Effect of Bimetallic Dimer-Embedded TiO2(101) Surface on CO2 Reduction: The First-Principles Calculation
Abstract
:1. Introduction
2. Computational Details
3. Results and Discussions
3.1. Bimetallic Sites on Anatase TiO2(101) Surface
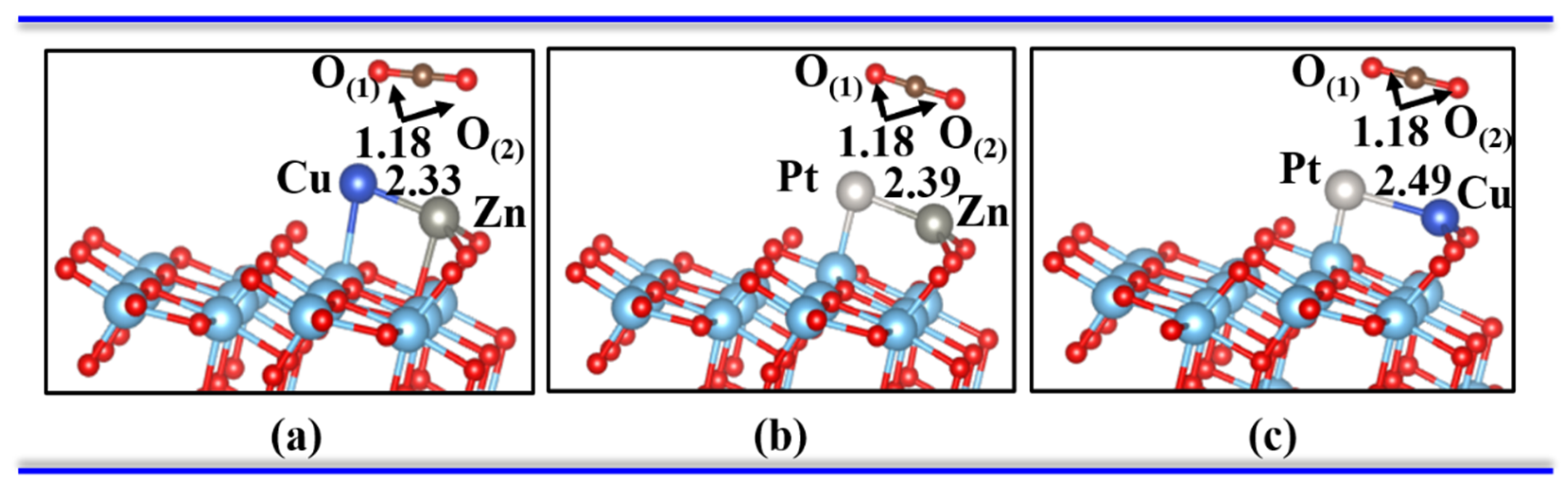
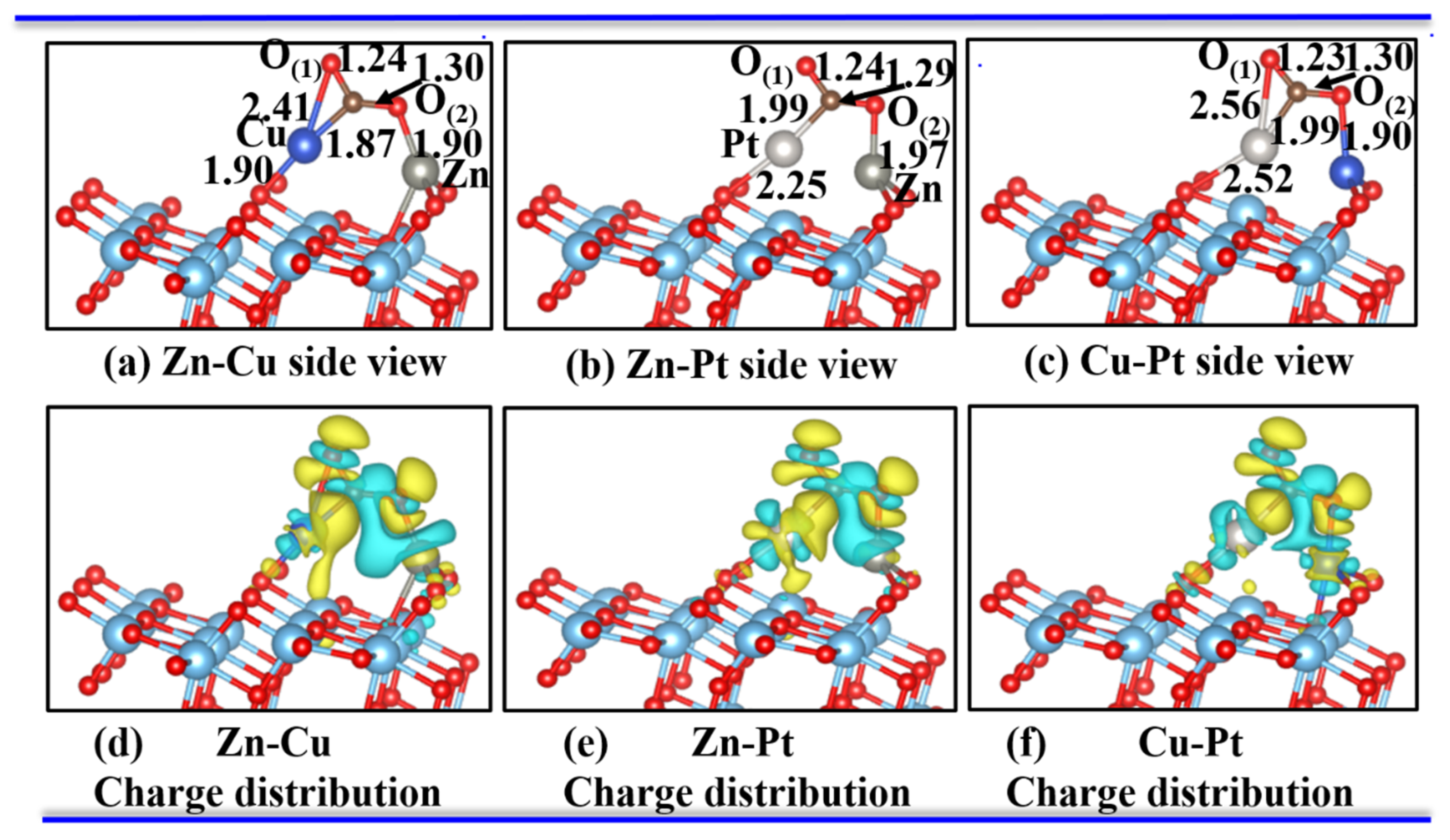
3.2. One CO2 Molecule Adsorbed on the Bimetal-TiO2(101) Surface
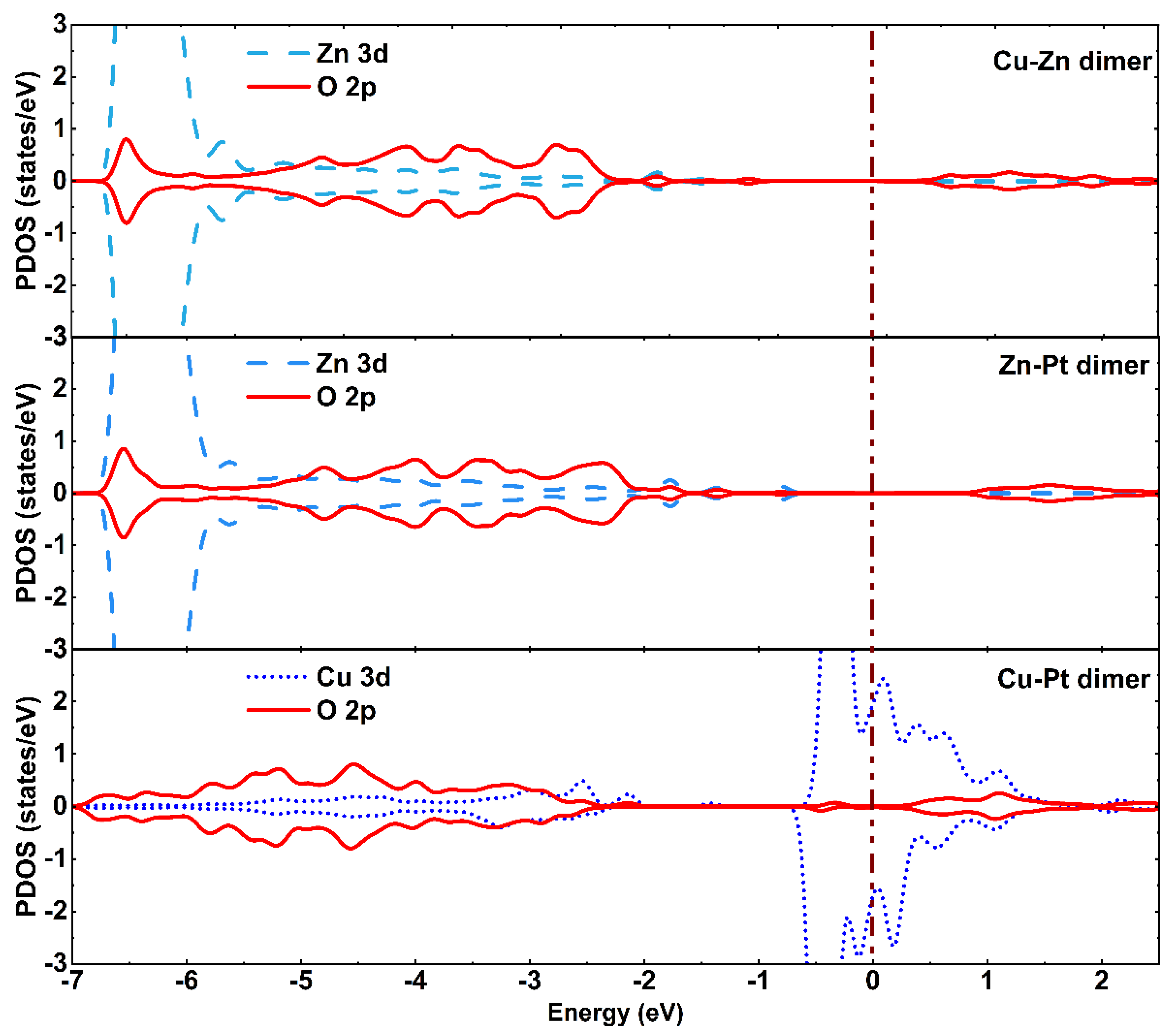
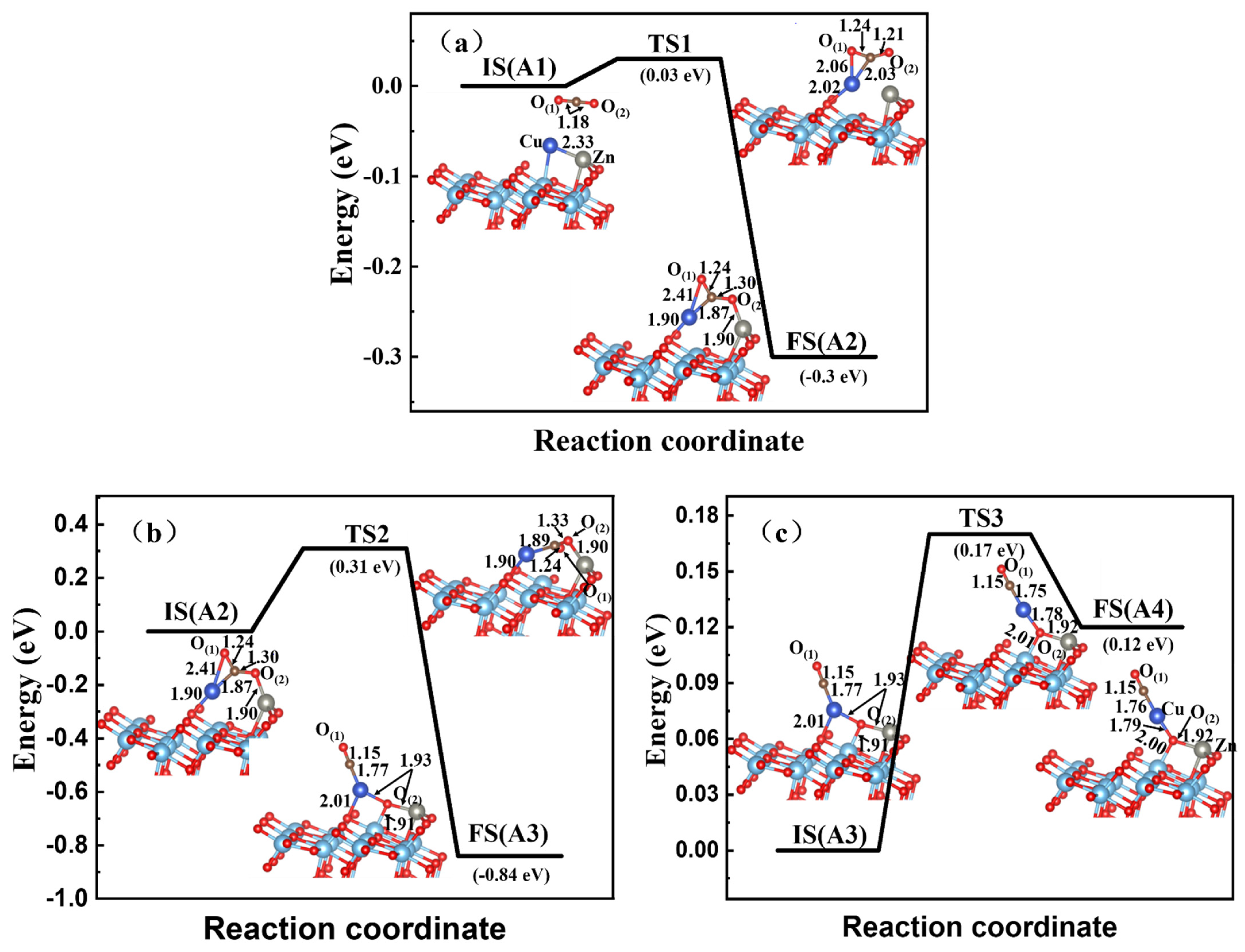
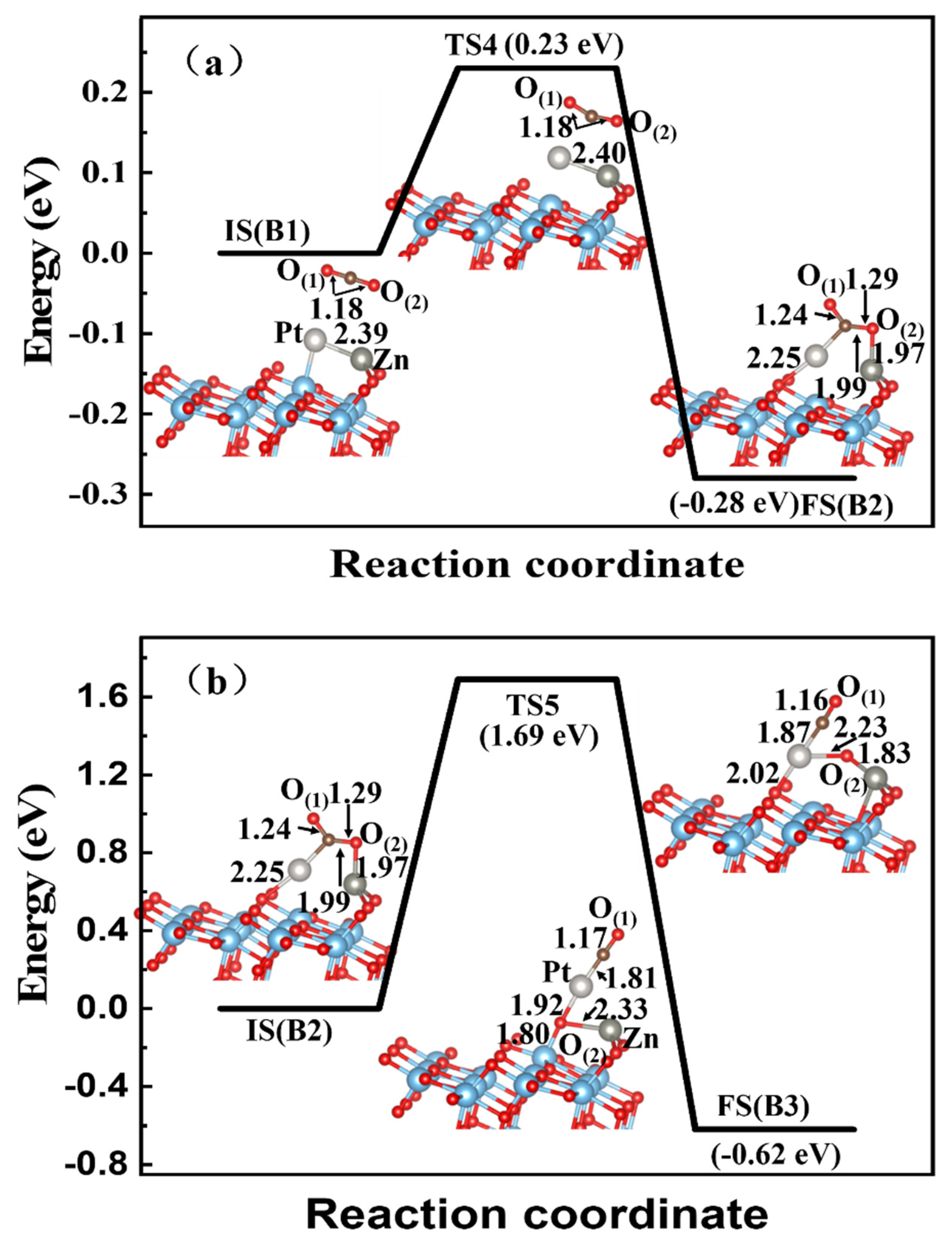
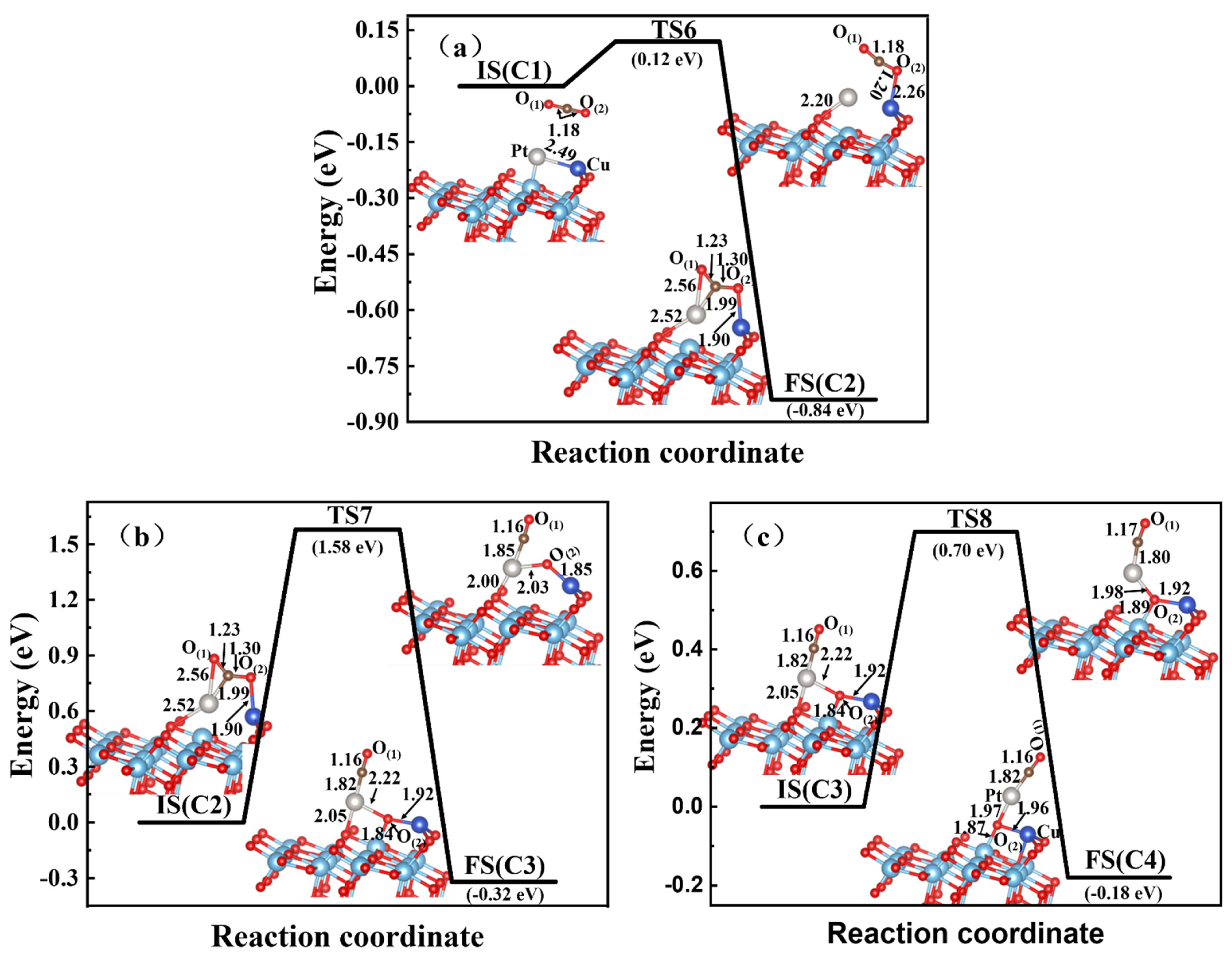
3.3. CO2 Reduction on the Zn-Cu, Zn-Pt, and Cu-Pt Dimer-Embedded Anatase TiO2(101) Surface
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- USDC. Trends in Atmospheric Carbon Dioxide, Monthly Average Mauna Loa CO2; National Oceanic and Atmospheric Administration: Silver Spring, MD, USA; Earth System Research Laboratory: Boulder, CO, USA; Global Monitoring Division: Boulder, CO, USA; United States Department of Commerce: Washington, DC, USA, 2021. Available online: https://gml.noaa.gov/ccgg/trends/ (accessed on 17 July 2021).
- Manojkumar, N.; Srimuruganandam, B. Size-segregated particulate matter and health effects in air pollution in India: A review. Environ. Chem. Lett. 2021, 19, 3837–3858. [Google Scholar] [CrossRef]
- Desa, U. World Population Prospects 2019: Highlights; United Nations Department for Economic and Social Affairs: New York, NY, USA, 2019. [Google Scholar]
- Millati, R.C.; Ariyanto, T.; Azzahrani, I.N.; Putri, R.U.; Taherzadeh, M.J. Sustainable Resource Recovery and Zero Waste Approaches; Elsevier: New York, NY, USA, 2019. [Google Scholar]
- Khanal, S.K.; Varjani, S.; Lin, C.S.K.; Awasthi, M.K. Waste-to-resources: Opportunities and challenges. Bioresour. Technol. 2020, 317, 123987. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Shafy, H.I.; Mansour, M.S.M. Solid waste issue: Sources, composition, disposal, recycling, and valorization. Egypt J. Petrol. 2018, 27, 1275–1290. [Google Scholar] [CrossRef]
- Hussain, M.; Liu, G.; Yousaf, B.; Ahmed, R.; Uzma, F.; Ali, M.U.; Ullah, H.; Butt, A.R. Regional and sectoral assessment on climate-change in Pakistan: Social norms and indigenous perceptions on climate-change adaptation and mitigation in relation to global context. J. Clean. Prod. 2018, 200, 791–808. [Google Scholar] [CrossRef]
- Mustafa, A.; Lougou, B.G.; Shuai, Y.; Wang, Z.; Tan, H. Current technology development for CO2 utilization into solar fuels and chemicals: A review. J. Energy Chem. 2020, 49, 96–123. [Google Scholar] [CrossRef]
- Tackett, B.M.; Gomez, E.; Chen, J.G. Net reduction of CO2 via its thermocatalytic and electrocatalytic transformation reactions in standard and hybrid processes. Nat. Catal. 2019, 2, 381–386. [Google Scholar] [CrossRef]
- Huang, B.; Wu, Y.; Luo, Y.; Zhou, N. Double atom-anchored Defective Boron Nitride catalyst for efficient electroreduction of CO2 to CH4: A first principles study. Chem. Phys. Lett. 2020, 756, 137852. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, Z. Reductive Transformation of Carbon Dioxide. Acta Phys. Chim. Sin. 2021, 37, 2012024. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, Y.; Zhou, Z.; Cai, F.; Zhao, X.; Huang, W.; Li, Y.; Zhu, J.; Liu, P.; Yang, F.; et al. Enhancing CO2 Electroreduction with the Metal–Oxide Interface. J. Am. Chem. Soc. 2017, 139, 5652–5655. [Google Scholar] [CrossRef]
- Kim, D.; Resasco, J.; Yu, Y.; Asiri, A.M.; Yang, P. Synergistic geometric and electronic effects for electrochemical reduction of carbon dioxide using gold–copper bimetallic nanoparticles. Nat. Commun. 2014, 5, 4948. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Yang, B.; Tyo, E.; Seifert, S.; DeBartolo, J.; von Issendorff, B.; Zapol, P.; Vajda, S.; Curtiss, L.A. Carbon Dioxide Conversion to Methanol over Size-Selected Cu4 Clusters at Low Pressures. J. Am. Chem. Soc. 2015, 137, 8676–8679. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Shao, Q.; Wang, P.; Dai, Q.; Wang, X.; Huang, X. Highly Active and Selective Hydrogenation of CO2 to Ethanol by Ordered Pd–Cu Nanoparticles. J. Am. Chem. Soc. 2017, 139, 6827–6830. [Google Scholar] [CrossRef]
- Lakshmidevi, J.; Ramesh Naidu, B.; Venkateswarlu, K. A rapid-room temperature synthesis of α-cyanoacrylates, α-cyanoacrylonitriles and 4H-pyrans using water extract of pomegranate ash as catalytic media. Sustain. Chem. Pharm. 2022, 25, 100610. [Google Scholar] [CrossRef]
- Wang, Z.; Hong, J.; Ng, S.-F.; Liu, W.; Huang, J.; Chen, P.; Ong, W.-J. Recent Progress of Perovskite Oxide in Emerging Photocatalysis Landscape: Water Splitting, CO2 Reduction, and N2 Fixation. Acta Phys. Chim. Sin. 2020, 37, 2011033. [Google Scholar] [CrossRef]
- Naidu, B.R.; Lakshmidevi, J.; Naik, B.S.S.; Venkateswarlu, K. Water extract of pomegranate ash as waste-originated biorenewable catalyst for the novel synthesis of chiral tert-butanesulfinyl aldimines in water. Mol. Catal. 2021, 511, 111719. [Google Scholar] [CrossRef]
- Appa, R.M.; Naidu, B.R.; Venkateswarlu, D.; Hanafiah, M.M.; Lakkaboyana, S.K.; Lakshmidevi, J.; Venkateswarlu, K. Water extract of pomegranate ash–I2 as sustainable system for external oxidant/metal/catalyst-free oxidative iodination of (hetero)arenes. Green Chem. Lett. Rev. 2021, 14, 700–712. [Google Scholar] [CrossRef]
- Di Giovannantonio, M.; Kosmala, T.; Bonanni, B.; Serrano, G.; Zema, N.; Turchini, S.; Catone, D.; Wandelt, K.; Pasini, D.; Contini, G.; et al. Surface-Enhanced Polymerization via Schiff-Base Coupling at the Solid–Water Interface under pH Control. J. Phys. Chem. C 2015, 119, 19228–19235. [Google Scholar] [CrossRef]
- Di Giovannantonio, M.; El Garah, M.; Lipton-Duffin, J.; Meunier, V.; Cardenas, L.; Revurat, Y.F.; Cossaro, A.; Verdini, A.; Perepichka, D.F.; Rosei, F.; et al. Insight into Organometallic Intermediate and Its Evolution to Covalent Bonding in Surface-Confined Ullmann Polymerization. ACS Nano 2013, 7, 8190–8198. [Google Scholar] [CrossRef]
- Klopp, J.M.; Pasini, D.; Byers, J.D.; Willson, C.G.; Frechet, J.M.J. Microlithographic assessment of a novel family of transparent and etch-resistant chemically amplified 193-nm resists eased on cyclopolymers. Chem. Mater. 2001, 13, 4147–4153. [Google Scholar] [CrossRef]
- Lakshmidevi, J.; Vakati, V.; Naidu, B.R.; Raghavender, M.; Rao, K.S.V.K.; Venkateswarlu, K. Pd(5%)-KIT-6, Pd(5%)-SBA-15 and Pd(5%)-SBA-16 catalysts in water extract of pomegranate ash: A case study in heterogenization of Suzuki-Miyaura reaction under external base and ligand free conditions. Sustain. Chem. Pharm. 2021, 19, 100371. [Google Scholar] [CrossRef]
- Zhou, X. TiO2 Supported Single-Atom Catalysts for Photocatalytic Reactions. Acta Phys. Chim. Sin. 2020, 37, 2008064. [Google Scholar] [CrossRef]
- Cai, S.; Wang, L.; Heng, S.; Li, H.; Bai, Y.; Dang, D.; Wang, Q.; Zhang, P.; He, C. Interaction of Single-Atom Platinum–Oxygen Vacancy Defects for the Boosted Photosplitting Water H2 Evolution and CO2 Photoreduction: Experimental and Theoretical Study. J. Phys. Chem. C 2020, 124, 24566–24579. [Google Scholar] [CrossRef]
- Huang, L.; Li, W.; Zeng, M.; He, G.; Shearing, P.R.; Parkin, I.P.; Brett, D.J.L. Metal-Nitrogen-doped carbon single-atom electrocatalysts for CO2 electroreduction. Compos. Part. B Eng. 2021, 220, 108986. [Google Scholar] [CrossRef]
- Liu, J. Catalysis by Supported Single Metal Atoms. ACS Catal. 2016, 7, 34–59. [Google Scholar] [CrossRef]
- Lang, Q.; Yang, Y.; Zhu, Y.; Hu, W.; Jiang, W.; Zhong, S.; Gong, P.; Teng, B.; Zhao, L.; Bai, S. High-index facet engineering of PtCu cocatalysts for superior photocatalytic reduction of CO2 to CH4. J. Mater. Chem. A 2017, 5, 6686–6694. [Google Scholar] [CrossRef]
- Li, A.; Wang, T.; Chang, X.; Zhao, Z.-J.; Li, C.; Huang, Z.; Yang, P.; Zhou, G.; Gong, J. Tunable syngas production from photocatalytic CO2 reduction with mitigated charge recombination driven by spatially separated cocatalysts. Chem. Sci. 2018, 9, 5334–5340. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Han, F.; Shi, B.; Farsinezhad, S.; Dechaine, G.P.; Shankar, K. Photocatalytic Conversion of Diluted CO2 into Light Hydrocarbons Using Periodically Modulated Multiwalled Nanotube Arrays. Angew. Chem. Int. Ed. 2012, 51, 12732–12735. [Google Scholar] [CrossRef]
- Lee, S.; Jeong, S.; Kim, W.D.; Lee, S.; Lee, K.; Bae, W.K.; Moon, J.H.; Lee, S.; Lee, D.C. Low-coordinated surface atoms of CuPt alloy cocatalysts on TiO2 for enhanced photocatalytic conversion of CO2. Nanoscale 2016, 8, 10043–10048. [Google Scholar] [CrossRef] [Green Version]
- Neaţu, Ş.; Maciá-Agulló, J.A.; Concepción, P.; Garcia, H. Gold–Copper Nanoalloys Supported on TiO2 as Photocatalysts for CO2 Reduction by Water. J. Am. Chem. Soc. 2014, 136, 15969–15976. [Google Scholar] [CrossRef]
- Kang, Q.; Wang, T.; Li, P.; Liu, L.; Chang, K.; Li, M.; Ye, J. Photocatalytic reduction of carbon dioxide by hydrous hydrazine over Au-Cu alloy nanoparticles supported on SrTiO3/TiO2 coaxial nanotube arrays. Angew. Chem. Int. Ed. 2015, 54, 841–845. [Google Scholar] [CrossRef]
- Baldoví, H.G.; Neaţu, Ş.; Khan, A.; Asiri, A.M.; Kosa, S.A.; Garcia, H. Understanding the Origin of the Photocatalytic CO2 Reduction by Au- and Cu-Loaded TiO2: A Microsecond Transient Absorption Spectroscopy Study. J. Phys. Chem. C 2015, 119, 6819–6827. [Google Scholar] [CrossRef]
- Zheng, M.; Jia, C.; Sharman, E.; Jiang, J.; Fan, W.; Zhao, X. Maximizing the Synergistic Effect of PdAu Catalysts on TiO2(101) for Robust CO2 Reduction:A DFT Study. Appl. Surf. Sci. 2021, 563, 150365. [Google Scholar] [CrossRef]
- Ren, X.; Gao, Y.; Zheng, L.; Wang, Z.; Wang, P.; Zheng, Z.; Liu, Y.; Cheng, H.; Dai, Y.; Huang, B. Oxygen vacancy enhancing CO2 electrochemical reduction to CO on Ce-doped ZnO catalysts. Surf. Interfaces 2021, 23, 100923. [Google Scholar] [CrossRef]
- Yui, T.; Kan, A.; Saitoh, C.; Koike, K.; Ibusuki, T.; Ishitani, O. Photochemical Reduction of CO2 Using TiO2: Effects of Organic Adsorbates on TiO2 and Deposition of Pd onto TiO2. ACS Appl. Mater. Interfaces 2011, 3, 2594–2600. [Google Scholar] [CrossRef]
- Kreft, S.; Wei, D.; Junge, H.; Beller, M. Recent advances on TiO2-based photocatalytic CO2 reduction. Energy Chem. 2020, 2, 100044. [Google Scholar] [CrossRef]
- Zhou, W.; Guo, J.-K.; Shen, S.; Pan, J.; Tang, J.; Chen, L.; Au, C.-T.; Yin, S.-F. Progress in Photoelectrocatalytic Reduction of Carbon Dioxide. Acta Phys. Chim. Sin. 2020, 36, 1906048. [Google Scholar] [CrossRef]
- Blochl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab-Iinitio molecular-dynamics for open-shell transition-metals. Phys. Rev. B 1993, 48, 13115–13118. [Google Scholar] [CrossRef]
- Kresse, G. Ab initio molecular dynamics for liquid metals. J. Non-Cryst. Solids 1995, 192–193, 222–229. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal-amorphous-semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Camellone, M.F.; Fabris, S. Reaction Mechanisms for the CO Oxidation on Au/CeO2 Catalysts: Activity of Substitutional Au3+/Au+ Cations and Deactivation of Supported Au+ Adatoms. J. Am. Chem. Soc. 2009, 131, 10473–10483. [Google Scholar] [CrossRef]
- He, Z.; Wen, L.; Wang, D.; Xue, Y.; Lu, Q.; Wu, C.; Chen, J.; Song, S. Photocatalytic Reduction of CO2 in Aqueous Solution on Surface-Fluorinated Anatase TiO2 Nanosheets with Exposed {001} Facets. Energy Fuel 2014, 28, 3982–3993. [Google Scholar] [CrossRef]
- Jónsson, H.; Mills, G.; Jacobsen, K.W. Nudged elastic band method for nding minimum energy paths of transitions. In Classical and Quantum Dynamics in Condensed Phase Simulations; Berne, B.J., Ciccotti, G., Coker, D.F., Eds.; World Scientific: Singapore, 1998; p. 385. [Google Scholar]
- Henkelman, G.; Uberuaga, B.P.; Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef] [Green Version]
- Henkelman, G.; Jónsson, H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 2000, 113, 9978–9985. [Google Scholar] [CrossRef] [Green Version]
- Henkelman, G.; Arnaldsson, A.; Jónsson, H. A fast and robust algorithm for Bader decomposition of charge density. Comput. Mater. Sci. 2006, 36, 354–360. [Google Scholar] [CrossRef]


| Dimer | dM-N (Å) | dM-O (Å) | ∠O2c-M-O2c (°) | dTi-N (Å) | Eb (eV) | ∆Q (e) |
|---|---|---|---|---|---|---|
| Zn-Cu | 2.32 | 2.07 | 108.42° | 2.62 | −2.34 | −0.85 |
| Zn-Pt | 2.39 | 2.02 | 114.96° | 2.28 | −3.60 | −0.52 |
| Cu-Pt | 2.48 | 1.92 | 135.90° | 2.29 | −2.90 | −0.60 |
| ∠O2c-M-O2c (°) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pure TiO2 | - | - | 1.18 | 1.18 | 180 | - | - | −0.31 | - |
| Zn-Cu | 3.45 | 1.90 | 1.24 | 1.30 | 125.92 | 1.87 | 1.90 | −0.36 | 0.82 |
| Zn-Pt | 2.68 | 1.97 | 1.24 | 1.29 | 130.90 | 1.99 | 2.25 | −0.46 | 0.67 |
| Cu-Pt | 2.63 | 1.90 | 1.23 | 1.30 | 132.32 | 1.99 | 2.52 | −0.97 | 0.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Shang, C.; Zhao, B.; Zhang, G.; Liu, L.; Yang, W.; Chen, Z. Effect of Bimetallic Dimer-Embedded TiO2(101) Surface on CO2 Reduction: The First-Principles Calculation. Materials 2022, 15, 2538. https://doi.org/10.3390/ma15072538
Li C, Shang C, Zhao B, Zhang G, Liu L, Yang W, Chen Z. Effect of Bimetallic Dimer-Embedded TiO2(101) Surface on CO2 Reduction: The First-Principles Calculation. Materials. 2022; 15(7):2538. https://doi.org/10.3390/ma15072538
Chicago/Turabian StyleLi, Chongyang, Cui Shang, Bin Zhao, Gang Zhang, Liangliang Liu, Wentao Yang, and Zhiquan Chen. 2022. "Effect of Bimetallic Dimer-Embedded TiO2(101) Surface on CO2 Reduction: The First-Principles Calculation" Materials 15, no. 7: 2538. https://doi.org/10.3390/ma15072538






