Synthesis and Characterization of Sn/SnO2/C Nano-Composite Structure: High-Performance Negative Electrode for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of Sn/SnO2/C in Carbon Matrix
2.2. Materials Characterization
2.3. Electrochemical Measurement
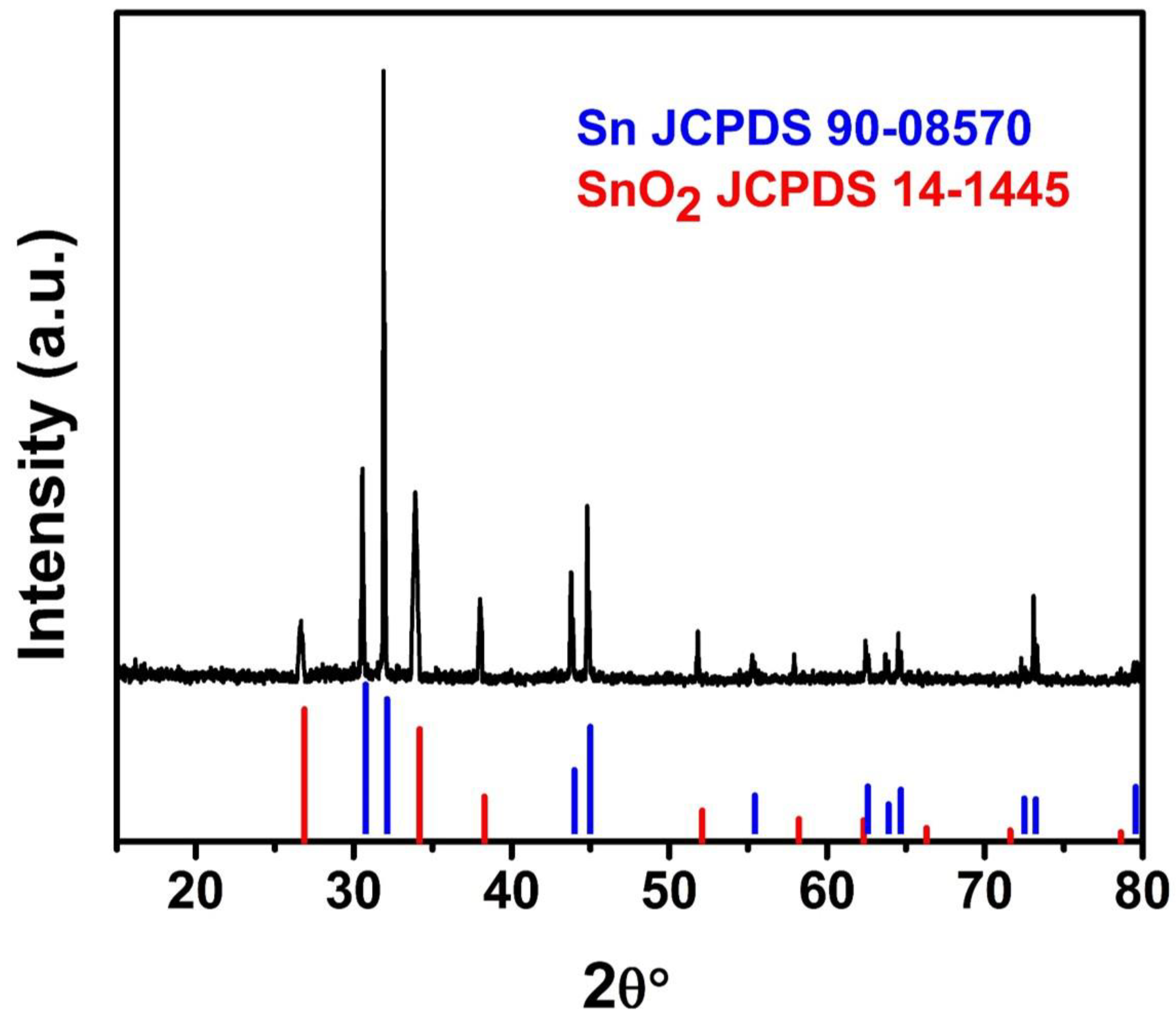
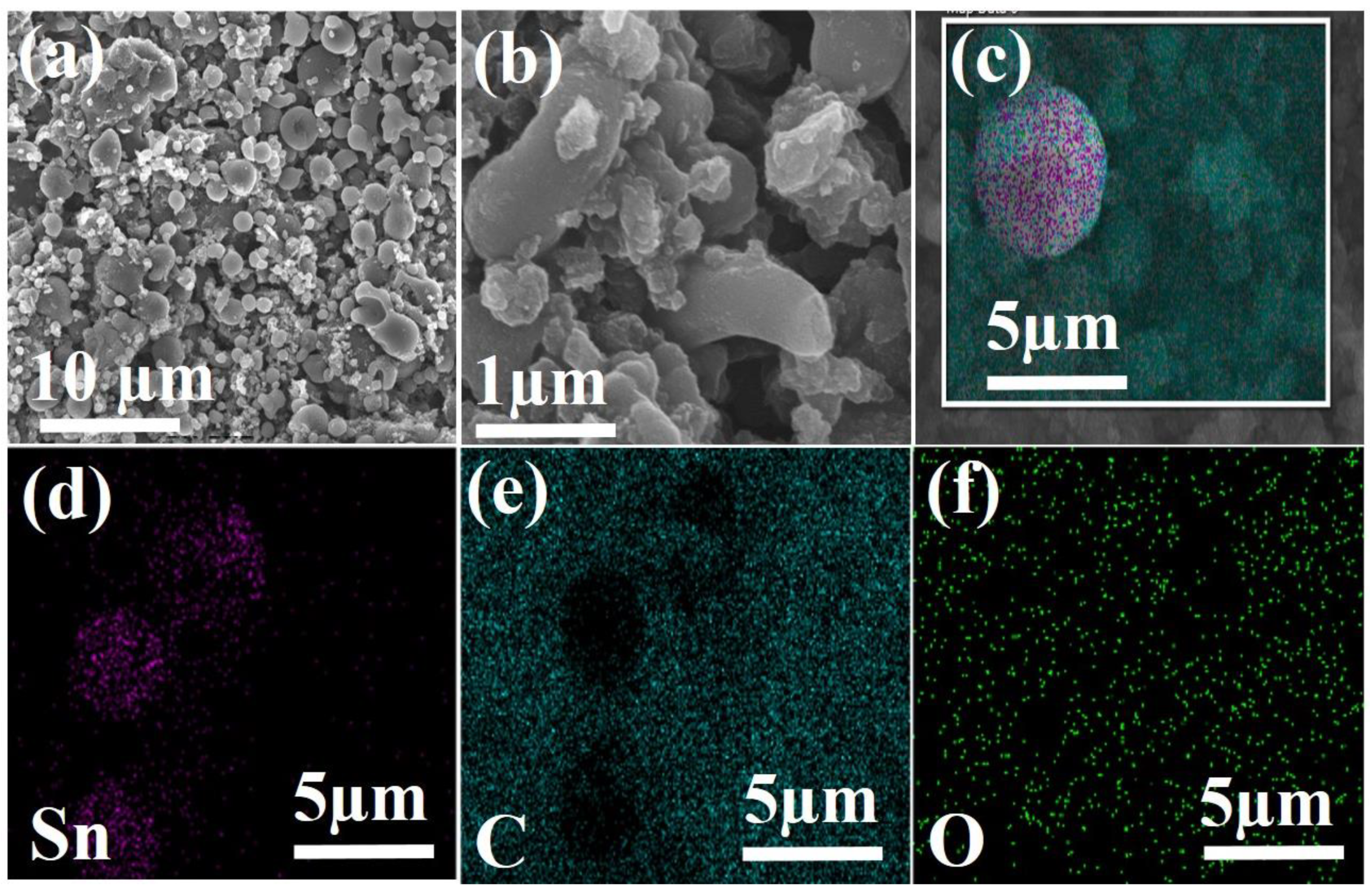
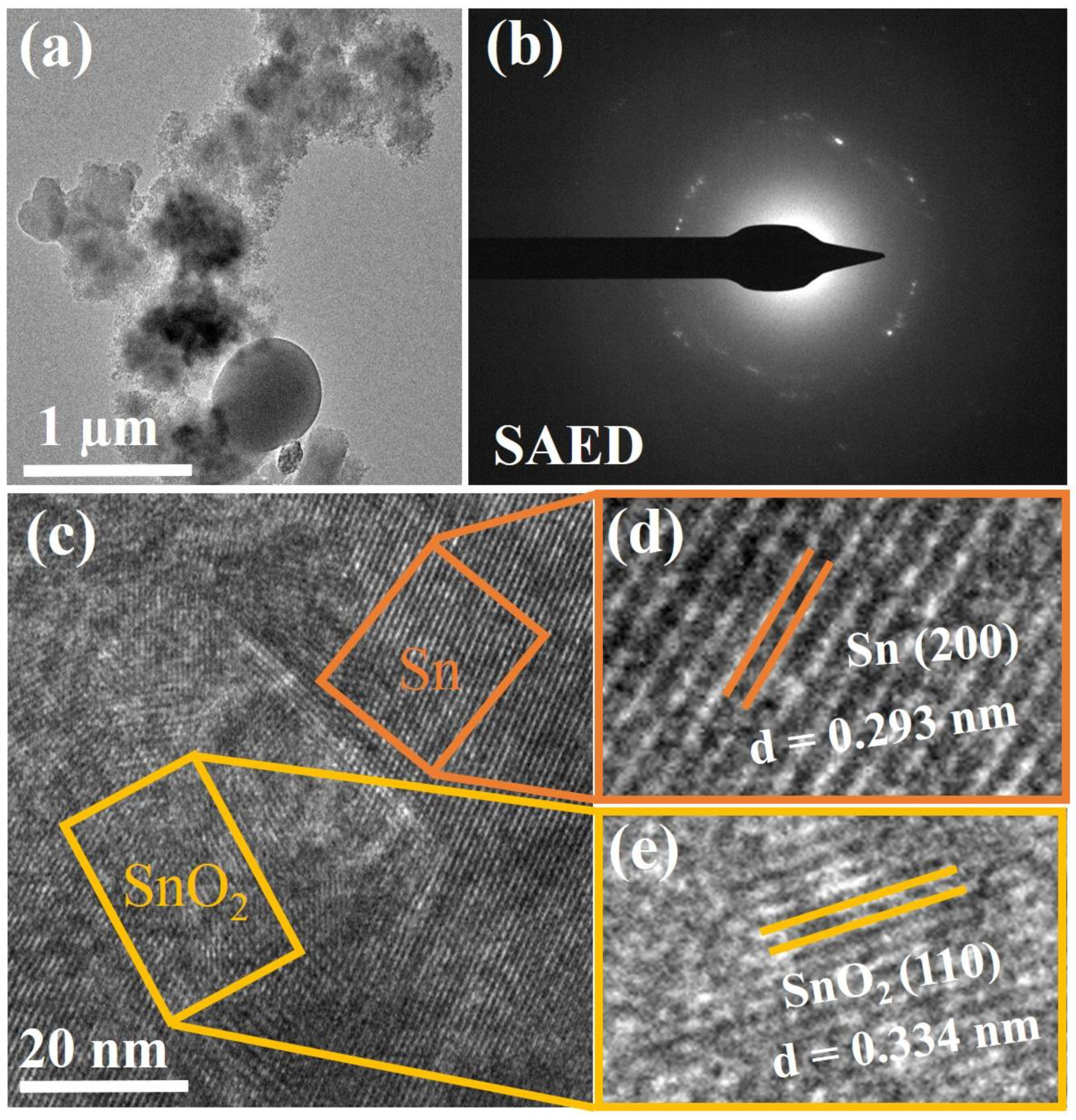
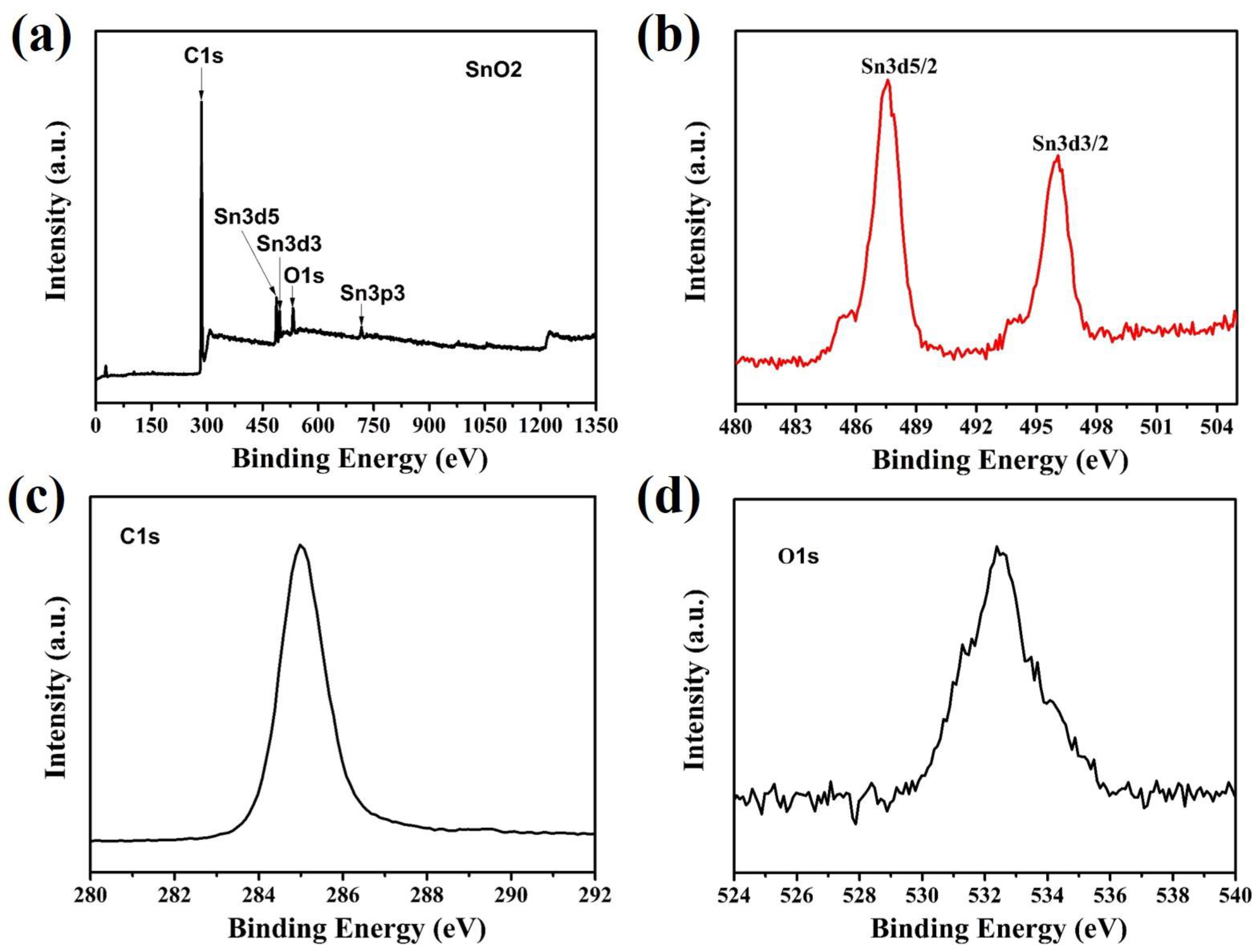
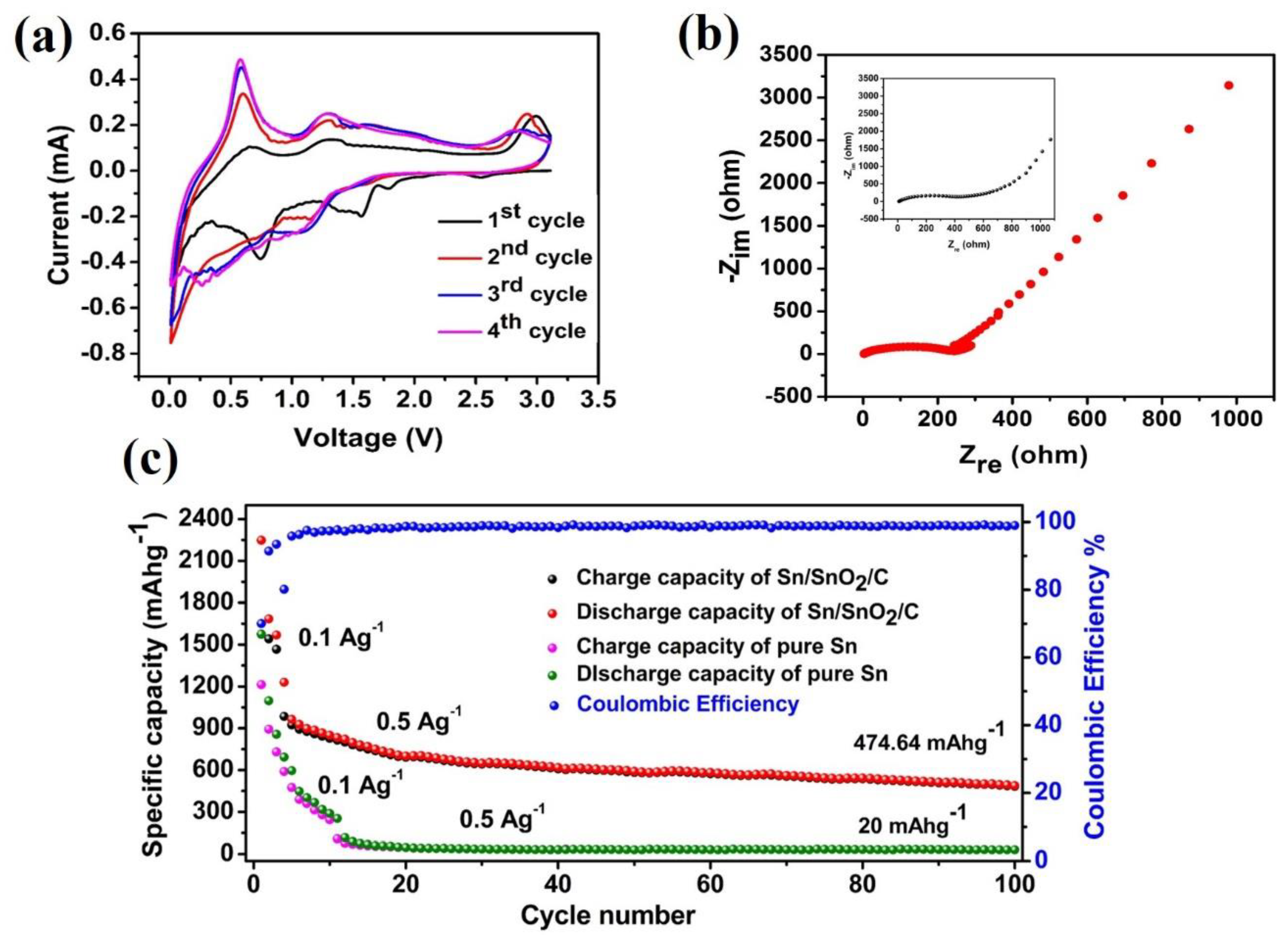
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ.Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, C.-H.; Shi, Y. A Tin-Based Amorphous Oxide Composite with a Porous, Spherical, Multideck-Cage Morphology as a Highly Reversible Anode Material for Lithium-Ion Batteries. Adv. Mater. 2007, 19, 993–997. [Google Scholar] [CrossRef]
- Poizot, P.; Laruelle, S.; Grugeon, S.; Dupont, L.; Tarascon, J.M. Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature 2000, 407, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-J. A review of the electrochemical performance of alloy anodes for lithium-ion batteries. J. Power Sources 2011, 196, 13–24. [Google Scholar] [CrossRef]
- Ali, S.; Jaffer, S.; Maitlo, I.; Shehzad, F.K.; Wang, Q.; Ali, S.; Akram, M.Y.; He, Y.; Nie, J. Photo cured 3D porous silica-carbon (SiO2–C) membrane as anode material for high performance rechargeable Li-ion batteries. J. Alloys Compd. 2020, 812, 152127. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Si, W.; Liu, X.; Deng, J.; Xi, L.; Liu, L.; Yan, C.; Schmidt, O.G. Multifunctional Ni/NiO hybrid nanomembranes as anode materials for high-rate Li-ion batteries. Nano Energy 2014, 9, 168–175. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, Q.; Shi, G. Graphene based new energy materials. Energy Environ. Sci. 2011, 4, 1113–1132. [Google Scholar] [CrossRef]
- Lian, P.; Dong, Y.; Wu, Z.-S.; Zheng, S.; Wang, X.; Sen, W.; Sun, C.; Qin, J.; Shi, X.; Bao, X. Alkalized Ti3C2 MXene nanoribbons with expanded interlayer spacing for high-capacity sodium and potassium ion batteries. Nano Energy 2017, 40, 1–8. [Google Scholar] [CrossRef]
- Yuan, C.; Wu, H.B.; Xie, Y.; Lou, X.W. Mixed Transition-Metal Oxides: Design, Synthesis, and Energy-Related Applications. Angew. Chem. Int. Ed. 2014, 53, 1488–1504. [Google Scholar] [CrossRef]
- Yin, L.; Chai, S.; Huang, J.; Kong, X.; Wang, J.; Bai, P. Influence of solvent on the structure and electrochemical performances of Sn-based anode for lithium-ion battery. Ceram. Int. 2017, 43, 12667–12674. [Google Scholar] [CrossRef]
- Du, Z.; Zhang, S.; Jiang, T.; Bai, Z. Preparation and characterization of three-dimensional tin thin-film anode with good cycle performance. Electrochim. Acta 2010, 55, 3537–3541. [Google Scholar] [CrossRef]
- Hassoun, J.; Derrien, G.; Panero, S.; Scrosati, B. A Nanostructured Sn–C Composite Lithium Battery Electrode with Unique Stability and High Electrochemical Performance. Adv.Mat. 2008, 20, 3169–3175. [Google Scholar] [CrossRef]
- Ao, X.; Jiang, J.; Ruan, Y.; Li, Z.; Zhang, Y.; Sun, J.; Wang, C. Honeycomb-inspired design of ultrafine SnO2@C nanospheres embedded in carbon film as anode materials for high performance lithium- and sodium-ion battery. J. Power Sources 2017, 359, 340–348. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Guo, J.; Gerasopoulos, K.; Langrock, A.; Brown, A.; Ghodssi, R.; Culver, J.N.; Wang, C. 3D tin anodes prepared by electrodeposition on a virus scaffold. J. Power Sources 2012, 211, 129–132. [Google Scholar] [CrossRef]
- Ahn, D.; Xiao, X.; Li, Y.; Sachdev, A.K.; Park, H.W.; Yu, A.; Chen, Z. Applying functionalized carbon nanotubes to enhance electrochemical performances of tin oxide composite electrodes for Li-ion battery. J. Power Sources 2012, 212, 66–72. [Google Scholar] [CrossRef]
- Hu, R.; Sun, W.; Liu, H.; Zeng, M.; Zhu, M. The fast filling of nano-SnO2 in CNTs by vacuum absorption: A new approach to realize cyclic durable anodes for lithium ion batteries. Nanoscale 2013, 5, 11971–11979. [Google Scholar] [CrossRef]
- Jiang, P.; Jing, J.; Wang, Y.; Li, H.; He, X.; Chen, Y.; Liu, W. Facilely transforming bulk materials to SnO/pristine graphene 2D-2D heterostructures for stable and fast lithium storage. J. Alloys Compd. 2020, 812, 152114. [Google Scholar] [CrossRef]
- Kim, H.; Kim, M.-C.; Kim, S.-b.; Kim, Y.-S.; Choi, J.-H.; Park, K.-W. Porous SnO2 nanostructure with a high specific surface area for improved electrochemical performance. RSC Adv. 2020, 10, 10519–10525. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Wan, L.-J.; Guo, Y.-G. Binding SnO2 Nanocrystals in Nitrogen-Doped Graphene Sheets as Anode Materials for Lithium-Ion Batteries. Adv. Mater. 2013, 25, 2152–2157. [Google Scholar] [CrossRef]
- Ma, B.; Lu, B.; Luo, J.; Deng, X.; Wu, Z.; Wang, X. The hollow mesoporous silicon nanobox dually encapsulated by SnO2/C as anode material of lithium ion battery. Electrochim. Acta 2018, 288, 61–70. [Google Scholar] [CrossRef]
- Lee, W.H.; Kim, H. Oxidized iridium nanodendrites as catalysts for oxygen evolution reactions. Catal. Commun. 2011, 12, 408–411. [Google Scholar] [CrossRef]
- Saikia, D.; Deka, J.R.; Chou, C.-J.; Kao, H.-M.; Yang, Y.-C. 3D interpenetrating cubic mesoporous carbon supported nanosized SnO2 as an efficient anode for high performance lithium-ion batteries. J. Alloys Compd. 2019, 791, 892–904. [Google Scholar] [CrossRef]
- Saddique, J.; Zhang, X.; Wu, T.; Su, H.; Liu, S.; Zhang, D.; Zhang, Y.; Yu, H. Sn4P3-induced crystalline/amorphous composite structures for enhanced sodium-ion battery anodes. J. Mater. Sci. Technol. 2020, 55, 73–80. [Google Scholar] [CrossRef]
- Chang, L.; Yi, Z.; Wang, Z.; Wang, L.; Cheng, Y. Ultrathin SnO2 nanosheets anchored on graphene with improved electrochemical kinetics for reversible lithium and sodium storage. Appl. Surf. Sci. 2019, 484, 646–654. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, Z.; Jiang, W.; Zou, Y.; Wang, Y.; Lu, C. Sandwich-like CNTs@SnO2/SnO/Sn anodes on three-dimensional Ni foam substrate for lithium ion batteries. J. Electroanal. Chem. 2016, 767, 49–55. [Google Scholar] [CrossRef]
- Wei, W.; Du, P.; Liu, D.; Wang, H.; Liu, P. Facile mass production of nanoporous SnO2 nanosheets as anode materials for high performance lithium-ion batteries. J. Colloid Interface Sci. 2017, 503, 205–213. [Google Scholar] [CrossRef]
- Mao, Y.; Tian, Q.; Chen, F.; Chen, J.; Yang, L. Improving the lithium storage performance of SnO2 nanoparticles by in-situ embedding into a porous carbon framework. J. Alloys Compd. 2019, 803. [Google Scholar] [CrossRef]
- Liu, D.; Wei, Z.; Liu, L.; Pan, H.; Duan, X.; Xia, L.; Zhong, B.; Wang, H.; Jia, D.; Zhou, Y.; et al. Ultrafine SnO2 anchored in ordered mesoporous carbon framework for lithium storage with high capacity and rate capability. Chem. Eng. J. 2021, 406, 126710. [Google Scholar] [CrossRef]
- Cheng, Y.; Yi, Z.; Wang, C.; Wu, Y.; Wang, L. Controllable fabrication of C/Sn and C/SnO/Sn composites as anode materials for high-performance lithium-ion batteries. Chem. Eng. J. 2017, 330, 1035–1043. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, D.-W.; Yin, L.-C.; Li, N.; Li, F.; Cheng, H.-M. Oxygen Bridges between NiO Nanosheets and Graphene for Improvement of Lithium Storage. ACS Nano 2012, 6, 3214–3223. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.J.K.; Ryu, S.H.; Shanmugharaj, A.M. Synthesis of SnO2 pillared carbon using long chain alkylamine grafted graphene oxide: An efficient anode material for lithium ion batteries. Nanoscale 2016, 8, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Dai, Z.; Liu, S.; Bao, J.; Guo, Y.-G. Ultra-Uniform SnOx/Carbon Nanohybrids toward Advanced Lithium-Ion Battery Anodes. Adv. Mater. 2014, 26, 3943–3949. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yang, Q.-R.; Ma, J.; Chou, S.-L.; Zhu, M.; Li, Y. Sn/SnO2@C composite nanofibers as advanced anode for lithium-ion batteries. Electrochim. Acta 2015, 186, 271–276. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saddique, J.; Shen, H.; Ge, J.; Huo, X.; Rahman, N.; Mushtaq, M.; Althubeiti, K.; Al-Shehri, H. Synthesis and Characterization of Sn/SnO2/C Nano-Composite Structure: High-Performance Negative Electrode for Lithium-Ion Batteries. Materials 2022, 15, 2475. https://doi.org/10.3390/ma15072475
Saddique J, Shen H, Ge J, Huo X, Rahman N, Mushtaq M, Althubeiti K, Al-Shehri H. Synthesis and Characterization of Sn/SnO2/C Nano-Composite Structure: High-Performance Negative Electrode for Lithium-Ion Batteries. Materials. 2022; 15(7):2475. https://doi.org/10.3390/ma15072475
Chicago/Turabian StyleSaddique, Jaffer, Honglie Shen, Jiawei Ge, Xiaomin Huo, Nasir Rahman, Muhammad Mushtaq, Khaled Althubeiti, and Hamza Al-Shehri. 2022. "Synthesis and Characterization of Sn/SnO2/C Nano-Composite Structure: High-Performance Negative Electrode for Lithium-Ion Batteries" Materials 15, no. 7: 2475. https://doi.org/10.3390/ma15072475
APA StyleSaddique, J., Shen, H., Ge, J., Huo, X., Rahman, N., Mushtaq, M., Althubeiti, K., & Al-Shehri, H. (2022). Synthesis and Characterization of Sn/SnO2/C Nano-Composite Structure: High-Performance Negative Electrode for Lithium-Ion Batteries. Materials, 15(7), 2475. https://doi.org/10.3390/ma15072475





