Mechanical Characteristics of the Flebogrif System—The New System of Mechano-Chemical Endovenous Ablation
Abstract
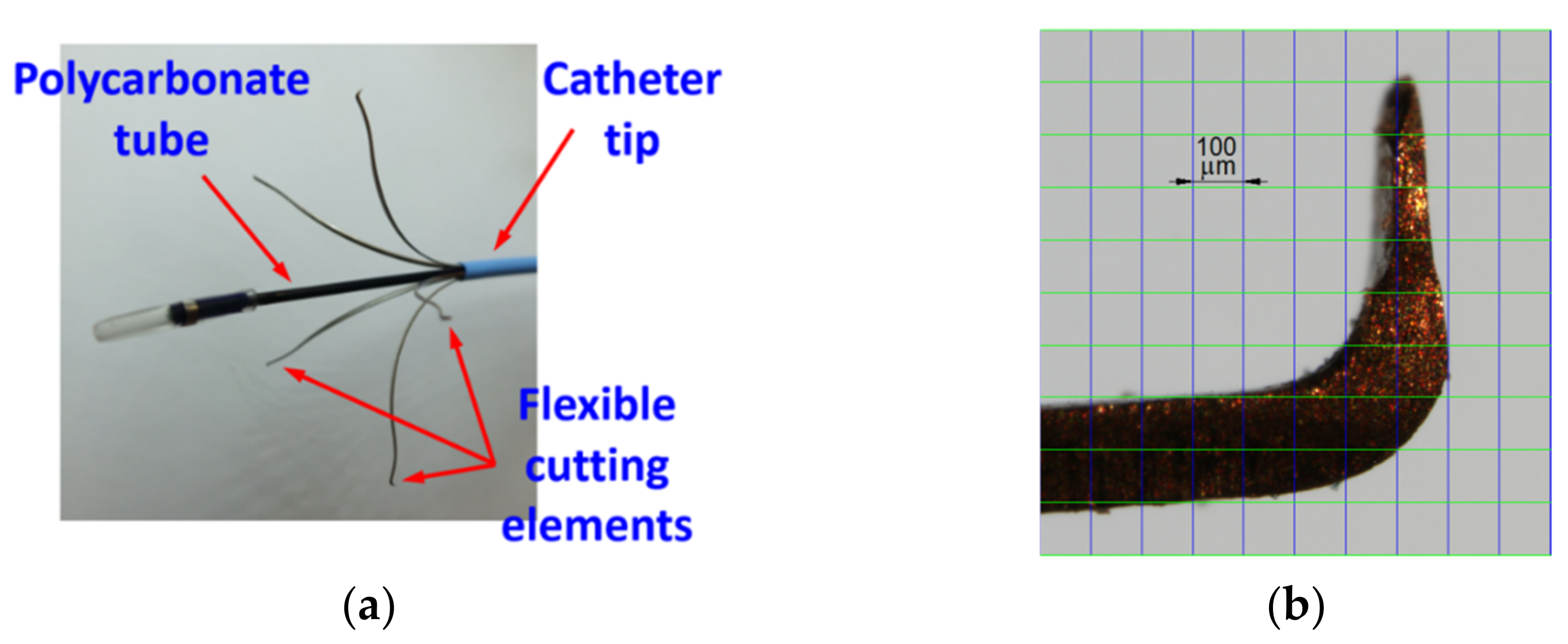
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
- The method established and used to measure the acting force both for the cutting element bundle and for a single cutting element allowed for an effective determination of their mechanical characteristics.
- The tests allowed for the determination of the value range for the Flebogrif cutting element contact force acting on the vein surface, depending on the protrusion of the system from the sheath introducer.
- Decreasing the working length of the Flebogrif cutting elements results in an increase in their rigidity, which is associated with the increase in contact forces acting on the internal surface of the vein. As a result, this leads to the determination of the force variability ranges and corresponding diameter ranges of operated veins.
- On the basis of Flebogrif testing, it may be stated that the values of contact force acting on a vein falling in the range of Ff ∈ 〈0.08–0.14〉 N can be achieved for a complete catheter protrusion of Lmax and vein diameters between 13.8 and 22.7 mm, the protrusion of 0.9⋅Lmax and vein diameters between 16.5 and 21.1 mm, and the protrusion of 0.8⋅Lmax and vein diameters between 12.5 and 15.2 mm.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eklof, B.G.; Perrin, M.; Delis, K.T.; Rutherford, R.B.; Gloviczki, P. Updated terminology of chronic venous disorders: The VEIN-TERM transatlantic interdisciplinary consensus document. J. Vasc. Surg. 2009, 49, 498–501. [Google Scholar] [CrossRef] [PubMed]
- Gloviczki, P.; Comerota, A.J.; Lohr, M.; Dalsing, M.C.; Eklöf, B.G.; Gillespie, D.L.; Gloviczki, M.L.; Lohr, J.M.; McLafferty, R.B.; Meissner, M.H.; et al. The care of patients with varicose veins and associated chronic venous diseases: Clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J. Vasc. Surg. 2011, 53, 2S–47S. [Google Scholar] [CrossRef] [PubMed]
- Wittens, C.; Davies, A.H.; Bwkgaard, N.; Broholm, R.; Cavezzi, A.; Chastanet, S.; De Wolf, M.; Eggen, C.; Giannoukas, A.; Gohel, M.; et al. Editor’s Choice—Management of Chronic Venous Disease: Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2015, 49, 678–737. [Google Scholar] [CrossRef] [PubMed]
- Elias, S.; Raines, J.K. Mechanochemical tumescentless endovenous ablation: Final results of the initial clinical trial. Phlebology 2012, 27, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Boersma, D.; van Eekeren, R.R.J.P.; Kelder, H.J.C.; Werson, D.A.B.; Holewijn, S.; Schreve, M.A.; Reijnen, M.M.P.J.; de Vries, J.P.P.M. Mechanochemical endovenous ablation versus radiofrequency ablation in the treatment of primary small saphenous vein insufficiency (MESSI trial): Study protocol for a randomized controlled trial. Trials 2014, 15, 421. [Google Scholar] [CrossRef]
- Whiteley, M.S.; Dos Santos, S.J.; Lee, C.T.; Li, J.M. Mechanochemical ablation causes endothelial and medial damage to the vein wall resulting in deeper penetration of sclerosant compared with sclerotherapy alone in extrafascial great saphenous vein using an ex vivo model. J. Vasc. Surg. Ven. Lymphat. Disord. 2017, 5, 370–377. [Google Scholar] [CrossRef]
- Boersma, D.; van Haelst, S.T.W.; van Eekeren, R.R.J.P.; Vink, A.; Reijnen, M.M.J.P.; de Vries, J.P.P.M.; de Borst, G.J. Macroscopic and Histologic Analysis of Vessel Wall Reaction After Mechanochemical Endovenous Ablation Using the ClariVein OC Device in an Animal Model. Eur. J. Vasc. Endovasc. Surg. 2017, 53, 290–298. [Google Scholar] [CrossRef][Green Version]
- Leung, C.C.M.; Carradice, D.; Wallace, T.; Chetter, I.C. Endovenous laser ablation versus mechanochemical ablation with ClariVein® in the management of superficial venous insufficiency (LAMA trial): Study protocol for a randomised controlled trial. Trials 2016, 17, 421. [Google Scholar] [CrossRef]
- Lane, T.; Bootun, R.; Dharmarajah, B.; Lim, C.S.; Najem, M.; Renton, S.; Sritharan, K.; Davies, A.H. A multi-centre randomised controlled trial comparing radiofrequency and mechanical occlusion chemically assisted ablation of varicose veins—Final results of the Venefit versus Clarivein for varicose veins trial. Phlebology 2017, 32, 89–98. [Google Scholar] [CrossRef]
- Tawfik, A.M.; Sorour, W.A.; El-Laboudy, M.E. Laser ablation versus mechanochemical ablation in the treatment of primary varicose veins: A randomized clinical trial. J. Vasc. Surg. Ven. Lymphat. Disord. 2020, 8, 211–215. [Google Scholar] [CrossRef]
- Iłżecki, M.; Terlecki, P.; Przywara, S.; Zubilewicz, T. Single-centre experience with mechanochemical ablation of insufficient veins with the Flebogrif® catheter in a 36-month follow-up. Phlebol. Rev. 2021, 29, 32–37. [Google Scholar] [CrossRef]
- Ciostek, P.; Kowalski, M.; Woźniak, W.; Miłek, T.; Myrcha, P.; Migda, B. Phlebogriffe—A new device for mechanochemical ablation of incompetent saphenous veins: A pilot study. Phlebol. Rev. 2015, 23, 72–77. [Google Scholar] [CrossRef]
- Zubilewicz, T.; Terlecki, P.; Terlecki, K.; Przywara, S.; Rybak, J.; Iłżecki, M. Application of endovenous mechanochemical ablation (MOCA) with FlebogrifTM to treat varicose veins of the lower extremities: A single center experience over 3 months of observation. Acta Angiol. 2017, 22, 137–142. [Google Scholar] [CrossRef]
- Rybak, Z.; Janeczek, M.; Dobrzynski, M.; Wujczyk, M.; Czerski, A.; Kuropka, P.; Noszczyk-Nowak, A.; Szymonowicz, M.; Sender-Janeczek, A.; Wiglusz, K.; et al. Study of Flebogrif®—A New Tool for Mechanical Sclerotherapy—Effectiveness Assessment Based on Animal Model. Nanomaterials 2021, 11, 544. [Google Scholar] [CrossRef]
- Bishawi, M.; Bernstein, R.; Boter, M.; Draughn, D.; Gould, C.F.; Hamilton, C.; Koziarski, J. Mechanochemical ablation in patients with chronic venous disease: A prospective multicenter report. Phlebology 2014, 29, 397–400. [Google Scholar] [CrossRef]
- Holewijn, S.; van Eekeren, R.R.J.P.; Vahl, A.; de Vries, J.P.P.M.; Reijnen, M.M.P.J.; Werson, D.; Cuijpers-Patist, B.; Boersma, D.; Bosma, J.; Kolkert, J.L.P.; et al. Two-year results of a multicenter randomized controlled trial comparing Mechanochemical endovenous Ablation to RADiOfrequeNcy Ablation in the treatment of primary great saphenous vein incompetence (MARADONA trial). J. Vasc. Surg. Ven. Lymphat. Disord. 2019, 7, 364–374. [Google Scholar] [CrossRef]
- Mohamed, A.H.; Leung, C.; Wallace, T.; Smith, G.; Carradice, D.; Chetter, I. A Randomized Controlled Trial of Endovenous Laser Ablation Versus Mechanochemical Ablation with ClariVein in the Management of Superficial Venous Incompetence (LAMA Trial). Ann. Surg. 2021, 273, e188–e195. [Google Scholar] [CrossRef]
- Baccellieri, D.; Apruzzi, L.; Ardita, V.; Favia, N.; Saracino, C.; Carta, N.; Melissano, G.; Chiesa, R. Early results of mechanochemical ablation for small saphenous vein incompetency using 2% polidocanol. J. Vasc. Surg. Ven. Lymphat. Disord. 2021, 9, 683–690. [Google Scholar] [CrossRef]
- Shaidakov, E.V.; Grigoryan, A.G.; Ilyukhin, E.A.; Bulatov, V.L.; Rosukhovskiy, D.A. Radiofrequency ablation or stripping of large-diameter incompetent great saphenous varicose veins with C2 or C3 disease. J. Vasc. Surg. Ven. Lymphat. Disord. 2016, 4, 45–50. [Google Scholar] [CrossRef]
- Alozai, T.; Huizing, E.; Schreve, M.; Mooij, M.C.; van Vlijmen, C.J.; Wisselink, W.; Ünlü, Ç. A systematic review and meta-analysis of mechanochemical endovenous ablation using Flebogrif for varicose veins. J. Vasc. Surg. Ven. Lymphat. Disord. 2022, 10, 248–257. [Google Scholar] [CrossRef]
- Vos, C.G.; Ünlü, Ç.; Bosma, J.; van Vlijmen, C.J.; de Nie, A.J.; Schreve, M.A. A systematic review and meta-analysis of two novel techniques of nonthermal endovenous ablation of the great saphenous vein. J. Vasc. Surg. Ven. Lymphat. Disord. 2017, 5, 880–896. [Google Scholar] [CrossRef]
- Kemaloğlu, C. Saphenous vein diameter is a single risk factor for early recanalization after endothermal ablation of incompetent great saphenous vein. Vascular 2019, 27, 537–541. [Google Scholar] [CrossRef]
- Soliman-Hamad, M.A. Mechano-Chemical Endo-Venous Ablation of Varicose Veins with Flebogrif Occlusion Catheter. Med. J. Cairo Univ. 2019, 87, 3749–3754. [Google Scholar] [CrossRef]








 | |||
| Protrusion Levels L | Side a (mm) | Diagonal p (mm) | Calculated Diameter df (mm) |
| L = Lmax | 20.6 | 33.2 | 34.5 |
| 21.5 | 32.5 | ||
| 19.8 | 31.8 | ||
| 20.5 | 32.9 | ||
| 19.6 | 32.5 | ||
| L = 0.9⋅Lmax | 16.3 | 25.2 | 27.3 |
| 17.2 | 25.1 | ||
| 16.5 | 24.8 | ||
| 17.1 | 25.2 | ||
| 16.4 | 24.6 | ||
| L = 0.8⋅Lmax | 11.0 | 17.7 | 18.6 |
| 11.3 | 17.8 | ||
| 10.8 | 17.6 | ||
| 11.2 | 17.7 | ||
| 10.1 | 17.7 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terlecki, P.; Boryga, M.; Kołodziej, P.; Gołacki, K.; Stropek, Z.; Janczak, D.; Antkiewicz, M.; Zubilewicz, T. Mechanical Characteristics of the Flebogrif System—The New System of Mechano-Chemical Endovenous Ablation. Materials 2022, 15, 2599. https://doi.org/10.3390/ma15072599
Terlecki P, Boryga M, Kołodziej P, Gołacki K, Stropek Z, Janczak D, Antkiewicz M, Zubilewicz T. Mechanical Characteristics of the Flebogrif System—The New System of Mechano-Chemical Endovenous Ablation. Materials. 2022; 15(7):2599. https://doi.org/10.3390/ma15072599
Chicago/Turabian StyleTerlecki, Piotr, Marek Boryga, Paweł Kołodziej, Krzysztof Gołacki, Zbigniew Stropek, Dariusz Janczak, Maciej Antkiewicz, and Tomasz Zubilewicz. 2022. "Mechanical Characteristics of the Flebogrif System—The New System of Mechano-Chemical Endovenous Ablation" Materials 15, no. 7: 2599. https://doi.org/10.3390/ma15072599
APA StyleTerlecki, P., Boryga, M., Kołodziej, P., Gołacki, K., Stropek, Z., Janczak, D., Antkiewicz, M., & Zubilewicz, T. (2022). Mechanical Characteristics of the Flebogrif System—The New System of Mechano-Chemical Endovenous Ablation. Materials, 15(7), 2599. https://doi.org/10.3390/ma15072599







