Improving the Properties of Porous Geopolymers Based on TPP Ash and Slag Waste by Adjusting Their Chemical Composition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Calculation of Compositions and Synthesis of Porous Geopolymers
2.3. Methods
3. Results and Discussion
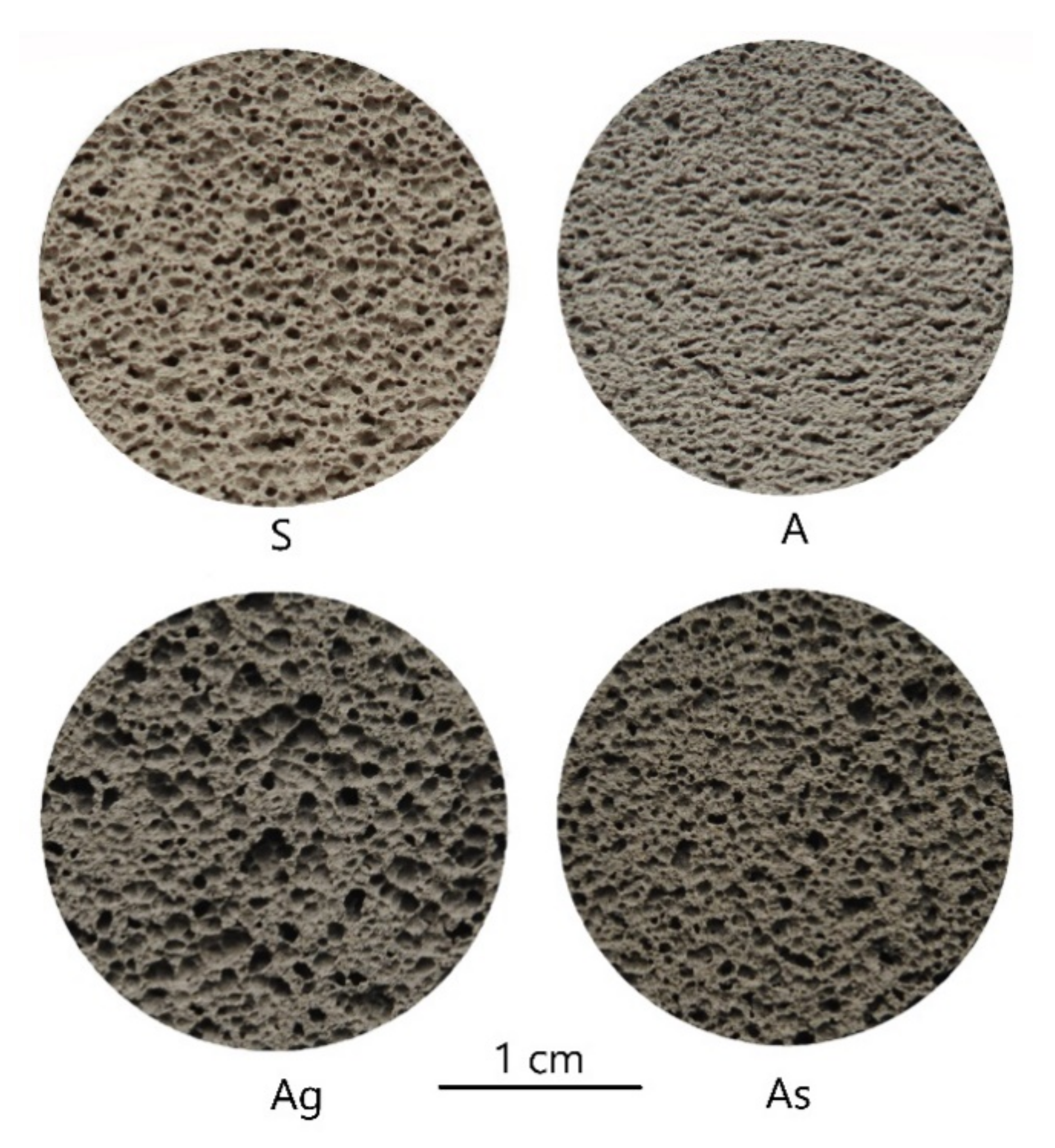
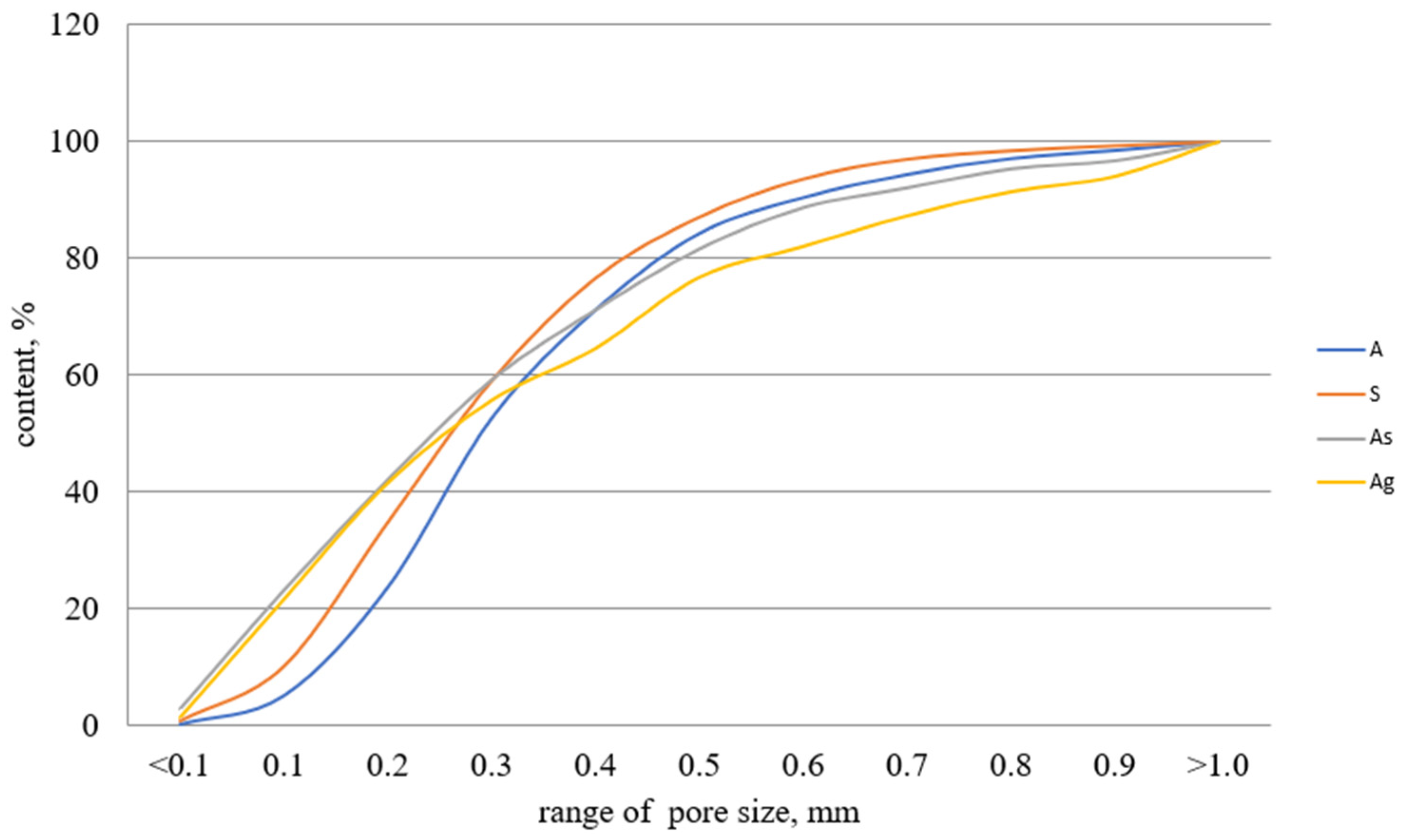
3.1. Macrostructure and Properties
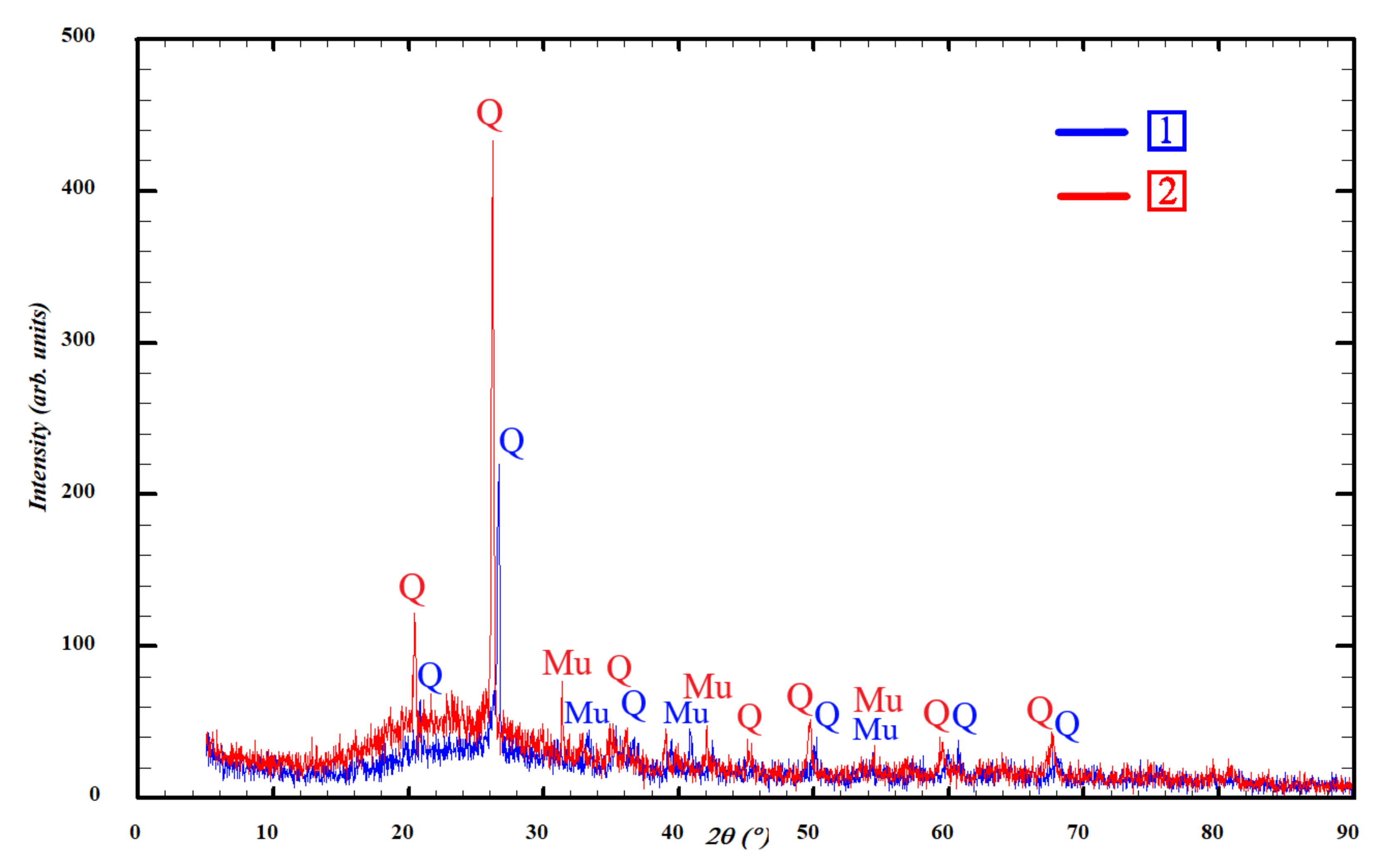
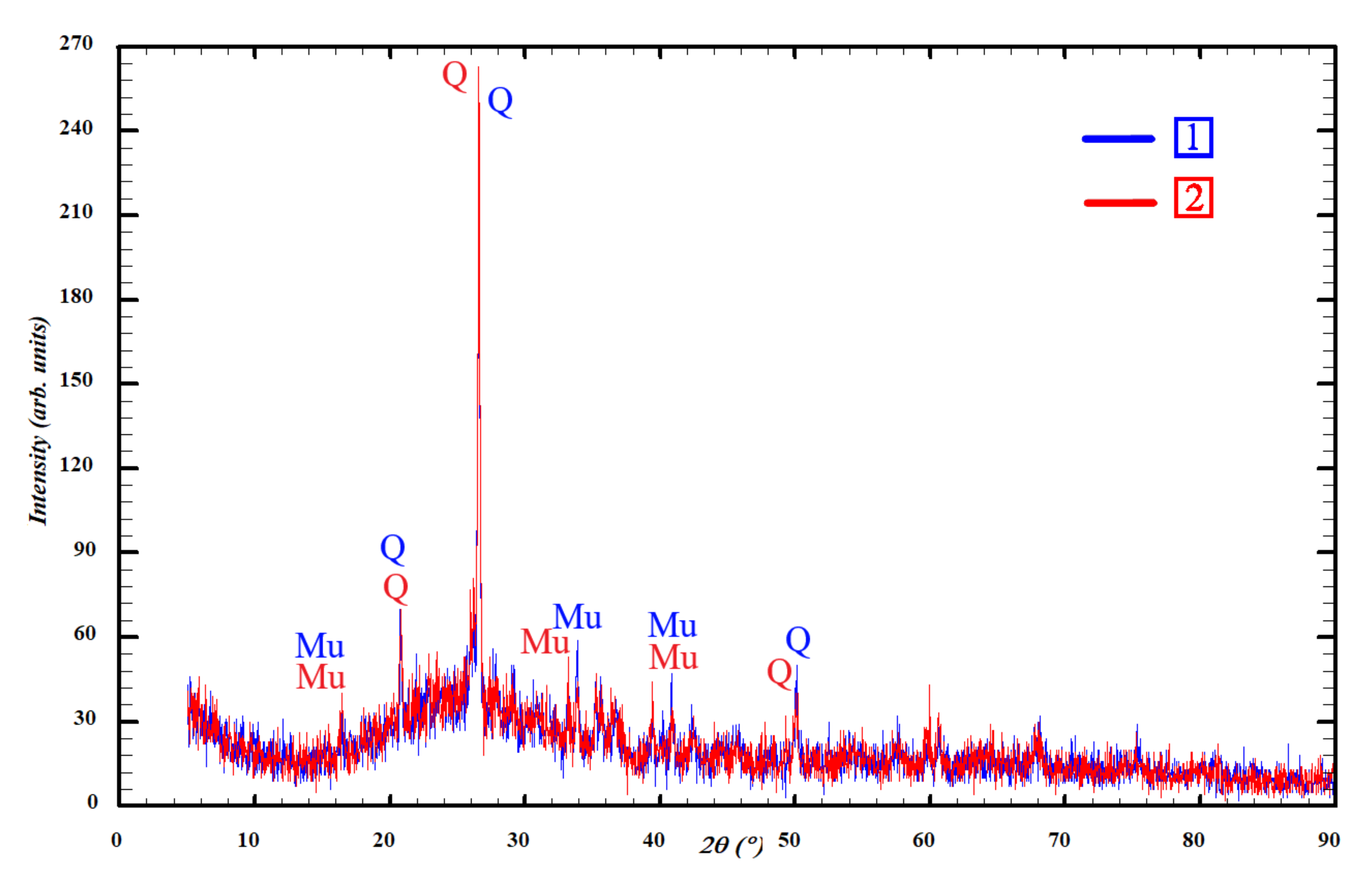
3.2. Phase Composition
3.3. Microstructure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yousuf, A.; Manzoor, S.O.; Youssouf, M.; Malik, Z.A.; Khawaja, K.S. Fly ash: Production and utilization in India—An overview. J. Mater. Environ. Sci. 2020, 11, 911–921. [Google Scholar]
- Production and Use Survey Results. Available online: https://acaa-usa.org/wp-content/uploads/2021/12/2020-Production-and-Use-Survey-Results-FINAL.pdf (accessed on 15 March 2022).
- Luo, Y.; Wu, Y.; Ma, S.; Zheng, S.; Zhang, Y.; Chu, P.K. Utilization of coal fly ash in China: A mini-review on challenges and future directions. Environ. Sci. Pollut. Res. 2021, 28, 18727–18740. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Zhao, Z.; Jiang, S.; Sun, J.; Pan, H.; Jiang, L. Comparison and summary of relevant standards for comprehensive utilization of fly ash at home and abroad. IOP Conf. Ser. Earth Environ. Sci. 2021, 621, 012006. [Google Scholar] [CrossRef]
- State Report on the Status and Protection of Environment in Russia in 2020; Ministry of Natural Resources of Russia, Moscow State University Named after M.V. Lomonosov: Moscow, Russia, 2021.
- Khagleev, E.P. Ash and slag dumps of annual regulation, differentiated flows of ash and slag from coal thermal power plants. News High. Educ. Inst. Energy Probl. 2017, 19, 21–32. [Google Scholar]
- Pichugin, E.A. Analytical review of the experience accumulated in the Russian Federation of involving ash and slag waste from thermal power plants in the economic turnover. Probl. Reg. Ecol. 2019, 4, 77–87. [Google Scholar]
- Zhang, Z.; Provis, J.L.; Reid, A.; Wang, H. Fly ash-based geopolymers: The relationship between composition, pore structure and efflorescence. Cem. Concr. Res. 2014, 61, 30–41. [Google Scholar] [CrossRef]
- Vatin, N.I.; Petrosov, D.V. Application of ashes and ash and slag waste in construction. Eng. Constr. J. 2011, 4, 16–21. [Google Scholar]
- Ahmed, M.F.; Nuruddin, M.F.; Shafiq, N. Compressive Strength and Workability Characteristics of Low-Calcium Fly ash-based Self-Compacting Geopolymer Concrete. Int. J. Civ. Environ. Eng. 2011, 3, 72–78. [Google Scholar]
- Thang, V.L.; Nguyen, Z.T.; Samchenko, S.V. Addition of additives of ash and slag waste to the properties of sulfoaluminate Portland cement. Vestnik MGSU 2019, 14, 991–1003. [Google Scholar] [CrossRef]
- Ivashina, M.A.; Krivoborodov, Y.R. The use of industrial waste in the technology of sulfoaluminate clinker. Adv. Chem. Chem. Technol. 2017, 31, 22–24. [Google Scholar]
- Van Lam, T.; Hung, N.X.; Bulgakov, B.I.; Alexandrova, O.V.; Larsen, O.A.; Orekhova, A.Y. The use of ash and slag waste as an additional cementing material. Vestnik BSTU VG Shukhov 2018, 8, 19–27. [Google Scholar]
- Wu, K.; Shi, H.; Guo, H. Utilization of municipal waste ash for the production of sulfoaluminate cement clinker. Waste Manag. 2011, 31, 2001–2008. [Google Scholar] [CrossRef] [PubMed]
- Lihach, S.A.; Ilyasova, A.S.; Kulesh, R.N.; Nikolaeva, V.I. Utilization direction of industrial raw products built-up in power station ash dumps. MATEC Web Conf. 2017, 92, 01074. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.; Akhai, S. Trends in Utilization of Coal Fly Ash in India: A Review. J. Eng. Des. Anal. 2019, 2, 13–16. [Google Scholar]
- Global Aspects on Coal Combustion Products. Available online: https://www.coaltrans.com/insights/article/global-aspects-oncoal-combustion-products (accessed on 15 February 2022).
- Zyryanov, V.V.; Zyryanov, D.V. Fly Ash—Technogenic Raw Materials; Mask: Moscow, Russia, 2009; pp. 120–166. [Google Scholar]
- ASTM D5759-12; Standard Guide for Characterization of Coal Fly Ash and Clean Coal Combustion Fly Ash for Potential Uses. ASTM International: West Conshohocken, PA, USA, 2020; p. 4.
- Duxson, P.; Fernandez-Jimenez, A.; Provis, J.L. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Eroshkina, N.A.; Korovkin, M.O. Geopolymer Building Materials Based on Industrial Waste; PGUAS: Penza, Russia, 2014; pp. 20–24. [Google Scholar]
- Menshova, P.V.; Khlupina, Y.V.; Nalesnika, O.I.; Makarovskikha, A.V. Ash and Slag Waste as a Secondary Raw Material. Procedia Chem. 2014, 10, 184–191. [Google Scholar] [CrossRef] [Green Version]
- Eroshkina, N.A.; Korovkin, M.O.; Tymchuk, E.I. Structure formation of geopolymers. Young Sci. 2015, 7, 123–126. [Google Scholar]
- Abdalrhman, M.; Ahmed, A.; Babalghaith, A.; Zubair, M.; Nuha, M.; Salaheddin, A.; Yusoff, M.; Izzi, N. Utilisation of Waste-Based Geopolymer in Asphalt Pavement Modification and Construction-A Review. Sustainability 2021, 13, 3330. [Google Scholar]
- Davidovits, J. Geopolymer Chemistry and Applications, 3rd ed.; Institute Geopolymer: Saint-Quentin, France, 2011. [Google Scholar]
- Feng, J.; Zhang, R.; Gong, L.; Li, Y.S.; Cao, W.; Cheng, X. Development of porous fly ash-based geopolymer with low thermal conductivity. Mater. Des. 2015, 65, 529–533. [Google Scholar] [CrossRef]
- Zawrah, M.F.; Farag, R.S.; Kohail, M.H. Improvement of physical and mechanical properties of geopolymer through addition of zircon. Mater. Chem. Phys. 2018, 217, 90–97. [Google Scholar] [CrossRef]
- Mucsi, G.; Ambrus, M. Raw materials for geopolymerisation. In Proceedings of the MultiScience—XXXI, microCAD International Multidisciplinary Scientific Conference, Miskolc, Hungary, 20–21 April 2017; pp. 21–22. [Google Scholar]
- Razorenov, Y.I.; Yatsenko, E.A.; Goltsman, B.M. Building materials based on manmade waste of the mining industry and solid fuel energy—An environmental trend of the modern time. Gornyi Zhurnal 2021, 11, 95–98. [Google Scholar] [CrossRef]
- Steinerova, M. Mechanical properties of geopolymer mortarsin relation to their porous structure. Ceram.—Silikáty 2011, 55, 362–372. [Google Scholar]
- Provis, J.L.; Van Deventer, J.S.J. Geopolymers Structure, Processing, Properties and Industrial Applications; Woodhead Publishing: Sawston, UK, 2009; pp. 267–311. [Google Scholar]
- Škvára, F.; Jílek, T.; Kopecký, L. Geopolymer materials based on fly ash. Ceram.—Silikáty 2005, 49, 195–204. [Google Scholar]
- Hamid, A.; Alfaidi, H.; Baaj, H.; El-Hakim, M. Evaluating Fly Ash-Based Geopolymers as a Modifier for Asphalt Binders. Adv. Mater. Sci. Eng. 2020, 4, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Khudyakova, L.I.; Zalutsky, A.B.; Paleev, P.L. The use of ash and slag waste from thermal power plants. Ecology 2019, 4, 375–391. [Google Scholar]
- Yatsenko, E.A.; Ryabova, A.V.; Vilbitskaya, N.A.; Kurdashov, V.M.; Trofimov, S.V. Ecogeopolymers based on ash and slag waste from thermal power plants—Promising materials for the construction of roads in the Arctic zone of the Russian Federation. Glass Ceram. 2021, 12, 32–37. [Google Scholar]
- Amin, M.; Elsakhawy, Y.; Abu el-hassan, K.; Abdelsalam, B.A. Behavior evaluation of sustainable high strength geopolymer concrete based on fly ash, metakaolin, and slag. Case Stud. Constr. Mater. 2022, 16, e00976. [Google Scholar] [CrossRef]
- Gómez-Casero, M.A.; De Dios-Arana, C.; Bueno-Rodríguez, J.S.; Pérez-Villarejo, L.; Eliche-Quesada, D. Physical, mechanical and thermal properties of metakaolin-fly ash geopolymers. Sustain. Chem. Pharm. 2022, 26, 100620. [Google Scholar] [CrossRef]
- Azad, N.M.; Samarakoon, S.M. Utilization of Industrial By-Products/Waste to Manufacture Geopolymer Cement/Concrete. Sustainability 2021, 13, 873. [Google Scholar] [CrossRef]
- Colangelo, F.; Farina, I.; Travaglioni, M.; Salzano, C.; Cioffi, R.; Petrillo, A. Eco-efficient industrial waste recycling for the manufacturing of fibre reinforced innovative geopolymer mortars: Integrated waste management and green product development through LCA. J. Clean. Prod. 2021, 312, 127777. [Google Scholar] [CrossRef]
- Yatsenko, E.A.; Smolii, V.A.; Klimova, L.V.; Gol’tsman, B.M.; Ryabova, A.V.; Golovko, D.A.; Chumakov, A.A. Solid Fuel Combustion Wastes at CHPP in the Arctic Zone of the Russian Federation: Utility in Eco-Geopolymer Technology. Glass Ceram. 2022, 78, 374–377. [Google Scholar] [CrossRef]
- Singh, N.B. Fly Ash-Based Geopolymer Binder: A Future Construction Material. Minerals 2018, 8, 299. [Google Scholar] [CrossRef] [Green Version]
- Fitria, F.; Nugroho, D.; Hidayati, R.E.; Nurlina, H.; Ridho, B.; Djoko, H.; Hamzah, F. Optimization of SiO2/Al2O3 Ratio in the Prepa-ration of Geopolymer from High Calcium Fly Ash. IOP Conf. Ser. Earth Environ. Sci. 2020, 616, 012051. [Google Scholar]
- Yatsenko, E.A.; Goltsman, B.M.; Ryabova, A.V.; Yatsenko, L.A. Study of the possibilities of synthesis of glass-ceramic geopolymer materials. In Proceedings of the “Glass: Science and Practice”—GlasSP2021—3rd Russian Conference with International Participation, Saint Petersburg, Russia, 13–17 September 2021; Institute of Chemistry of Silicates Named after IV Grebenshchikov: Saint Petersburg, Russia, 2021; pp. 25–27. [Google Scholar]
- Hlaváček, P.; Šmilauer, V.; Škvára, F.; Kopecký, L.; Šulc, R. Inorganic foams made from alkali-activated fly ash: Mechanical, chemical and physical properties. J. Eur. Ceram. Soc. 2015, 35, 703–709. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; Ngo, T.; Mendis, P.; Sanjayan, J. Regulating the chemical foaming reaction to control the porosity of geopolymer foams. Mater. Des. 2017, 120, 255–265. [Google Scholar] [CrossRef]
- Papa, E.; Landi, E.; Miccio, F.; Medri, V. K2O-Metakaolin-Based Geopolymer Foams: Production, Porosity Characterization and Permeability Test. Materials 2022, 15, 1008. [Google Scholar] [CrossRef]



| Component | SiO2 | Al2O3 | Fe2O3 | MgO | Na2O | K2O | CaO | TiO2 | MnO | P2O5 | SO3 | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ASW (Apatitskaya CHPP) | 52.86 | 22.35 | 7.8 | 2.65 | 0.79 | 1.96 | 3.62 | 1.06 | 0.07 | 0.36 | 0.37 | 6.11 |
| ASW (Severodvinskaya CHPP-1) | 61.57 | 17.91 | 6.01 | 2.75 | 3.59 | 2.32 | 2.1 | 0.83 | 0.07 | 0.21 | 0.32 | 2.32 |
| Glass | 71.2 | 2.7 | 0.8 | 7.6 | 13.2 | 0.8 | 3.4 | – | – | – | 0.2 | 0.1 |
| Quartz sand | 98.91 | 0.29 | 0.07 | – | – | – | – | – | – | – | – | 0.73 |
| Waterglass | 29.2 | 0.61 | 0.1 | – | 14.26 | – | 0.2 | – | – | – | 0.11 | 55.52 |
| NaOH | – | – | – | – | 77.5 | – | – | – | – | – | – | 22.5 |
| # | ASW | Addition | NaOH (Powder) | Water | Waterglass | Aluminum Powder, over 100 |
|---|---|---|---|---|---|---|
| S | 70.0(S) | - | 2.5 | 5.0 | 22.5 | 2.0 |
| A | 70.0(A) | - | 2.5 | 5.0 | 22.5 | 2.0 |
| Ag | 49.0(A) | 21.0 (glass) | 2.5 | 5.0 | 22.5 | 2.0 |
| As | 56.0(A) | 14.0 (sand) | 2.5 | 5.0 | 22.5 | 2.0 |
| # | SiO2 | Al2O3 | Fe2O3 | MgO | Na2O | K2O | CaO | TiO2 | MnO | P2O5 | SO3 | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | 49.67 | 12.67 | 4.23 | 1.93 | 7.66 | 1.62 | 1.52 | 0.58 | 0.05 | 0,15 | 0.25 | 19.67 |
| A | 43.57 | 15.78 | 5.48 | 1.86 | 5.70 | 1.37 | 2.58 | 0.74 | 0.05 | 0.25 | 0.28 | 22.34 |
| Ag | 47.42 | 11.66 | 4.01 | 2.89 | 8.31 | 1.13 | 2.53 | 0.52 | 0.03 | 0.18 | 0.25 | 21.07 |
| As | 49.91 | 12.75 | 4.42 | 1.49 | 5.59 | 1.10 | 2.08 | 0.60 | 0.04 | 0.20 | 0.23 | 21.59 |
| # | Foam Expansion,% | Density, kg/m3 | Compressive Strength, MPa | Porosity, % | Thermal Conductivity, W/(m·K) |
|---|---|---|---|---|---|
| S | 89.00 ± 2.96 | 510 ± 18 | 1.39 ± 0.05 | 74.93 ± 2.24 | 0.1057 ± 0.0004 |
| A | 74.88 ± 2.08 | 568 ± 23 | 0.61 ± 0.03 | 68.99 ± 2.84 | 0.1247 ± 0.0005 |
| Ag | 85.83 ± 1.06 | 516 ± 3 | 1.22 ± 0.06 | 71.83 ± 0.14 | 0.1408 ± 0.0002 |
| As | 77.37 ± 3.86 | 484 ± 12 | 1.10 ± 0.03 | 73.57 ± 2.27 | 0.1439 ± 0.0004 |
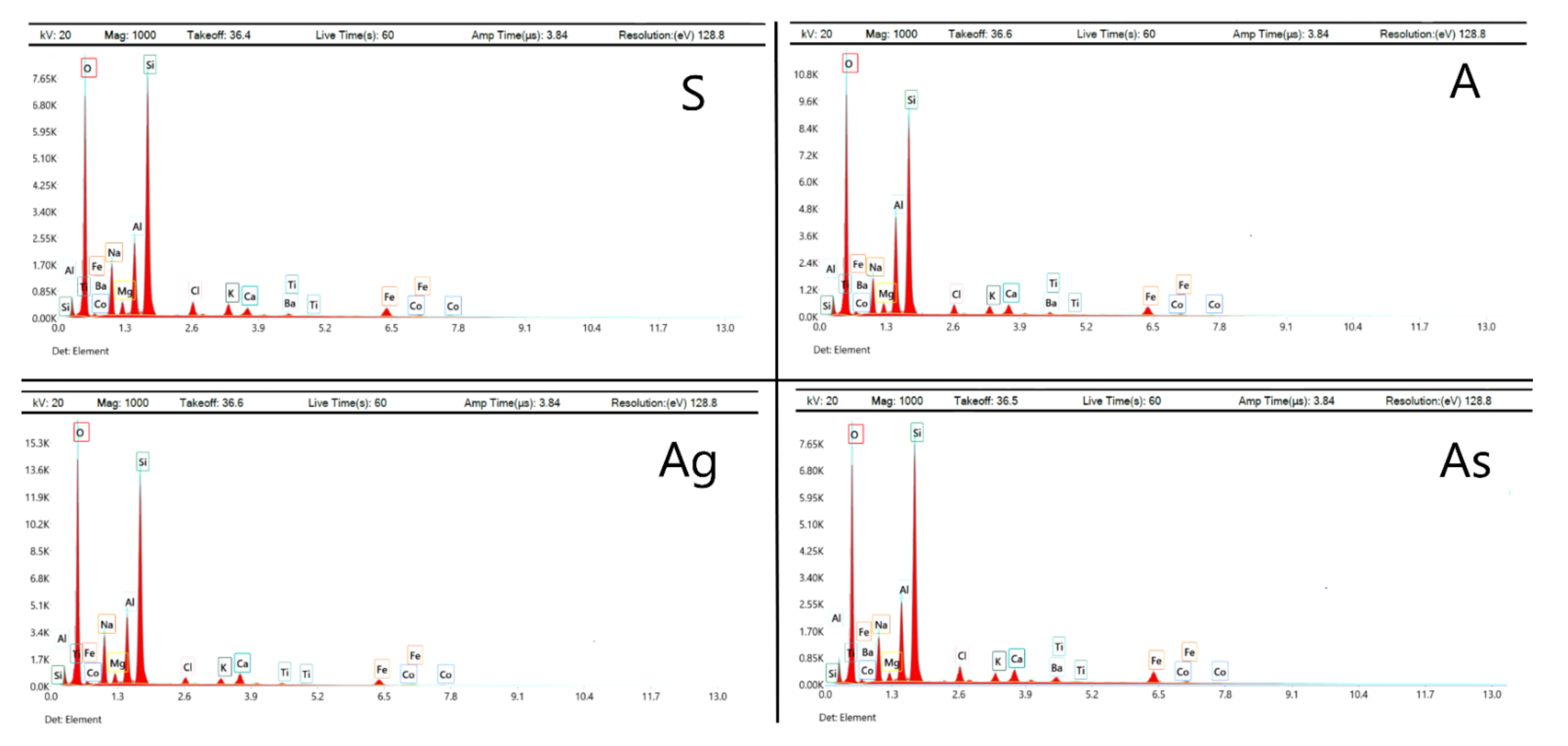
| Element | S | A | Ag | As |
|---|---|---|---|---|
| O | 49.31 | 51.61 | 52.73 | 49.89 |
| Na | 9.16 | 7.05 | 9.55 | 8.16 |
| K | 1.92 | 1.50 | 1.08 | 1.32 |
| Ca | 1.53 | 1.97 | 2.22 | 2.26 |
| Mg | 1.59 | 1.46 | 1.48 | 1.07 |
| Al | 7.51 | 10.20 | 7.62 | 7.76 |
| Si | 22.06 | 19.85 | 21.00 | 21.22 |
| Fe | 3.66 | 3.67 | 2.48 | 4.41 |
| Ba | 0.75 | 0.75 | – | 0.77 |
| Co | 0.32 | 0.32 | 0.33 | 0.33 |
| Ti | 0.26 | 0.26 | 0.53 | 0.81 |
| Cl | 1.93 | 1.36 | 0.98 | 2.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yatsenko, E.A.; Goltsman, B.M.; Trofimov, S.V.; Kurdashov, V.M.; Novikov, Y.V.; Smoliy, V.A.; Ryabova, A.V.; Klimova, L.V. Improving the Properties of Porous Geopolymers Based on TPP Ash and Slag Waste by Adjusting Their Chemical Composition. Materials 2022, 15, 2587. https://doi.org/10.3390/ma15072587
Yatsenko EA, Goltsman BM, Trofimov SV, Kurdashov VM, Novikov YV, Smoliy VA, Ryabova AV, Klimova LV. Improving the Properties of Porous Geopolymers Based on TPP Ash and Slag Waste by Adjusting Their Chemical Composition. Materials. 2022; 15(7):2587. https://doi.org/10.3390/ma15072587
Chicago/Turabian StyleYatsenko, Elena A., Boris M. Goltsman, Sergei V. Trofimov, Viktor M. Kurdashov, Yuri V. Novikov, Victoria A. Smoliy, Anna V. Ryabova, and Lyudmila V. Klimova. 2022. "Improving the Properties of Porous Geopolymers Based on TPP Ash and Slag Waste by Adjusting Their Chemical Composition" Materials 15, no. 7: 2587. https://doi.org/10.3390/ma15072587
APA StyleYatsenko, E. A., Goltsman, B. M., Trofimov, S. V., Kurdashov, V. M., Novikov, Y. V., Smoliy, V. A., Ryabova, A. V., & Klimova, L. V. (2022). Improving the Properties of Porous Geopolymers Based on TPP Ash and Slag Waste by Adjusting Their Chemical Composition. Materials, 15(7), 2587. https://doi.org/10.3390/ma15072587








