Effects of Inorganic Metabolites of Sulphate-Reducing Bacteria on the Corrosion of AZ31B and AZ63B Magnesium Alloy in 3.5 wt.% NaCl Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Materials
2.2. Experimental Medium
2.3. Electrochemical Corrosion Analyses
2.4. Weight-Loss Testing
2.5. Surface Characterisation
3. Results and Discussion
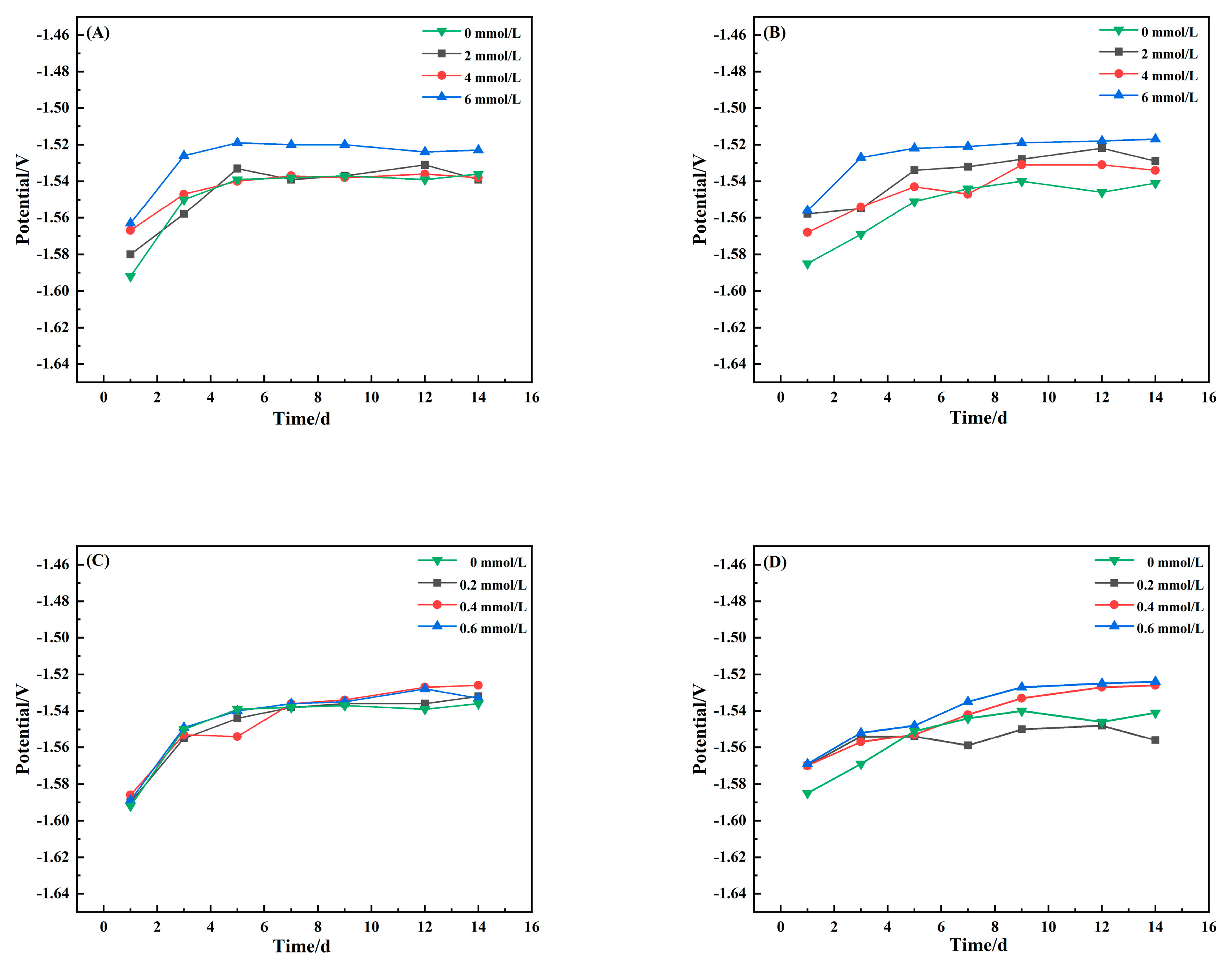
3.1. Open-Circuit Potential
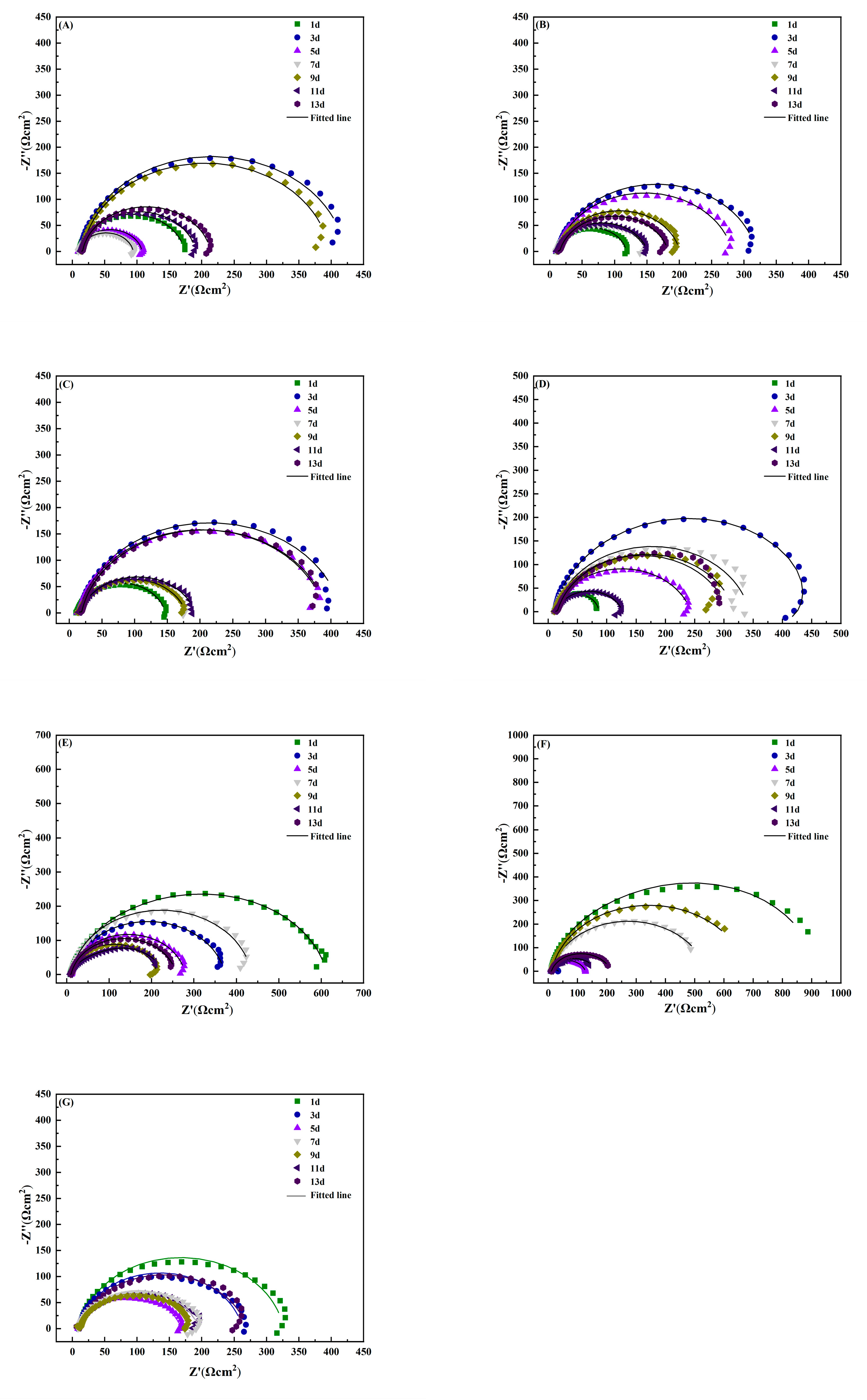
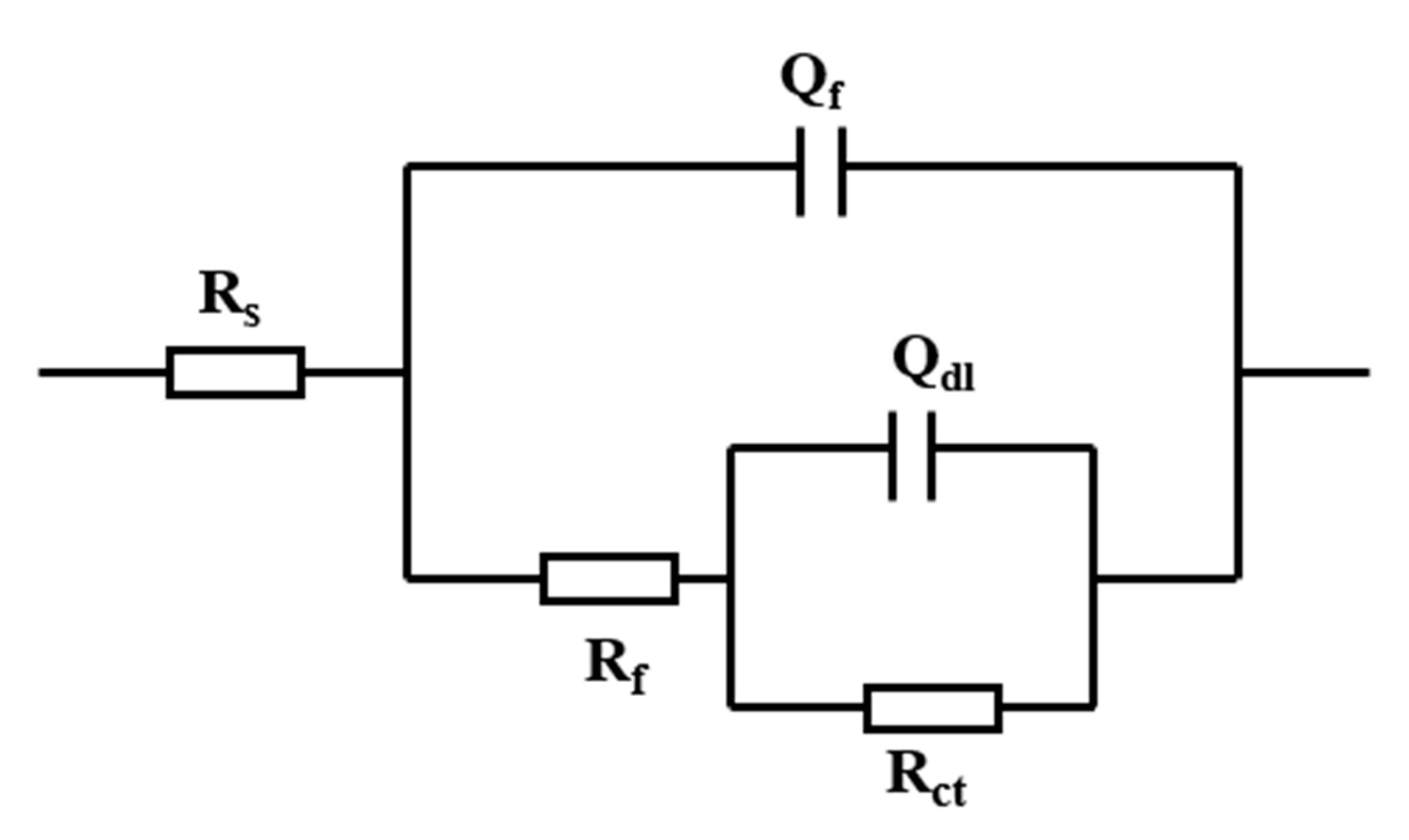
3.2. Electrochemical Impedance Spectrum
3.3. Characterisation of Electrochemical Polarisation Curves
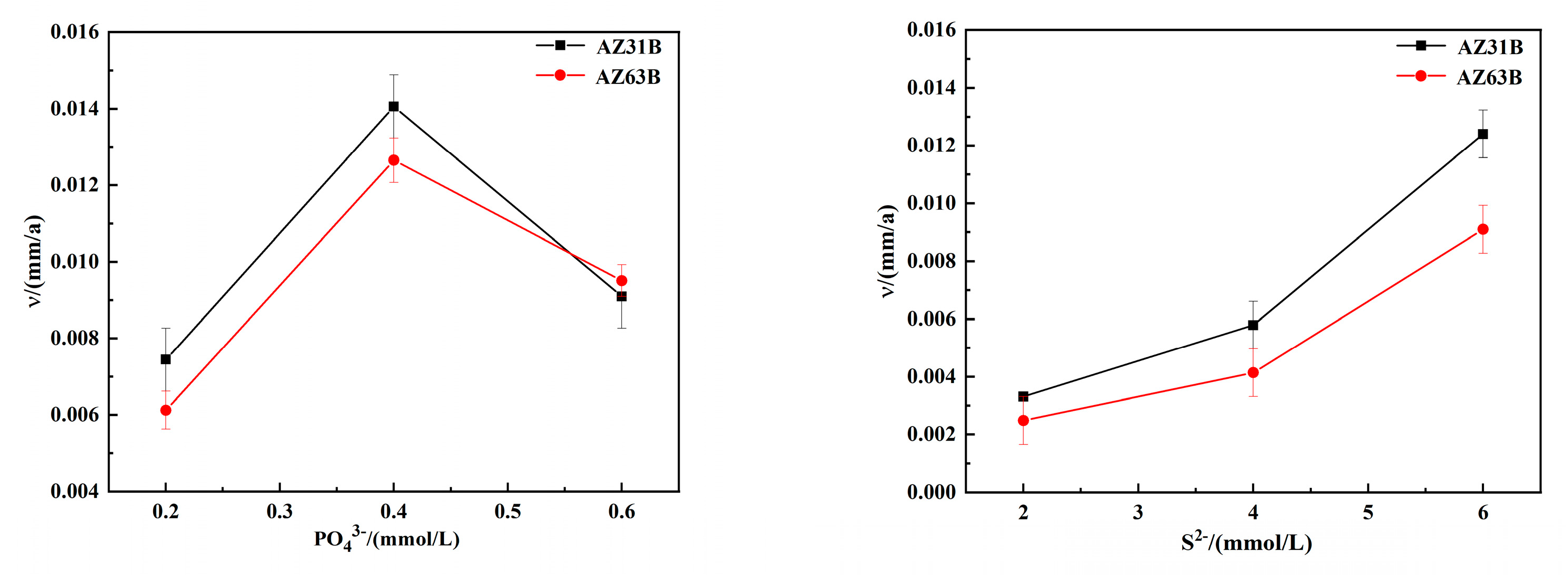
3.4. Weight Loss Data Analysis
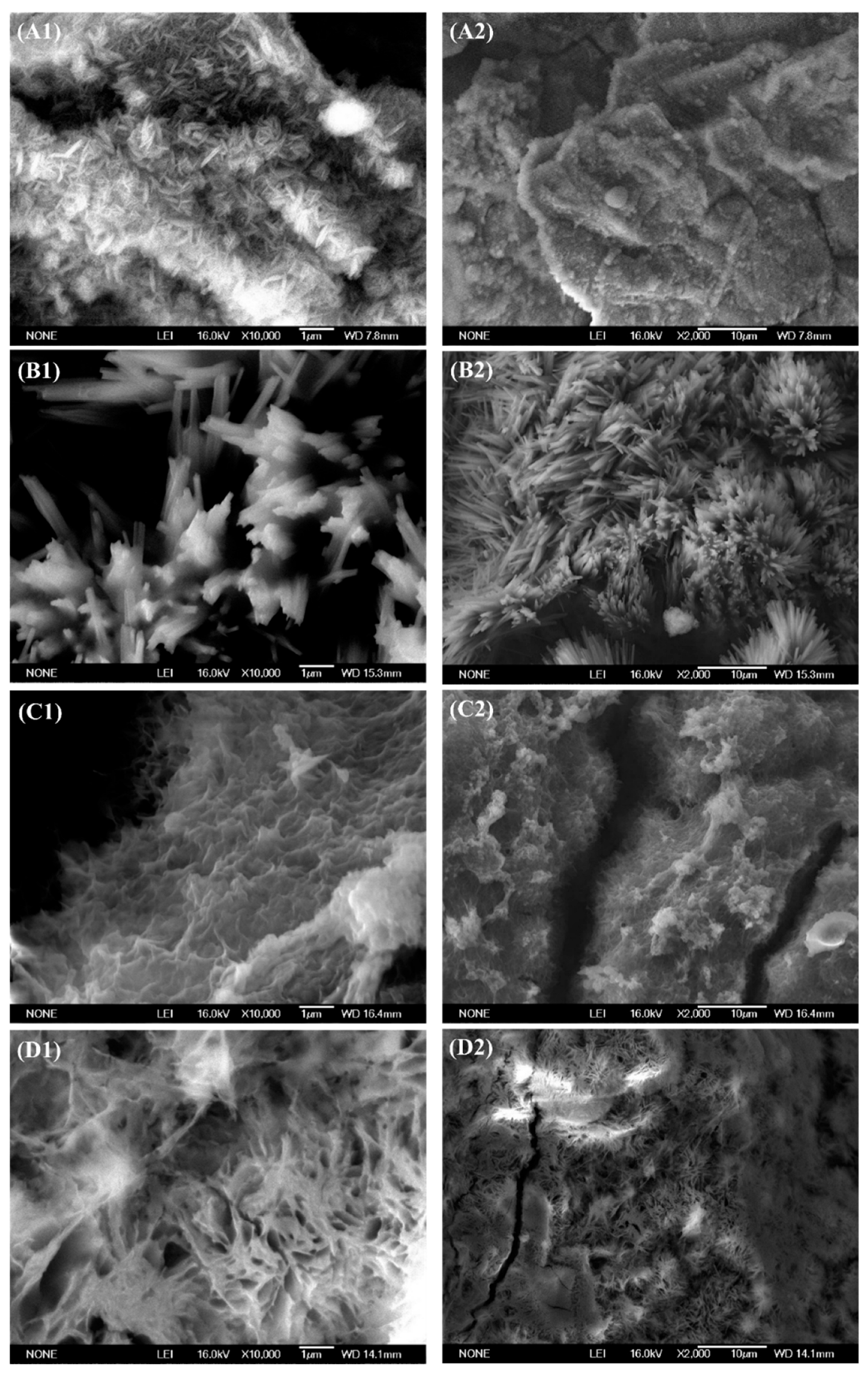
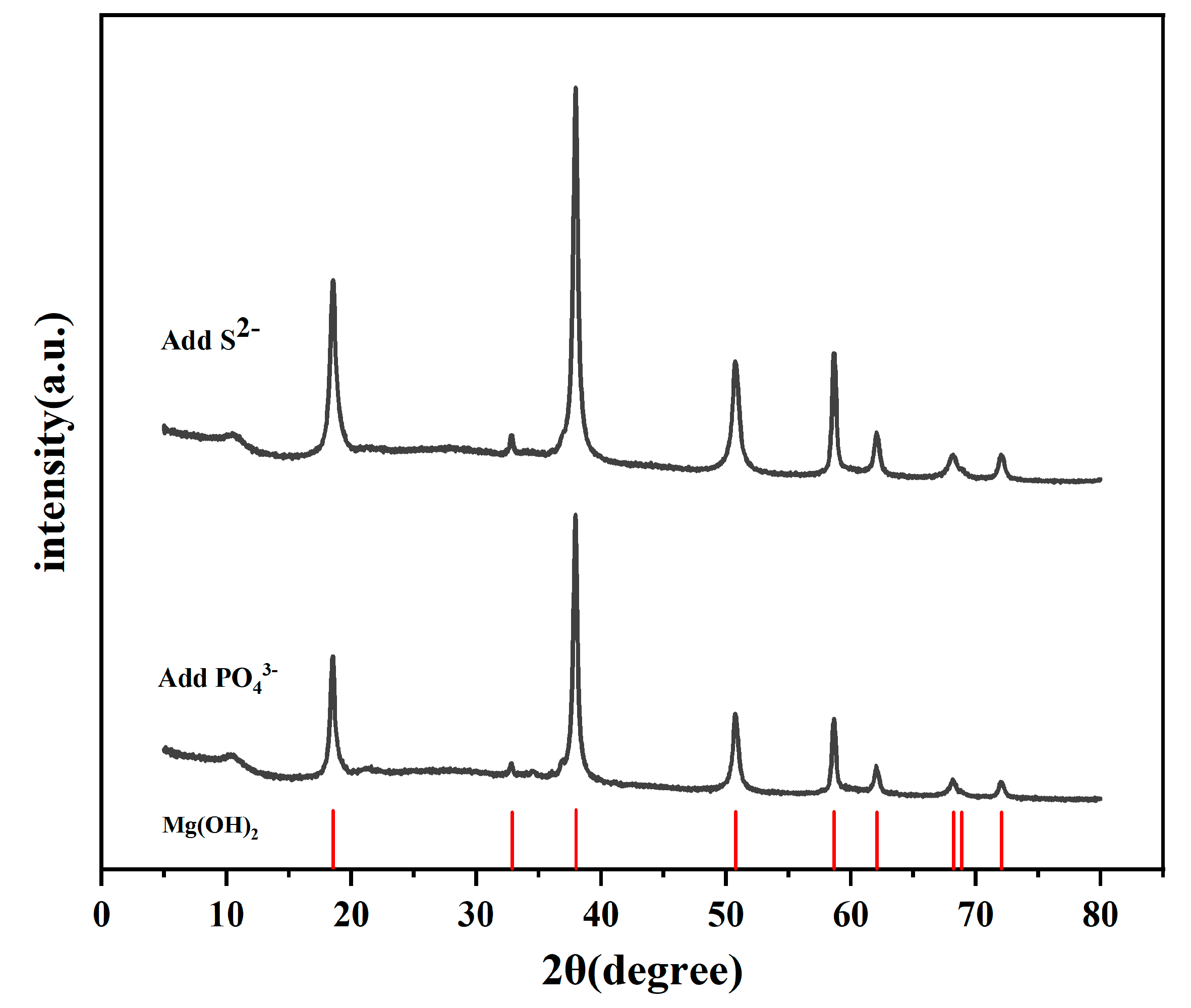
3.5. Corrosion Product Analysis
4. Conclusions
- A certain amount of SRB inorganic metabolites can accelerate the corrosion of magnesium alloys AZ31B and AZ63B. If the phosphate concentration is 0.4 mmol/L or the sulphide ion concentration is 6 mmol/L, the corrosion is most significant.
- At the beginning of the immersion test, the corrosion product film can prevent corrosion from continuing. However, it was not stable. About 1–3 d after the experiment, the corrosion products start to fall off from the surface of the specimen. Even the magnesium blocks on the metal surface of the sample may fall off, thus accelerating corrosion. Over time, the corrosion products build up on the metal substrate surface again; however, they cannot provide effective protection.
- The corrosion product layer attached to the surface is loose and accumulates microcracks and voids. This layer does not provide long-term and effective protection against corrosion.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sherar, B.W.; Power, I.M.; Keech, P.G.; Mitlin, S.; Southam, G.; Shoesmith, D.W. Characterizing the effect of carbon steel exposure in sulfide containing solutions to microbially induced corrosion. Corros. Sci. 2011, 53, 955–960. [Google Scholar] [CrossRef]
- Rao, T.S.; Kora, A.J.; Anupkumar, B.; Narasimhan, S.V.; Feser, R. Pitting corrosion of titanium by a freshwater strain of sulphate reducing bacteria (Desulfovibrio vulgaris). Corros. Sci. 2005, 47, 1071–1084. [Google Scholar] [CrossRef]
- Wu, J.J.; Zhang, D.; Wang, P.; Cheng, Y.; Sun, S.M.; Sun, Y.; Chen, S. The influence of Desulfovibrio sp. and Pseudoalteromonas sp. on the corrosion of Q235 carbon steel in natural seawater. Corros. Sci. 2016, 112, 552–562. [Google Scholar] [CrossRef]
- Zhang, P.Y.; Xu, D.K.; Li, Y.C.; Yang, K.; Gu, T.Y. Electron mediators accelerate the microbiologically influenced corrosion of 304 stainless steel by the Desulfovibrio vulgaris biofilm. Bioelectrochemistry 2015, 101, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.Y.; Jia, R.; Unsal, T.; Xu, D.K. Toward a better understanding of microbiologically influenced corrosion cause by sulfate reducing bacteria. J. Mater. Sci. Technol. 2018, 35, 631–636. [Google Scholar] [CrossRef]
- Li, Y.C.; Xu, D.K.; Chen, C.F.; Li, X.G.; Jia, R.; Zhang, D.W.; Wolfgang, S.; Wang, F.H.; Gu, T.Y. Anaerobic microbiologically influenced corrosion mechanisms interpreted using bioenergetics and bioelectrochemistry: A review. J. Mater. Sci. Technol. 2018, 34, 1713–1718. [Google Scholar] [CrossRef]
- Jia, R.; Jie, L.T.; Jin, P.; Daniel, J.B.; Xu, D.K.; Gu, T.Y. Effects of biogenic H2S on the microbiologically influenced corrosion of C1018 carbon steel by sulfate reducing Desulfovibrio vulgaris biofilm. Corros. Sci. 2018, 130, 1–11. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, E.Z.; Jiang, C.Y.; Jia, R.; Liu, S.J.; Xu, D.K.; Gu, T.Y.; Wang, F.H. Endogenous phenazine-1-carboxamide encoding gene PhzH regulated the extracellular electron transfer in biocorrosion of stainless steel by marine Pseudomonas aeruginosa. Electrochem. Commun. 2018, 94, 9–13. [Google Scholar] [CrossRef]
- Dou, W.W.; Liu, J.L.; Cai, W.Z.; Wang, D.; Jia, R.; Chen, S.G.; Gu, T.Y. Electrochemical investigation of increased carbon steel corrosion via extracellular electron transfer by a sulfate reducing bacterium under carbon source starvation. Corros. Sci. 2019, 150, 258–267. [Google Scholar] [CrossRef]
- Sheng, X.X.; Ting, Y.P.; Pehkonen, S.O. The influence of sulphate-reducing bacteria biofilm on the corrosion of stainless steel AISI 316. Corros. Sci. 2007, 49, 2159–2176. [Google Scholar] [CrossRef]
- Videla, H.A.; Herrera, L.K. Understanding microbial inhibition of corrosion. A comprehensive overview. Int. Biodeterior. Biodegrad. 2009, 63, 896–900. [Google Scholar] [CrossRef]
- Voordouw, G.; Niviere, V.; Ferris, F.G.; Fedorak, P.M.; Westlake, D.W. Distribution of hydrogenase genes in Desulfovibrio spp. and their use in identification of species from the oil field environment. Appl. Environ. Microbiol. 1990, 56, 3748–3754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iverson, W.P. Direct evidence for the cathodic depolarization theory of bacterial Corrosion. Science 1996, 151, 986–988. [Google Scholar] [CrossRef] [PubMed]
- Glindeman, D.; Eismann, F.; Bergmann, A.; Kuschk, P.; Stottmeister, U. Phosphine by bio-corrosion of phosphide-rich iron. Environ. Sci. Pollut. Res. 1998, 5, 71–74. [Google Scholar] [CrossRef]
- Yin, L.Y.; Huang, R.N.; Zhou, W.; Li, L. Analysis of factors affecting hydrogen evolution in Magnesium seawater battery. Chin. J. Power Sources 2011, 21, 259–268. [Google Scholar]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Abbasi, S.; Aliofkhazraei, M.; Mojiri, H.; Mina, A.; Mohammad, A.; Masoud, S. Corrosion behavior of pure Mg and AZ31 magnesium alloy. Prot. Met. Phys. Chem. Surf. 2017, 53, 573–578. [Google Scholar] [CrossRef]
- Gray-Munro, J.E.; Seguin, C.; Strong, M. Influence of surface modification on the in vitro corrosion rate of magnesium alloy AZ31. J. Biomed. Mater. Res. Part A 2010, 91, 221–230. [Google Scholar]
- Zhang, T.C.; Zhang, K.M.; Zou, J.X.; Yan, P.; Yang, H.Y.; Song, L.X.; Zhang, X. Surface microstructure and property modifications in a Mg-8Gd-3Y-0.5Zr magnesium alloy treated by high current pulsed electron beam. J. Alloys Compd. 2019, 788, 231–239. [Google Scholar] [CrossRef]
- Yang, G.Z.; Yang, H.W.; Shi, L.; Wang, T.L.; Zhou, W.C.; Zhou, T.; Han, W.; Zhang, Z.Y.; Lu, W.; Hu, J.Z. Enhancing Corrosion Resistance, Osteoinduction and Antibacterial Properties by Zn/Sr Additional Surface Modification of Magnesium Alloy. ACS Biomater. Sci. Eng. 2018, 4, 4289–4298. [Google Scholar] [CrossRef]
- Duan, J.Z.; Wu, S.R.; Zhang, X.J.; Huang, G.Q.; Du, M.; Hou, B. Corrosion of carbon steel influenced by anaerobic biofilm in natural seawater. Electrochim. Acta 2009, 54, 22–28. [Google Scholar] [CrossRef]
- Chen, S.Q.; Zhang, D. Study of corrosion behavior of copper in 3.5 wt.% NaCl solution containing extracellular polymeric substances of an aerotolerant sulphate-reducing bacteria. Corros. Sci. 2018, 136, 275–284. [Google Scholar] [CrossRef]
- Qi, B.; Zhang, D.; Lv, D.D.; Wang, P. Effects of two main metabolites of sulphate-reducing bacteria on the corrosion of Q235 steels in 3.5 wt.% NaCl media. Corros. Sci. 2012, 65, 405–413. [Google Scholar]
- Chen, S.Q.; Wang, P.; Zhang, D. Corrosion behavior of copper under biofilm of sulfate-reducing bacteria. Corros. Sci. 2014, 87, 407–415. [Google Scholar] [CrossRef]
- Wan, Y.; Zhang, D.; Liu, H.Q.; Li, Y.J.; Hou, B.R. Influence of sulphate-reducing bacteria on environmental parameters and marine corrosion behavior of Q235 steel in aerobic conditions. Electrochim. Acta 2010, 55, 1528–1534. [Google Scholar] [CrossRef]
- Xu, D.; Li, Y.C.; Gu, T.Y. Mechanistic modeling of biocorrosion caused by biofilms of sulfate. Bioelectrochemistry 2016, 110, 52–58. [Google Scholar] [CrossRef]
- Wang, N.G.; Wang, R.C.; Peng, C.Q.; Feng, Y.; Chen, B. Effect of hot rolling and subsequent annealing on electrochemical discharge behavior of AP65 magnesium alloy as anode for seawater activated battery. Corros. Sci. 2012, 64, 17–27. [Google Scholar] [CrossRef]
- Wang, N.G.; Wang, R.C.; Peng, C.Q.; Feng, Y. Enhancement of the discharge performance of AP65 magnesium alloy anodes by hot extrusion. Corros. Sci. 2014, 81, 85–95. [Google Scholar] [CrossRef]
- Liu, H.W.; Gu, T.Y.; Asif, M.; Zhang, G.A.; Liu, H.F. The corrosion behavior and mechanism of carbon steel induced by extracellular polymeric substances of iron-oxidizing bacteria. Corros. Sci. 2017, 114, 102–111. [Google Scholar] [CrossRef]
- Yu, L.; Duan, J.Z.; Du, X.Q.; Huang, Y.L.; Hou, B.R. Accelerated anaerobic corrosion of electroactive sulfate-reducing bacteria by electrochemical impedance spectroscopy and chronoamperometry. Electrochem. Commun. 2013, 26, 101–104. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Li, X.G.; Cheng, Y.F. Electrochemical state conversion model for occurrence of pitting corrosion on a cathodically polarized carbon steel in a near-neutral pH solution. Electrochim. Acta 2011, 56, 4167–4175. [Google Scholar] [CrossRef]
- LI, Y.; Shi, Z.M.; Chen, X.R.; Atrens, A. Anodic hydrogen evolution on Mg. J. Magnes. Alloy. 2021, 9, 2049–2062. [Google Scholar] [CrossRef]


| Composition | Al | Be | Si | Ca | Zn | Mn | Cu | Fe | Ce | Mg |
|---|---|---|---|---|---|---|---|---|---|---|
| AZ31B | 3.19 | 0.100 | 0.020 | 0.040 | 0.810 | 0.334 | 0.050 | 0.005 | — | 95.5 |
| AZ63B | 5.30 | — | — | — | 2.50 | 0.150 | — | — | trace | 92.1 |
| Immersion Time (d) | 2 mmol·L−1 | 4 mmol·L−1 | 6 mmol·L−1 |
|---|---|---|---|
| 1 | 201.8 | 764.4 | 554.2 |
| 3 | 83.30 | 249.6 | 203.8 |
| 5 | 83.16 | 241.3 | 160.8 |
| 7 | 157.7 | 47.21 | 159.6 |
| 9 | 138.0 | 41.43 | 132.7 |
| 11 | 409.8 | 346.5 | 312.3 |
| 13 | 121.4 | 123.1 | 144.4 |
| Immersion Time (d) | 2 mmol·L−1 | 4 mmol·L−1 | 6 mmol·L−1 |
|---|---|---|---|
| 1 | 62.63 | 605.5 | 885.9 |
| 3 | 430.4 | 358.8 | 39.94 |
| 5 | 227.5 | 268.8 | 113.9 |
| 7 | 329.3 | 422.4 | 526.1 |
| 9 | 292.5 | 201.5 | 662.1 |
| 11 | 110.4 | 60.71 | 149.8 |
| 13 | 301.2 | 246.0 | 198.6 |
| Immersion Time (d) | 0.2 mmol·L−1 | 0.4 mmol·L−1 | 0.6 mmol·L−1 |
|---|---|---|---|
| 1 | 389.4 | 219.0 | 381.8 |
| 3 | 191.4 | 140.9 | 184.8 |
| 5 | 96.47 | 102.3 | 104.9 |
| 7 | 83.56 | 112.8 | 74.18 |
| 9 | 73.56 | 118.4 | 74.90 |
| 11 | 63.05 | 89.03 | 70.85 |
| 13 | 58.01 | 99.82 | 84.81 |
| Immersion Time (d) | 0.2 mmol·L−1 | 0.4 mmol·L−1 | 0.6 mmol·L−1 |
|---|---|---|---|
| 1 | 158.6 | 105.0 | 133.6 |
| 3 | 381.3 | 305.6 | 399.4 |
| 5 | 93.51 | 268.2 | 375.9 |
| 7 | 79.29 | 134.8 | 163.1 |
| 9 | 398.5 | 185.4 | 163.3 |
| 11 | 192.0 | 135.1 | 175.3 |
| 13 | 197.0 | 165.7 | 371.0 |
| Magnesium Alloy | PO43− (mmol/L) | S2− (mmol/L) | icorr (Acm−2) | Ecorr (V) vs. SCE | βa(mv/Decade) | βc(mv/Decade) |
|---|---|---|---|---|---|---|
| AZ31B | 0.0 | 0.0 | 2.90 × 10−5 | −1.513 | 94.32 | −91.18 |
| 0.2 | 4.26 × 10−5 | −1.509 | 38.94 | −36.86 | ||
| 0.4 | 1.19 × 10−4 | −1.538 | 52.11 | −43.47 | ||
| 0.6 | 4.02 × 10−5 | −1.522 | 60.95 | −49.70 | ||
| 2.0 | 7.27 × 10−5 | −1.506 | 24.91 | −24.70 | ||
| 4.0 | 5.85 × 10−5 | −1.515 | 35.86 | −33.26 | ||
| 6.0 | 1.25 × 10−4 | −1.514 | 52.21 | −47.00 | ||
| AZ63B | 0 | 0 | 1.06 × 10−5 | −1.539 | 48.90 | −45.90 |
| 0.2 | 2.57 × 10−4 | −1.524 | 47.16 | −45.40 | ||
| 0.4 | 3.96 × 10−4 | −1.515 | 52.84 | −50.14 | ||
| 0.6 | 1.72 × 10−4 | −1.526 | 55.41 | −54.25 | ||
| 2.0 | 1.31 × 10−4 | −1.512 | 44.07 | −39.01 | ||
| 4.0 | 1.75 × 10−4 | −1.515 | 59.54 | −55.75 | ||
| 6.0 | 2.80 × 10−4 | −1.505 | 55.94 | −49.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Liu, X.; Zhang, J.; Zhang, R.; Wang, M.; Sand, W.; Duan, J.; Zhu, Q.; Zhai, S.; Hou, B. Effects of Inorganic Metabolites of Sulphate-Reducing Bacteria on the Corrosion of AZ31B and AZ63B Magnesium Alloy in 3.5 wt.% NaCl Solution. Materials 2022, 15, 2212. https://doi.org/10.3390/ma15062212
Li J, Liu X, Zhang J, Zhang R, Wang M, Sand W, Duan J, Zhu Q, Zhai S, Hou B. Effects of Inorganic Metabolites of Sulphate-Reducing Bacteria on the Corrosion of AZ31B and AZ63B Magnesium Alloy in 3.5 wt.% NaCl Solution. Materials. 2022; 15(6):2212. https://doi.org/10.3390/ma15062212
Chicago/Turabian StyleLi, Jinrong, Xin Liu, Jie Zhang, Ruiyong Zhang, Mingxing Wang, Wolfgang Sand, Jizhou Duan, Qingjun Zhu, Shenbao Zhai, and Baorong Hou. 2022. "Effects of Inorganic Metabolites of Sulphate-Reducing Bacteria on the Corrosion of AZ31B and AZ63B Magnesium Alloy in 3.5 wt.% NaCl Solution" Materials 15, no. 6: 2212. https://doi.org/10.3390/ma15062212
APA StyleLi, J., Liu, X., Zhang, J., Zhang, R., Wang, M., Sand, W., Duan, J., Zhu, Q., Zhai, S., & Hou, B. (2022). Effects of Inorganic Metabolites of Sulphate-Reducing Bacteria on the Corrosion of AZ31B and AZ63B Magnesium Alloy in 3.5 wt.% NaCl Solution. Materials, 15(6), 2212. https://doi.org/10.3390/ma15062212







