temperature-Controlled-Release Herbicide Using a Nanocomposite. ACS Sustain. Chem. Eng. 2017, 5, 4969–4975. [Google Scholar] [CrossRef]
- Gao, Y.; Xiao, Y.; Mao, K.; Qin, X.; Zhang, Y.; Li, D.; Zhang, Y.; Li, J.; Wan, H.; He, S. Thermoresponsive polymer-encapsulated hollow mesoporous silica nanoparticles and their application in insecticide delivery. Chem. Eng. J. 2020, 383, 123169. [Google Scholar] [CrossRef]
- Xiao, D.; Liang, W.; Xie, Z.; Cheng, J.; Du, Y.; Zhao, J. A temperature-responsive release cellulose-based microcapsule loaded with chlorpyrifos for sustainable pest control. J. Hazard. Mater. 2021, 403, 123654. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ren, S.; Chen, C.; Wang, D.; Liu, B.; Cai, D.; Wu, Z. Near infrared light-driven release of pesticide with magnetic collectability using gel-based nanocomposite. Chem. Eng. J. 2021, 411, 127881. [Google Scholar] [CrossRef]
- Abdelrahman, T.M.; Qin, X.; Li, D.; Senosy, I.A.; Mmby, M.; Wan, H.; Li, J.; He, S. Pectinase-responsive carriers based on mesoporous silica nanoparticles for improving the translocation and fungicidal activity of prochloraz in rice plants. Chem. Eng. J. 2021, 404, 126440. [Google Scholar] [CrossRef]
- Kaziem, A.E.; Gao, Y.; Zhang, Y.; Qin, X.; Xiao, Y.; Zhang, Y.; You, H.; Li, J.; He, S. alpha-Amylase triggered carriers based on cyclodextrin anchored hollow mesoporous silica for enhancing insecticidal activity of avermectin against Plutella xylostella. J. Hazard. Mater. 2018, 359, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Fan, C.; Dong, H.; Zhang, W.; Tang, G.; Yang, J.; Jiang, N.; Cao, Y. Preparation of MSNs-Chitosan@Prochloraz Nanoparticles for Reducing Toxicity and Improving Release Properties of Prochloraz. ACS Sustain. Chem. Eng. 2018, 6, 10211–10220. [Google Scholar] [CrossRef]
- Fan, C.; Guo, M.; Liang, Y.; Dong, H.; Ding, G.; Zhang, W.; Tang, G.; Yang, J.; Kong, D.; Cao, Y. Pectin-conjugated silica microcapsules as dual-responsive carriers for increasing the stability and antimicrobial efficacy of kasugamycin. Carbohydr. Polym. 2017, 172, 322–331. [Google Scholar] [CrossRef]
- Gao, Y.; Liang, Y.; Dong, H.; Niu, J.; Tang, J.; Yang, J.; Tang, G.; Zhou, Z.; Tang, R.; Shi, X.; et al. A Bioresponsive System Based on Mesoporous Organosilica Nanoparticles for Smart Delivery of Fungicide in Response to Pathogen Presence. ACS Sustain. Chem. Eng. 2020, 8, 5716–5723. [Google Scholar] [CrossRef]
- Liang, Y.; Gao, Y.; Wang, W.; Dong, H.; Tang, R.; Yang, J.; Niu, J.; Zhou, Z.; Jiang, N.; Cao, Y. Fabrication of smart stimuli-responsive mesoporous organosilica nano-vehicles for targeted pesticide delivery. J. Hazard. Mater. 2020, 389, 122075. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, G.; Dai, Z.; Xiang, Y.; Liu, B.; Bian, P.; Zheng, K.; Wu, Z.; Cai, D. Fabrication of light-responsively controlled-release herbicide using a nanocomposite. Chem. Eng. J. 2018, 349, 101–110. [Google Scholar] [CrossRef]
- Ye, Z.; Guo, J.; Wu, D.; Tan, M.; Xiong, X.; Yin, Y.; He, G. Photo-responsive shell cross-linked micelles based on carboxymethyl chitosan and their application in controlled release of pesticide. Carbohydr. Polym. 2015, 132, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Bai, B.; Zhao, H.; Xu, X.; Hu, N.; Wang, H. Stimuli-responsive Ca-alginate-based photothermal system with enhanced foliar adhesion for controlled pesticide release. Colloids Surf. B Biointerfaces 2021, 207, 112004. [Google Scholar] [CrossRef] [PubMed]
- Sato, I.; Shimatani, K.; Fujita, K.; Abe, T.; Shimizu, M.; Fujii, T.; Hoshino, T.; Takaya, N. Glutathione Reductase/Glutathione Is Responsible for Cytotoxic Elemental Sulfur Tolerance via Polysulfide Shuttle in Fungi. J. Biol. Chem. 2011, 286, 20283–20291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, I.; Shimizu, M.; Hoshino, T.; Takaya, N. The Glutathione System of Aspergillus nidulans Involves a Fungus-specific Glutathione S-Transferase. J. Biol. Chem. 2009, 284, 8042–8053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carelli, S.; Ceriotti, A.; Cabibbo, A.; Fassina, G.; Ruvo, M.; Sitia, R. Cysteine and glutathione secretion in response to protein disulfide bond formation in the ER. Science 1997, 277, 1681–1684. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Xie, Z.; Liu, M.; Zhu, H.; Sun, H. Redox-sensitive mesoporous silica nanoparticles functionalized with PEG through a disulfide bond linker for potential anticancer drug delivery. RSC Adv. 2015, 5, 59576–59582. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, T.; Cheng, C.; Wang, Q.; Lin, S.; Liu, C.; Han, X. Research Progress on Synthesis and Application of Cyclodextrin Polymers. Molecules 2021, 26, 1090. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-L.; Xu, S.-H.; Zhou, H.; Wang, X.; Dong, B.; Gao, H.; Tang, J.; Yang, Y.-W. pH and Glutathione Dual-Responsive Dynamic Cross-Linked Supramolecular Network on Mesoporous Silica Nanoparticles for Controlled Anticancer Drug Release. ACS Appl. Mater. Interfaces 2015, 7, 28656–28664. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-S.; Chen, Z.; Chen, T.-Q.; Fu, C.-Y. Hollow Mesoporous Silica Nanoparticles Decorated with Cyclodextrin for Inhibiting the Corrosion of Mg Alloys. ACS Appl. Nano Mater. 2020, 3, 4542–4552. [Google Scholar] [CrossRef]
- Liang, W.; Xie, Z.; Cheng, J.; Xiao, D.; Xiong, Q.; Wang, Q.; Zhao, J.; Gui, W. A Light-Triggered pH-Responsive Metal-Organic Framework for Smart Delivery of Fungicide to Control Sclerotinia Diseases of Oilseed Rape. ACS Nano 2021, 15, 6987–6997. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Sun, J.; Li, Y.; Zhang, L. Bimodal mesoporous silicas functionalized with different level and species of the amino groups for adsorption and controlled release of aspirin. J. Nanosci. Nanotechnol. 2011, 11, 6690–6697. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Wang, Q.; Sun, J.; Panezai, H.; Bai, S.; Wu, X. P(NIPAM-co-AA)@BMMs with mesoporous silica core and controlled copolymer shell and its fractal characteristics for dual pH- and temperature-responsive performance of ibuprofen release. Int. J. Polym. Mater. Polym. Biomater. 2017, 67, 131–142. [Google Scholar] [CrossRef]
- Pan, H.; Li, W.; Wu, L.; Huang, W.; Zhang, F. β-Cyclodextrin-Modified Mesoporous Silica Nanoparticles with Photo-Responsive Gatekeepers for Controlled Release of Hexaconazole. Coatings 2021, 11, 1489. [Google Scholar] [CrossRef]
- Kai, M.; Effmert, U.; Berg, G.; Piechulla, B. Volatiles of bacterial antagonists inhibit mycelial growth of the plant pathogen Rhizoctonia solani. Arch. Microbiol. 2007, 187, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Bist, V.; Srivastava, S.; Singh, P.C.; Trivedi, P.K.; Asif, M.H.; Chauhan, P.S.; Nautiyal, C.S. Unraveling Aspects of Bacillus amyloliquefaciens Mediated Enhanced Production of Rice under Biotic Stress of Rhizoctonia solani. Front. Plant Sci. 2016, 7, 587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbas, A.; Khan, S.U.; Khan, W.U.; Saleh, T.A.; Khan, M.H.U.; Ullah, S.; Ali, A.; Ikram, M. Antagonist effects of strains of Bacillus spp. against Rhizoctonia solani for their protection against several plant diseases: Alternatives to chemical pesticides. C. R. Biol. 2019, 342, 124–135. [Google Scholar] [CrossRef]
- Baumann, L.; Knoerr, S.; Keiter, S.; Nagel, T.; Segner, H.; Braunbeck, T. Prochloraz causes irreversible masculinization of zebrafish (Danio rerio). Environ. Sci. Pollut. Res. 2015, 22, 16417–16422. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Cao, L.; Ma, D.; Zhou, Z.; Huang, Q.; Pan, C. Translocation, distribution and degradation of prochloraz-loaded mesoporous silica nanoparticles in cucumber plants. Nanoscale 2018, 10, 1798–1806. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, C.; Liu, Y.; Cao, L.; Wu, Y.; Huang, Q. Size-Dependent Effect of Prochloraz-Loaded mPEG-PLGA Micro- and Nanoparticles. J. Nanosci. Nanotechnol. 2016, 16, 6231–6237. [Google Scholar] [CrossRef]
- Lin, G.; Zhou, H.; Lian, J.; Chen, H.; Xu, H.; Zhou, X. Preparation of pH-responsive avermectin/feather keratin-hyaluronic acid with anti-UV and sustained-release properties. Colloids Surf. B Biointerfaces 2019, 175, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Song, J.; Dong, H.; Huo, Z.; Gao, Y.; Zhou, Z.; Tian, Y.; Li, Y.; Cao, Y. Fabrication of pH-responsive nanoparticles for high efficiency pyraclostrobin delivery and reducing environmental impact. Sci. Total Environ. 2021, 787, 147422. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Huang, W.; Wu, L.; Hong, Q.; Hu, Z.; Wang, M.; Zhang, F. A pH Dual-Responsive Multifunctional Nanoparticle Based on Mesoporous Silica with Metal-Polymethacrylic Acid Gatekeeper for Improving Plant Protection and Nutrition. Nanomaterials 2022, 12, 687. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; He, S.; Liu, Y.; Geng, Q.; Ding, G.; Guo, M.; Deng, Y.; Zhu, J.; Li, J.; Cao, Y. Preparation and characterization of novel functionalized prochloraz microcapsules using silica-alginate-elements as controlled release carrier materials. ACS Appl. Mater. Interfaces 2014, 6, 11783–11790. [Google Scholar] [CrossRef] [PubMed]

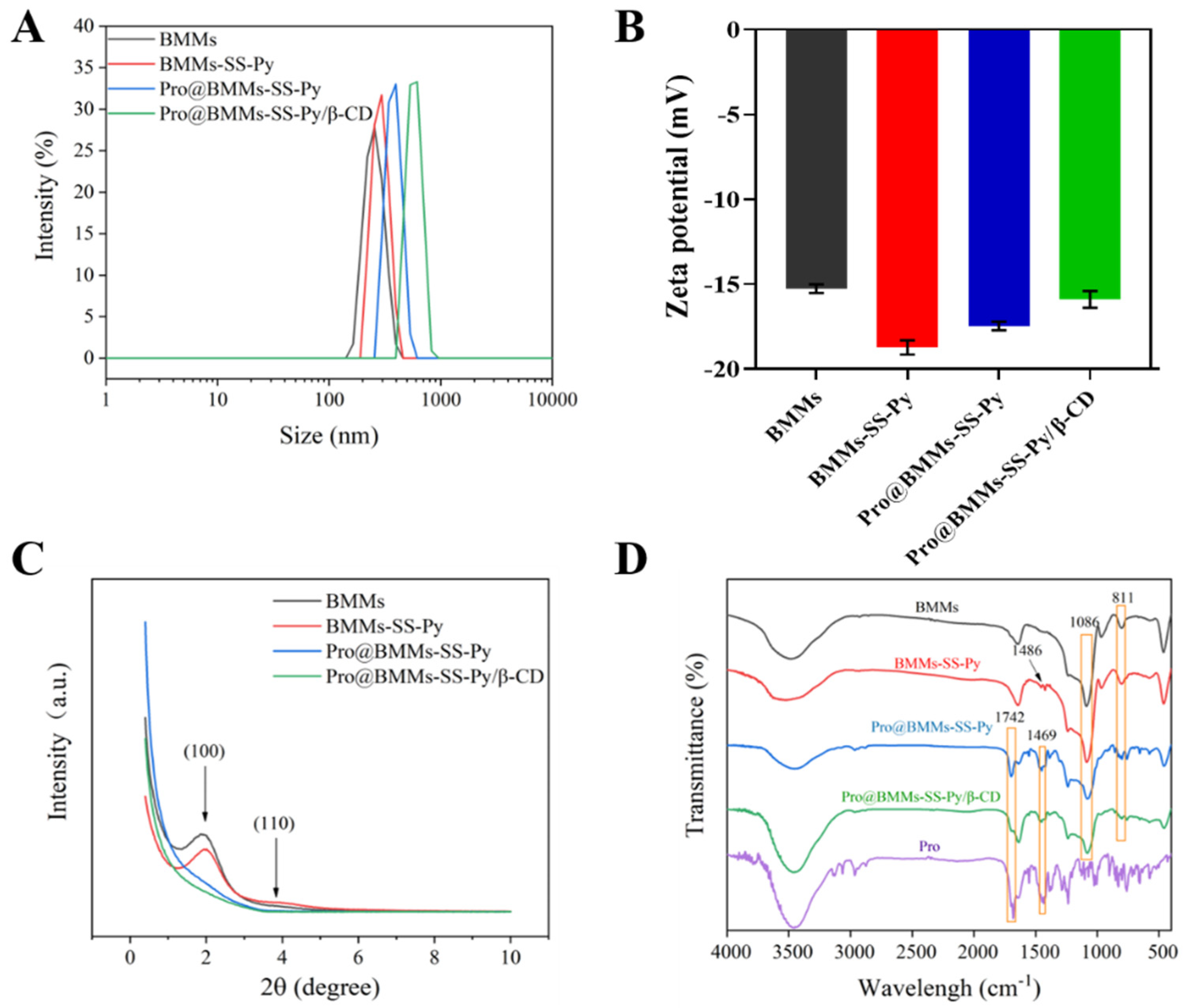
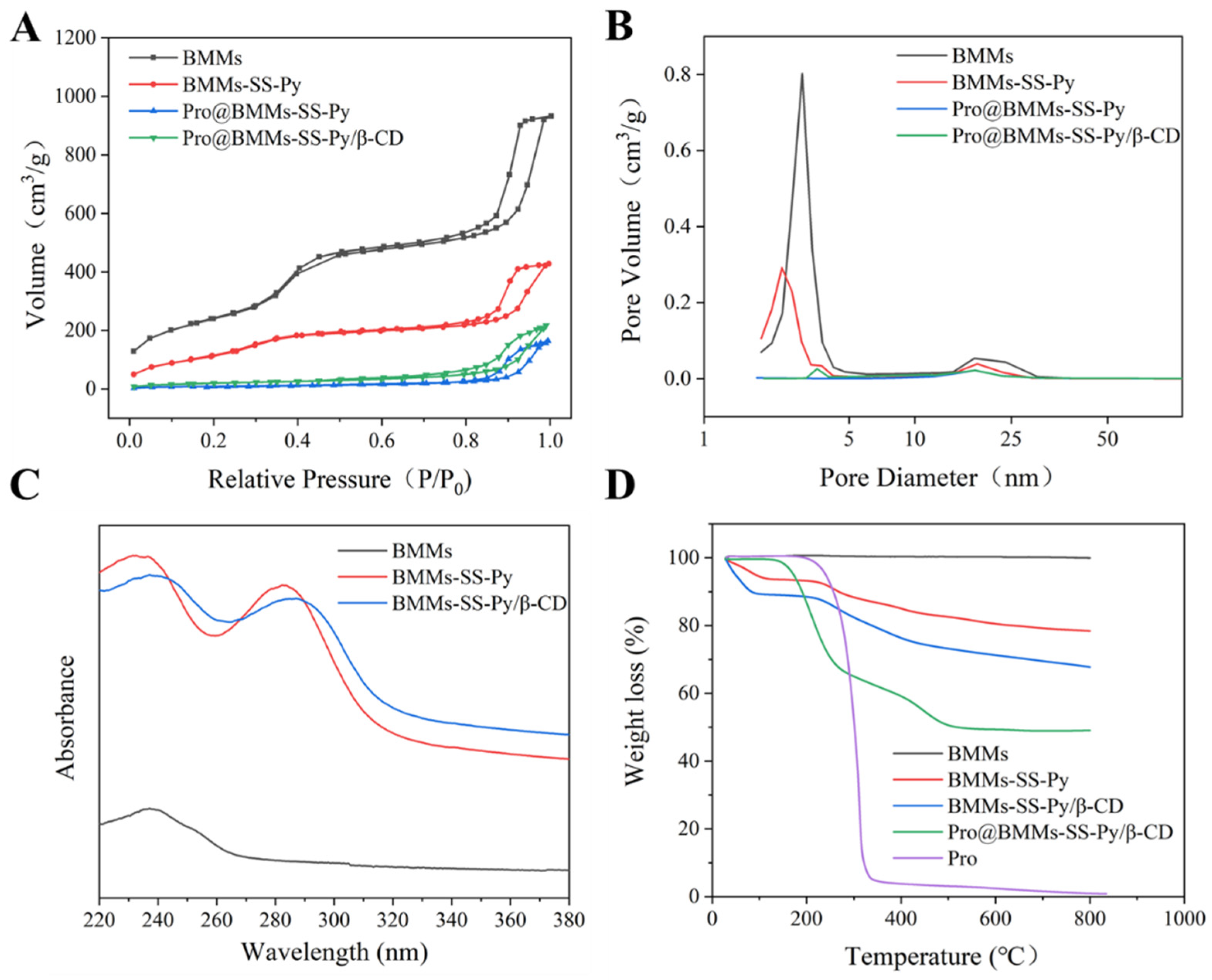
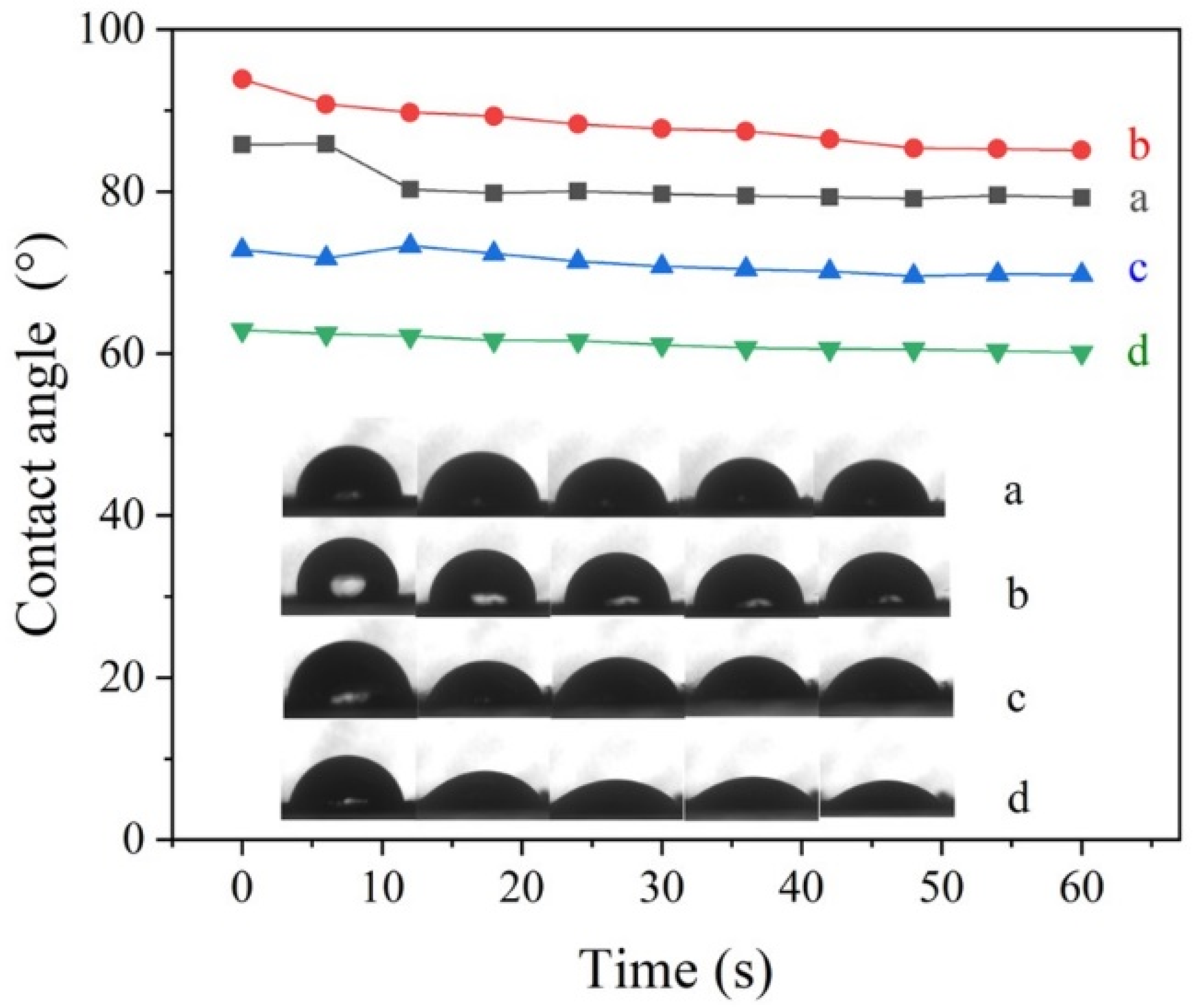


| Sample | SBET (m2/g) | Vt (cm3/g) | Pore Size (nm) | Size (nm) | PDI | Zeta (mV) |
|---|---|---|---|---|---|---|
| BMMs | 1126.57 | 1.48 | 5.26 | 295.3 ± 3.9 | 0.52 ± 0.02 | −15.3 ± 0.3 |
| BMMs-SS-Py | 546.93 | 0.69 | 5.07 | 360.6 ± 2.8 | 0.66 ± 0.04 | −18.7 ± 0.4 |
| Pro@BMMs-SS-Py | 56.15 | 0.25 | — | 466.6 ± 3.5 | 0.61 ± 0.03 | −17.5 ± 0.3 |
| Pro@BMMs-SS-Py/β-CD | 98.08 | 0.34 | — | 546.4 ± 3.0 | 0.32 ± 0.02 | −15.9 ± 0.5 |
| Nanocarrier | Stimuli | Release Time (h) | Stability (Pro Loss) | Adhesion | EC50 (mg·L−1) | Reference |
|---|---|---|---|---|---|---|
| Zif-8 | Light and pH | 36 | 60.2 ± 4.6% after 24 h | Pro retention 175.6 ± 1.6 μg·cm2 (before leaf) 155.6 ± 11.7 μg·cm2 (after leaf) | 0.122 ± 0.02 (S. sclerotiorum) | [31] |
| MSN | — | 96 | — | — | 0.3058 (Botrytis cinerea) | [39] |
| MON-CaC | pH and reduction | 120 | Less than 15% after 24 h | — | 0.142 (S. sclerotiorum) | [19] |
| BMMs-PMAA/Fe3+ | pH | 144 | 54.2% after 7 d, good thermal stability | — | 0.184 ± 0.013 (Rhizoctonia solani) | [43] |
| MSNs-chitosan | Esterase and pH | 720 | About 6.3% after 72 h | 12.468 (11.274–13.900) at 96 h (zebrafish) | [17] | |
| Silica-Alginate | — | 1440 | Good stability under different pH, temperature and light | — | — | [44] |
| mPEG-PLGA | — | 384 | — | — | Best germicidal efficacy (Fusarium graminearum) | [40] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Pan, H.; Huang, W.; Hu, Z.; Wang, M.; Zhang, F. pH and Redox Dual-Responsive Mesoporous Silica Nanoparticle as Nanovehicle for Improving Fungicidal Efficiency. Materials 2022, 15, 2207. https://doi.org/10.3390/ma15062207
Wu L, Pan H, Huang W, Hu Z, Wang M, Zhang F. pH and Redox Dual-Responsive Mesoporous Silica Nanoparticle as Nanovehicle for Improving Fungicidal Efficiency. Materials. 2022; 15(6):2207. https://doi.org/10.3390/ma15062207
Chicago/Turabian StyleWu, Litao, Hua Pan, Weilan Huang, Zhongxuan Hu, Meijing Wang, and Fang Zhang. 2022. "pH and Redox Dual-Responsive Mesoporous Silica Nanoparticle as Nanovehicle for Improving Fungicidal Efficiency" Materials 15, no. 6: 2207. https://doi.org/10.3390/ma15062207






