Corrosion Resistance of Selective Laser Melted Ti6Al4V3Cu Alloy Produced Using Pre-Alloyed and Mixed Powder
Abstract
:1. Introduction
2. Materials and Methods
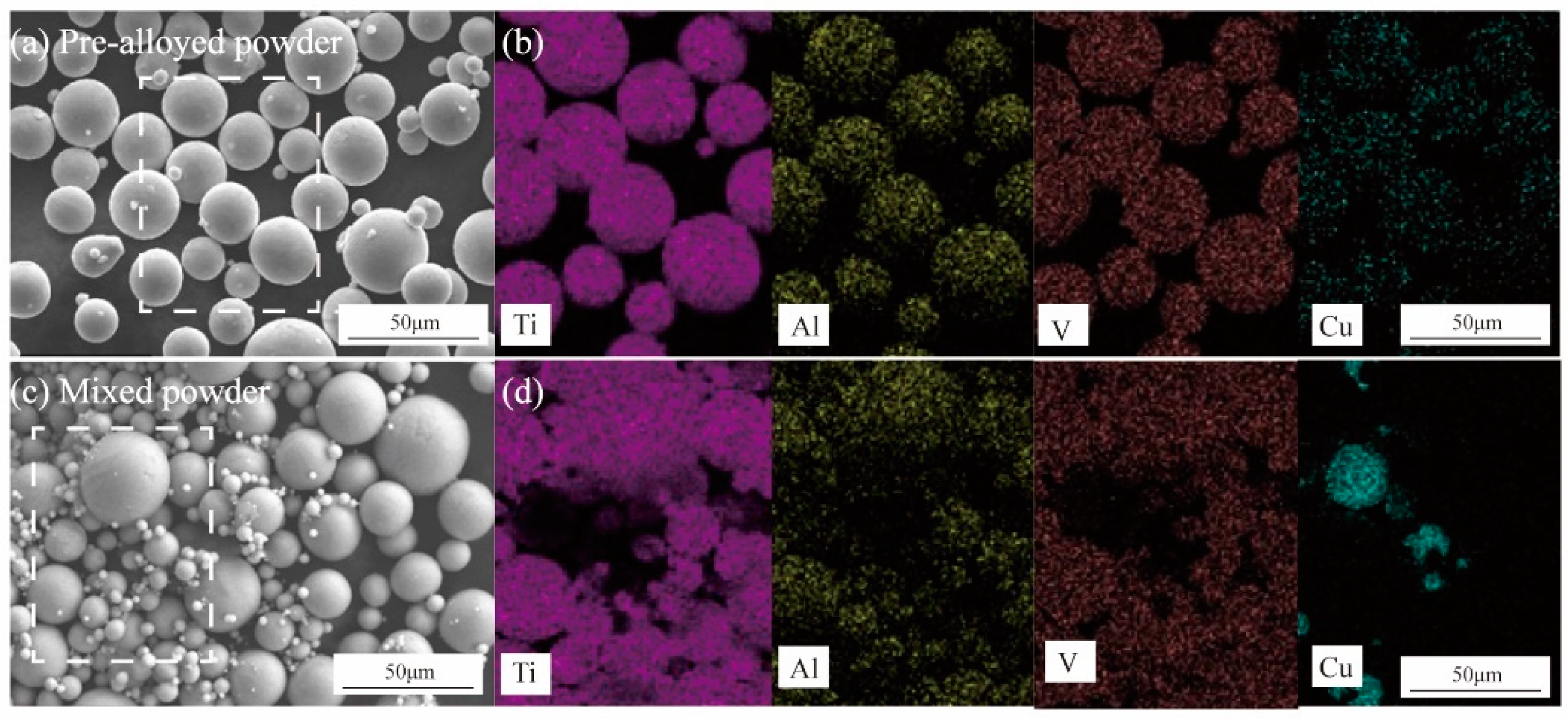
2.1. Materials Preparation
2.2. Materials Preparation
2.3. Electrochemical Test
2.4. Static Immersion Test
3. Results
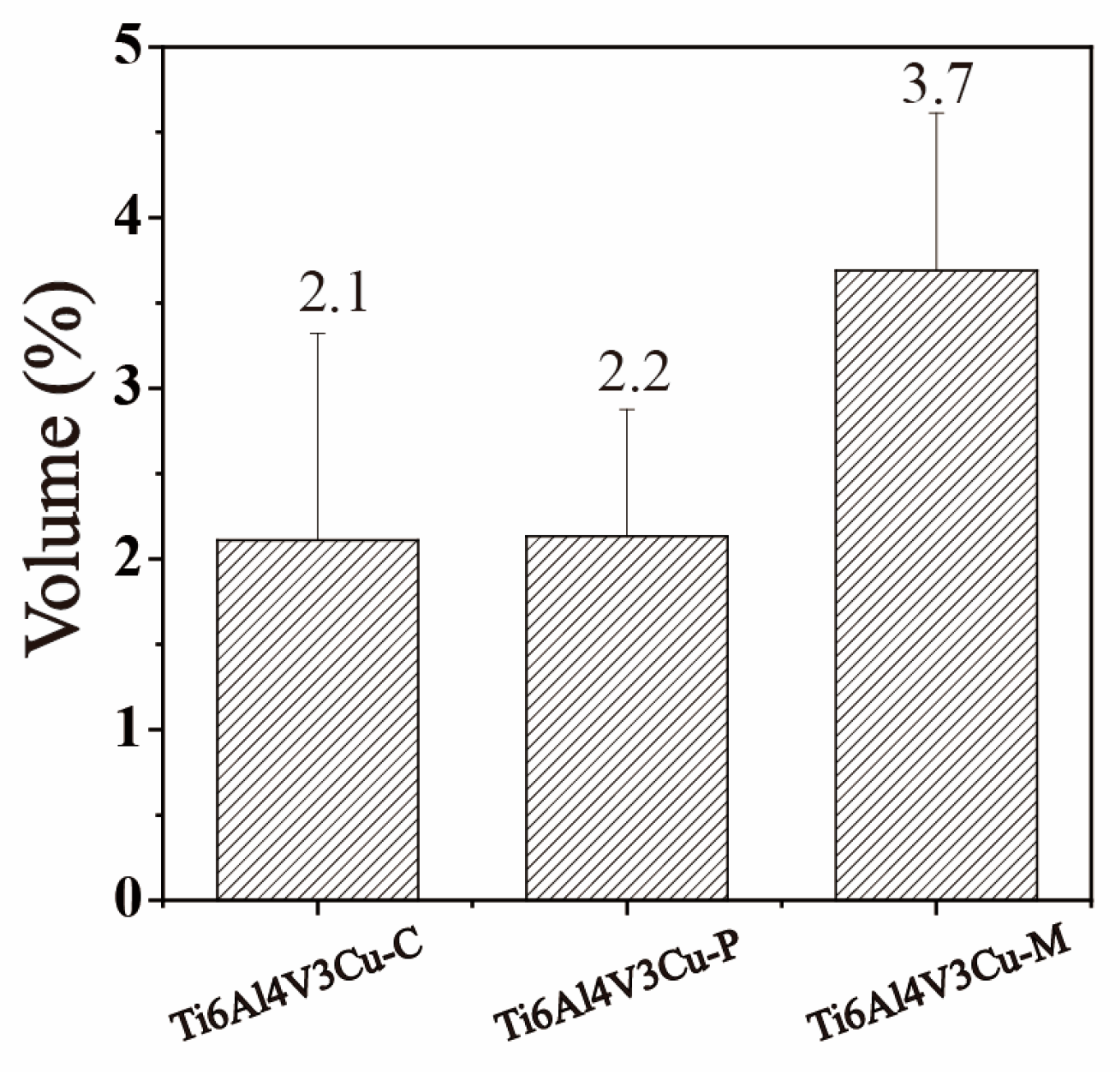
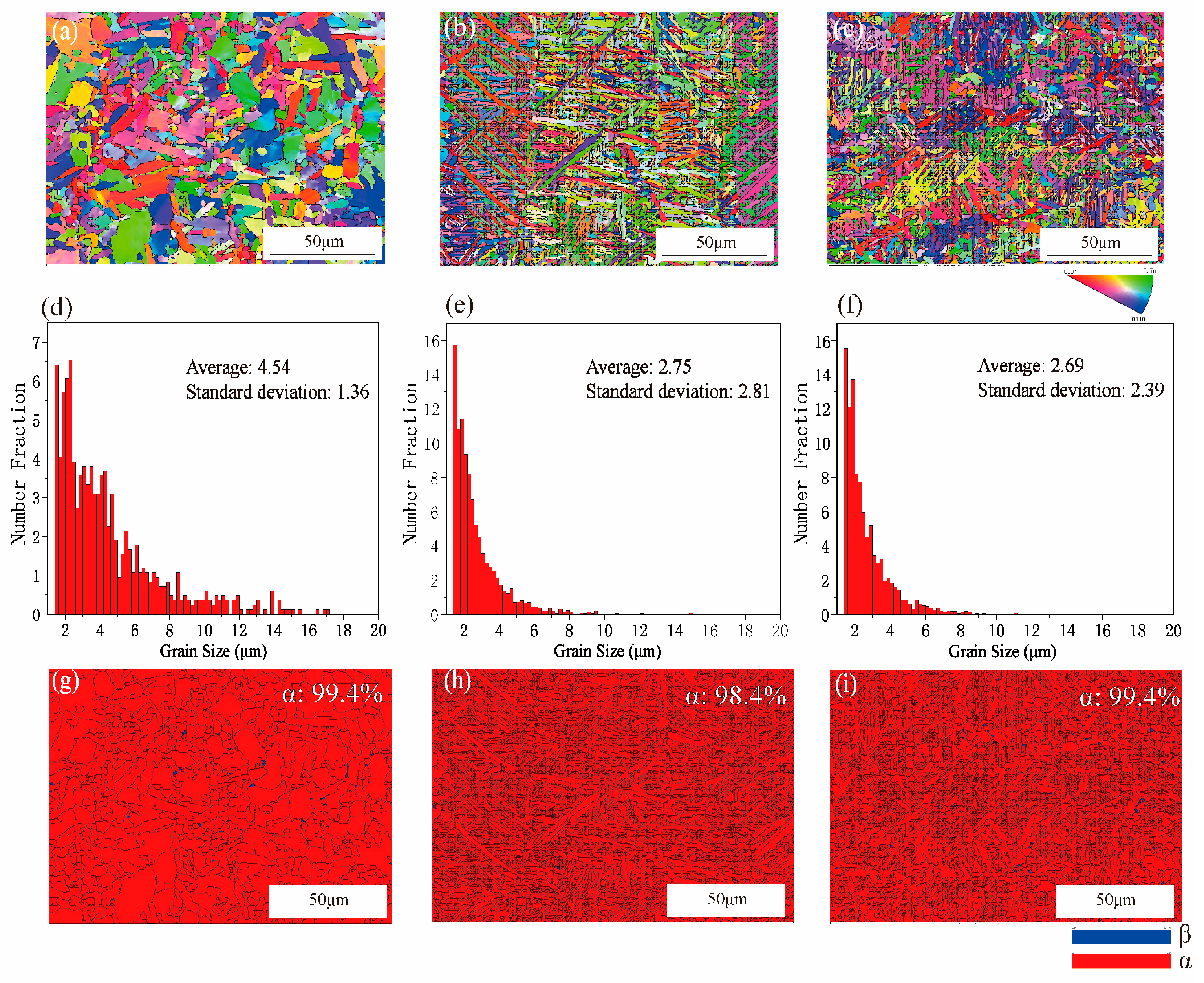
3.1. Microstructure Analysis
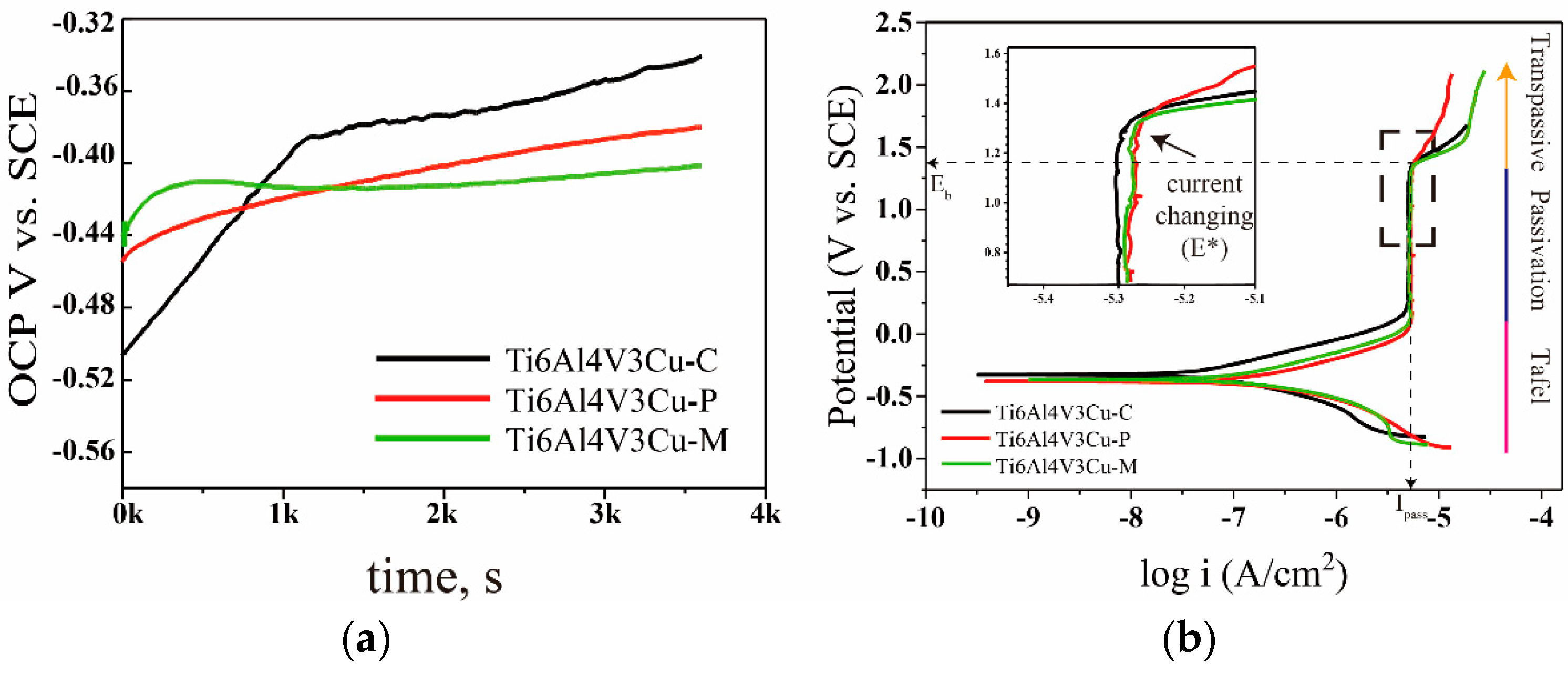
3.2. Electrochemical Analysis
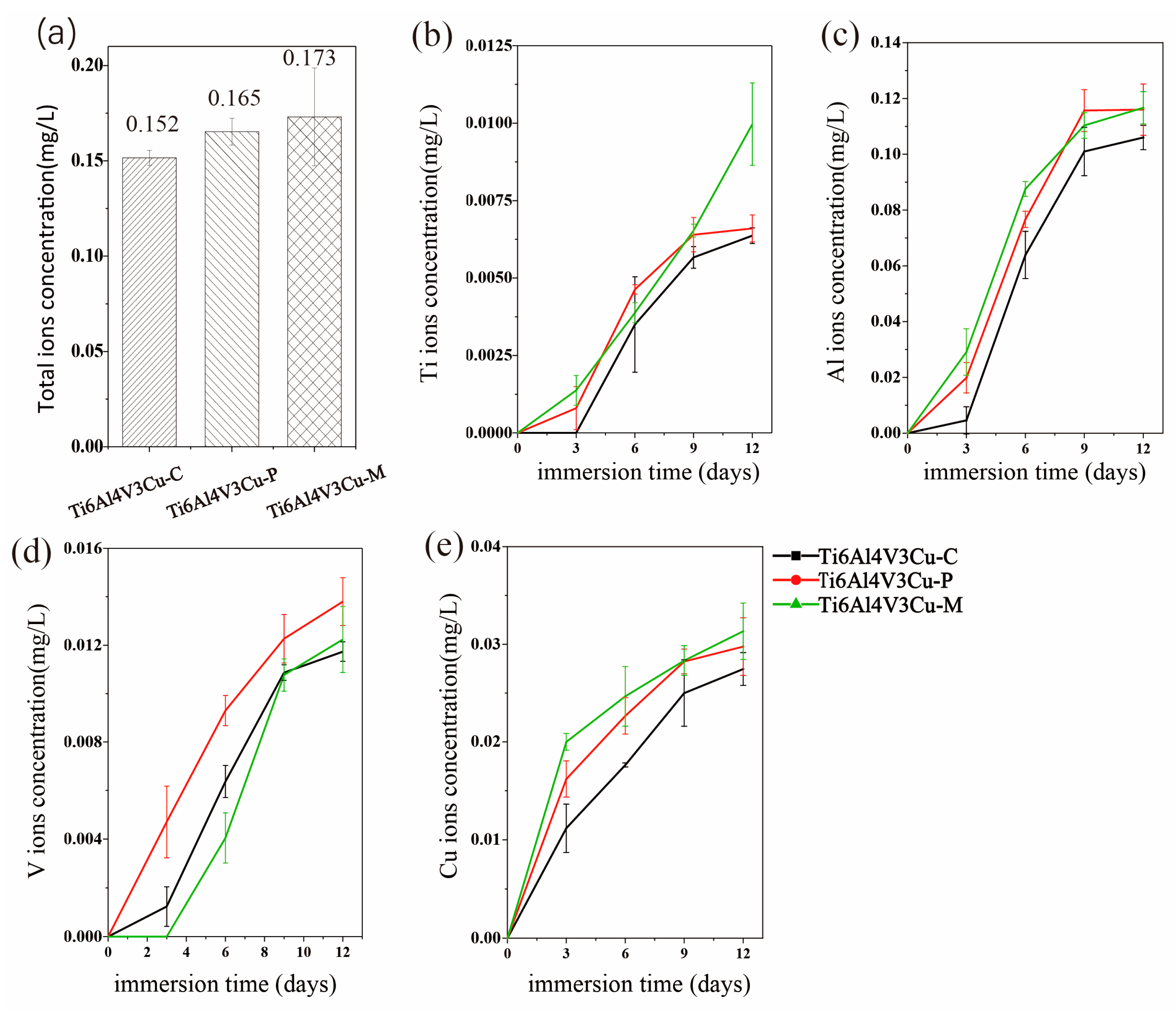
3.3. ICP Analysis
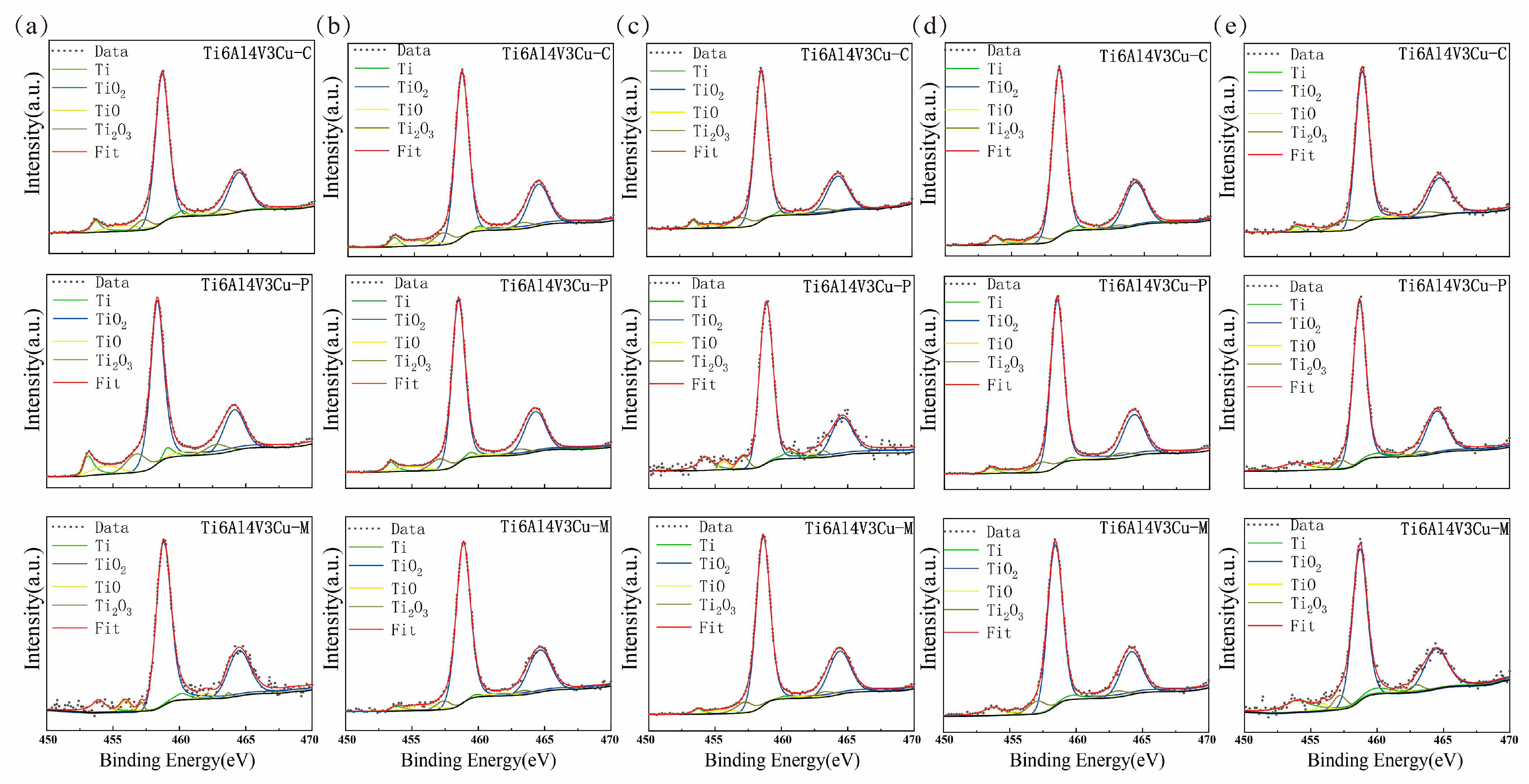
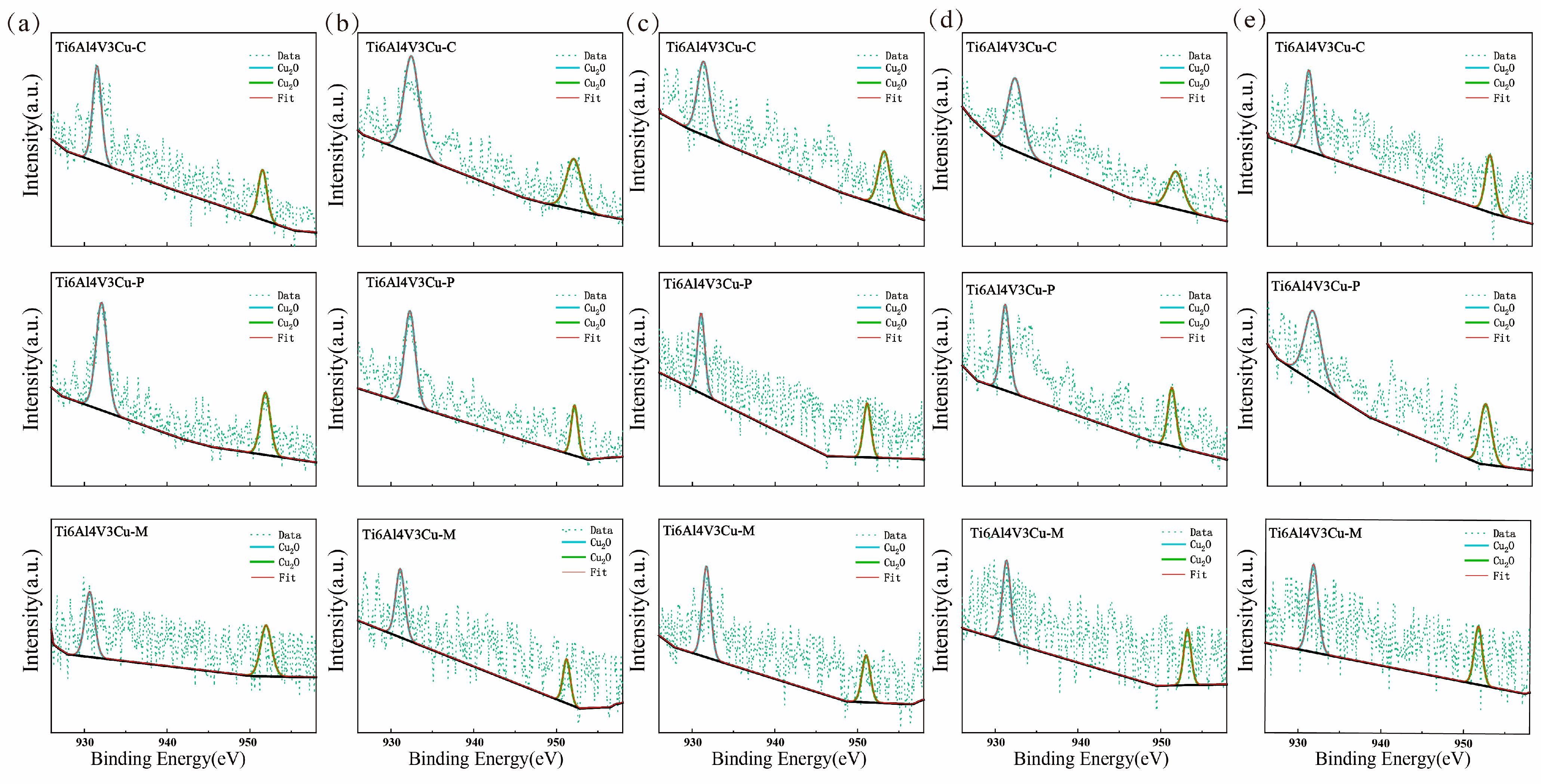
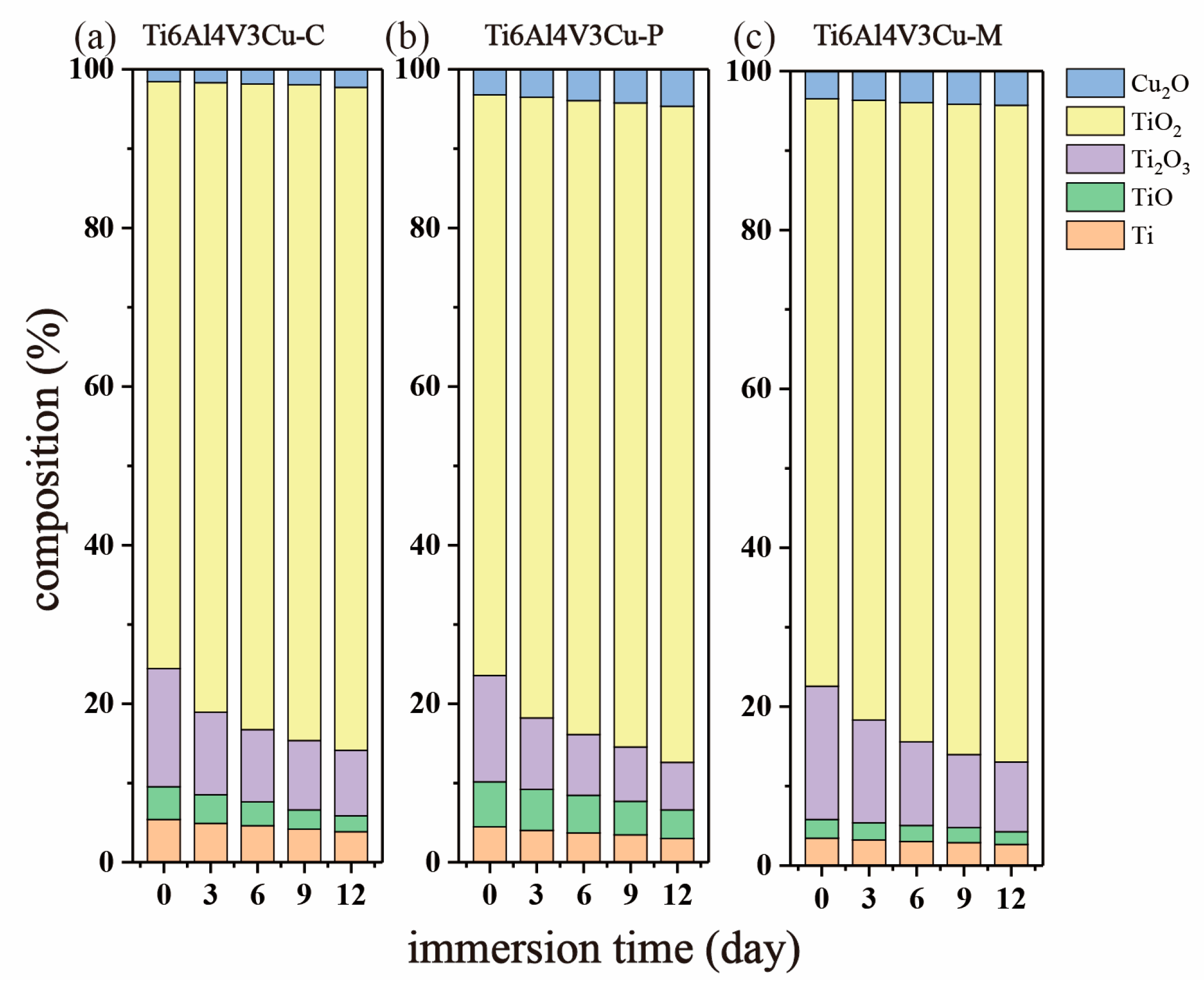
3.4. XPS Study
4. Discussion
5. Conclusions
- The intermetallic compound Ti2Cu could be found in all microstructures of Ti6Al4V3Cu alloys, regardless of the processing method. However, the processing method could affect the distribution form of the Ti2Cu, and the more uniform distribution Ti2Cu could be found in the Ti6Al4V3Cu-P alloys.
- Compared to the microstructures of the casting alloy, the SLM-produced Ti6Al4V3Cu alloys exhibited finer martensite and small grain size.
- The corrosion experiment revealed that, due to the grain refinement of the SLM process, SLM-produced Ti6Al4V3Cu performs better than the casting sample, in terms of anti-corrosion capability.
- The corrosion resistance of Ti6Al4V3Cu alloys, fabricated by pre-alloyed metallic powder, is superior to that fabricated by the mixed elemental powder because of the uniform Cu element in the matrix.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zitzmann, N.U.; Berglundh, T. Definition and prevalence of peri-implant diseases. J. Clin. Periodontol. 2008, 35 (Suppl. S8), 286–291. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.X.; Qi, X.; Huang, S.Y.; Chen, S.S.; Wu, Y.F.; Zhao, L. Theaflavins inhibit pathogenic properties of P. gingivalis and MMPs production in P. gingivalis-stimulated human gingival fibroblasts. Arch. Oral Biol. 2015, 60, 12–22. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, A.M.; McManus, B.A.; Kinnevey, P.M.; Brennan, G.I.; Fleming, T.E.; Cashin, P.J.; O’Sullivan, M.; Palyzois, I.; Coleman, D.C. Significant Enrichment and Diversity of the Staphylococcal Arginine Catabolic Mobile Element ACME in Staphylococcus epidermidis Isolates From Subgingival Peri-implantitis Sites and Periodontal Pockets. Front. Microbiol. 2018, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.L. Peri-implantitis. J. Clin. Periodontol. 2018, 45, S246–S266. [Google Scholar] [CrossRef] [Green Version]
- Norowski, P.A.; Bumgardner, J.D. Biomaterial and Antibiotic Strategies for Peri-implantitis. J. Biomed. Mater. Res. Part B 2009, 88, 530–543. [Google Scholar] [CrossRef]
- Barie, P.S. Multidrug-Resistant Organisms and Antibiotic Management. Surg. Clin.-N. Am. 2012, 92, 345–391. [Google Scholar] [CrossRef]
- Liu, R.; Memarzadeh, K.; Chang, B.; Zhang, Y.; Ma, Z.; Allaker, R.P.; Ren, L.; Yang, K. Antibacterial effect of copper-bearing titanium alloy (Ti-Cu) against Streptococcus mutans and Porphyromonas gingivalis. Sci. Rep. 2016, 6, 29985. [Google Scholar] [CrossRef] [Green Version]
- Zhang, E.; Li, F.; Wang, H.; Liu, J.; Wang, C.; Li, M.; Yang, K. A new antibacterial titanium-copper sintered alloy: Preparation and antibacterial property. Mater. Sci. Eng. 2013, 33, 4280–4287. [Google Scholar] [CrossRef]
- Bao, M.; Liu, Y.; Wang, X.; Yang, L.; Li, S.; Ren, J.; Qin, G.; Zhang, E. Optimization of mechanical properties, biocorrosion properties and antibacterial properties of wrought Ti-3Cu alloy by heat treatment. Bioact. Mater. 2018, 3, 28–38. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, G.; Li, H.; Yang, L.; Wang, X.; Qin, G.; Zhang, E. Anti-bacterium influenced corrosion effect of antibacterial Ti-3Cu alloy in Staphylococcus aureus suspension for biomedical application. Mater. Sci. Eng. C 2019, 94, 376–384. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, X.; Yang, C.; Ren, L.; Luo, K.; Yang, K.; Lin, J. In vitro insights into the role of copper ions released from selective laser melted CoCrW-xCu alloys in the potential attenuation of inflammation and osteoclastogenesis. J. Mater. Sci. Technol. 2020, 41, 56–67. [Google Scholar] [CrossRef]
- Lu, Y.; Yang, C.; Liu, Y.; Yang, K.; Lin, J. Characterization of lattice defects and tensile deformation of biomedical Co29Cr9W3Cu alloy produced by selective laser melting. Addit. Manuf. 2019, 30, 100908. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, J.; Liu, L.; Xi, T.; Yang, C.; Li, Q.; Yang, K. Passivation potential regulating corrosion resistance and antibacterial property of 316L-Cu stainless steel in different simulated body fluids. Mater. Technol. 2021, 36, 118–130. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, W.; Yang, C.; Liu, Y.; Xiang, H.; Yang, K.; Lin, J. Improving mechanical properties of selective laser melted Co29Cr9W3Cu alloy by eliminating mesh-like random high-angle grain boundary. Mater. Sci. Eng. A 2020, 793, 139895. [Google Scholar] [CrossRef]
- Vrancken, B.; Thijs, L.; Kruth, J.-P.; Van Humbeeck, J. Heat treatment of Ti6Al4V produced by Selective Laser Melting: Microstructure and mechanical properties. J. Alloys Compd. 2012, 541, 177–185. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.C.; Chen, L.Y. A Review on Biomedical Titanium Alloys: Recent Progress and Prospect. Adv. Eng. Mater. 2019, 21, 1801215. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; Abanteriba, S.; Wong, S.; Brkljaca, R.; Houshyar, S. Optimisation of grafted phosphorylcholine-based polymer on additively manufactured titanium substrate for hip arthroplasty. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 101, 696–706. [Google Scholar] [CrossRef]
- Bartolomeu, F.; Buciumeanu, M.; Pinto, E.; Alves, N.; Silva, F.S.; Carvalho, O.; Miranda, G. Wear behavior of Ti6Al4V biomedical alloys processed by selective laser melting, hot pressing and conventional casting. Trans. Nonferrous Met. Soc. China 2017, 27, 829–838. [Google Scholar] [CrossRef]
- Fischer, M.; Joguet, D.; Robin, G.; Peltier, L.; Laheurte, P. In situ elaboration of a binary Ti-26Nb alloy by selective laser melting of elemental titanium and niobium mixed powders. Mater. Sci. Eng. C-Mater. Biol. Appl. 2016, 62, 852–859. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.C.; Liu, Y.J.; Qin, P.; Liang, S.X.; Sercombe, T.B.; Zhang, L.C. Selective laser melting of Ti-35Nb composite from elemental powder mixture: Microstructure, mechanical behavior and corrosion behavior. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2019, 760, 214–224. [Google Scholar] [CrossRef]
- Wang, J.C.; Liu, Y.J.; Rabadia, C.D.; Liang, S.X.; Sercombe, T.B.; Zhang, L.C. Microstructural homogeneity and mechanical behavior of a selective laser melted Ti-35Nb alloy produced from an elemental powder mixture. J. Mater. Sci. Technol. 2021, 61, 221–233. [Google Scholar] [CrossRef]
- Qin, P.; Liu, Y.J.; Sercombe, T.B.; Li, Y.H.; Zhang, C.W.; Cao, C.D.; Sun, H.Q.; Zhang, L.C. Improved Corrosion Resistance on Selective Laser Melting Produced Ti-5Cu Alloy after Heat Treatment. Acs Biomater. Sci. Eng. 2018, 4, 2633–2642. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.Y.; Yi, Z.; Feng, X.; Tian, W.Z.; Xu, D.K.; Valentino, A.M.C.; Wang, Q.; Sun, H.C. Antibacterial property of a gradient Cu-bearing titanium alloy by laser additive manufacturing. Rare Met. 2022, 41, 580–593. [Google Scholar] [CrossRef]
- Luo, J.; Guo, S.; Lu, Y.; Xu, X.; Zhao, C.; Wu, S.; Lin, J. Cytocompatibility of Cu-bearing Ti6Al4V alloys manufactured by selective laser melting. Mater. Charact. 2018, 143, 127–136. [Google Scholar] [CrossRef]
- Guo, S.; Lu, Y.; Wu, S.; Liu, L.; He, M.; Zhao, C.; Gan, Y.; Lin, J.; Luo, J.; Xu, X.; et al. Preliminary study on the corrosion resistance, antibacterial activity and cytotoxicity of selective-laser-melted Ti6Al4V-xCu alloys. Mater. Sci. Eng. C 2017, 72, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, Y.X.; Lu, Y.J.; Qin, S.J.; Huang, G.H.; Huang, T.T.; Lin, J.X. Effect of heat treatment on the corrosion resistance of selective laser melted Ti6Al4V3Cu alloy. J. Mater. Res. Technol. 2021, 12, 904–915. [Google Scholar] [CrossRef]
- Qin, P.; Chen, L.Y.; Zhao, C.H.; Liu, Y.J.; Cao, C.D.; Sun, H.; Zhang, L.C. Corrosion behavior and mechanism of selective laser melted Ti35Nb alloy produced using pre-alloyed and mixed powder in Hank’s solution. Corros. Sci. 2021, 189, 109609. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Z.; Li, D.; Xie, M.; Kollo, L.; Luo, Z.; Zhang, W.; Prashanth, K.G. Comparison of additively manufacturing samples fabricated from pre-alloyed and mechanically mixed powders. J. Alloys Compd. 2020, 830, 154603. [Google Scholar] [CrossRef]
- Zong, W.; Zhang, S.; Zhang, C.; Ren, L.; Wang, Q. Design and characterization of selective laser-melted Ti6Al4V–5Cu alloy for dental implants. Mater. Corros. 2020, 71, 1697–1710. [Google Scholar] [CrossRef]
- Takahashi, M.; Kikuchi, M.; Takada, Y.; Okuno, O.; Okabe, T. Corrosion behavior and microstructures of experimental Ti-Au alloys. Dent. Mater. J. 2004, 23, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Harris, E.D. Basic and clinical aspects of copper. Crit. Rev. Clin. Lab. Sci. 2003, 40, 547–586. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, H.; Yu, H.; Wang, Z.; Zeng, X. Corrosion Behavior of Additive Manufactured Ti-6Al-4V Alloy in NaCl Solution. Metall. Mater. Trans. A 2017, 48, 3583–3593. [Google Scholar] [CrossRef]
- Hemmasian Ettefagh, A.; Zeng, C.; Guo, S.; Raush, J. Corrosion behavior of additively manufactured Ti-6Al-4V parts and the effect of post annealing. Addit. Manuf. 2019, 28, 252–258. [Google Scholar] [CrossRef]
- Dai, N.; Zhang, J.; Chen, Y.; Zhang, L.-C. Heat Treatment Degrading the Corrosion Resistance of Selective Laser Melted Ti-6Al-4V Alloy. J. Electrochem. Soc. 2017, 164, C428–C434. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, Z.; Zhang, J.; Wang, X.; Qin, G.; Zhang, E. Enhanced antibacterial activity of Ti-Cu alloy by selective acid etching. Surf. Coat. Technol. 2021, 421, 127478. [Google Scholar] [CrossRef]
- Osório, W.R.; Cremasco, A.; Andrade, P.N.; Garcia, A.; Caram, R. Electrochemical behavior of centrifuged cast and heat treated Ti–Cu alloys for medical applications. Electrochim. Acta 2010, 55, 759–770. [Google Scholar] [CrossRef]
- Zhang, E.; Wang, X.; Chen, M.; Hou, B. Effect of the existing form of Cu element on the mechanical properties, bio-corrosion and antibacterial properties of Ti-Cu alloys for biomedical application. Mater. Sci. Eng. C 2016, 69, 1210–1221. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, S.; Sun, Z.; Wang, H.; Ren, L.; Yang, K. Optimization of mechanical property, antibacterial property and corrosion resistance of Ti-Cu alloy for dental implant. J. Mater. Sci. Technol. 2019, 35, 2336–2344. [Google Scholar] [CrossRef]



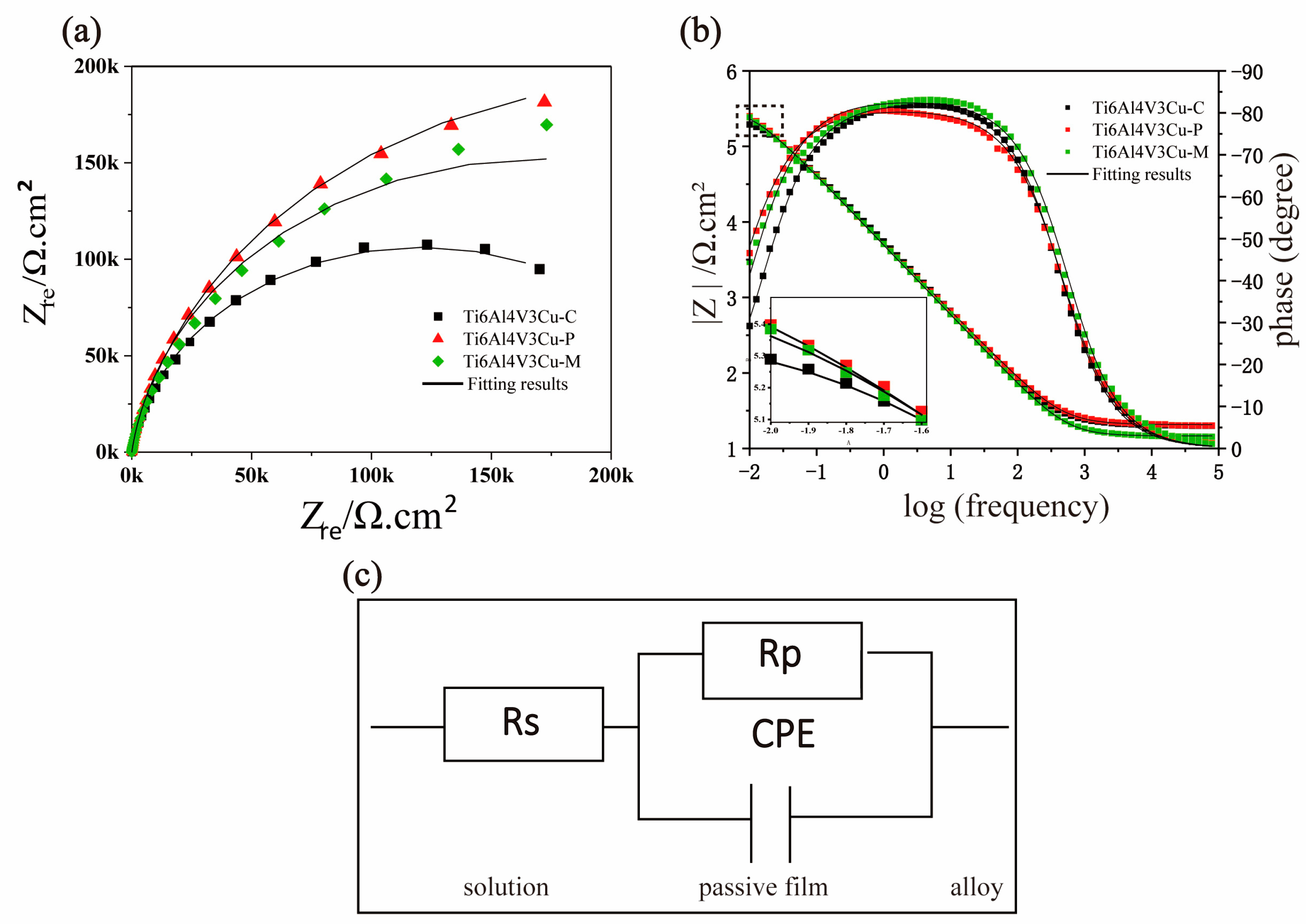
| Samples | Icorr (nA) | Ecorr (mV) | Corrosion Rate (μg∙cm−2∙yr−1) | Eb (mV) |
|---|---|---|---|---|
| Ti6Al4V3Cu-C | 215 ± 22.51 | −321.7 ± 21.2 | 174.2 ± 18.1 | 1270 ± 4 |
| Ti6Al4V3Cu-P | 66.6 ± 17.6 | −388.5 ± 10.2 | 53.9 ± 14.3 | 1359 ± 6 |
| Ti6Al4V3Cu-M | 84.2 ± 15.5 | −354.0 ± 42.1 | 68.3 ± 12.6 | 1336 ± 4 |
| Samples | Rs (Ω·cm2) | Rp (K·Ωcm2) | Y0 (μΩ−1∙sn∙cm−2) | n |
|---|---|---|---|---|
| Ti6Al4V3Cu-C | 19.91 ± 1.76 | 231.52 ± 25.94 | 29.12 ± 4.9 | 0.89 ± 0.03 |
| Ti6Al4V3Cu-P | 21.81 ± 2.36 | 371.67 ± 103.60 | 35.87 ± 2.86 | 0.91 ± 0.01 |
| Ti6Al4V3Cu-M | 17.7 ± 2.95 | 354.17 ± 141.78 | 34.09 ± 2.81 | 0.89 ± 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, G.; Fan, Z.; Li, L.; Lu, Y.; Lin, J. Corrosion Resistance of Selective Laser Melted Ti6Al4V3Cu Alloy Produced Using Pre-Alloyed and Mixed Powder. Materials 2022, 15, 2487. https://doi.org/10.3390/ma15072487
Huang G, Fan Z, Li L, Lu Y, Lin J. Corrosion Resistance of Selective Laser Melted Ti6Al4V3Cu Alloy Produced Using Pre-Alloyed and Mixed Powder. Materials. 2022; 15(7):2487. https://doi.org/10.3390/ma15072487
Chicago/Turabian StyleHuang, Gonghao, Zefeng Fan, Liu Li, Yanjin Lu, and Jinxin Lin. 2022. "Corrosion Resistance of Selective Laser Melted Ti6Al4V3Cu Alloy Produced Using Pre-Alloyed and Mixed Powder" Materials 15, no. 7: 2487. https://doi.org/10.3390/ma15072487
APA StyleHuang, G., Fan, Z., Li, L., Lu, Y., & Lin, J. (2022). Corrosion Resistance of Selective Laser Melted Ti6Al4V3Cu Alloy Produced Using Pre-Alloyed and Mixed Powder. Materials, 15(7), 2487. https://doi.org/10.3390/ma15072487







