Lanthanide-Doped Upconversion Luminescent Nanoparticles—Evolving Role in Bioimaging, Biosensing, and Drug Delivery
Abstract
:1. Introduction
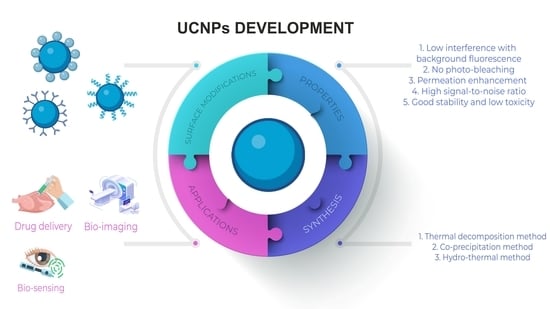
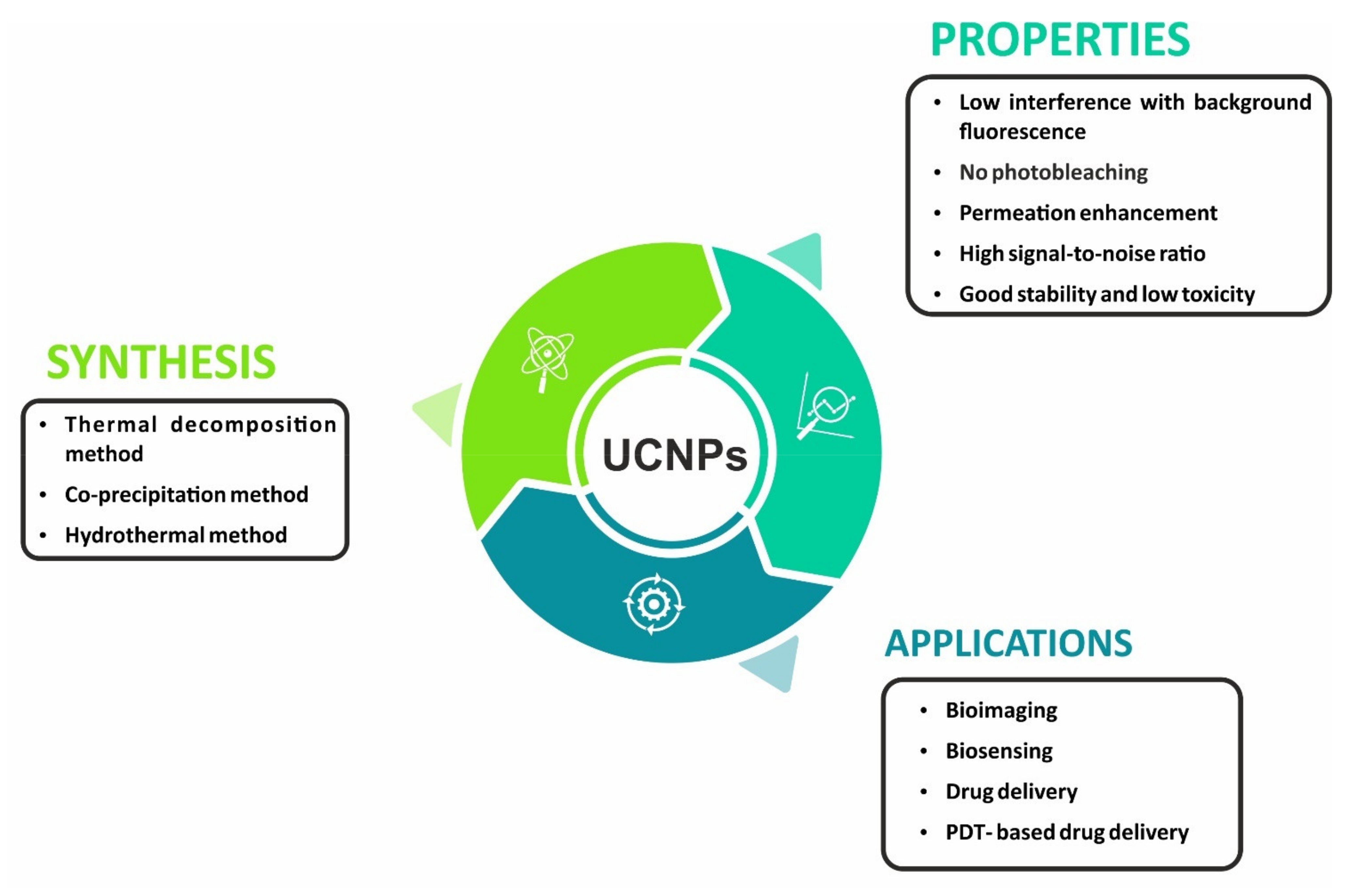
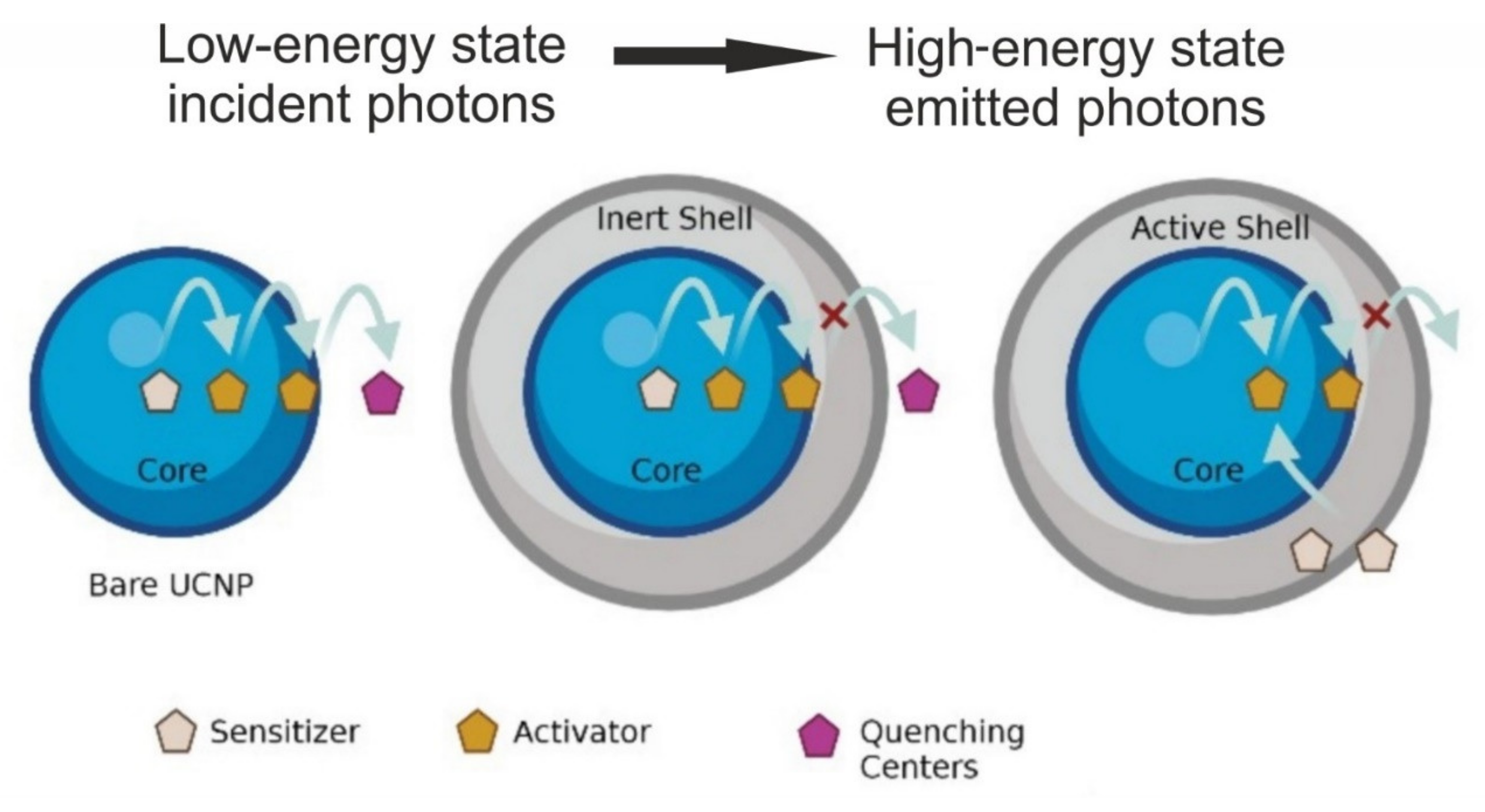
2. Properties and Composition of UCNPs
3. Synthesis of UCNPs
3.1. Thermal Decomposition Method
3.2. Co-Precipitation Method
3.3. Hydrothermal Method
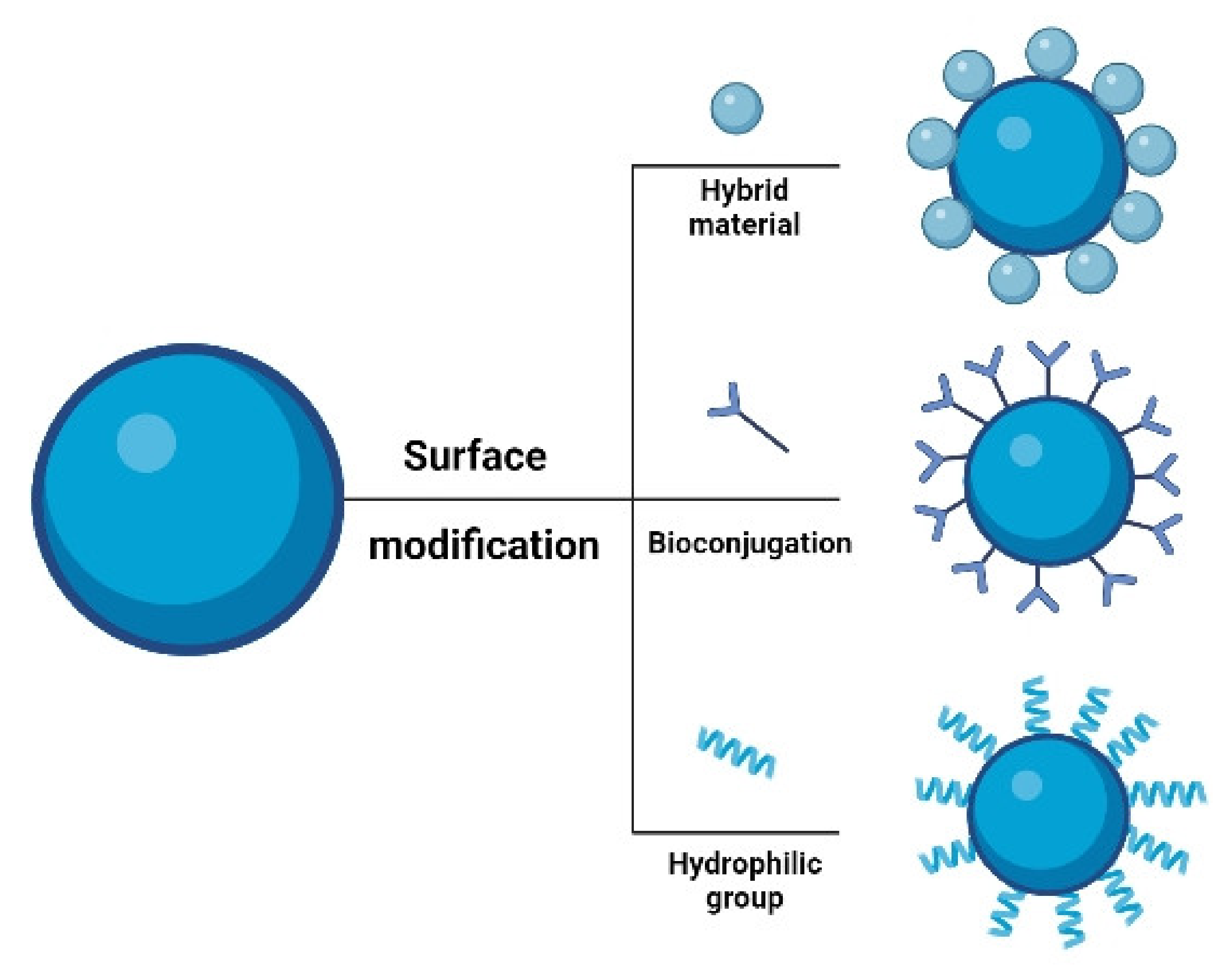
4. Surface Modification of UCNPs
5. Applications of UCNPs
5.1. UCNPs in Bioimaging
5.2. UCNPs in Biosensing
5.3. UCNPs in Drug Delivery
5.4. UCNPs in Photodynamic Therapy (PDT)-Based Drug Delivery
6. Pharmacokinetics (PK) of UCNPs
7. Outlook and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The history of nanoscience and nanotechnology: From chemical-physical applications to nanomedicine. Molecules 2020, 25, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasrollahzadeh, M.; Sajadi, S.M.; Sajjadi, M.; Issaabadi, Z. An Introduction to Nanotechnology, 1st ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; Volume 28, ISBN 9780128135860. [Google Scholar]
- Talapin, D.V.; Shevchenko, E.V. Introduction: Nanoparticle chemistry. Chem. Rev. 2016, 116, 10343–10345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerrini, L.; Alvarez-Puebla, R.A.; Pazos-Perez, N. Surface modifications of nanoparticles for stability in biological fluids. Materials 2018, 11, 1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, K.; Sun, K. Upconversion nanoparticles. In Photonanotechnology for Therapeutics and Imaging; Elsevier: Amsterdam, The Netherlands, 2020; pp. 147–176. [Google Scholar]
- Haase, M.; Schäfer, H. Upconverting nanoparticles. Angew. Chem.-Int. Ed. 2011, 50, 5808–5829. [Google Scholar] [CrossRef]
- Das, A.; Bae, K.; Park, W. Enhancement of upconversion luminescence using photonic nanostructures. Nanophotonics 2020, 9, 1359–1371. [Google Scholar] [CrossRef]
- Chen, X.; Peng, D.; Ju, Q.; Wang, F. Photon upconversion in core-shell nanoparticles. Chem. Soc. Rev. 2015, 44, 1318–1330. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.; Liu, X.; Wu, F.; Zhang, H. Excitation energy migration dynamics in upconversion nanomaterials. Chem. Soc. Rev. 2015, 44, 1331–1345. [Google Scholar] [CrossRef] [Green Version]
- Wen, S.; Zhou, J.; Zheng, K.; Bednarkiewicz, A.; Liu, X.; Jin, D. Advances in highly doped upconversion nanoparticles. Nat. Commun. 2018, 9, 2415. [Google Scholar] [CrossRef]
- Wei, W.; Chen, G.; Baev, A.; He, G.S.; Shao, W.; Damasco, J.; Prasad, P.N. Alleviating Luminescence Concentration Quenching in Upconversion Nanoparticles through Organic Dye Sensitization. J. Am. Chem. Soc. 2016, 138, 15130–15133. [Google Scholar] [CrossRef]
- Viger, M.L.; Live, L.S.; Therrien, O.D.; Boudreau, D. Reduction of self-quenching in fluorescent silica-coated silver Nnanoparticles. Plasmonics 2008, 3, 33–40. [Google Scholar] [CrossRef]
- Li, X.; Zhang, F.; Zhao, D. Lab on upconversion nanoparticles: Optical properties and applications engineering via designed nanostructure. Chem. Soc. Rev. 2015, 44, 1346–1378. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Guo, X.; Zhang, X.; Chen, S.; Wang, Y.; Chen, T.; Huang, G.; Gao, Y.; Tian, Z.; Yang, Z. Multifunctional Phototheranostic Nanomedicine for Cancer Imaging and Treatment; Elsevier Ltd.: Amsterdam, The Netherlands, 2020; Volume 5, ISBN 0086298266328. [Google Scholar]
- Liu, B.; Li, C.; Xing, B.; Yang, P.; Lin, J. Multifunctional UCNPs@PDA-ICG nanocomposites for upconversion imaging and combined photothermal/photodynamic therapy with enhanced antitumor efficacy. J. Mater. Chem. B 2016, 4, 4884–4894. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Shi, B.; Jin, D.; Liu, X. Controlling upconversion nanocrystals for emerging applications. Nat. Nanotechnol. 2015, 10, 924–936. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Ge, H.; Wei, Y.; Zhang, K.; Su, W.; Zhou, J.; Yin, L.; Zhan, Q.; Jing, S.; Huang, L. Design for Brighter Photon Upconversion Emissions via Energy Level Overlap of Lanthanide Ions. ACS Nano 2018, 12, 10992–10999. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Meijerink, A. Concentration Quenching in Upconversion Nanocrystals. J. Phys. Chem. C 2018, 122, 26298–26306. [Google Scholar] [CrossRef] [Green Version]
- Eliseeva, S.V.; Bünzli, J.C.G. Lanthanide luminescence for functional materials and bio-sciences. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar] [CrossRef]
- Vu, D.T.; Tsai, Y.C.; Le, Q.M.; Kuo, S.W.; Lai, N.D.; Benisty, H.; Lin, J.Y.; Kan, H.C.; Hsu, C.C. A synergy approach to enhance upconversion luminescence emission of rare-earth nanophosphors with million-fold enhancement factor. Crystals 2021, 11, 1187. [Google Scholar] [CrossRef]
- Kavand, A.; Serra, C.A.; Blanck, C.; Lenertz, M.; Anton, N.; Vandamme, T.F.; Mély, Y.; Przybilla, F.; Chan-Seng, D. Controlled Synthesis of NaYF4:Yb, Er Upconversion Nanocrystals as Potential Probe for Bioimaging: A Focus on Heat Treatment. ACS Appl. Nano Mater. 2021, 4, 5319–5329. [Google Scholar] [CrossRef]
- Nie, Z.; Ke, X.; Li, D.; Zhao, Y.; Zhu, L.; Qiao, R.; Zhang, X.L. NaYF4:Yb,Er,Nd@NaYF4:Nd Upconversion Nanocrystals Capped with Mn:TiO2 for 808 nm NIR-Triggered Photocatalytic Applications. J. Phys. Chem. C 2019, 123, 22959–22970. [Google Scholar] [CrossRef]
- Unal, F.; Kaya, F.; Kazmanli, K. Synthesis, characterization and radioluminescence properties of erbium-doped yttria phosphors. Int. J. Miner. Metall. Mater. 2021, 28, 1983–1990. [Google Scholar] [CrossRef]
- Xie, J.; Liu, B.; Qong, Q.; Xu, Z.; Jin, Z.; Ma, W. Eu3+/Eu2+/Tb3+ co-activated single-phase Gd2O2S: A high-performance white light emitting phosphor for light emitting diode. Mater. Express 2021, 11, 54–62. [Google Scholar] [CrossRef]
- Lin, H.; Luo, Q.; Tong, W.Y.; Jiang, C.; Huang, R.; Peng, H.; Zhang, L.C.; Travas-Sejdic, J.; Duan, C.G. Facile preparation of rare-earth semiconductor nanocrystals and tuning of their dimensionalities. RSC Adv. 2015, 5, 86885–86890. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, S.; Xu, Z.; Zhang, L.; Zuo, P.; Wu, Q. Near-infrared light-driven photocatalytic NaYF4:Yb,Tm@ZnO core/shell nanomaterials and their performance. RSC Adv. 2019, 9, 3688–3692. [Google Scholar] [CrossRef] [Green Version]
- Hehlen, M.P.; Frei, G.; Güdel, H.U. Dynamics of infrared-to-visible up-conversion in Cs3Lu2Br9: 1%Er3+. Phys. Rev. B Condens. Matter. 1994, 50, 16264–16273. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.C.; Vetrone, F.; Cuccia, L.A.; Capobianco, J.A. Synthesis of colloidal upconverting NaYF4 nanocrystals doped with Er3+, Yb3+ and Tm3+, Yb3+ via thermal decomposition of lanthanide trifluoroacetate precursors. J. Am. Chem. Soc. 2006, 128, 7444–7445. [Google Scholar] [CrossRef] [PubMed]
- Mai, H.X.; Zhang, Y.W.; Sun, L.D.; Yan, C.H. Size- and phase-controlled synthesis of monodisperse NaYF4:Yb,Er nanocrystals from a unique delayed nucleation pathway monitored with upconversion spectroscopy. J. Phys. Chem. C 2007, 111, 13730–13739. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Sun, X.; Si, R.; You, L.P.; Yan, C.H. Single-crystalline and monodisperse LaF3 triangular nanoplates from a single-source precursor. J. Am. Chem. Soc. 2005, 127, 3260–3261. [Google Scholar] [CrossRef]
- Wang, M.; Abbineni, G.; Clevenger, A.; Mao, C.; Xu, S. Upconversion nanoparticles: Synthesis, surface modification and biological applications. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 710–729. [Google Scholar] [CrossRef] [Green Version]
- Ibarra-Ruiz, A.M.; Rodríguez Burbano, D.C.; Capobianco, J.A. Photoluminescent nanoplatforms in biomedical applications. Adv. Phys. X 2016, 1, 194–225. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Deng, R.; Liu, X. Preparation of core-shell NaGdF4 nanoparticles doped with luminescent lanthanide ions to be used as upconversion-based probes. Nat. Protoc. 2014, 9, 1634–1644. [Google Scholar] [CrossRef]
- Chen, H.; Wang, W.; Ji, C.; Wang, L. Dye-sensitized core–shell NaGdF4:Yb,Er@NaGdF4:Yb,Nd upconversion nanoprobe for determination of H2S. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2021, 248, 119281. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yan, J.; Wang, K.; Wang, Y.; Luo, G. Continuous synthesis of ultrasmall core-shell upconversion nanoparticles via a flow chemistry method. Nano Res. 2022, 15, 1199–1204. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Xu, X.; Huang, J.; Lu, Z.; Qiu, D. One-step synthesis and upconversion luminescence properties of hierarchical In2O3:Yb3+,Er3+ nanorod flowers. RSC Adv. 2017, 7, 54500–54505. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Lu, F.; Zhang, X.; Chen, D. Synthesis and characterization of efficient near-infrared upconversion Yb and Tm codoped NaYF4 nanocrystal reporter. J. Alloys Compd. 2007, 427, 333–340. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, Y.; Zang, Y.; Han, J.; Xiong, Q.; Xiong, J. SiO2 coated up-conversion nanomaterial doped with ag nanoparticles for micro-CT imaging. Nanomaterials 2021, 11, 3395. [Google Scholar] [CrossRef]
- Nampi, P.P.; Vakurov, A.; Viswambharan, H.; Schneider, J.E.; Brydson, R.; Millner, P.A.; Saha, S.; Jose, G. Barium yttrium fluoride based upconversion nanoparticles as dual mode image contrast agents. Mater. Sci. Eng. C 2021, 124, 111937. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, Y.; Tian, L.; Yu, Y.; Kong, X.; Zhao, J.; Zhang, H. Controlled synthesis and morphology dependent upconversion luminescence of NaYF4:Yb, Er nanocrystals. Nanotechnology 2007, 18, 275609. [Google Scholar] [CrossRef]
- Li, C.; Yang, J.; Quan, Z.; Yang, P.; Kong, D.; Lin, J. Different microstructures of β-NaYF4 fabricated by hydrothermal process: Effects of pH values and fluoride sources. Chem. Mater. 2007, 19, 4933–4942. [Google Scholar] [CrossRef]
- Yi, M.; Liu, Y.; Gao, H.; Huang, Z.; Liang, J.; Mao, Y. Upconversion effective enhancement of NaYF4:Yb3+/Er3+ nanoparticles by Ni2+ doping. J. Mater. Sci. 2018, 53, 1395–1403. [Google Scholar] [CrossRef]
- Reddy, K.L.; Prabhakar, N.; Rosenholm, J.M.; Krishnan, V. Core-shell structures of upconversion nanocrystals coated with silica for near infrared light enabled optical imaging of cancer cells. Micromachines 2018, 9, 400. [Google Scholar] [CrossRef] [Green Version]
- MacKenzie, L.E.; Alvarez-Ruiz, D.; Pal, R. Low-temperature open-air synthesis of PVP-coated NaYF 4 :Yb,Er,Mn upconversion nanoparticles with strong red emission. R. Soc. Open Sci. 2022, 9, 211508. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Huang, X.; Zhong, J.; Wang, Z.; Cheng, X. Upconversion luminescence and temperature sensing properties of NaGd(WO4)2:Yb3+/Er3+@SiO2 core-shell nanoparticles. RSC Adv. 2021, 11, 3981–3989. [Google Scholar] [CrossRef]
- Liu, G.; Sun, Z.; Jia, M.; Fu, Z.; Zhang, A.; Li, P. One pot synthesis and optimized luminescent intensity of Gd2(WO4)3: Yb3+/Ho3+@SiO2 nanoparticles for biological application. J. Lumin. 2019, 206, 1–5. [Google Scholar] [CrossRef]
- Mohan, M.; Poddar, R. Polymerically engineered upconversion nanoparticles (UCNPs) as a contrast agent for functionally modified optical coherence tomography (OCT). Mater. Sci. Eng. C 2021, 121, 111841. [Google Scholar] [CrossRef]
- Rafique, R.; Baek, S.H.; Phan, L.M.T.; Chang, S.J.; Gul, A.R.; Park, T.J. A facile hydrothermal synthesis of highly luminescent NaYF4:Yb3+/Er3+ upconversion nanoparticles and their biomonitoring capability. Mater. Sci. Eng. C 2019, 99, 1067–1074. [Google Scholar] [CrossRef]
- Du, P.; Zhang, P.; Kang, S.H.; Yu, J.S. Hydrothermal synthesis and application of Ho3+-activated NaYbF4 bifunctional upconverting nanoparticles for in vitro cell imaging and latent fingerprint detection. Sens. Actuators B Chem. 2017, 252, 584–591. [Google Scholar] [CrossRef]
- Lakshmanan, A.; Akasov, R.A.; Sholina, N.V.; Demina, P.A.; Generalova, A.N.; Gangadharan, A.; Sardar, D.K.; Lankamsetty, K.B.; Khochenkov, D.A.; Khaydukov, E.V.; et al. Nanocurcumin-loaded UCNPs for cancer theranostics: Physicochemical properties, in vitro toxicity, and in vivo imaging studies. Nanomaterials 2021, 11, 2234. [Google Scholar] [CrossRef]
- Guo, X.; Wu, W.; Li, Y.; Zhang, J.; Wang, L.; Ågren, H. Recent research progress for upconversion assisted dye-sensitized solar cells. Chin. Chem. Lett. 2021, 32, 1834–1846. [Google Scholar] [CrossRef]
- Liang, X.; Fan, J.; Zhao, Y.; Jin, R. Core-Shell Structured NaYF4:Yb,Er Nanoparticles with Excellent Upconversion Luminescent for Targeted Drug Delivery. J. Clust. Sci. 2021, 32, 1683–1691. [Google Scholar] [CrossRef]
- Liu, S.; Huang, J.; Yan, L.; Song, N.; Zhang, P.; He, J.; Zhou, B. Multiphoton ultraviolet upconversion through selectively controllable energy transfer in confined sensitizing sublattices towards improved solar photocatalysis. J. Mater. Chem. A 2021, 9, 4007–4017. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Ohulchanskyy, T.Y.; Chen, G. Lanthanide-Doped Near-Infrared Nanoparticles for Biophotonics. Adv. Mater. 2021, 33, 2000678. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Zheng, P.; Liu, Q.; Han, S.; Li, D.; Luo, S.; Temple, H.; Xing, C.; Wang, J.; Wei, Y.; et al. Recent advances of upconversion nanomaterials in the biological field. Nanomaterials 2021, 11, 2474. [Google Scholar] [CrossRef] [PubMed]
- Zha, S.; Chau, H.F.; Chau, W.Y.; Chan, L.S.; Lin, J.; Lo, K.W.; Cho, W.C.; Yip, Y.L.; Tsao, S.W.; Farrell, P.J.; et al. Dual-Targeting Peptide-Guided Approach for Precision Delivery and Cancer Monitoring by Using a Safe Upconversion Nanoplatform. Adv. Sci. 2021, 8, 2002919. [Google Scholar] [CrossRef] [PubMed]
- Nehra, M.; Uthappa, U.T.; Kumar, V.; Kumar, R.; Dixit, C.; Dilbaghi, N.; Mishra, Y.K.; Kumar, S.; Kaushik, A. Nanobiotechnology-assisted therapies to manage brain cancer in a personalized manner. J. Control. Release 2021, 338, 224–243. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, Y.; Li, J.; Huo, B.; Huang, H.; Bai, J.; Peng, Y.; Li, S.; Han, D.; Ren, S.; et al. A fluorescence aptasensor for the sensitive detection of T-2 toxin based on FRET by adjusting the surface electric potentials of UCNPs and MIL-101. Anal. Chim. Acta 2021, 1160, 338450. [Google Scholar] [CrossRef] [PubMed]
- Raiko, K.; Lyytikäinen, A.; Ekman, M.; Nokelainen, A.; Lahtinen, S.; Soukka, T. Supersensitive photon upconversion based immunoassay for detection of cardiac troponin I in human plasma. Clin. Chim. Acta 2021, 523, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; De La Rosa, E.; Oliva, J.; Solis, D.; Kar, A.; Patra, A. Influence of surface coating on the upconversion emission properties of LaPO4:Yb/Tm core-shell nanorods. J. Appl. Phys. 2009, 105, 113532. [Google Scholar] [CrossRef]
- Singh, R.; Dumlupinar, G.; Andersson-Engels, S.; Melgar, S. Emerging applications of upconverting nanoparticles in intestinal infection and colorectal cancer. Int. J. Nanomed. 2019, 14, 1027–1038. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Liang, T.; Wang, Q.; Liu, Z. Strategies for Constructing Upconversion Luminescence Nanoprobes to Improve Signal Contrast. Small 2020, 16, 1905084. [Google Scholar] [CrossRef]
- Sedlmeier, A.; Gorris, H.H. Surface modification, and characterization of photon-upconverting nanoparticles for bioanalytical applications. Chem. Soc. Rev. 2015, 44, 1526–1560. [Google Scholar] [CrossRef] [Green Version]
- Kostiv, U.; Farka, Z.; Mickert, M.J.; Gorris, H.H.; Velychkivska, N.; Pop-Georgievski, O.; Pastucha, M.; Odstrčilíková, E.; Skládal, P.; Horák, D. Versatile Bioconjugation Strategies of PEG-Modified Upconversion Nanoparticles for Bioanalytical Applications. Biomacromolecules 2020, 21, 4502–4513. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Deng, Q.; Fang, G.; Gu, D.; Yang, Y.; Wang, S. Upconversion fluorescence metal-organic frameworks thermo-sensitive imprinted polymer for enrichment and sensing protein. Biosens. Bioelectron. 2016, 79, 341–346. [Google Scholar] [CrossRef]
- Liu, H.; Li, J.; Hu, P.; Sun, S.; Shi, L.; Sun, L. Facile synthesis of Er3+/Tm3+ co-doped magnetic/luminescent nanosystems for possible bioimaging and therapy applications. J. Rare Earths 2022, 40, 11–19. [Google Scholar] [CrossRef]
- Liu, G.; Jiang, F.; Chen, Y.; Yu, C.; Ding, B.; Shao, S.; Jia, M.; Ma, P.; Fu, Z.; Lin, J. Superior Temperature Sensing of Small-Sized Upconversion Nanocrystals for Simultaneous Bioimaging and Enhanced Synergetic Therapy; Elsevier Inc.: Amsterdam, The Netherlands, 2020; Volume 24, ISBN 8643185262614. [Google Scholar]
- Zhou, J.; Liu, Z.; Li, F. Upconversion nanophosphors for small-animal imaging. Chem. Soc. Rev. 2012, 41, 1323–1349. [Google Scholar] [CrossRef] [PubMed]
- González-Béjar, M.; Francés-Soriano, L.; Pérez-Prieto, J. Upconversion nanoparticles for bioimaging and regenerative medicine. Front. Bioeng. Biotechnol. 2016, 4, 47. [Google Scholar] [CrossRef] [Green Version]
- Bartosik, P.B.; Fitzgerald, J.E.; El Khatib, M.; Yaseen, M.A.; Vinogradov, S.A.; Niedre, M. Prospects for the use of upconverting nanoparticles as a contrast agent for enumeration of circulating cells in vivo. Int. J. Nanomed. 2020, 15, 1709–1719. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Wen, S.; Kong, M.; Liu, Y.; Hu, W.; Shi, B.; Shi, X.; Jin, D. Highly Doped Upconversion Nanoparticles for in Vivo Applications under Mild Excitation Power. Anal. Chem. 2020, 92, 10913–10919. [Google Scholar] [CrossRef]
- Mohan, M.; Maurya, S.K.; Kumar, K.; Poddar, R. In Vitro Imaging of Animal Tissue with Upconversion Nanoparticles (UCNPs) as a Molecular Probing Agent Using Swept Source Optical Coherence Tomography (SSOCT). J. Med. Biol. Eng. 2020, 40, 251–263. [Google Scholar] [CrossRef]
- Gerelkhuu, Z.; Huy, B.T.; Sharipov, M.; Jung, D.; Phan, T.L.; Conte, E.D.; Lee, Y.I. One-step synthesis of NaLu80−xGdxF4:Yb183+/Er23+(Tm3+) upconversion nanoparticles for in vitro cell imaging. Mater. Sci. Eng. C 2018, 86, 56–61. [Google Scholar] [CrossRef]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.; Hong, H.; Cai, W. Radio-nanomaterials for biomedical applications: State of the art. Eur. J. Nanomed. 2016, 8, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Zhao, S.; Liu, G.; Chen, H.; Ma, L.; You, H.; Liu, C.; Wang, Z. Construction of lanthanide-doped upconversion nanoparticle-Uelx Europaeus Agglutinin-I bioconjugates with brightness red emission for ultrasensitive in vivo imaging of colorectal tumor. Biomaterials 2019, 212, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zhang, C.; Ahmed, A.; Zhao, Y.; Deng, Y.; Ding, Y.; Cai, J.; Hu, Y. H2O2-Sensitive Upconversion Nanocluster Bomb for Tri-Mode Imaging-Guided Photodynamic Therapy in Deep Tumor Tissue. Adv. Healthc. Mater. 2019, 8, 1900972. [Google Scholar] [CrossRef]
- Zheng, X.; Zhu, X.; Lu, Y.; Zhao, J.; Feng, W.; Jia, G.; Wang, F.; Li, F.; Jin, D. High-Contrast Visualization of Upconversion Luminescence in Mice Using Time-Gating Approach. Anal. Chem. 2016, 88, 3449–3454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolesov, R.; Reuter, R.; Xia, K.; Stöhr, R.; Zappe, A.; Wrachtrup, J. Super-resolution upconversion microscopy of praseodymium-doped yttrium aluminum garnet nanoparticles. Phys. Rev. B-Condens. Matter Mater. Phys. 2011, 84, 153413. [Google Scholar] [CrossRef]
- Dong, H.; Sun, L.D.; Yan, C.H. Lanthanide-Doped Upconversion Nanoparticles for Super-Resolution Microscopy. Front. Chem. 2021, 8, 1231. [Google Scholar] [CrossRef]
- Xing, H.; Bu, W.; Zhang, S.; Zheng, X.; Li, M.; Chen, F.; He, Q.; Zhou, L.; Peng, W.; Hua, Y.; et al. Multifunctional nanoprobes for upconversion fluorescence, MR and CT trimodal imaging. Biomaterials 2012, 33, 1079–1089. [Google Scholar] [CrossRef]
- Zhao, N.; Wu, B.; Hu, X.; Xing, D. NIR-triggered high-efficient photodynamic and chemo-cascade therapy using caspase-3 responsive functionalized upconversion nanoparticles. Biomaterials 2017, 141, 40–49. [Google Scholar] [CrossRef]
- Ren, F.; Ding, L.; Liu, H.; Huang, Q.; Zhang, H.; Zhang, L.; Zeng, J.; Sun, Q.; Li, Z.; Gao, M. Ultra-small nanocluster mediated synthesis of Nd3+-doped core-shell nanocrystals with emission in the second near-infrared window for multimodal imaging of tumor vasculature. Biomaterials 2018, 175, 30–43. [Google Scholar] [CrossRef]
- Mimun, L.C.; Ajithkumar, G.; Rightsell, C.; Langloss, B.W.; Therien, M.J.; Sardar, D.K. Synthesis and characterization of Na(Gd0.5Lu0.5)F4: Nd3+, a core-shell free multifunctional contrast agent. J. Alloys Compd. 2017, 695, 280–285. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zhang, Y. An efficient and user-friendly method for the synthesis of hexagonal-phase NaYF4:Yb, Er/Tm nanocrystals with controllable shape and upconversion fluorescence. Nanotechnology 2008, 19, 345606. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Bu, W.; Ren, Q.; Zheng, X.; Li, M.; Zhang, S.; Qu, H.; Wang, Z.; Hua, Y.; Zhao, K.; et al. A NaYbF4: Tm3+ nanoprobe for CT and NIR-to-NIR fluorescent bimodal imaging. Biomaterials 2012, 33, 5384–5393. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, W.; Gai, S.; Yang, G.; Zhong, C.; Dai, Y.; He, F.; Yang, P.; Suh, Y.D. A smart tumor microenvironment responsive nanoplatform based on upconversion nanoparticles for efficient multimodal imaging guided therapy. Biomater. Sci. 2019, 7, 951–962. [Google Scholar] [CrossRef] [PubMed]
- De Guereñu, A.L.; Bastian, P.; Wessig, P.; John, L.; Kumke, M.U. Energy transfer between TM-doped upconverting nanoparticles and a small organic dye with large stokes shift. Biosensors 2019, 9, 9. [Google Scholar] [CrossRef] [Green Version]
- Su, Q.; Feng, W.; Yang, D.; Li, F. Resonance energy transfer in upconversion nanoplatforms for selective biodetection. Acc. Chem. Res. 2017, 50, 32–40. [Google Scholar] [CrossRef]
- Xu, S.; Dong, B.; Zhou, D.; Yin, Z.; Cui, S.; Xu, W.; Chen, B.; Song, H. Paper-based upconversion fluorescence resonance energy transfer biosensor for sensitive detection of multiple cancer biomarkers. Sci. Rep. 2016, 6, 23406. [Google Scholar] [CrossRef] [Green Version]
- Jo, E.J.; Mun, H.; Kim, M.G. Homogeneous Immunosensor Based on Luminescence Resonance Energy Transfer for Glycated Hemoglobin Detection Using Upconversion Nanoparticles. Anal. Chem. 2016, 88, 2742–2746. [Google Scholar] [CrossRef]
- Li, Z.; Lv, S.; Wang, Y.; Chen, S.; Liu, Z. Construction of LRET-based nanoprobe using upconversion nanoparticles with confined emitters and bared surface as a luminophore. J. Am. Chem. Soc. 2015, 137, 3421–3427. [Google Scholar] [CrossRef]
- Mo, J.; Shen, L.; Xu, Q.; Zeng, J.; Sha, J.; Hu, T.; Bi, K.; Chen, Y. An Nd3+-Sensitized Upconversion Fluorescent Sensor for Epirubicin Detection. Nanomaterials 2019, 9, 1700. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Xu, L.; Ma, W.; Wu, X.; Sun, M.; Kuang, H.; Wang, L.; Kotov, N.A.; Xu, C. Dual-Mode Ultrasensitive Quantification of MicroRNA in Living Cells by Chiroplasmonic Nanopyramids Self-Assembled from Gold and Upconversion Nanoparticles. J. Am. Chem. Soc. 2016, 138, 306–312. [Google Scholar] [CrossRef]
- Gong, Z.; Wu, T.; Chen, X.; Guo, J.; Zhang, Y.; Li, Y. Upconversion Nanoparticle Decorated Spider Silks as Single-Cell Thermometers. Nano Lett. 2021, 21, 1469–1476. [Google Scholar] [CrossRef]
- Lin, X.; Kong, M.; Wu, N.; Gu, Y.; Qiu, X.; Chen, X.; Li, Z.; Feng, W.; Li, F. Measurement of Temperature Distribution at the Nanoscale with Luminescent Probes Based on Lanthanide Nanoparticles and Quantum Dots. ACS Appl. Mater. Interfaces 2020, 12, 52393–52401. [Google Scholar] [CrossRef] [PubMed]
- Sedlmeier, A.; Achatz, D.E.; Fischer, L.H.; Gorris, H.H.; Wolfbeis, O.S. Photon upconverting nanoparticles for luminescent sensing of temperature. Nanoscale 2012, 4, 7090–7096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Qian, C.; Shen, R.; Xiao, H.; Zhao, W.; Ye, S. Fluorescence fiber optic temperature sensor based on fused upconversion luminescent nanoparticles. Opt. Express 2018, 26, 30753. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.; Li, Z.; Ren, Z.; Li, X.; Han, G. Rare-earth-doped upconversion nanocrystals embedded mesoporous silica nanoparticles for multiple microRNA detection. Chem. Eng. J. 2019, 374, 863–869. [Google Scholar] [CrossRef]
- Cheng, Z.H.; Liu, X.; Zhang, S.Q.; Yang, T.; Chen, M.L.; Wang, J.H. Placeholder Strategy with Upconversion Nanoparticles-Eriochrome Black T Conjugate for a Colorimetric Assay of an Anthrax Biomarker. Anal. Chem. 2019, 91, 12094–12099. [Google Scholar] [CrossRef]
- Wei, R.; Wei, Z.; Sun, L.; Zhang, J.Z.; Liu, J.; Ge, X.; Shi, L. Nile Red Derivative-Modified Nanostructure for Upconversion Luminescence Sensing and Intracellular Detection of Fe3+ and MR Imaging. ACS Appl. Mater. Interfaces 2016, 8, 400–410. [Google Scholar] [CrossRef]
- Ren, H.; Long, Z.; Shen, X.; Zhang, Y.; Sun, J.; Ouyang, J.; Na, N. Sandwich DNA Hybridization Fluorescence Resonance Energy-Transfer Strategy for miR-122 Detection by Core-Shell Upconversion Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 25621–25628. [Google Scholar] [CrossRef]
- Qiu, Z.; Shu, J.; Tang, D. Near-Infrared-to-Ultraviolet Light-Mediated Photoelectrochemical Aptasensing Platform for Cancer Biomarker Based on Core-Shell NaYF4:Yb,Tm@TiO2 Upconversion Microrods. Anal. Chem. 2018, 90, 1021–1028. [Google Scholar] [CrossRef]
- Ma, L.; Liu, F.; Lei, Z.; Wang, Z. A novel upconversion@polydopamine core@shell nanoparticle based aptameric biosensor for biosensing and imaging of cytochrome c inside living cells. Biosens. Bioelectron. 2017, 87, 638–645. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Deng, H.; Xiong, X.; Zhang, H.; Liang, T.; Li, C. An aptamer biosensor for CA125 quantification in human serum based on upconversion luminescence resonance energy transfer. Microchem. J. 2021, 161, 105761. [Google Scholar] [CrossRef]
- Hu, S.; Xu, H.; Zhou, B.; Xu, S.; Shen, B.; Dong, B.; Yin, Z.; Xu, S.; Sun, L.; Lv, J.; et al. Double Stopband Bilayer Photonic Crystal Based Upconversion Fluorescence PSA Sensor. Sens. Actuators B Chem. 2021, 326, 128816. [Google Scholar] [CrossRef]
- Navya, P.N.; Kaphle, A.; Srinivas, S.P.; Bhargava, S.K.; Rotello, V.M.; Daima, H.K. Current trends, and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019, 6, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects 10 Technology 1007 Nanotechnology 03 Chemical Sciences 0306 Physical Chemistry (incl. Structural) 03 Chemical Sciences 0303 Macromolecular and Materials Chemistry 11 Medical and He. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, S.; Samanta, A.; Xie, X.; Huang, L.; Peng, J.; Park, S.J.; Teh, D.B.L.; Choi, Y.; Chang, Y.T.; All, A.H.; et al. Gold and Hairpin DNA Functionalization of Upconversion Nanocrystals for Imaging and In Vivo Drug Delivery. Adv. Mater. 2017, 29, 1700244. [Google Scholar] [CrossRef]
- Gargas, D.J.; Chan, E.M.; Ostrowski, A.D.; Aloni, S.; Altoe, M.V.P.; Barnard, E.S.; Sanii, B.; Urban, J.J.; Milliron, D.J.; Cohen, B.E.; et al. Engineering bright sub-10-nm upconverting nanocrystals for single-molecule imaging. Nat. Nanotechnol. 2014, 9, 300–305. [Google Scholar] [CrossRef]
- Liu, F.; He, X.; Lei, Z.; Liu, L.; Zhang, J.; You, H.; Zhang, H.; Wang, Z. Facile preparation of doxorubicin-loaded upconversion@polydopamine nanoplatforms for simultaneous in vivo multimodality imaging and chemophotothermal synergistic therapy. Adv. Healthc. Mater. 2015, 4, 559–568. [Google Scholar] [CrossRef]
- Bao, W.; Liu, X.; Lv, Y.; Lu, G.H.; Li, F.; Zhang, F.; Liu, B.; Li, D.; Wei, W.; Li, Y. Nanolongan with Multiple On-Demand Conversions for Ferroptosis-Apoptosis Combined Anticancer Therapy. ACS Nano 2019, 13, 260–273. [Google Scholar] [CrossRef]
- Song, X.; Yan, T.; Tian, F.; Li, F.; Ren, L.; Li, Q.; Zhang, S. Aptamer Functionalized Upconversion Nanotheranostic Agent With Nuclear Targeting as the Highly Localized Drug-Delivery System of Doxorubicin. Front. Bioeng. Biotechnol. 2021, 9, 38. [Google Scholar] [CrossRef]
- Xiang, J.; Tong, X.; Shi, F.; Yan, Q.; Yu, B.; Zhao, Y. Near-infrared light-triggered drug release from UV-responsive diblock copolymer-coated upconversion nanoparticles with high monodispersity. J. Mater. Chem. B 2018, 6, 3531–3540. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.; Liu, H.; Li, Y.; Xu, Z.; Li, L.; Whittaker, A. Controllable synthesis of up-conversion nanoparticles UCNPs@MIL-PEG for pH-responsive drug delivery and potential up-conversion luminescence/magnetic resonance dual-mode imaging. J. Alloys Compd. 2018, 749, 939–947. [Google Scholar] [CrossRef]
- Zhang, R.; Yao, R.; Ding, B.; Shen, Y.; Shui, S.; Wang, L.; Li, Y.; Yang, X.; Tao, W. Fabrication of upconverting hybrid nanoparticles for near-infrared light triggered drug release. Adv. Mater. Sci. Eng. 2014, 2014, 169210. [Google Scholar] [CrossRef] [Green Version]
- Lin, M.; Gao, Y.; Diefenbach, T.J.; Shen, J.K.; Hornicek, F.J.; Park, Y.I.; Xu, F.; Lu, T.J.; Amiji, M.; Duan, Z. Facial Layer-by-Layer Engineering of Upconversion Nanoparticles for Gene Delivery: Near-Infrared-Initiated Fluorescence Resonance Energy Transfer Tracking and Overcoming Drug Resistance in Ovarian Cancer. ACS Appl. Mater. Interfaces 2017, 9, 7941–7949. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Y.; Liu, C.; Guo, H.; Chen, Z.; Xia, J.; Liao, Y.; Tang, C.Y.; Law, W.C. Photo- and pH-responsive drug delivery nanocomposite based on o-nitrobenzyl functionalized upconversion nanoparticles. Polymer 2021, 229, 123961. [Google Scholar] [CrossRef]
- Liu, J.N.; Bu, W.; Pan, L.M.; Zhang, S.; Chen, F.; Zhou, L.; Zhao, K.L.; Peng, W.; Shi, J. Simultaneous nuclear imaging and intranuclear drug delivery by nuclear-targeted multifunctional upconversion nanoprobes. Biomaterials 2012, 33, 7282–7290. [Google Scholar] [CrossRef]
- Chen, G.; Jaskula-Sztul, R.; Esquibel, C.R.; Lou, I.; Zheng, Q.; Dammalapati, A.; Harrison, A.; Eliceiri, K.W.; Tang, W.; Chen, H.; et al. Neuroendocrine Tumor-Targeted Upconversion Nanoparticle-Based Micelles for Simultaneous NIR-Controlled Combination Chemotherapy and Photodynamic Therapy, and Fluorescence Imaging. Adv. Funct. Mater. 2017, 27, 1604671. [Google Scholar] [CrossRef]
- Osuchowski, M.; Osuchowski, F.; Latos, W.; Kawczyk-Krupka, A. The use of upconversion nanoparticles in prostate cancer photodynamic therapy. Life 2021, 11, 360. [Google Scholar] [CrossRef]
- Gheewala, T.; Skwor, T.; Munirathinam, G. Photosensitizers in prostate cancer therapy. Oncotarget 2017, 8, 30524–30538. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Cheng, L.; Liu, Y.; Wang, X.; Ma, X.; Deng, Z.; Li, Y.; Liu, Z. Imaging-guided pH-sensitive photodynamic therapy using charge reversible upconversion nanoparticles under near-infrared light. Adv. Funct. Mater. 2013, 23, 3077–3086. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, L.; Liu, Z. Upconversion nanoparticles for photodynamic therapy and other cancer therapeutics. Theranostics 2013, 3, 317–330. [Google Scholar] [CrossRef] [Green Version]
- Xie, A.; Li, H.; Hao, Y.; Zhang, Y. Tuning the Toxicity of Reactive Oxygen Species into Advanced Tumor Therapy. Nanoscale Res. Lett. 2021, 16, 142. [Google Scholar] [CrossRef]
- Jin, F.; Liu, D.; Xu, X.; Ji, J.; Du, Y. Nanomaterials-based photodynamic therapy with combined treatment improves antitumor efficacy through boosting immunogenic cell death. Int. J. Nanomed. 2021, 16, 4693–4712. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.C.; Vijayaraghavan, P.; Chiang, W.H.; Chen, H.H.; Liu, T.I.; Shen, M.Y.; Omoto, A.; Kamimura, M.; Soga, K.; Chiu, H.C. Targeted delivery of functionalized upconversion nanoparticles for externally triggered photothermal/photodynamic therapies of brain glioblastoma. Theranostics 2018, 8, 1435–1448. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Ge, X.; Ke, D.M.; Tang, H.; Zhang, J.Z.; Calvaresi, M.; Gao, B.; Sun, L.; Su, Q.; Wang, H. The bioavailability, biodistribution, and toxic effects of silica-coated upconversion nanoparticles in vivo. Front. Chem. 2019, 7, 218. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Yang, T.; Yang, Y.; Xu, C.; Li, F. Long-term in vivo biodistribution imaging and toxicity of polyacrylic acid-coated upconversion nanophosphors. Biomaterials 2010, 31, 7078–7085. [Google Scholar] [CrossRef] [PubMed]




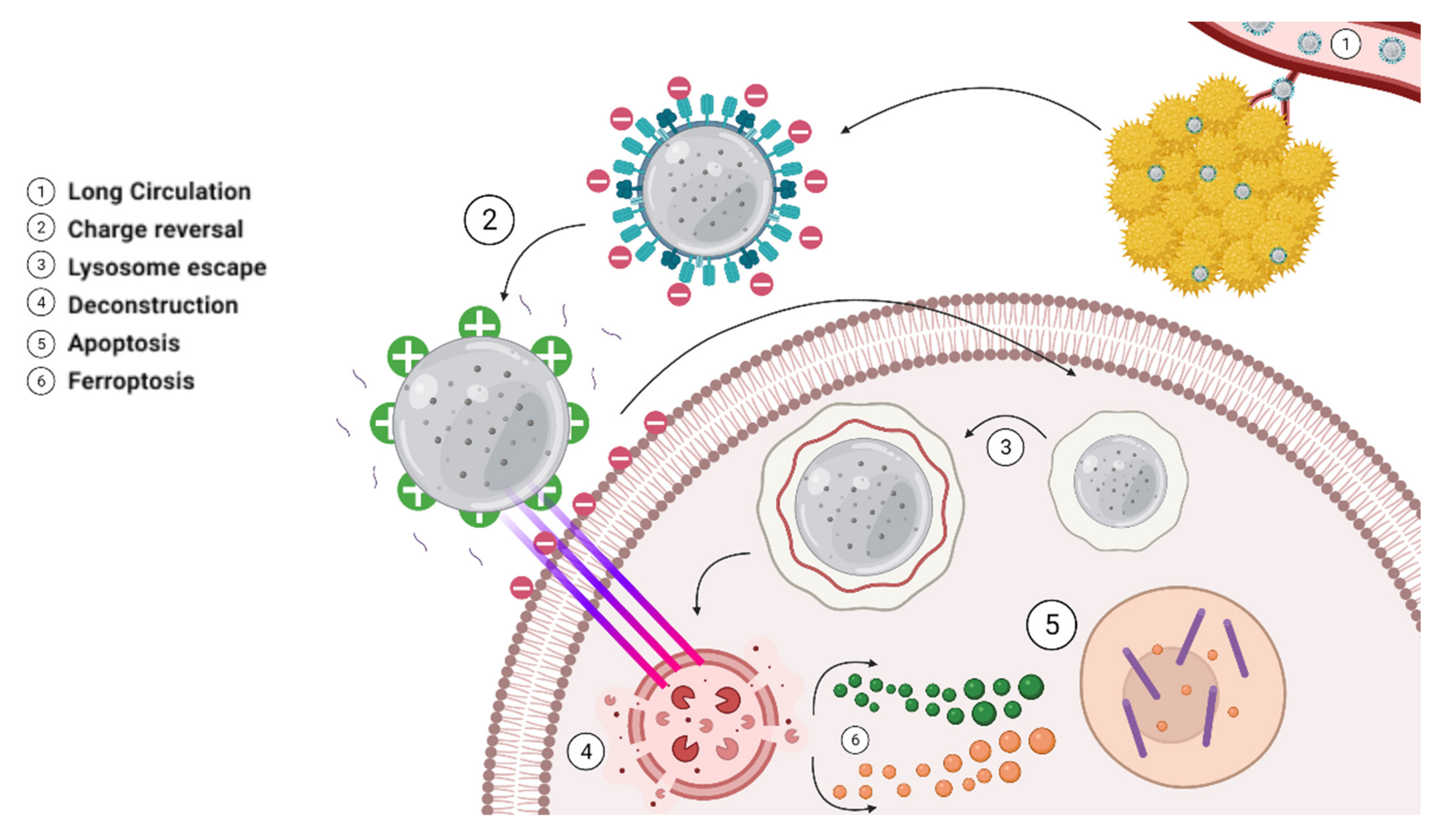
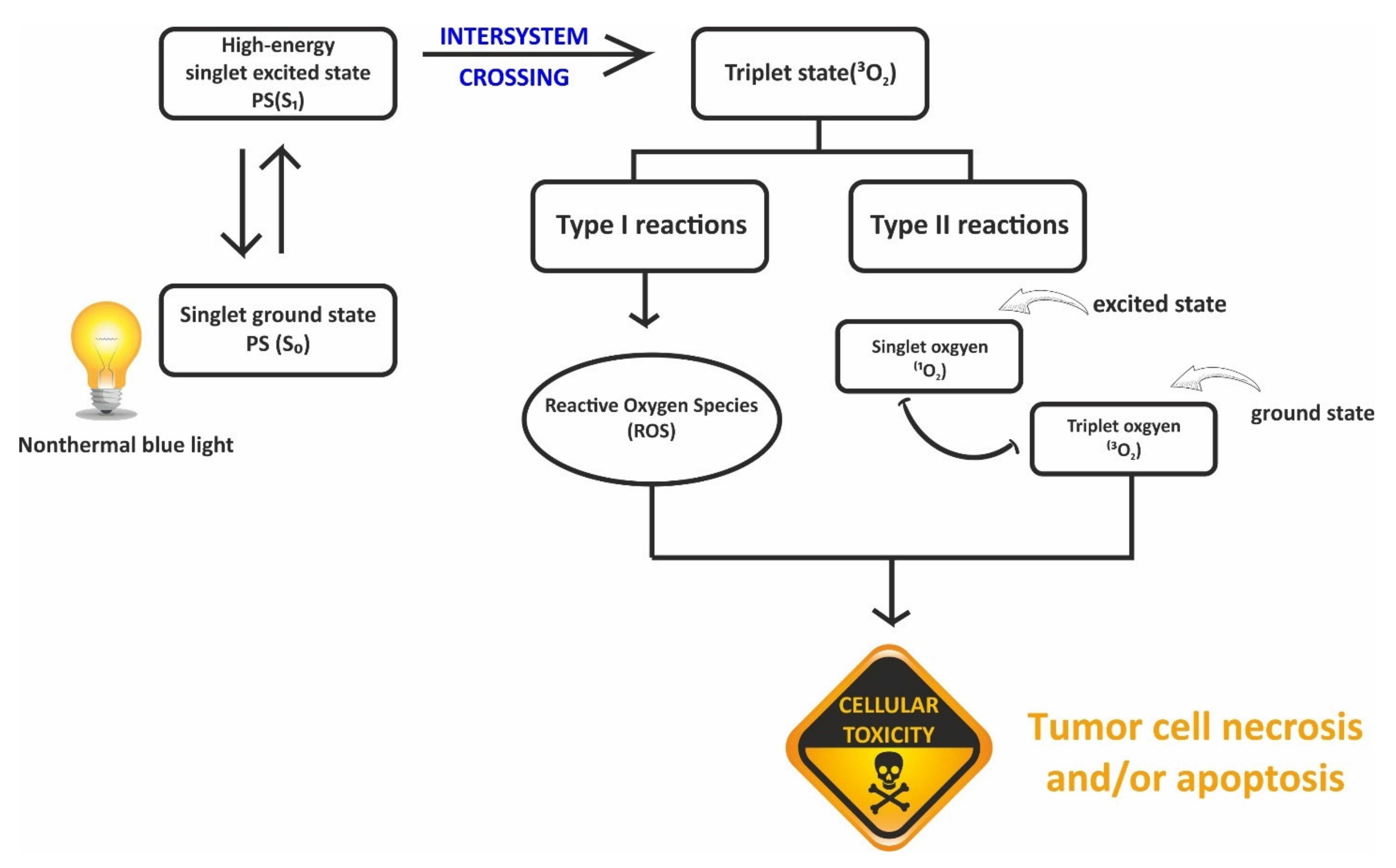
| Methods | Conditions | Advantages | Disadvantages | Examples | References |
|---|---|---|---|---|---|
| Thermal decomposition | Higher temperature with an anhydrous anaerobic environment | High uniformity and monodisperse crystals, high luminous efficiency | Expensive, toxic byproducts are formed | NaYF4, NaYbF4, LiYF4 | [44,45] |
| Co-precipitation method | Soluble salt solution, precipitant, coordinating ligand | Cost-effective with a simple operation process, ultrasmall UCNPs can be formed, usually requiring post treatment | Lack of particle size control | NaGdF4, LaF3, BaY5 | [46,47] |
| Hydrothermal method | Higher temperature and pressure conditions | Simple and inexpensive, good control of morphology and the size of crystals can control the shape and size of the product | Nanocrystal growth process cannot be observed | NaYF4, NaYbF4, YVO4, BaYF2 | [48,49] |
| Microemulsion method | Appropriate surfactant to stabilize a micelle and/or to control the growth of nanocrystals | Simple operation process, narrow size, high stability | In most cases, calcination or annealing is usually required | LaF3, NaYF4 | [50] |
| Combustion method | Explosive reaction by heating, the reaction temperature is generally 500–3000 °C | Faster reaction time and less utilization of energy; controllable product quantity | Poor product purity and luminescence | Ba5(PO4)3OH: Er3+/Yb3+ Na3Y(PO4)2: Er3+/Yb3+ | [51] |
| Sol-gel processing method | High luminescence intensity due to high crystallinity at high annealing temperature | Inexpensive precursors; small product size and simple procedures | Broad particle size and unsuitable for bioapplication | GdVO4 | [52] |
| Composition/Modifier | Results | Route of Synthesis | References |
|---|---|---|---|
| β-NaY/GdF4: Yb, Er, Tm (UCNP) | Targets the lymphatic node, used for MR and CT imaging | Thermal decomposition | [81] |
| NaYF4:Yb3+, Er3+ /DEVD peptide | In vitro and in vivo fluorescence results demonstrated the potency of tumor cell killing and significant suppression of tumor growth without any detectable side effects | Hydrothermal method | [82] |
| NaYF4:5%Nd@NaGdF4/DSPE-PEG2000 | Strongest photoluminescence among the resultant NCs for NIR-II fluorescence imaging, and possess strong paramagnetism and X-ray attenuation for MRI and CT imaging | Liquid–solid-solution | [83] |
| NaLuF4:Gd3+/Yb3+/Tm3+/Oleic acid | Used for fluorescence imaging/MRI | Solvothermal method | [84] |
| NaYbF4:Tm3+/PEG | CT and strong NIR-fluorescent imaging that demonstrates both high in vitro and in vivo performances in the dual-bioimaging; very low cytotoxicity | User-friendly method [85] | [86] |
| NaYF4: Yb, Er@NaYF4: Yb, Nd UCNPs /Folate–chitosan | Effective UCL/CT imaging and combined chemotherapy and photothermal therapy | - | [87] |
| Mechanism | Biomarker | Probes | Limit of Detection (LOD) | Applications | Reference |
|---|---|---|---|---|---|
| Fluorescence | CaF2:RE3+@MSN+ Fe3O4 | Oligonucleotide | 100 nM | Multiple breast cancer-related miRNA biomarkers. | [99] |
| Fluorescence | Dipicolinic acid (DPA) | UCNPs−TPP/EBT | 0.9 μM | Analysis of DPA in human serum. | [100] |
| Luminescence resonance energy transfer | Fe3+ | NaYF4:Yb,Er,Tm@NaGdF4/ Nile red derivative (NRD) fluorescent | 106.2 nM | Development of mPEG-UCNPs-NRD nanostructure used for detecting the intracellular Fe3+. | [101] |
| Fluorescence resonance energy transfer | Microrna-122 | NaGdF4@NaGdF4: Yb,Er@DNA | 10−13 M | Sandwich-hybridization observed between miR-122 and the designed DNAs. | [102] |
| Photoelectrochemical (PEC) aptasensing | Carcinoembryonic antigen | NaYF4:Yb, Tm@TiO2 upconversion microrods | 3.6 pg/mL | NIR light-mediated PEC aptasensing system exhibiting a PEC response towards target CEA and its detection. | [103] |
| Fluorescence | Cyt c aptamer | NaYF4:Yb,Er@ NaGdF4@PDA@AP | 20 nM | Intracellular Cyt c evaluation using UCNP@PDA@AP. | [104] |
| Luminescence resonance energy transfer | Carbohydrate antigen125 (CA125) | Polyacrylic acid (PAA)-coated UCNPs | 9.0 × 10−3 U/mL−1 | CA125 quantification in human serum, construction of point-of-care testing (POCT) devices. | [105] |
| Fluorescence | Prostate-specific antigen (PSA) | Anti-PSA antibodies | 0.01 ng/mL | Biochip sensor for early diagnosis of cancer markers. | [106] |
| Material Composition | Payload Drug in UCNPs | UCL Excitation (nm) | Therapeutic Efficacy/Drug Loading Efficiency | Release Profile | Results | References |
|---|---|---|---|---|---|---|
| UCNPs@PDL PDL-poly-D-lysine | DOX | 980 nm | - | - | Nanotheranostic agent developed to achieve highly localized therapy with great therapeutic efficacy against malignant tumors | [113] |
| NaYF4:Yb3+, Tm3+ | DOX | 980 nm | - | Increase in DOX release by activation of NIR light | Development of NIR light-triggered drug release of encapsulated DOX molecules by using UCNP/polymer nanomaterials in diblock copolymer self-assembly | [114] |
| UCNPs@MIL-PEG | DOX | 980 nm | Therapeutic efficacy-60% | Less than 20% at pH = 7.4 UCNPs@MIL-100–60% after 30 h at pH 7.4 and 80% after 50 h; UCNPs@MIL-PEG reaches less than 20% at pH 7.4 | Application of multifunctional UCNPs@MIL-PEG nanoparticles for UCL/MR dual-mode imaging and pH-responsive anticancer drug delivery | [115] |
| NaYF4: Tm3+, Yb3+ | Nile Red | 980 nm | - | - | Synthesized hybrid nanoparticles release the Nile red in response to a NIR-triggered drug release stimulus | [116] |
| NaYF4: Yb,Er/PAA/PEI nanoparticles | MDR1-siRNA | 980 nm | Drug loading rate: 34.1% | 50% MDR1-siRNA released from UCNP/PAA/PEI/MDR1-siRNA complex | UCNP nano complex—effective in gene silencing in paclitaxel-resistant ovarian cancer cells and resensitizes them to paclitaxel treatment | [117] |
| UCNPs@SiO2@PNBAM/MAA | DOX | 980 nm | Drug loading rate: 7.23 wt% | Release rate constants and the correlation coefficients 4.15 × 10−6 and 0.98 (pH 7.4 and visible light), 2.64 × 10−5 and 0.99 (NIR light), 3.26 × 10−5 and 0.97 (pH 4.5 and visible light), 2.59 × 10−4 and 0.99 (pH 4.5 and NIR light), respectively | NIR irradiation and acidic conditions are beneficial to drug release; this controlled release feature makes the nanocomposite a promising carrier of drugs | [118] |
| NaYF4:Er/Yb@NaGdF4 ePEG | DOX | 980 nm | - | - | Nuclear-targeted UCNPs-based theranostic systems combined with MR/optical imaging for cell nuclei and direct nuclear drug delivery functionalities to deliver drugs into the cell nuclei more efficiently | [119] |
| NaYF4:Yb/Tm/Er | hydrophobic AB3 | 980 nm | Loading efficiency: 16.7 wt% | Released without the 980 nm laser (<14 wt%) after 16 h. With a 10 min irradiation of 980 nm laser—nearly 75 wt% of drugs released after 16 h | A superior chemotherapy efficacy, whereas in vivo studies demonstrated that AB3-loaded UCNP-based micelles capable of targeted combination chemotherapy and PDT—provides a better antitumor efficacy compared to chemotherapy or PDT alone, without any apparent systemic toxicity | [120] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jethva, P.; Momin, M.; Khan, T.; Omri, A. Lanthanide-Doped Upconversion Luminescent Nanoparticles—Evolving Role in Bioimaging, Biosensing, and Drug Delivery. Materials 2022, 15, 2374. https://doi.org/10.3390/ma15072374
Jethva P, Momin M, Khan T, Omri A. Lanthanide-Doped Upconversion Luminescent Nanoparticles—Evolving Role in Bioimaging, Biosensing, and Drug Delivery. Materials. 2022; 15(7):2374. https://doi.org/10.3390/ma15072374
Chicago/Turabian StyleJethva, Palak, Munira Momin, Tabassum Khan, and Abdelwahab Omri. 2022. "Lanthanide-Doped Upconversion Luminescent Nanoparticles—Evolving Role in Bioimaging, Biosensing, and Drug Delivery" Materials 15, no. 7: 2374. https://doi.org/10.3390/ma15072374