Acacia nilotica Pods’ Extract Assisted-Hydrothermal Synthesis and Characterization of ZnO-CuO Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
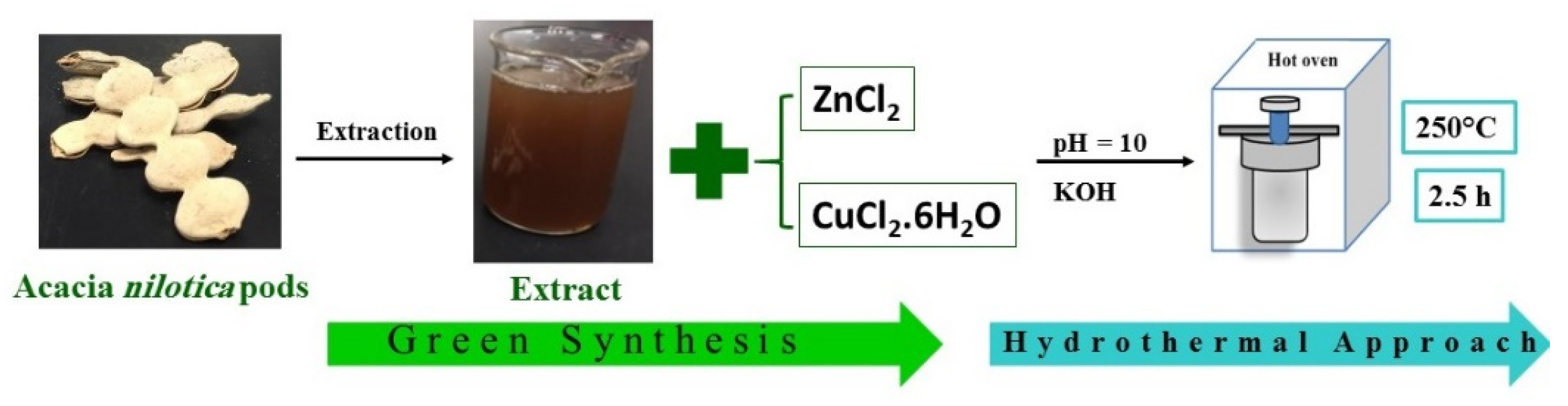
2.2. Acacia nilotica Extraction
2.3. Green Synthesis
2.4. Hydrothermal Synthesis
3. Results
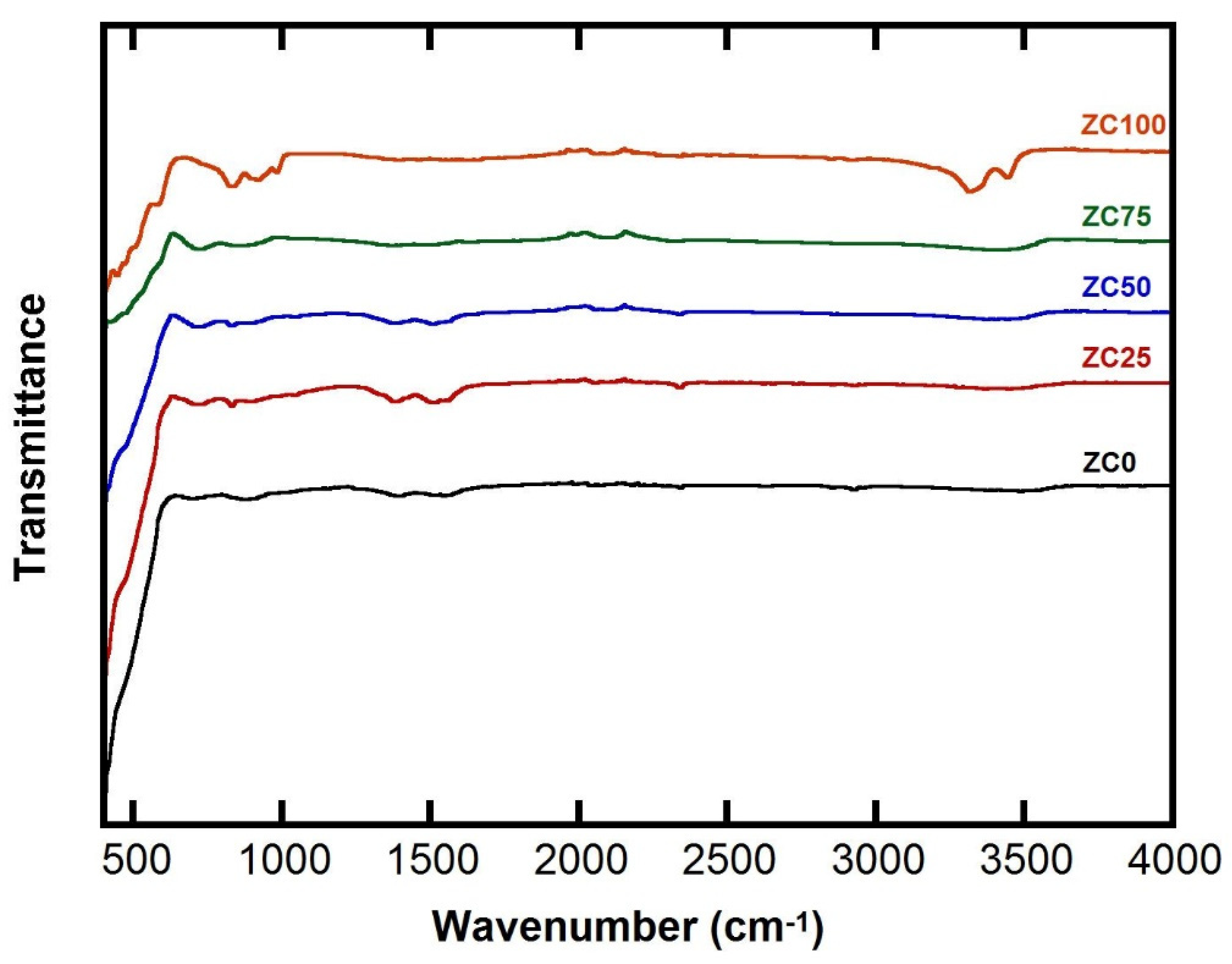
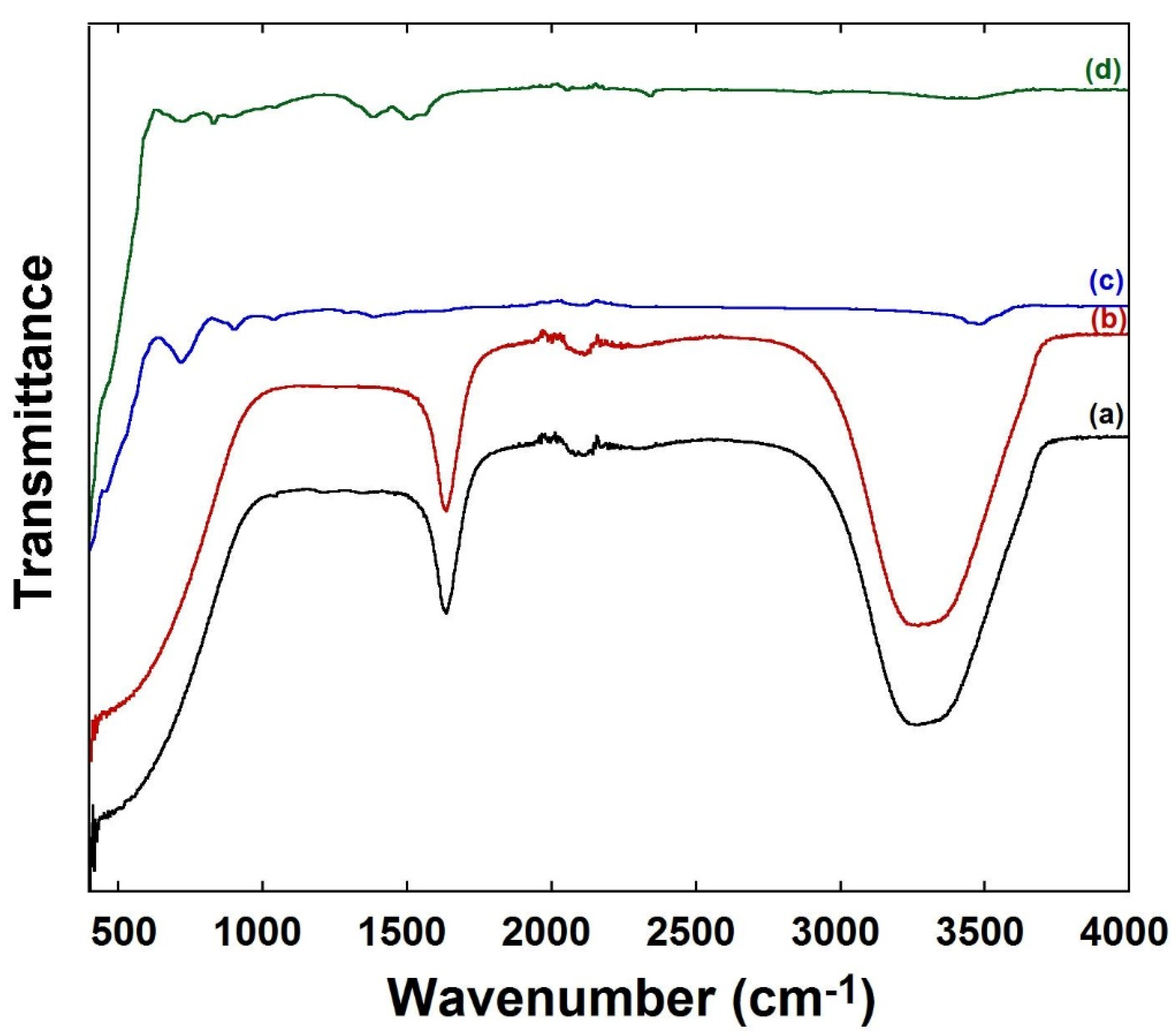
3.1. FTIR Characterization
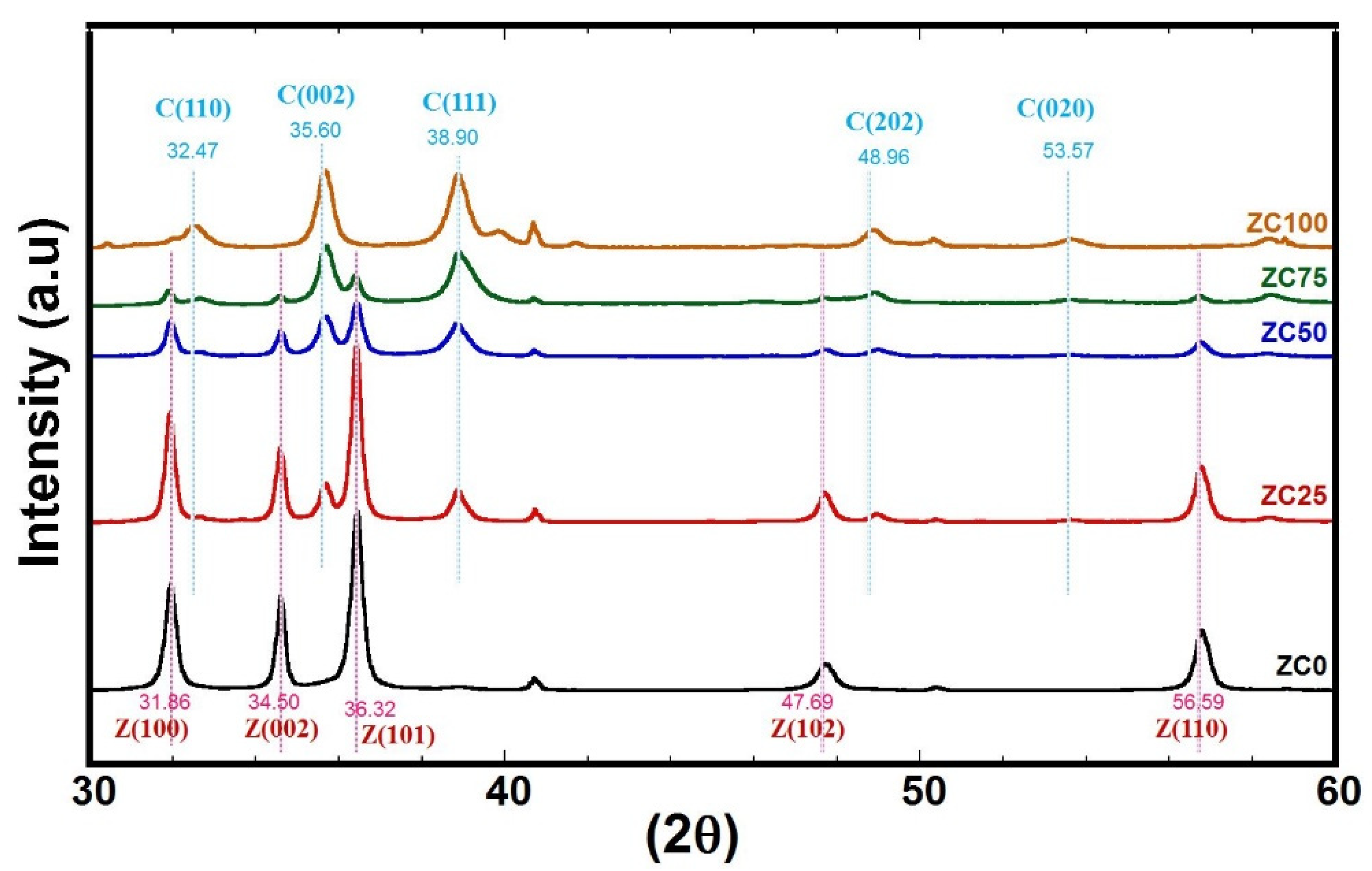
3.2. XRD Characterization
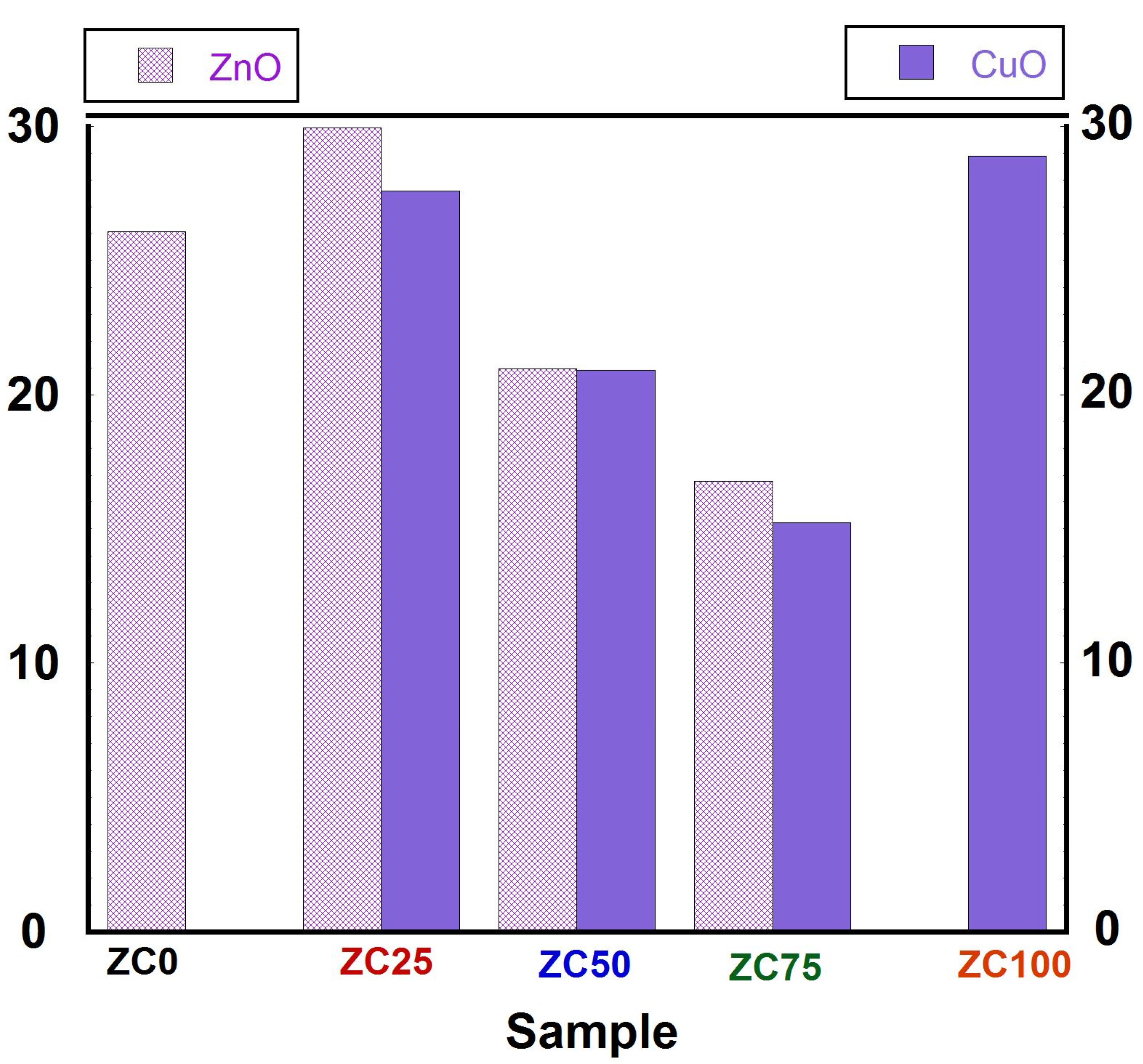
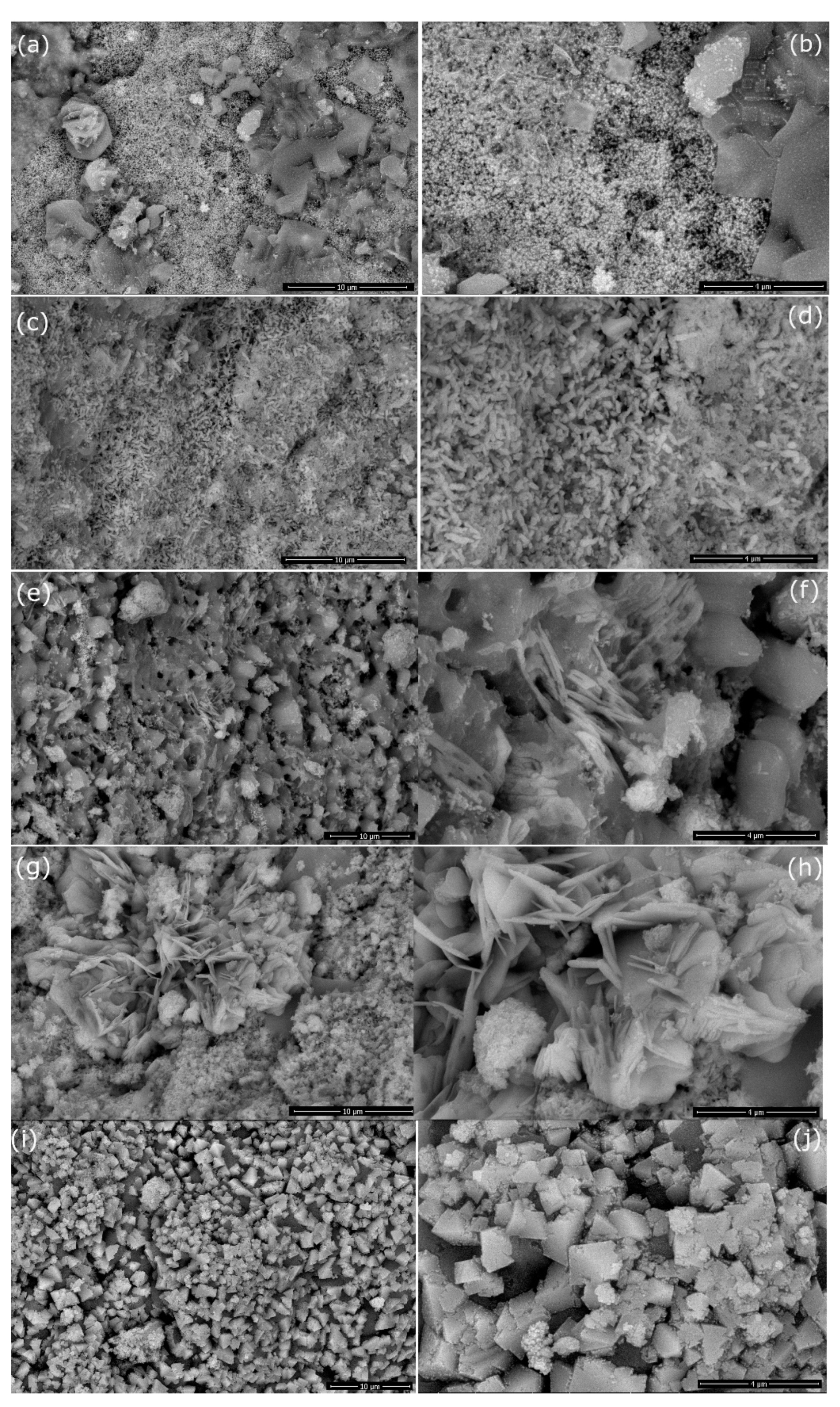
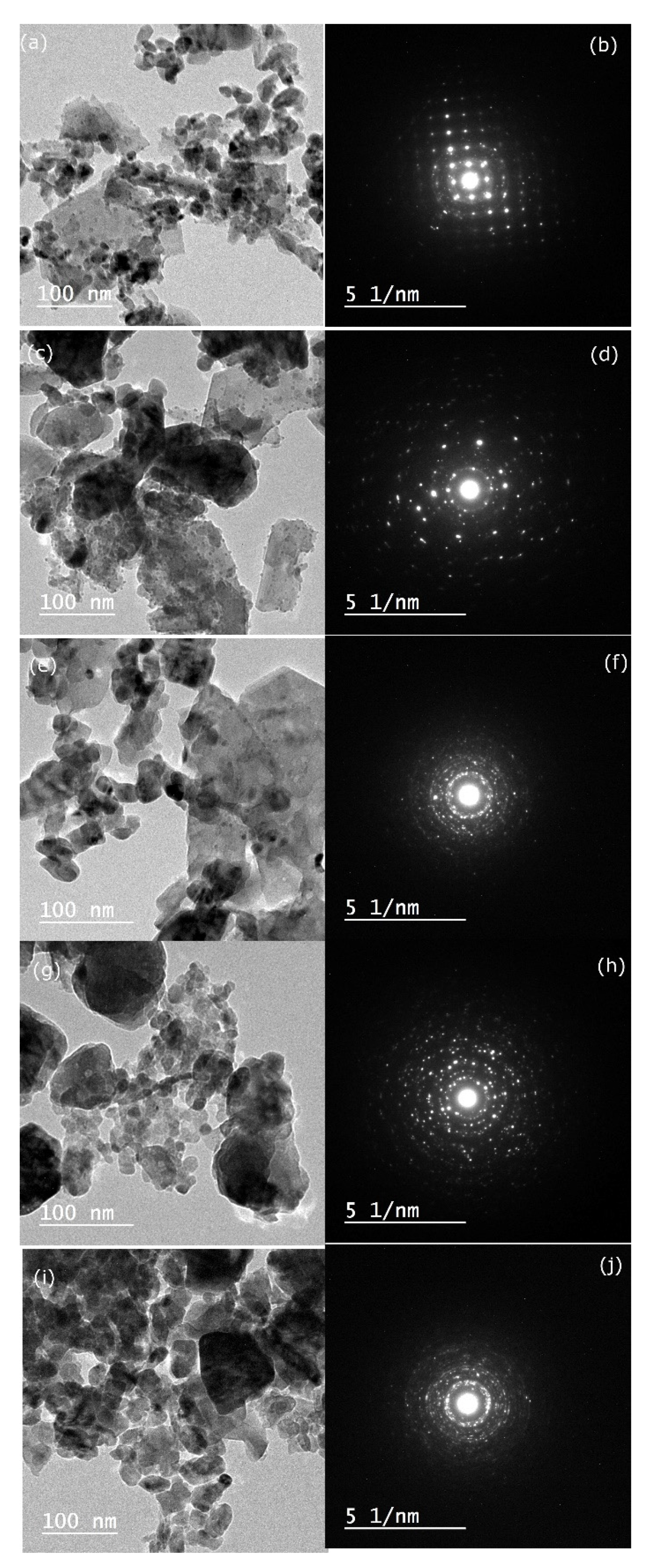
3.3. Morphology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yarahmadi, M.; Maleki-Ghaleh, H.; Mehr, M.E.; Dargahi, Z.; Rasouli, F.; Siadati, M.H. Synthesis and characterization of Sr-doped ZnO nanoparticles for photocatalytic applications. J. Alloys Compd. 2021, 853, 157000. [Google Scholar] [CrossRef]
- Truong, T.T.; Pham, T.T.; Truong, T.T.T.; Pham, T.D. Synthesis, characterization of novel ZnO/CuO nanoparticles, and the applications in photocatalytic performance for rhodamine B dye degradation. Environ. Sci. Pollut. Res. Int. 2021, 29, 22576–22588. [Google Scholar] [CrossRef] [PubMed]
- Azar, B.E.; Ramazani, A.; Fardood, S.T.; Morsali, A. Green synthesis and characterization of ZnAl2O4@ZnO nanocomposite and its environmental applications in rapid dye degradation. Optik 2020, 208, 164129. [Google Scholar] [CrossRef]
- Náfrádi, M.; Alapi, T.; Farkas, L.; Bencsik, G.; Kozma, G.; Hernádi, K. Wavelength Dependence of the Transformation Mechanism of Sulfonamides Using Different LED Light Sources and TiO2 and ZnO Photocatalysts. Materials 2021, 15, 49. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.R.R.; Betageri, V.S.; Nagaraju, G.; Pujar, G.H.; Onkarappa, H.S.; Latha, M.S. Synthesis of Core/Shell (ZnO/Ag) Nanoparticles Using Calotropis gigantea and Their Applications in Photocatalytic and Antibacterial Studies. J. Inorg. Organomet. Polym. Mater. 2020, 30, 3410–3417. [Google Scholar] [CrossRef]
- Wang, J.; Chen, R.; Xiang, L.; Komarneni, S. Synthesis, properties and applications of ZnO nanomaterials with oxygen vacancies: A review. Ceram. Int. 2018, 44, 7357–7377. [Google Scholar] [CrossRef]
- Achouri, F.; Merlin, C.; Corbel, S.; Alem, H.; Mathieu, L.; Balan, L.; Medjahdi, G.; Ben Said, M.; Ghrabi, A.; Schneider, R. ZnO Nanorods with High Photocatalytic and Antibacterial Activity under Solar Light Irradiation. Materials 2018, 11, 2158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murali, S.; Dammala, P.K.; Rani, B.; Santhosh, R.; Jadhao, C.; Sahu, N.K. Polyol mediated synthesis of anisotropic ZnO nanomaterials and composite with rGO: Application towards hybrid supercapacitor. J. Alloys Compd. 2020, 844, 156149. [Google Scholar] [CrossRef]
- Ponnamma, D.; Cabibihan, J.-J.; Rajan, M.; Pethaiah, S.S.; Deshmukh, K.; Gogoi, J.P.; Pasha, S.K.K.; Ahamed, M.B.; Krishnegowda, J.; Chandrashekar, B.N.; et al. Synthesis, optimization and applications of ZnO/polymer nanocomposites. Mater. Sci. Eng. C 2019, 98, 1210–1240. [Google Scholar] [CrossRef] [PubMed]
- Shetti, N.P.; Bukkitgar, S.D.; Reddy, K.R.; Reddy, C.V.; Aminabhavi, T.M. ZnO-based nanostructured electrodes for electrochemical sensors and biosensors in biomedical applications. Biosens. Bioelectron. 2019, 141, 111417. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Wang, Z.; Wu, K. Evolution of Intrinsic and Extrinsic Electron Traps at Grain Boundary during Sintering ZnO Based Varistor Ceramics. Materials 2022, 15, 1098. [Google Scholar] [CrossRef] [PubMed]
- Saboor, A.; Shah, S.M.; Hussain, H. Band gap tuning and applications of ZnO nanorods in hybrid solar cell: Ag-doped verses Nd-doped ZnO nanorods. Mater. Sci. Semicond. Process. 2019, 93, 215–225. [Google Scholar] [CrossRef]
- Mishra, Y.K.; Adelung, R. ZnO tetrapod materials for functional applications. Mater. Today 2018, 21, 631–651. [Google Scholar] [CrossRef]
- Qiu, Y.; Chen, W.; Yang, S. Facile hydrothermal preparation of hierarchically assembled, porous single-crystalline ZnO nanoplates and their application in dye-sensitized solar cells. J. Mater. Chem. 2010, 20, 1001–1006. [Google Scholar] [CrossRef]
- Akhmedov, A.; Abduev, A.; Murliev, E.; Asvarov, A.; Muslimov, A.; Kanevsky, V. The ZnO-In2O3 Oxide System as a Material for Low-Temperature Deposition of Transparent Electrodes. Materials 2021, 14, 6859. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.; Xia, C.; Cai, Y.; Afzal, M.; Wang, H.; Qiao, J.; Zhu, B. Electrochemical and electrical properties of doped CeO2-ZnO composite for low-temperature solid oxide fuel cell applications. J. Power Sources 2018, 392, 33–40. [Google Scholar] [CrossRef]
- Pearton, S.; Norton, D.; Ip, K.; Heo, Y.; Steiner, T. Recent progress in processing and properties of ZnO. Superlattices Microstruct. 2003, 34, 3–32. [Google Scholar] [CrossRef]
- Nava, O.; Soto-Robles, C.; Gómez-Gutiérrez, C.; Vilchis-Nestor, A.; Castro-Beltrán, A.; Olivas, A.; Luque, P. Fruit peel extract mediated green synthesis of zinc oxide nanoparticles. J. Mol. Struct. 2017, 1147, 1–6. [Google Scholar] [CrossRef]
- Zeng, Q.-R.; Feng, J.-T.; Lin, X.-C.; Zhao, Y.; Liu, H.; Wang, S.-Z.; Dong, Z.-J.; Feng, W. One-step facile synthesis of a NiO/ZnO biomorphic nanocomposite using a poplar tree leaf template to generate an enhanced gas sensing platform to detect n-butanol. J. Alloys Compd. 2020, 815, 150550. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Yi, G.; Li, H.; Shi, C.; Sun, G.; Zhang, Z. Hydrothermal Synthesis of Co3O4/ZnO Hybrid Nanoparticles for Triethylamine Detection. Nanomaterials 2019, 9, 1599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Hao, X.; Wang, Z.; Dong, M.; Cui, L. Facile fabrication of Mn2+ -doped ZnO photocatalysts by electrospinning. R. Soc. Open Sci. 2020, 7, 191050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toe, M.Z.; Pung, S.-Y.; Le, A.T.; Yaccob, K.A.B.; Matsuda, A.; Tan, W.K.; Han, S.S. Morphology and optical properties of ZnO nanorods coupled with metal oxides of various bandgaps by photo-oxidation. J. Lumin. 2021, 229, 117649. [Google Scholar] [CrossRef]
- Kaenphakdee, S.; Putthithanas, P.; Yodyingyong, S.; Leelawattanachai, J.; Triampo, W.; Sanpo, N.; Jitputti, J.; Triampo, D. Zinc Oxide Synthesis from Extreme Ratios of Zinc Acetate and Zinc Nitrate: Synergistic Morphology. Materials 2022, 15, 570. [Google Scholar] [CrossRef] [PubMed]
- Youcef, R.; Benhadji, A.; Zerrouki, D.; Fakhakh, N.; Djelal, H.; Ahmed, M.T. Electrochemical synthesis of CuO–ZnO for enhanced the degradation of Brilliant Blue (FCF) by sono-photocatalysis and sonocatalysis: Kinetic and optimization study. React. Kinet. Mech. Catal. 2021, 133, 541–561. [Google Scholar] [CrossRef]
- Krishnakumar, B.; Alsalme, A.; Alharthi, F.A.; Mani, D.; Anandan, K.; Amutha, P.; Sobral, A.J. Synthesis, characterization of gelatin assisted ZnO and its effective utilization of toxic azo dye degradation under direct sunlight. Opt. Mater. 2021, 113, 110854. [Google Scholar] [CrossRef]
- Thatikayala, D.; Min, B. Ginkgo leaves extract-assisted synthesis of ZnO/CuO nanocrystals for efficient UV-induced photodegradation of organic dyes and antibacterial activity. J. Mater. Sci. Mater. Electron. 2021, 32, 17154–17169. [Google Scholar] [CrossRef]
- Hua, J. Synthesis and characterization of gold nanoparticles (AuNPs) and ZnO decorated zirconia as a potential adsorbent for enhanced arsenic removal from aqueous solution. J. Mol. Struct. 2020, 1228, 129482. [Google Scholar] [CrossRef]
- Cao, Y.; Dhahad, H.A.; El-Shorbagy, M.A.; Alijani, H.Q.; Zakeri, M.; Heydari, A.; Bahonar, E.; Slouf, M.; Khatami, M.; Naderifar, M.; et al. Dehkordi, Green synthesis of bimetallic ZnO-CuO nanoparticles and their cytotoxicity properties. Sci. Rep. 2021, 11, 23479. [Google Scholar] [CrossRef]
- Kumar, C.R.R.; Betageri, V.S.; Nagaraju, G.; Pujar, G.H.; Onkarappa, H.S.; Latha, M.S. One-pot green synthesis of ZnO–CuO nanocomposite and their enhanced photocatalytic and antibacterial activity. Adv. Nat. Sci. Nanosci. Nanotechnol. 2020, 11, 015009. [Google Scholar] [CrossRef]
- Muthukrishnaraj, A.; Kalaivani, S.S.; Manikandan, A.; Kavitha, H.P.; Srinivasan, R.; Balasubramanian, N. Sonochemical synthesis and visible light induced photocatalytic property of reduced graphene oxide@ZnO hexagonal hollow rod nanocomposite. J. Alloys Compd. 2020, 836, 155377. [Google Scholar] [CrossRef]
- Arun, V.; Prabhu, S.; Priyadharsan, A.; Maadeswaran, P.; Sohila, S.; Ramesh, R.; Kumar, A.S. Facile, low cost synthesis of cauliflower-shaped ZnO with MWCNT/rGO nanocomposites and their photocatalytic activity. J. Mater. Sci. Mater. Electron. 2021, 32, 15763–15777. [Google Scholar] [CrossRef]
- Nundy, S.; Eom, T.-Y.; Song, K.-Y.; Park, J.-S.; Lee, H.-J. Hydrothermal synthesis of mesoporous ZnO microspheres as NOX gas sensor materials—Calcination effects on microstructure and sensing performance. Ceram. Int. 2020, 46, 19354–19364. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L. Green synthesis of zinc oxide nanoparticles using Phoenix dactylifera waste as bioreductant for effective dye degradation and antibacterial performance in wastewater treatment. J. Hazard. Mater. 2020, 402, 123560. [Google Scholar] [CrossRef]
- Rahman, A.; Harunsani, M.H.; Tan, A.L.; Khan, M.M. Zinc oxide and zinc oxide-based nanostructures: Biogenic and phytogenic synthesis, properties and applications. Bioprocess Biosyst. Eng. 2021, 44, 1333–1372. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.M.; Ismail, A.A. Photocatalytic reduction and removal of mercury ions over mesoporous CuO/ZnO S-scheme heterojunction photocatalyst. Ceram. Int. 2021, 47, 9659–9667. [Google Scholar] [CrossRef]
- Prajapati, A.K.; Mondal, M.K. Novel green strategy for CuO–ZnO–C nanocomposites fabrication using marigold (Tagetes spp.) flower petals extract with and without CTAB treatment for adsorption of Cr(VI) and Congo red dye. J. Environ. Manag. 2021, 290, 112615. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Nayan, N.; Ahmad, M.K.; Fhong, S.C.; Ali, M.S.M.; Mustafa, M.K.; Tahir, M. Interface study of hybrid CuO nanoparticles embedded ZnO nanowires heterojunction synthesized by controlled vapor deposition approach for optoelectronic devices. Opt. Mater. 2021, 117, 111132. [Google Scholar] [CrossRef]
- Saratale, R.G.; Saratale, G.D.; Cho, S.-K.; Ghodake, G.; Kadam, A.; Kumar, S.; Mulla, S.I.; Kim, D.-S.; Jeon, B.-H.; Chang, J.S.; et al. Phyto-fabrication of silver nanoparticles by Acacia nilotica leaves: Investigating their antineoplastic, free radical scavenging potential and application in H2O2 sensing. J. Taiwan Inst. Chem. Eng. 2019, 99, 239–249. [Google Scholar] [CrossRef]
- Rather, L.J.; Islam, S.-U.; Mohammad, F. Acacia nilotica (L.): A review of its traditional uses, phytochemistry, and pharmacology. Sustain. Chem. Pharm. 2015, 2, 12–30. [Google Scholar] [CrossRef]
- Ramesh, S.; Vinitha, U.; Anthony, S.P.; Muthuraman, M.S. Pods of Acacia nilotica mediated synthesis of copper oxide nanoparticles and it’s in vitro biological applications. Mater. Today Proc. 2021, 47, 751–756. [Google Scholar] [CrossRef]
- Rao, T.N.; Babji, P.; Ahmad, N.; Khan, R.A.; Hassan, I.; Shahzad, S.A.; Husain, F.M. Green synthesis and structural classification of Acacia nilotica mediated-silver doped titanium oxide (Ag/TiO2) spherical nanoparticles: Assessment of its antimicrobial and anticancer activity. Saudi J. Biol. Sci. 2019, 26, 1385–1391. [Google Scholar] [CrossRef] [PubMed]
- Da’Na, E.; Taha, A.; Afkar, E. Green Synthesis of Iron Nanoparticles by Acacia nilotica Pods Extract and Its Catalytic, Adsorption, and Antibacterial Activities. Appl. Sci. 2018, 8, 1922. [Google Scholar] [CrossRef] [Green Version]
- Sakhaei, Z.; Rezaei, M. Mechanochemical synthesis of ZnO.Al2O3 powders with various Zn/Al molar ratios and their applications in reverse water-gas shift reaction. Environ. Sci. Pollut. Res. 2021, 28, 13790–13799. [Google Scholar] [CrossRef] [PubMed]
- Gajendiran, J.; Rajendran, V. Synthesis and characterization of coupled semiconductor metal oxide (ZnO/CuO) nanocomposite. Mater. Lett. 2014, 116, 311–313. [Google Scholar] [CrossRef]
- Kampara, R.K.; Sonia, T.; Jeyaprakash, B.G. CuO/ZnO Heterojunction Nanograins: Methanol Vapour Detection. J. Electron. Mater. 2021, 50, 2482–2495. [Google Scholar] [CrossRef]
- Mali, S.M.; Narwade, S.S.; Navale, Y.H.; Tayade, S.B.; Digraskar, R.V.; Patil, V.B.; Kumbhar, A.S.; Sathe, B.R. Heterostructural CuO-ZnO Nanocomposites: A Highly Selective Chemical and Electrochemical NO2 Sensor. ACS Omega 2019, 4, 20129–20141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavín, A.; Sivasamy, R.; Mosquera, E.; Morel, M.J. High proportion ZnO/CuO nanocomposites: Synthesis, structural and optical properties, and their photocatalytic behavior. Surfaces Interfaces 2019, 17, 100367. [Google Scholar] [CrossRef]
- Wilson, D.A.; Gurung, K.; Langell, M.A. Effect of zinc substitution on the growth morphology of ZnO-CuO tenorite solid solutions. J. Cryst. Growth 2021, 562, 126062. [Google Scholar] [CrossRef]
- Yakout, S.M.; El-Sayed, A.M. Enhanced ferromagnetic and photocatalytic properties in Mn or Fe doped p-CuO/n-ZnO nanocomposites. Adv. Powder Technol. 2019, 30, 2841–2850. [Google Scholar] [CrossRef]
- Kumari, V.; Yadav, S.; Jindal, J.; Sharma, S.; Kumari, K.; Kumar, N. Synthesis and characterization of heterogeneous ZnO/CuO hierarchical nanostructures for photocatalytic degradation of organic pollutant. Adv. Powder Technol. 2020, 31, 2658–2668. [Google Scholar] [CrossRef]
- Mansournia, M.; Ghaderi, L. CuO@ZnO core-shell nanocomposites: Novel hydrothermal synthesis and enhancement in photocatalytic property. J. Alloys Compd. 2017, 691, 171–177. [Google Scholar] [CrossRef]
- Hessien, M.; Da’Na, E.; Al-Amer, K.; Khalaf, M.M.; D’Na, E. Nano ZnO (hexagonal wurtzite) of different shapes under various conditions: Fabrication and characterization. Mater. Res. Express 2019, 6, 085057. [Google Scholar] [CrossRef]
- Quirino, M.R.; Lucena, G.L.; Medeiros, J.A.; dos Santos, I.M.G.; De Oliveira, M.J.C. CuO Rapid Synthesis with Different Morphologies by the Microwave Hydrothermal Method. Mater. Res. 2018, 21, e20180227. [Google Scholar] [CrossRef]
- Gao, P.; Liu, D. Facile synthesis of copper oxide nanostructures and their application in non-enzymatic hydrogen peroxide sensing. Sens. Actuators B Chem. 2015, 208, 346–354. [Google Scholar] [CrossRef]
- Chen, H.; Shin, D.-W.; Lee, J.-H.; Park, S.-M.; Kwon, K.-W.; Yoo, J.-B. Three-dimensional CuO nanobundles consisted of nanorods: Hydrothermal synthesis, characterization, and formation mechanism. J. Nanosci. Nanotechnol. 2010, 10, 5121–5128. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Mushtaq, L.; Ullah, Z.; Fazal, A.; Khan, A.M. Effect of vegetable waste extract on microstructure, morphology, and photocatalytic efficiency of ZnO–CuO nanocomposites. Inorg. Nano-Metal Chem. 2021, 51, 963–975. [Google Scholar] [CrossRef]
- Kumari, V.; Sharma, S.; Sharma, A.; Kumari, K.; Kumar, N. Hydrothermal synthesis conditions effect on hierarchical ZnO/CuO hybrid materials and their photocatalytic activity. J. Mater. Sci. Mater. Electron. 2021, 32, 9596–9610. [Google Scholar] [CrossRef]
- Lu, P.; Zhou, W.; Li, Y.; Wang, J.; Wu, P. Abnormal room temperature ferromagnetism in CuO/ZnO nanocomposites via hydrothermal method. Appl. Surf. Sci. 2017, 399, 396–402. [Google Scholar] [CrossRef]
- Chang, T.; Li, Z.; Yun, G.; Jia, Y.; Yang, H. Enhanced Photocatalytic Activity of ZnO/CuO Nanocomposites Synthesized by Hydrothermal Method. Nano-Micro Lett. 2013, 5, 163–168. [Google Scholar] [CrossRef]
- Bordbar, M.; Negahdar, N.; Nasrollahzadeh, M. Melissa officinalis L. leaf extract assisted green synthesis of CuO/ZnO nanocomposite for the reduction of 4-nitrophenol and Rhodamine B. Sep. Purif. Technol. 2018, 191, 295–300. [Google Scholar] [CrossRef]
| Sample Code | ZC0 | ZC25 | ZC50 | ZC75 | ZC100 |
|---|---|---|---|---|---|
| ZnCl2 | 100 | 75 | 50 | 25 | 0 |
| CuCl2.6H2O | - | 25 | 50 | 75 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hessien, M.; Taha, A.; Da’na, E. Acacia nilotica Pods’ Extract Assisted-Hydrothermal Synthesis and Characterization of ZnO-CuO Nanocomposites. Materials 2022, 15, 2291. https://doi.org/10.3390/ma15062291
Hessien M, Taha A, Da’na E. Acacia nilotica Pods’ Extract Assisted-Hydrothermal Synthesis and Characterization of ZnO-CuO Nanocomposites. Materials. 2022; 15(6):2291. https://doi.org/10.3390/ma15062291
Chicago/Turabian StyleHessien, Manal, Amel Taha, and Enshirah Da’na. 2022. "Acacia nilotica Pods’ Extract Assisted-Hydrothermal Synthesis and Characterization of ZnO-CuO Nanocomposites" Materials 15, no. 6: 2291. https://doi.org/10.3390/ma15062291
APA StyleHessien, M., Taha, A., & Da’na, E. (2022). Acacia nilotica Pods’ Extract Assisted-Hydrothermal Synthesis and Characterization of ZnO-CuO Nanocomposites. Materials, 15(6), 2291. https://doi.org/10.3390/ma15062291







