Waste Originating from the Cleaning of Flue Gases from the Combustion of Industrial Wastes as a Lime Partial Replacement in Autoclaved Aerated Concrete
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Mix Proportions
2.3. Testing Procedure
3. Results
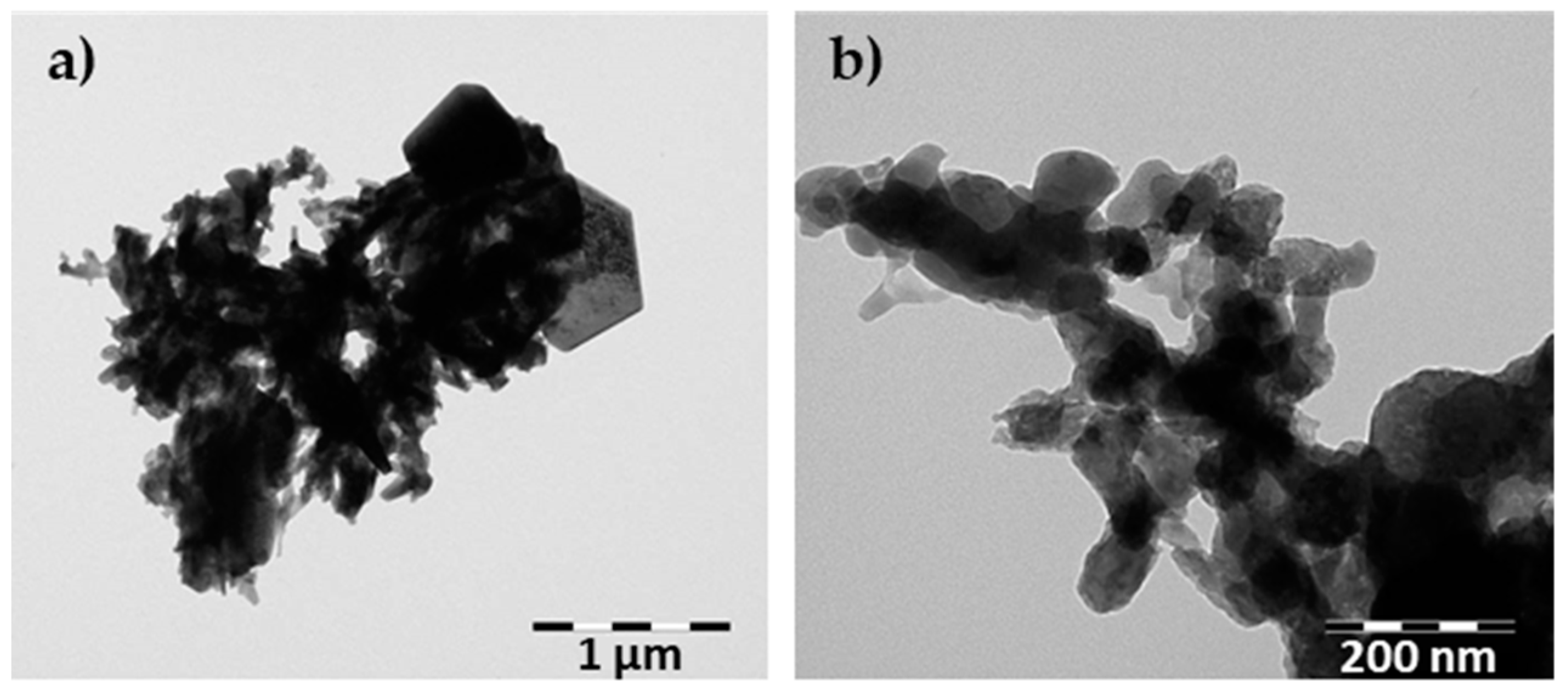
3.1. Characterization of Waste
3.2. Influence of Calcareous Waste on the Properties of AAC
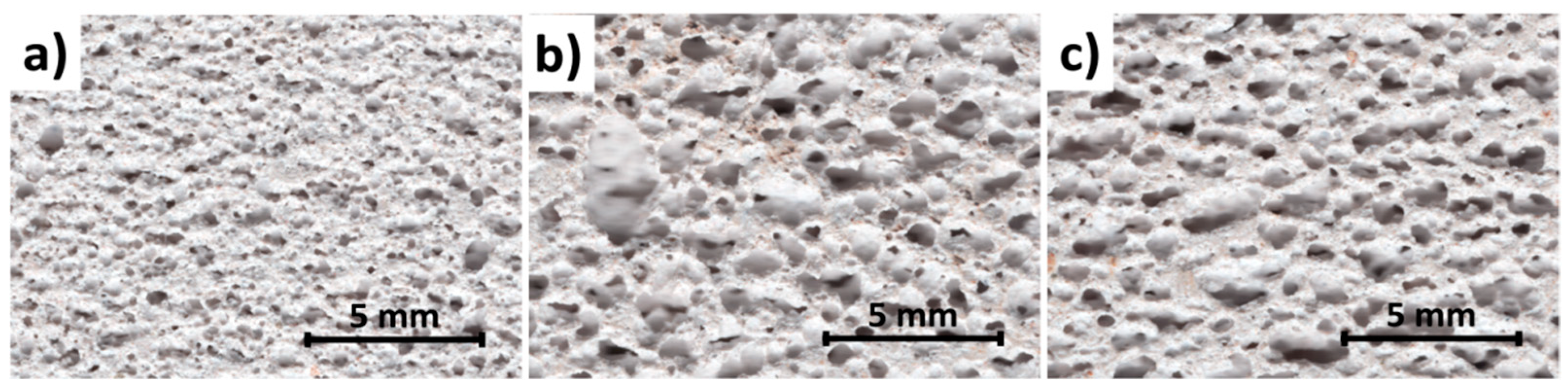
3.2.1. Bulk Density
3.2.2. Compressive Strength
3.2.3. Thermal Conductivity
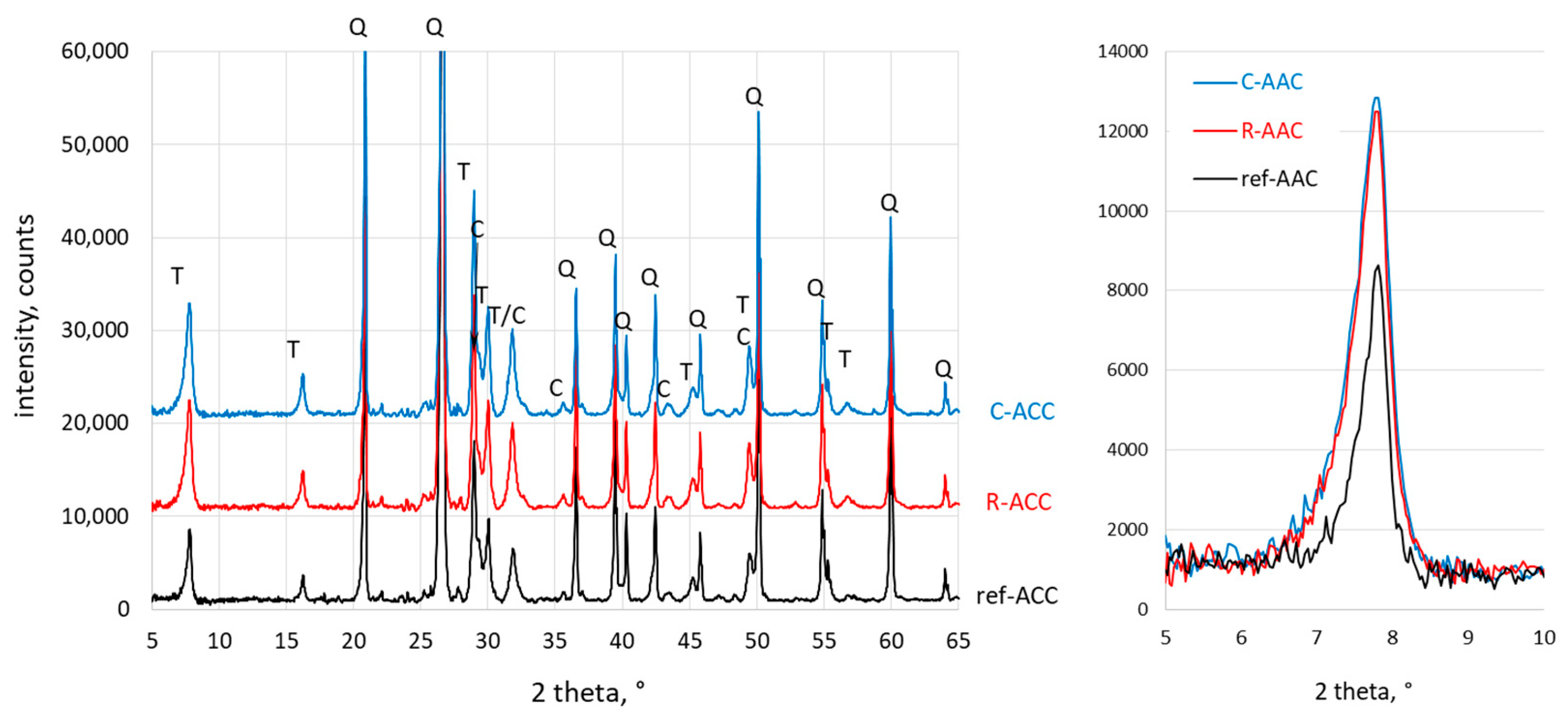
3.3. X-ray Diffraction Analysis
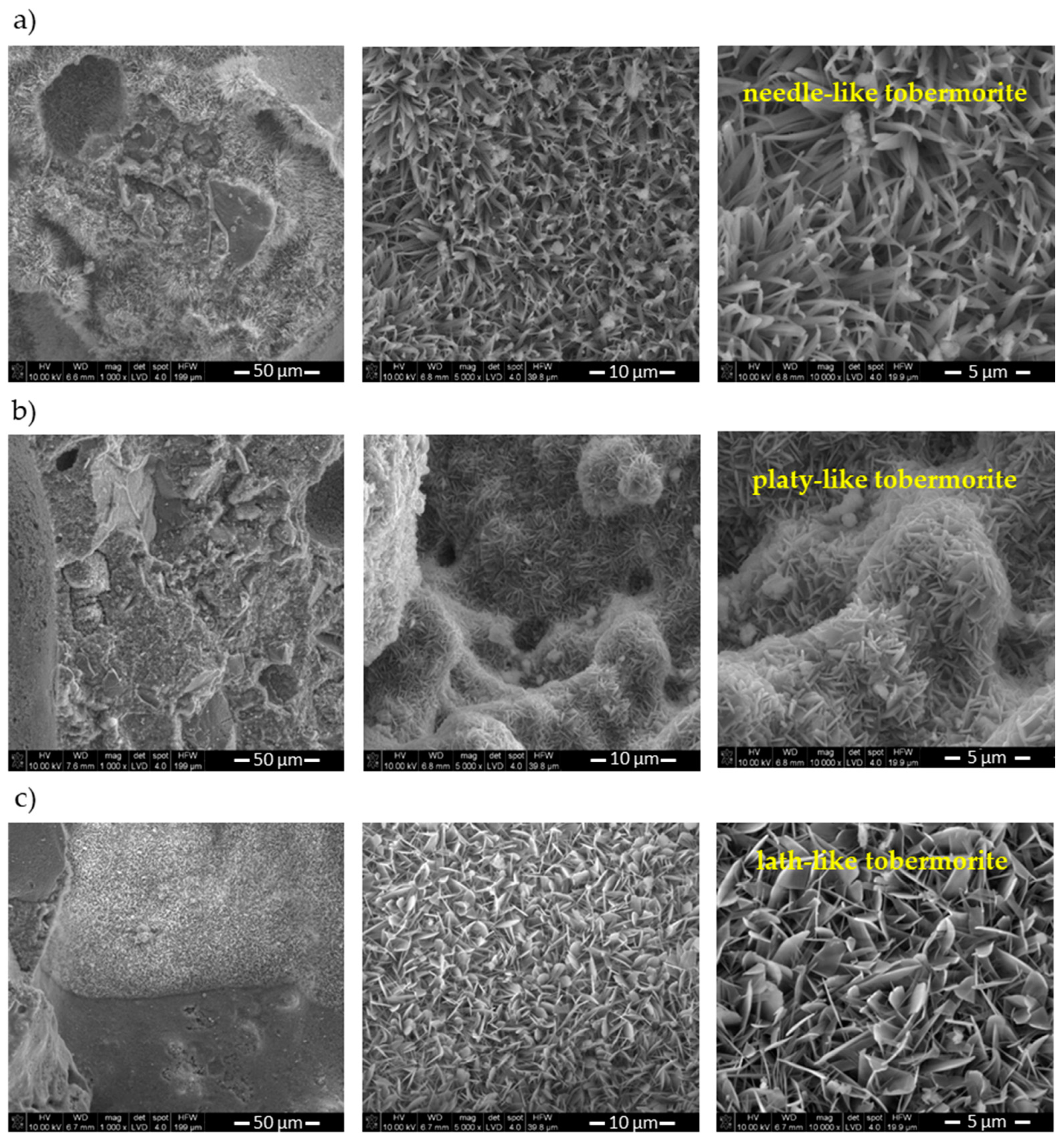
3.4. Microstructure Characterization
4. Conclusions
- Waste can be potentially used as a substitution of lime in AAC technology. This allows reducing the emissions of CO2 related to the limestone calcination process.
- It was found that waste affects the properties of AAC by altering the morphology of 1.1 nm tobermorite and pore structure of AAC.
- The compressive strength was found to increase with waste addition. The SEM analysis showed that the addition of waste resulted in densely packed plate 1.1 nm tobermorite crystals. The densified microstructure improved the mechanical properties of AAC. Additionally, it was shown that dense microstructure could compensate for the loss of strength associated with the increase in pore size distribution. The role of chlorine-bearing compounds present in the waste should be the subject of further tests, since it is possible that they influence the strength gain of AAC.
- The thermal conductivity was found to increase with waste addition. The main reason for the increase in thermal conductivity was the pore size distribution significantly influenced by the waste addition.
- The replacement of lime by waste promoted the formation and changed the morphology of 1.1 nm tobermorite.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Misiewicz, L. Polish market of building materials for erecting walls in 2020. Mater. Bud. 2021, 4, 8–9. (In Polish) [Google Scholar]
- Zapotoczna-Sytek, G.; Balkovic, S. Autoclaved Aerated Concrete; PWN: Warszawa, Poland, 2013. (In Polish) [Google Scholar]
- Kunchariyakun, K.; Asavapisit, S.; Sombatsompop, K. Properties of autoclaved aerated concrete incorporating rice husk ash as partial replacement for fine aggregate. Cem. Concr. Comp. 2015, 55, 11–16. [Google Scholar] [CrossRef]
- Song, Y.; Li, B.; Yang, E.; Liu, Y.; Ding, Y. Feasibility study on utilization of municipal solid waste incineration bottom ash as aerating agent for the production of autoclaved aerated concrete. Cem. Concr. Comp. 2015, 56, 51–58. [Google Scholar] [CrossRef]
- Wongkeo, W.; Chaipanich, A. Compressive strength, microstructure and thermal analysis of autoclaved and air cured structural lightweight concrete made with coal bottom ash and silica fume. Mater. Sci. Eng. A 2010, 527, 3676–3684. [Google Scholar] [CrossRef]
- Łagosz, A.; Szymanski, P.; Walczak, P. Influence of the Fly Ash Type on Properties of Autoclaved Aerated Concrete. Cem. Wapno Beton 2011, 16, 22–25. [Google Scholar]
- Walczak, P.; Szymanski, P.; Różycka, A. Autoclaved aerated concrete based on fly ash in density 350 kg/m3 as an environmentally friendly material for energy—Efficient constructions. Proc. Eng. 2015, 122, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Łagosz, A.; Szymanski, P.; Walczak, P. Influence of the fly ash properties on properties of autoclaved aerated concrete. In Proceedings of the 5th International Conference on Autoclaved Aerated Concrete “Securing a Sustainable Future”, Bydgoszcz, Poland, 14–17 September 2011; pp. 111–118. [Google Scholar]
- Różycka, R.; Pichór, W. Effect of perlite waste addition on the properties of autoclaved aerated concrete. Constr. Build. Mater. 2016, 120, 65–71. [Google Scholar] [CrossRef]
- Li, X.; Liu, Z.; Lv, Y.; Cai, L.; Jiang, D.; Jian, S. Utilization of municipal solid waste incineration bottom ash in autoclaved aerated concrete. Constr. Build. Mater. 2018, 178, 175–182. [Google Scholar] [CrossRef]
- Huang, X.; Ni, W.; Cui, W.; Wang, Z.; Zhu, L. Preparation of autoclaved aerated concrete using copper tailings and blast furnace slag. Constr. Build. Mater. 2012, 27, 1–5. [Google Scholar] [CrossRef]
- Wu, R.; Dai, S.; Jian, S.; Huang, J.; Tan, H.; Li, B. Utilization of solid waste high-volume calcium coal gangue in autoclaved aerated concrete: Physico-mechanical properties, hydration products and economic costs. J. Clean. Prod. 2021, 278, 1234416. [Google Scholar] [CrossRef]
- Zafar, M.; Javed, U.; Khushnood, R.; Nawaz, A.; Zafar, T. Sustainable incorporation of waste granite dust as partial replacement of sand in autoclave aerated concrete. Constr. Build. Mater. 2020, 250, 118878. [Google Scholar] [CrossRef]
- El-Didamony, H.; Amer, A.; Mohammed, M.; El–Hakim, M. Fabrication and properties of autoclaved aerated concrete containing agriculture and industrial solid wastes. J. Build. Eng. 2019, 22, 528–538. [Google Scholar] [CrossRef]
- Jiang, J.; Cai, Q.; Ma, B.; Hu, Y.; Quian, B.; Ma, F.; Shao, Z.; Xu, Z.; Wang, L. Effect of ZSM-5 waste dosage on the properties of autoclaved aerated concrete. Constr. Build. Mater. 2021, 278, 122114. [Google Scholar] [CrossRef]
- Peng, Y.; Liou, Y.; Zhan, B.; Xu, G. Preparation of autoclaved aerated concrete by using graphite tailings as an alternative silica source. Constr. Build. Mater. 2021, 267, 121792. [Google Scholar] [CrossRef]
- Yuan, B.; Straub, C.; Segers, S.; Lu, Q.; Brouwers, H.J.H. Sodium carbonate activated slag as cement replacement in autoclaved aerated concrete. Ceram. Int. 2017, 43, 6039–6047. [Google Scholar] [CrossRef]
- Wongkeo, W.; Thongsanitgarn, P.; Pimraksa, K.; Chaipanich, A. Compressive strength, flexural strength and thermal conductivity of autoclaved concrete block made using bottom ash as cement replacement materials. Mater. Des. 2012, 35, 434–439. [Google Scholar] [CrossRef]
- Hauser, A.; Eggenberger, U.; Mumenthaler, T. Fly ash from cellulose industry as secondary raw material in autoclaved aerated concrete. Cem. Concr. Res. 1999, 29, 297–302. [Google Scholar] [CrossRef]
- Kurdowski, W.; Pawluk, J. Limestone meal as active mineral additive for production of aerated autoclaved concrete. Cem. Wapno Beton 2019, 24, 154–160. [Google Scholar] [CrossRef]
- Gucluer, K.; Demir, I. Utilization of metakaolin and blast furnace slag in autoclaved aerated concrete production. Rev. Romana Mater. 2019, 49, 388–393. [Google Scholar]
- Wang, K.S.; Chiang, K.Y.; Lin, S.M.; Tsai, C.C.; Sun, C.J. Effects of Chlorides on Emissions of Hydrogen Chloride Formation in Waste Incineration. Chemosphere 1999, 38, 1571–1582. [Google Scholar] [CrossRef]
- Niessen, W.R. Combustion and incineration processes. In Application in Environmental Engineering; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Chin, T.; Yan, R.; Liang, D.T.; Tay, J.H. Hydrated Lime Reaction with HCl under Simulated Flue Gas Conditions. Ind. Eng. Chem. Res. 2005, 44, 3742–3748. [Google Scholar] [CrossRef]
- Dal Pozzo, A.; Moricone, R.; Antonioni, G.; Tugnoli, A.; Cozzani, V. Hydrogen Chloride Removal from Flue Gas by Low-Temperature Reaction with Calcium Hydroxide. Energy Fuels 2018, 32, 747–756. [Google Scholar] [CrossRef]
- Środa, B. Innovative technologies in the cement industry. Cem. Wapno Beton 2021, 26, 444–451. [Google Scholar] [CrossRef]
- Baran, T. The use of waste and industrial by-products and possibilities of reducing CO2 emission in the cement industry—industrial trials. Cem. Wapno Beton 2021, 26, 169–184. [Google Scholar] [CrossRef]
- Forster, A.M.; Valek, J.; Hughes, J.J.; Pilcher, N. Lime binders for the repair of historic buildings: Considerations for CO2 abatement. J. Clean. Prod. 2020, 252, 119802. [Google Scholar] [CrossRef]
- PN-EN 196-6:2019-01; Methods of Testing Cement—Part 6: Determination of Finneness. Polish Committee for Standardization: Warsaw, Poland, 2019.
- PN-77/H-04949; Powder Metallurgy. Determination of the Water Coverage of Flake Powders. Polish Committee for Standardization: Warsaw, Poland, 1977.
- Guidance on Classification of Waste According to EWC-Stat Categories. 2013. Available online: https://ec.europa.eu/eurostat/documents/342366/351806/Guidance-on-EWCStat-categories-2010.pdf/0e7cd3fc-c05c-47a7-818f-1c2421e55604 (accessed on 21 March 2022).
- Halstead, P.E.; Moore, A.E. The thermal dissociation of calcium hydroxide. J. Chem. Soc. 1957, 769, 3873–3875. [Google Scholar] [CrossRef]
- Birss, F.W.; Thorvaldson, T. The mechanism of the hydration of calcium oxide. Canad. J. Chem. 1955, 33, 881–886. [Google Scholar] [CrossRef] [Green Version]
- EN 772-13:2001; Methods of Test for Masonry Units—Part 13: Determination of Net and Gross Dry Density of Masonry Units (Except for Natural Stone). Polish Committee for Standardization: Warsaw, Poland, 2001.
- EN 772-1+A1:2015-10; Methods of Test for Masonry Units—Part 1: Determination of Compressive Strength. Polish Committee for Standardization: Warsaw, Poland, 2015.
- User’s Guide ISOMET Heat Transfer Analyser Model. Available online: https://www.appliedp.com/download/manual/isomet2114_ug_en.pdf (accessed on 21 March 2022).
- Chen, Y.; Ko, M.; Chang, J.; Lin, C.-T. Recycling of desulfurization slag for the production of autoclaved aerated concrete. Constr. Build. Mater. 2018, 158, 132–140. [Google Scholar] [CrossRef]
- Narayanan, N.; Ramamurthy, K. Structure and properties of aerated concrete: A review. Cem. Concr. Res. 2000, 22, 321–329. [Google Scholar] [CrossRef]
- Shamas, T.; Schober, G.; Heinz, G.; Seifert, S. Production of autoclaved aerated concrete with silica raw materials of a higher solubility than quartz part I: Influence of calcined diatomaceous earth. Constr. Build. Mater. 2021, 272, 122014. [Google Scholar] [CrossRef]
- Wan, H.; Hu, Y.; Liu, G.; Qu, Y. Study on the structure and properties of autoclaved aerated concrete produced with the stone-sawing mud. Constr. Build. Mater. 2018, 184, 20–26. [Google Scholar] [CrossRef]
- Qu, M.-L.; Tian, S.-Q.; Fan, L.-W.; Yu, Z.-T.; Ge, J. An experimental investigation and fractal modeling on the effective thermal conductivity of novel autoclaved aerated concrete (AAC)-based composites with silica aerogels (SA). Appl. Therm. Eng. 2020, 179, 115770. [Google Scholar] [CrossRef]
- Jin, H.; Yao, X.; Fan, L.; Xu, X.; Yu, Z. Experimental determination and fractal modeling of the effective thermal conductivity of autoclaved aerated concrete: Effects of moisture content. Int. J. Heat Mass Transf. 2016, 92, 589–602. [Google Scholar] [CrossRef]
- Chen, G.; Li, F.; Jing, P.; Geng, J.; Si, Z. Effect of pore structure on thermal conductivity and mechanical properties of autoclaved aerated concrete. Materials 2021, 14, 339. [Google Scholar] [CrossRef] [PubMed]
- Straube, B.; Walther, H. AAC with low thermal conductivity. In Proceedings of the 5th International Conference on Autoclaved Aerated Concrete “Securing a Sustainable Future” to Be Held at Bydgoszcz to Celebrate 60 Years of AAC Experience in Poland, Bydgoszcz, Poland, 14–17 September 2011. [Google Scholar]
- Kreft, O.; Hausmann, J.; Hubalkowa, J.; Aneziris, C.G.; Straube, B.; Schoch, T. Pore size distribution effects on the thermal conductivity of light weight autoclaved aerated concrete. Cem. Wapno Beton 2011, 16, 49–52. [Google Scholar]
- Asadi, I.; Shafigh, P.; Hassan, Z.; Mahyuddin, N. Thermal conductivity of concrete—A review. J. Build. Eng. 2018, 20, 81–93. [Google Scholar] [CrossRef]
- Wakili, K.; Hugi, E.; Karvonen, L.; Schnewnil, P.; Winnefeld, F. Thermal behaviour of autoclaved aerated concrete exposed to fire. Cem. Concr. Comp. 2015, 62, 52–58. [Google Scholar] [CrossRef]
- Muthu Kumar, E.; Ramamurthy, K. Effect of fineness and dosage of aluminum powder on the properties of moist-cured aerated concrete. Constr. Build. Mater. 2015, 95, 486–496. [Google Scholar] [CrossRef]
- Różycka, A.; Kotwica, Ł.; Małolepszy, J. Synthesis of single phase gyrolite in the CaO-quartz-Na2O-H2O system. Mater. Lett. 2014, 120, 166–169. [Google Scholar] [CrossRef]
- Guo, X.; Meng, F.; Shi, H. Microstructure and characterization of hydrothermal synthesis of Al-substituted tobermorite. Constr. Build. Mater. 2017, 133, 253–260. [Google Scholar] [CrossRef]
- Nocun-Wczelik, W. Effect of Na and Al on the phase composition and morphology of autoclaved calcium silicate hydrates. Cem. Concr. Res. 1999, 29, 1759–1767. [Google Scholar] [CrossRef]
- Różycka, A.; Kotwica, Ł. Effect of alkali on the synthesis of single phase gyrolite in the system CaO-quartz-H2O. Constr. Build. Mater. 2020, 239, 117799. [Google Scholar] [CrossRef]
- Kapeluszna, E.; Kotwica, Ł.; Różycka, A.; Gołek, Ł. Incorporation of Al in C-A-S-H gels with various Ca/Si and Al/Si ratio: Microstructural and structural characteristics with DTA/TG, XRD, FTIR and TEM analysis. Constr. Build. Mater. 2017, 155, 643–653. [Google Scholar] [CrossRef]
- Maeda, H.; Ishida, E.; Kasuda, T. Hydrothermal preparation of tobermorite incorporating phosphate species. Mater. Lett. 2012, 68, 382–384. [Google Scholar] [CrossRef]
- Skawińska, A.; Owsiak, Z.; Baran, T.; Hernik, K. The influence of halloysite addition on tobermorite formation in CaO and quartz mix under hydrothermal conditions. Cem. Wapno Beton 2017, 22, 426–434. [Google Scholar]
- Drochytka, R.; Cerny, V. Influence of fluidized bed combustion fly ash admixture on hydrothermal synthesis of tobermorite in the mixture with quartz sand, high temperature fly ash and lime. Constr. Build. Mater. 2020, 230, 117033. [Google Scholar] [CrossRef]
- Majdinasab, A.; Yuan, Q. Synthesis of Al-substituted 11 Å tobermorite using waste glass cullet: A study on the microstructure. Mater. Chem. Phys. 2020, 250, 123069. [Google Scholar] [CrossRef]
- Gou, X.; Song, M. Micro-nanostructures of tobermorite hydrothermal-synthesized from fly ash and municipal solid waste incineration fly ash. Constr. Build. Mater. 2018, 191, 431–439. [Google Scholar] [CrossRef]
- Tsutsumi, T.; Nishimoto, S.; Kameshima, Y.; Miyake, M. Hydrothermal preparation of tobermorite from blast furnace slag for Cs+ and Sr2+ sorption. J. Hazard. Mater. 2014, 226, 174–181. [Google Scholar] [CrossRef]
- Gou, X.; Zhang, T.; Song, M. Hydrothermal synthesized and nano-modified wall materials from solid wastes. Constr. Build. Mater. 2019, 217, 242–250. [Google Scholar] [CrossRef]
- Coleman, N.J.; Brassington, D.S. Synthesis of Al-substituted 11 Å tobermorite from newsprint recycling residue: A feasibility study. Mater. Res. Bull. 2003, 38, 485–497. [Google Scholar] [CrossRef]
- Cao, P.; Li, G.; Lou, J.; Rao, M.; Jiang, H.; Peng, Z.; Jiang, T. Alkali-reinforced hydrothermal synthesis of lathy tobermorite fibers using mixture of coal fly ash and lime. Constr. Build. Mater. 2020, 238, 117655. [Google Scholar] [CrossRef]
- Ding, J.; Tang, Z.; Ma, S.; Wang, Y.; Sheng, S.; Zang, Y.; Shen, S.; Xie, Z. A novel process for synthesis of tobermorite fiber from high-alumina fly ash. Cem. Concr. Comp. 2016, 65, 11–18. [Google Scholar] [CrossRef]
- Smalakys, G. The hydrothermal synthesis of 1.13 nm tobermorite from granite sawing powder waste. Ceram. Silik. 2020, 64, 239–248. [Google Scholar] [CrossRef]


| Composition | SiO2 | Al2O3 | Fe2O3 | Na2O | K2O | CaO | MgO | TiO2 | SO3 | P2O5 | Cl | Br | F |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2.03 | 0.43 | 0.55 | 3.15 | 0.75 | 67.45 | 0.39 | 0.14 | 5.09 | 0.07 | 18.43 | 0.14 | 0.08 |
| Sample | Mix Proportion (kg) | |||||
|---|---|---|---|---|---|---|
| OPC | Quartz Sand | Lime | Aluminum Powder | Waste | Water | |
| Ref. | 78 | 531 | 99 | 0.4 | 0 | 347 |
| R-AAC | 78 | 531 | 89 | 0.4 | 27 | 347 |
| C-AAC | 78 | 531 | 89 | 0.4 | 25 | 347 |
| Sample | Bulk Density (kg/m3) | Relative Bulk Density (%) |
|---|---|---|
| ref. AAC | 703 | 100 |
| R-AAC | 667 | 95 |
| C-AAC | 677 | 96 |
| Sample | Compressive Strength (MPa) | Relative Strength (%) |
|---|---|---|
| ref. AAC | 2.6 | 100 |
| R-AAC | 3.9 | 150 |
| C-AAC | 3.4 | 131 |
| Sample | Thermal Conductivity (W/(m·K)) | Relative Thermal Conductivity (%) |
|---|---|---|
| ref. AAC | 0.148 | 100 |
| R-AAC | 0.165 | 112 |
| C-AAC | 0.168 | 114 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Różycka, A.; Kotwica, Ł. Waste Originating from the Cleaning of Flue Gases from the Combustion of Industrial Wastes as a Lime Partial Replacement in Autoclaved Aerated Concrete. Materials 2022, 15, 2576. https://doi.org/10.3390/ma15072576
Różycka A, Kotwica Ł. Waste Originating from the Cleaning of Flue Gases from the Combustion of Industrial Wastes as a Lime Partial Replacement in Autoclaved Aerated Concrete. Materials. 2022; 15(7):2576. https://doi.org/10.3390/ma15072576
Chicago/Turabian StyleRóżycka, Agnieszka, and Łukasz Kotwica. 2022. "Waste Originating from the Cleaning of Flue Gases from the Combustion of Industrial Wastes as a Lime Partial Replacement in Autoclaved Aerated Concrete" Materials 15, no. 7: 2576. https://doi.org/10.3390/ma15072576
APA StyleRóżycka, A., & Kotwica, Ł. (2022). Waste Originating from the Cleaning of Flue Gases from the Combustion of Industrial Wastes as a Lime Partial Replacement in Autoclaved Aerated Concrete. Materials, 15(7), 2576. https://doi.org/10.3390/ma15072576





