Polyoxomolybdate Layered Crystals Constructed from a Heterocyclic Surfactant: Syntheses, Pseudopolymorphism and Introduction of Metal Cations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Genaral Procedures and Instrumental Methods
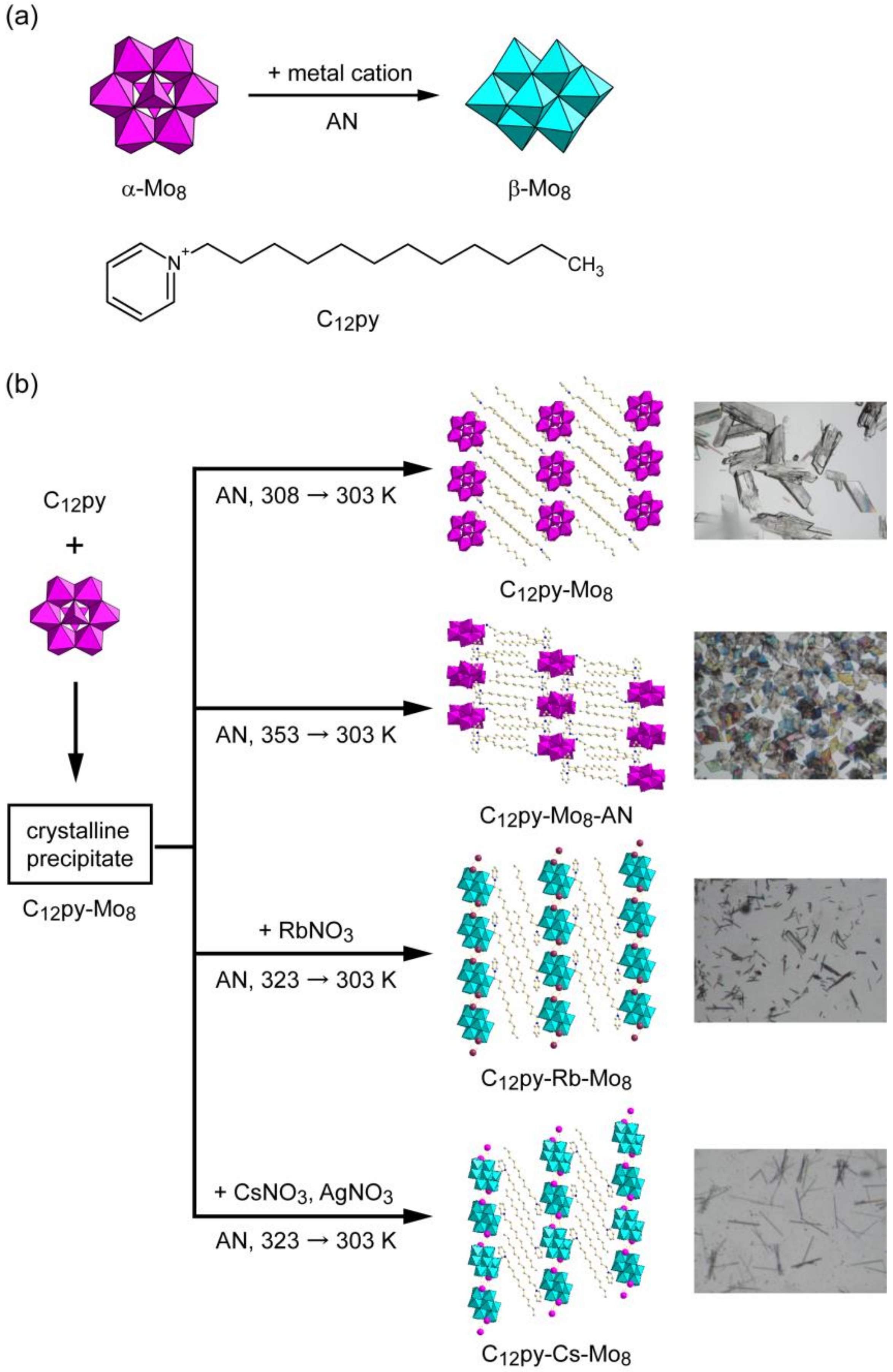
2.2. Syntheses of C12py-Mo8 and Related Hybrid Crystals
2.2.1. C12py-Mo8
2.2.2. C12py-Mo8-AN
2.2.3. C12py-Rb-Mo8
2.2.4. C12py-Cs-Mo8
2.3. X-ray Crystallography
3. Results
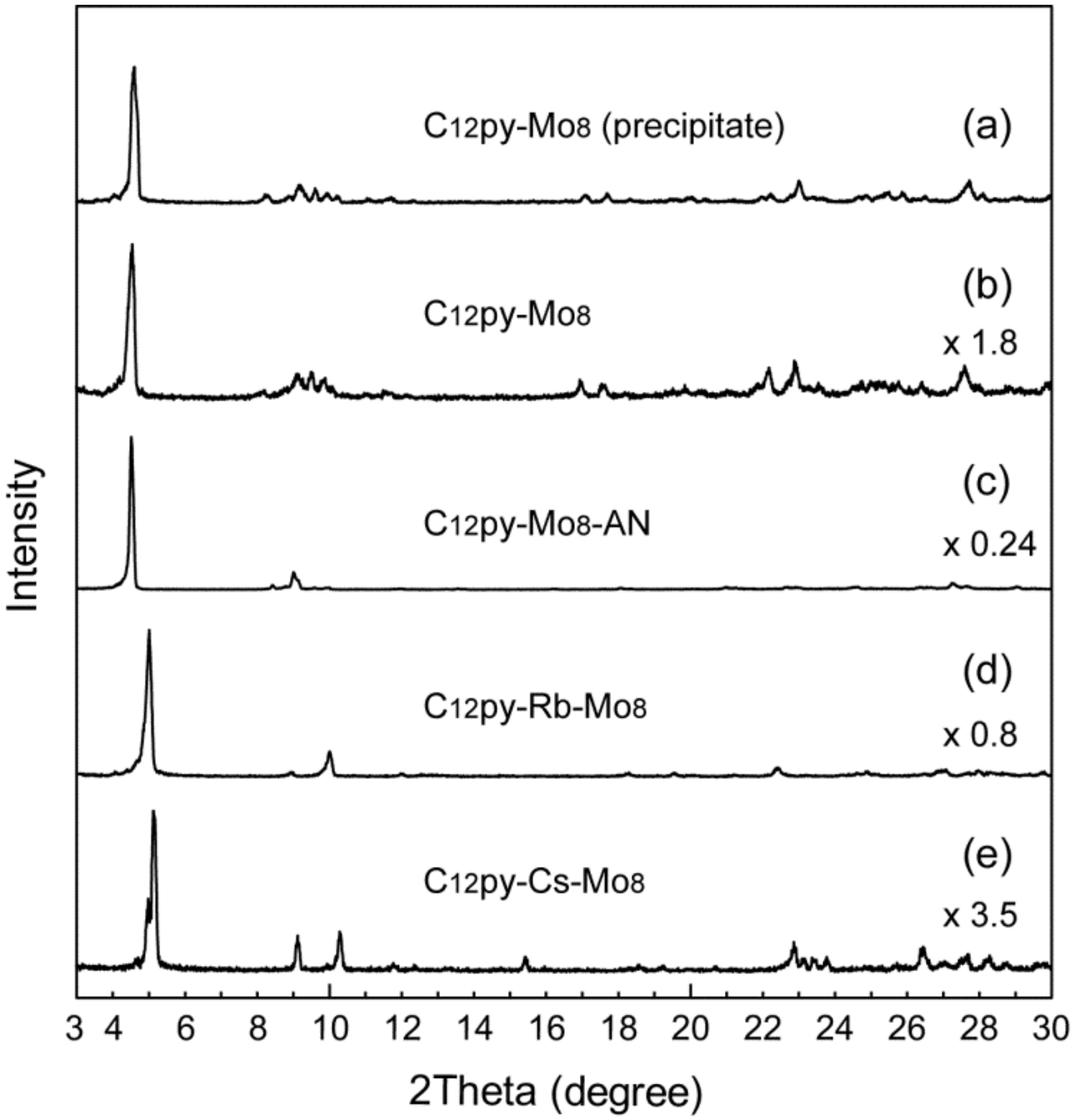
3.1. Syntheses of a Series of C12py-Mo8 Hybrid Crystals
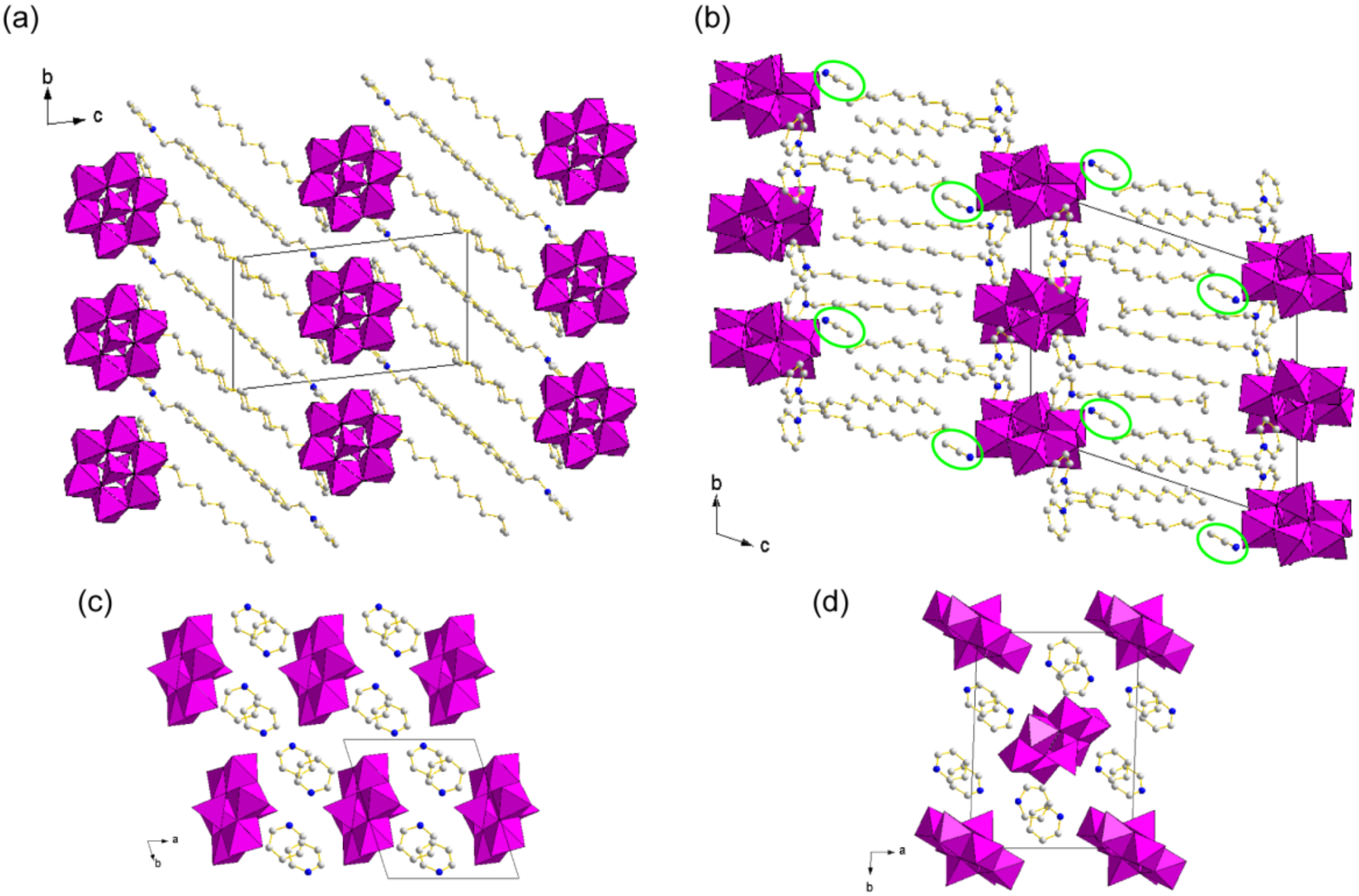
3.2. Crystal Structures of C12py-Mo8 Hybrid Crystals
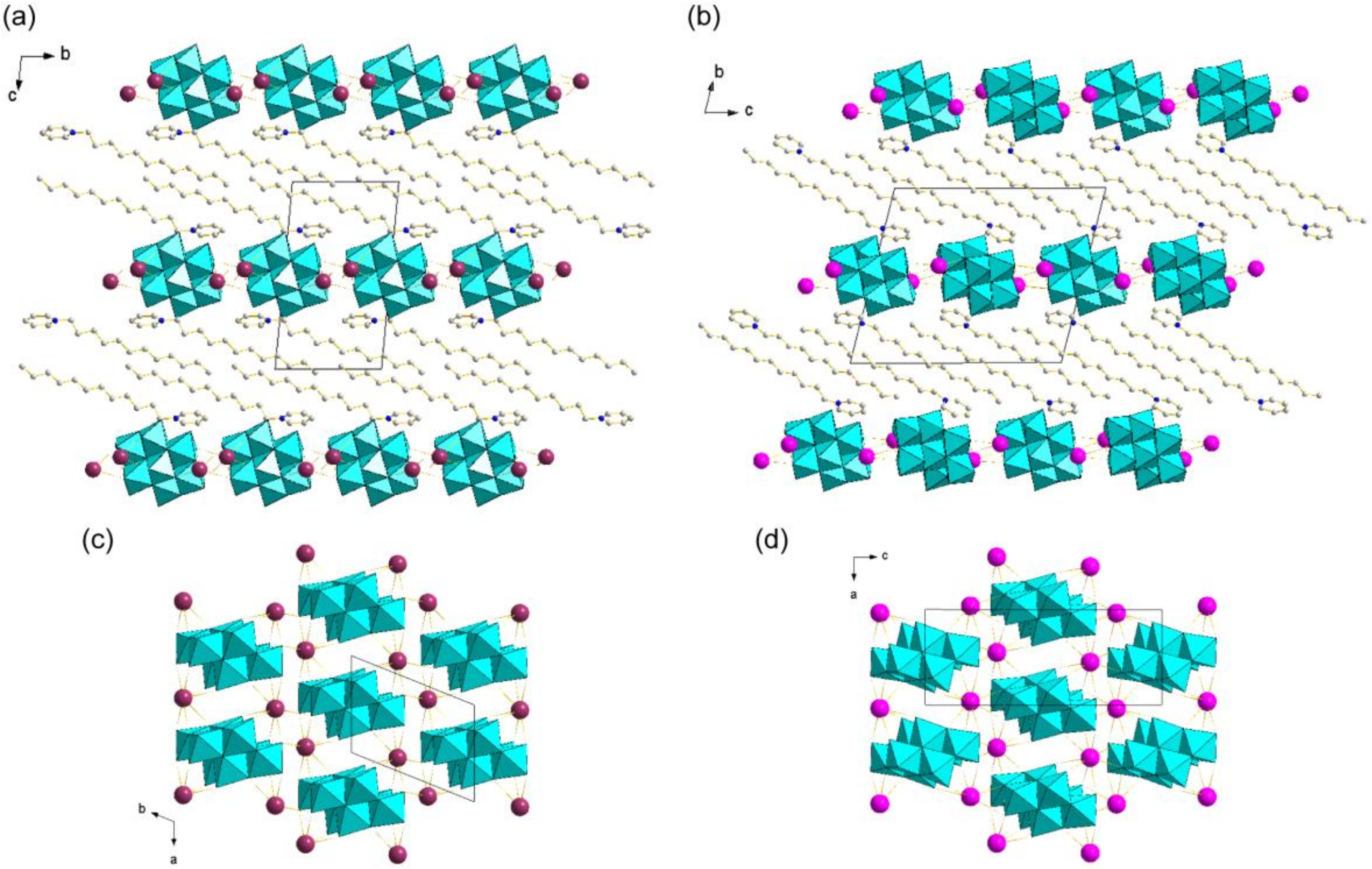
3.3. Crystal Structures of Metal Cation-Introduced Hybrid Crystals Derived from C12py-Mo8
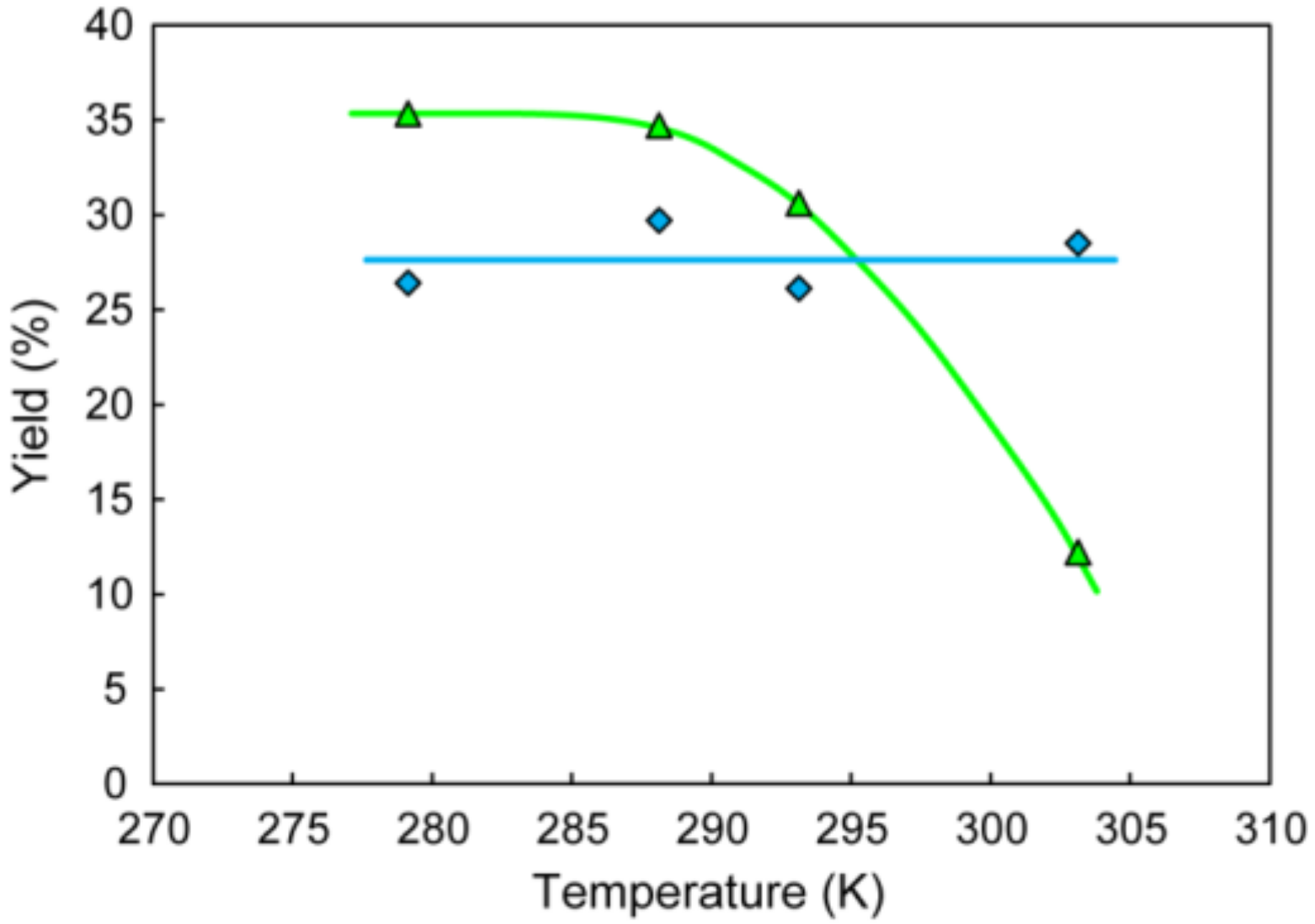
3.4. Yields of Metal Cation-Introduced Hybrid Crystals Relecant to Ion-Capturing Property
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kanatzidis, M.G. Discovery-Synthesis, Design, and prediction of chalcogenide phases. Inorg. Chem. 2017, 56, 3158–3173. [Google Scholar] [CrossRef] [PubMed]
- Akinwande, D.; Huyghebaert, C.; Wang, C.-H.; Serna, M.I.; Goossens, S.; Li, L.-J.; Wong, H.-S.P.; Koppens, F.H.L. Graphene and two-dimensional materials for silicon technology. Nature 2019, 573, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Uchida, J.; Ichikawa, T.; Sakamoto, T. Functional liquid crystals towards the next generation of materials. Angew. Chem. Int. Ed. 2018, 57, 4355–4371. [Google Scholar] [CrossRef]
- Ogawa, M.; Kuroda, K. Photofunctions of intercalation compounds. Chem. Rev. 1995, 95, 399–438. [Google Scholar] [CrossRef]
- Clemente-León, M.; Coronado, E.; Martí-Gastaldo, C.; Romero, F.M. Multifunctionality in hybrid magnetic materials based on bimetallic oxalate complexes. Chem. Soc. Rev. 2011, 40, 473–497. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.D.; Connor, B.A.; Karunadasa, H.I. Tuning the luminescence of layered halide perovskites. Chem. Rev. 2019, 119, 3104–3139. [Google Scholar] [CrossRef] [PubMed]
- Whittingham, M.S. Lithium batteries and cathode materials. Chem. Rev. 2004, 104, 4271–4301. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.-S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef]
- Coronado, E.; Gómez-García, C.J. Polyoxometalate-based molecular materials. Chem. Rev. 1998, 98, 273–296. [Google Scholar] [CrossRef]
- Vargas, B.; Rodríguez-López, G.; Solis-Ibarra, D. The emergence of halide layered double perovskites. ACS Energy Lett. 2020, 5, 3591–3608. [Google Scholar] [CrossRef]
- Manos, M.J.; Kanatzidis, M.G. Metal sulfide ion exchangers: Superior sorbents for the capture of toxic and nuclear waste-related metal ions. Chem. Sci. 2016, 7, 4804–4824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchida, S. Frontiers and progress in cation-uptake and exchange chemistry of polyoxometalate-based compounds. Chem. Sci. 2019, 10, 7670–7679. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, M.; Akiba, S.; Ohtani, A.; Hoshi, Y.; Ono, K.; Matsuba, M.; Togashi, T.; Kananizuka, K.; Sakamoto, M.; Takahashi, A.; et al. Proton-exchange mechanism of specific Cs+ adsorption via lattice defect sites of Prussian blue filled with coordination and crystallization water molecules. Dalton Trans. 2013, 42, 16049–16055. [Google Scholar] [CrossRef]
- Long, D.-L.; Burkholder, E.; Cronin, L. Polyoxometalate clusters, nanostructures and materials: From self assembly to designer materials and devices. Chem. Soc. Rev. 2007, 36, 105–121. [Google Scholar] [CrossRef]
- Proust, A.; Matt, B.; Villanneau, R.; Guillemot, G.; Gouzerh, P.; Izzet, G. Functionalization and post-functionalization: A step towards polyoxometalate-based materials. Chem. Soc. Rev. 2012, 41, 7605–7622. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.; Kozma, K.; Streb, C.; Nyman, M. Beyond charge balance: Counter-cations in polyoxometalate chemistry. Angew. Chem. Int. Ed. 2020, 59, 596–612. [Google Scholar] [CrossRef] [Green Version]
- Sadakane, M.; Steckhan, E. Electrochemical properties of polyoxometalates as electrocatalysts. Chem. Rev. 1998, 98, 219–237. [Google Scholar] [CrossRef]
- Martín-Caballero, J.; Wéry, A.S.J.; Reinoso, S.; Artetxe, B.; San Felices, L.; El Bakkali, B.; Trautwein, G.; Alcañiz-Monge, J.; Vilas, J.L.; Gutiérrez-Zorrilla, J.M. A robust open·framework formed by decavanadate clusters and copper(ii) complexes of macrocyclic polyamines: Permanent microporosity and catalytic oxidation of cycloalkanes. Inorg. Chem. 2016, 55, 4970–4979. [Google Scholar] [CrossRef] [Green Version]
- Rosen, M.J.; Kunjappu, J.T. Surfactants and Interfacial Phenomena, 4th ed.; Wiley: Hoboken, NJ, USA, 2012; pp. 126–132. [Google Scholar]
- Kronberg, B.; Holmberg, K.; Lindman, B. Surface Chemistry of Surfactants and Polymers, 1st ed.; Wiley: Chichester, UK, 2014; pp. 113–136. [Google Scholar]
- Yin, P.; Li, D.; Liu, T. Solution behaviors and self-assembly of polyoxometalates as models of macroions and amphiphilic polyoxometalate-organic hybrids as novel surfactants. Chem. Soc. Rev. 2012, 41, 7368–7383. [Google Scholar] [CrossRef]
- Wu, H.; Yang, H.-K.; Wang, W. Covalently-linked polyoxometalate–polymer hybrids: Optimizing synthesis, appealing structures and prospective applications. New J. Chem. 2016, 40, 886–897. [Google Scholar] [CrossRef]
- Song, Y.-F.; Long, D.-L.; Ritchie, C.; Cronin, L. Nanoscale Polyoxometalate-Based Inorganic/Organic Hybrids. Chem. Rec. 2011, 11, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, W.; Li, H.; Wu, L. Ionic complexes of metal oxide clusters for versatile self-assemblies. Acc. Chem. Res. 2017, 50, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Nisar, A.; Wang, X. Surfactant-encapsulated polyoxometalate building blocks: Controlled assembly and their catalytic properties. Dalton Trans. 2012, 41, 9832–9845. [Google Scholar] [CrossRef] [PubMed]
- Clemente-León, M.; Coronado, E.; Soriano-Portillo, A.; Mingotaud, C.; Dominguez-Vera, J.M. Langmuir–Blodgett films based on inorganic molecular complexes with magnetic or optical properties. Adv. Colloid Interface Sci. 2005, 116, 193–203. [Google Scholar] [CrossRef]
- Zhang, G.; Ke, H.; He, T.; Xiao, D.; Chen, Z.; Yang, W.; Yao, J. Synthesis and characterization of new layered polyoxometallates-1,10-decanediamine intercalative nanocomposites. J. Mater. Res. 2004, 19, 496–500. [Google Scholar] [CrossRef]
- Janauer, G.G.; Dobley, A.D.; Zavalij, P.Y.; Whittingham, M.S. Evidence for decavanadate clusters in the lamellar surfactant ion phase. Chem. Mater. 1997, 9, 647–649. [Google Scholar] [CrossRef]
- Spahr, M.E.; Nesper, R. Anhydrous octamolybdate with trimethyl hexadecyl ammonium cations. Z. Anorg. Allg. Chem. 2001, 627, 2133–2138. [Google Scholar] [CrossRef]
- Ito, T.; Sawada, K.; Yamase, T. Crystal structure of bis(dimethyldioctadecylammonium) hexamolybdate: A molecular model of Langmuir-Blodgett films. Chem. Lett. 2003, 32, 938–939. [Google Scholar] [CrossRef]
- Ito, T. Inorganic–organic hybrid surfactant crystals: Structural aspects and functions. Crystals 2016, 6, 24. [Google Scholar] [CrossRef] [Green Version]
- Nyman, M.; Ingersoll, D.; Singh, S.; Bonhomme, F.; Alam, T.M.; Brinker, C.J.; Rodriguez, M.A. Comparative study of inorganic cluster-surfactant arrays. Chem. Mater. 2005, 17, 2885–2895. [Google Scholar] [CrossRef]
- Nyman, M.; Rodriguez, M.A.; Anderson, T.M.; Ingersoll, D. Two structures toward understanding evolution from surfactant-polyoxometalate lamellae to surfactant-encapsulated polyoxometalates. Cryst. Growth Des. 2009, 9, 3590–3597. [Google Scholar] [CrossRef]
- Yin, P.; Wu, P.; Xiao, Z.; Li, D.; Bitterlich, E.; Zhang, J.; Cheng, P.; Vezenov, D.V.; Liu, T.; Wei, Y. A Double-tailed fluorescent surfactant with a hexavanadate cluster as the head group. Angew. Chem. Int. Ed. 2011, 50, 2521–2525. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Chen, K.; Hao, J.; Wei, Z.; Zhang, H.; Yin, P.; Wei, Y. Synthesis and crystallization behavior of surfactants with hexamolybdate as the polar headgroup. Inorg. Chem. 2015, 54, 6075–6077. [Google Scholar] [CrossRef] [PubMed]
- Rosnes, M.H.; Musumeci, C.; Yvon, C.; Macdonell, A.; Pradeep, C.P.; Sartorio, C.; Long, D.-L.; Pignataro, B.; Cronin, L. Exploring the interplay between ligand derivatisation and cation type in the assembly of hybrid polyoxometalate Mn-Andersons. Small 2013, 9, 2316–2324. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, X.; Huang, B.; Chen, W.; Xiao, Z.; Wu, P. The crystal packing, morphology and hydrophobicity of polyoxometalate-based amphiphilic materials. Cryst. Eng. Comm. 2020, 22, 2434–2438. [Google Scholar] [CrossRef]
- Mubeena, S.; Annapareddy, G.; Meghana, N.; Sarma, M. Crystallographic and spectroscopic analysis of 9,10-bis-alkyl imidazolium anthracene hexatungstate supramolecular complexes. J. Mol. Struct. 2021, 1243, 130876. [Google Scholar] [CrossRef]
- Ito, T.; Mikurube, K.; Abe, Y.; Koroki, T.; Saito, M.; Iijima, J.; Naruke, H.; Ozeki, T. Hybrid inorganic-organic crystals composed of octamolybdate isomers and pyridinium surfactant. Chem. Lett. 2010, 39, 1323–1325. [Google Scholar] [CrossRef]
- Ito, T.; Mikurube, K.; Hasegawa, K.; Kurasawa, M.; Naruke, H.; Ozeki, T. Polyoxomolybdate-surfactant hybrid layered crystal with unusually long periodicity. Chem. Lett. 2011, 40, 626–628. [Google Scholar] [CrossRef]
- Mikurube, K.; Hasegawa, K.; Matsumoto, T.; Kobayashi, J.; Naruke, H.; Ito, T. Isomerization-induced introduction of metal cations into polyoxomolybdate-surfactant hybrid crystals. Inorg. Chem. Commun. 2016, 73, 45–48. [Google Scholar] [CrossRef]
- Klemperer, W.G.; Shum, W. Synthesis and interconversion of the isomeric α- and β-Mo8O264− ions. J. Am. Chem. Soc. 1976, 98, 8291–8293. [Google Scholar] [CrossRef]
- Himeno, S.; Niiya, H.; Ueda, T. Raman studies on the identification of isopolymolybdates in aqueous solution. Bull. Chem. Soc. Jpn. 1997, 70, 631–637. [Google Scholar] [CrossRef]
- Cruywagen, J.J. Protonation, oligomerization, and condensation reactions of vanadate(V), molybdate(VI),and tungstate(VI). Adv. Inorg. Chem. 1999, 49, 127–182. [Google Scholar]
- CrystalClear; Rigaku Corporation: Tokyo, Japan, 2014.
- CrysAlisPro; Rigaku Oxford Diffraction: Tokyo, Japan, 2015.
- PROCESS-AUTO; Rigaku Corporation: Tokyo, Japan, 2002.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altomare, A.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A. Completion and refinement of crystal structures with SIR92. J. Appl. Crystallogr. 1993, 26, 343–350. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr., Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- CrystalStructure 4.3; Rigaku Corporation: Tokyo, Japan, 2017.
- Román, P.; Gutiérrez-Zorrilla, J.M.; Esteban-Calderón, C.; Martínez-Ripoll, M.; García-Blanco, S. Synthesis, characterization and crystal structure of the anilinium β-octamolybdate dihydrate. Polyhedron 1985, 4, 1043–1046. [Google Scholar] [CrossRef]
- Román, P.; San José, A.; Aranzabe, A.; Luque, A. Synthesis, solid state characterization and thermal behavior of some tert-butylammonium polymolybdates. Thermochim. Acta 1992, 206, 137–147. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, X.; Zhang, H.; Huang, C.; Cao, Y.; Sun, R. Synthesis, solid state characterization and thermal behavior of some tert-butylammonium polymolybdates. The structure and two-dimensional correlation infrared spectroscopy study of a new 2D polyoxomolybdate complex containing b-octamolybdate linked up by potassium ions: (4,4’-Hbpy)2(K2Mo8O26). Vib. Spectrosc. 2006, 40, 142–147. [Google Scholar]
- Courcot, B.; Adam, J.; Bridgeman, A.J. Structural and vibrational study of [Mo7O24]6– and [W7O24]6–. J. Phys. Chem. A 2009, 113, 10540–10548. [Google Scholar] [CrossRef]

| Compound | C12py-Mo8 | C12py-Mo8-AN | C12py-Rb-Mo8 | C12py-Cs-Mo8 |
|---|---|---|---|---|
| Chemical formula | C68H120N4Mo8O26 | C70H123N5Mo8O26 | C34H60N2Rb2Mo8O26 | C34H60N2Cs2Mo8O26 |
| Formula weight | 2177.23 | 2218.28 | 1851.30 | 1946.18 |
| Crystal system | triclinic | triclinic | triclinic | triclinic |
| Space group | P (No. 2) | P (No. 2) | P (No. 2) | P (No. 2) |
| a (Å) | 10.3430(5) | 12.8469(8) | 7.8864(4) | 7.9663(3) |
| b (Å) | 11.3843(6) | 18.0395(10) | 10.6302(4) | 17.4100(5) |
| c (Å) | 19.7519(11) | 20.5427(12) | 17.7431(10) | 20.3300(6) |
| α (°) | 79.308(5) | 108.6372(12) | 91.918(3) | 75.492(3) |
| β (°) | 76.042(4) | 94.6749(15) | 98.1055(19) | 88.785(3) |
| γ (°) | 70.315(4) | 90.4647(14) | 111.357(3) | 85.313(3) |
| V (Å3) | 2111.3(2) | 4493.2(5) | 1365.68(12) | 2720.59(16) |
| Z | 1 | 2 | 1 | 2 |
| ρcalcd (g∙cm−3) | 1.712 | 1.639 | 2.251 | 2.376 |
| T (K) | 93 | 193 | 193 | 193 |
| Wavelength (Å) | 0.71073 | 0.71075 | 0.71075 | 0.71073 |
| μ (mm−1) | 1.219 | 1.148 | 3.619 | 3.178 |
| No. of reflections measured | 32,803 | 66,871 | 22,036 | 21,492 |
| No. of independent reflections | 16,306 | 20,485 | 6242 | 12,476 |
| Rint | 0.0830 | 0.0485 | 0.0485 | 0.0445 |
| No. of parameters | 480 | 1315 | 418 | 652 |
| R1 (I > 2σ(I)) | 0.0554 | 0.0494 | 0.0313 | 0.0543 |
| wR2 (all data) | 0.0906 | 0.0904 | 0.0606 | 0.1395 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, J.; Shimura, K.; Mikurube, K.; Otobe, S.; Matsumoto, T.; Ishikawa, E.; Naruke, H.; Ito, T. Polyoxomolybdate Layered Crystals Constructed from a Heterocyclic Surfactant: Syntheses, Pseudopolymorphism and Introduction of Metal Cations. Materials 2022, 15, 2429. https://doi.org/10.3390/ma15072429
Kobayashi J, Shimura K, Mikurube K, Otobe S, Matsumoto T, Ishikawa E, Naruke H, Ito T. Polyoxomolybdate Layered Crystals Constructed from a Heterocyclic Surfactant: Syntheses, Pseudopolymorphism and Introduction of Metal Cations. Materials. 2022; 15(7):2429. https://doi.org/10.3390/ma15072429
Chicago/Turabian StyleKobayashi, Jun, Keisuke Shimura, Keisuke Mikurube, Saki Otobe, Takashi Matsumoto, Eri Ishikawa, Haruo Naruke, and Takeru Ito. 2022. "Polyoxomolybdate Layered Crystals Constructed from a Heterocyclic Surfactant: Syntheses, Pseudopolymorphism and Introduction of Metal Cations" Materials 15, no. 7: 2429. https://doi.org/10.3390/ma15072429






