3D-Printed Hydroxyapatite and Tricalcium Phosphates-Based Scaffolds for Alveolar Bone Regeneration in Animal Models: A Scoping Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection of the Studies
2.3. Data Extraction and Analysis
3. Results
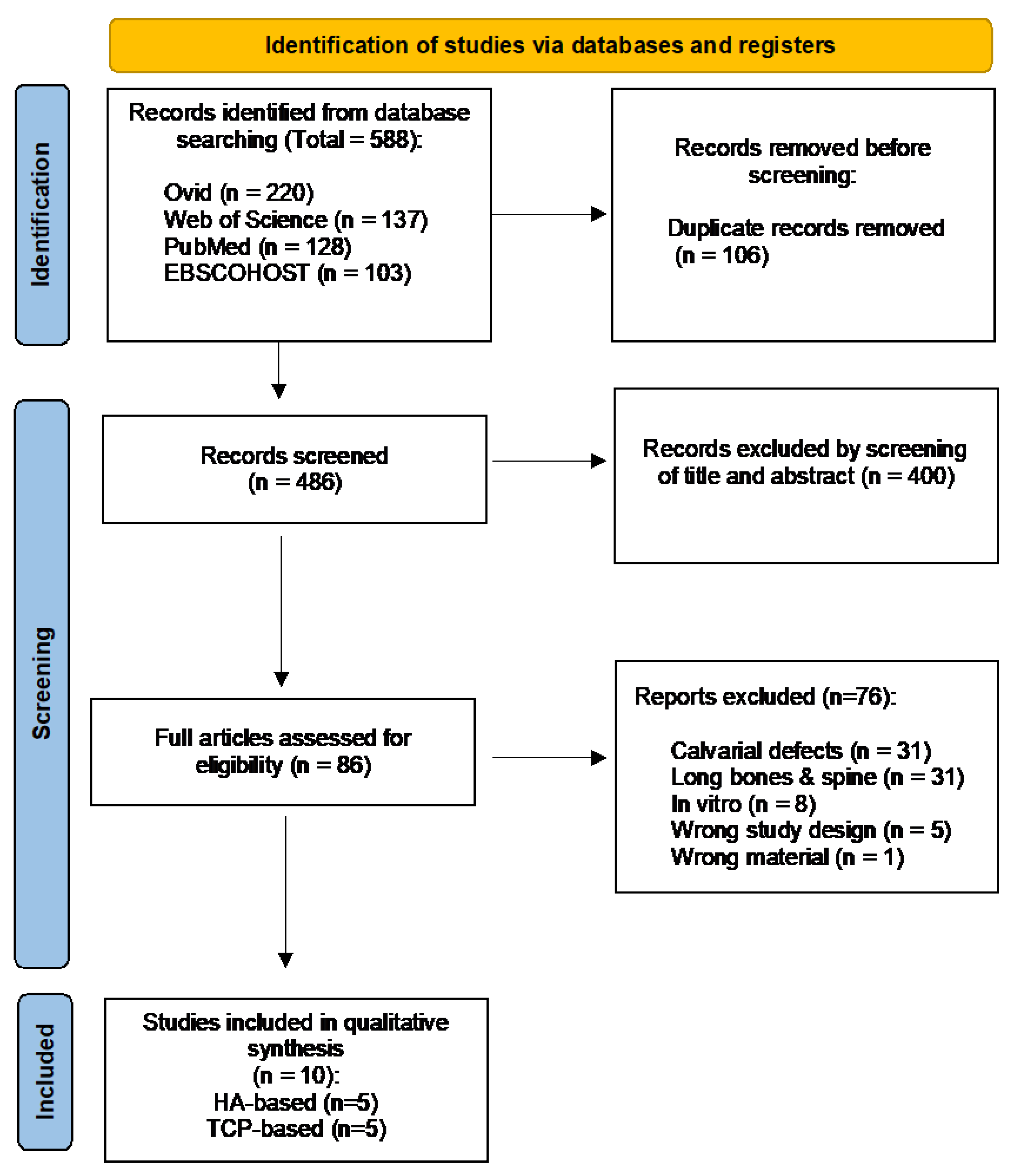
3.1. Study Selection and Characteristics
3.2. Characteristics of Included Studies
3.3. Study Design and Osseous Defects
3.4. Three-Dimensional-Printed HA- and TCP-Based Bone Scaffolds
3.5. Study Outcome Measures
3.5.1. Clinical Evaluation
3.5.2. Measurement of the Bone Regenerative Outcomes
3.5.3. Bone Regenerative Outcomes of the 3D-Printed HA- and TCP-Based Scaffolds
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Javed, F.; Ahmed, H.B.; Crespi, R.; Romanos, G.E. Role of primary stability for successful osseointegration of dental implants: Factors of influence and evaluation. Interv. Med. Appl. Sci. 2013, 5, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Li, S.; Zhang, T.; Wang, C.; Cai, X. Titanium mesh for bone augmentation in oral implantology: Current application and progress. Int. J. Oral. Sci. 2020, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- Milinkovic, I.; Cordaro, L. Are there specific indications for the different alveolar bone augmentation procedures for implant placement? A systematic review. Int. J. Oral. Maxillofac. Surg. 2014, 43, 606–625. [Google Scholar] [CrossRef]
- Jensen, S.S.; Terheyden, H. Bone augmentation procedures in localized defects in the alveolar ridge: Clinical results with different bone grafts and bone-substitute materials. Int. J. Oral. Maxillofac. Implants 2009, 24, 218–236. [Google Scholar] [PubMed]
- Larsson, L.; Decker, A.M.; Nibali, L.; Pilipchuk, S.P.; Berglundh, T.; Giannobile, W.V. Regenerative Medicine for Periodontal and Peri-implant Diseases. J. Dent. Res. 2016, 95, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Merli, M.; Mariotti, G.; Mazzoni, A.; Moscatelli, M.; Pagliaro, U.; Nieri, M. The Wafer Technique: Histomorphometric Results. Int. J. Periodontics Restor. Dent. 2020, 40, 815–823. [Google Scholar] [CrossRef]
- Tay, J.R.H.; Ng, E.; Lu, X.J.; Lai, W.M.C. Healing complications and their detrimental effects on bone gain in vertical-guided bone regeneration: A systematic review and meta-analysis. Clin. Implant. Dent. Relat Res. 2022, 24, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Proussaefs, P.; Lozada, J. The use of intraorally harvested autogenous block grafts for vertical alveolar ridge augmentation: A human study. Int. J. Periodontics Restor. Dent. 2005, 25, 351–363. [Google Scholar]
- Kainulainen, V.T.; Sandor, G.K.; Carmichael, R.P.; Oikarinen, K.S. Safety of zygomatic bone harvesting: A prospective study of 32 consecutive patients with simultaneous zygomatic bone grafting and 1-stage implant placement. Int. J. Oral. Maxillofac. Implant. 2005, 20, 245–252. [Google Scholar]
- Nkenke, E.; Weisbach, V.; Winckler, E.; Kessler, P.; Schultze-Mosgau, S.; Wiltfang, J.; Neukam, F.W. Morbidity of harvesting of bone grafts from the iliac crest for preprosthetic augmentation procedures: A prospective study. Int. J. Oral. Maxillofac. Surg. 2004, 33, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Raghoebar, G.M.; Louwerse, C.; Kalk, W.W.; Vissink, A. Morbidity of chin bone harvesting. Clin. Oral. Implant. Res. 2001, 12, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kerns, D.G. Mechanisms of guided bone regeneration: A review. Open Dent. J. 2014, 8, 56–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damien, C.J.; Parsons, J.R. Bone graft and bone graft substitutes: A review of current technology and applications. J. Appl. Biomater. 1991, 2, 187–208. [Google Scholar] [CrossRef]
- von Arx, T.; Buser, D. Horizontal ridge augmentation using autogenous block grafts and the guided bone regeneration technique with collagen membranes: A clinical study with 42 patients. Clin. Oral. Implant. Res. 2006, 17, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone substitutes: An update. Injury 2005, 36 (Suppl. S3), S20–S27. [Google Scholar] [CrossRef] [PubMed]
- Boyce, T.; Edwards, J.; Scarborough, N. Allograft bone. The influence of processing on safety and performance. Orthop. Clin. N. Am. 1999, 30, 571–581. [Google Scholar] [CrossRef]
- Skoglund, A.; Hising, P.; Young, C. A clinical and histologic examination in humans of the osseous response to implanted natural bone mineral. Int. J. Oral. Maxillofac. Implant. 1997, 12, 194–199. [Google Scholar]
- Hassan, M.N.; Yassin, M.A.; Suliman, S.; Lie, S.A.; Gjengedal, H.; Mustafa, K. The bone regeneration capacity of 3D-printed templates in calvarial defect models: A systematic review and meta-analysis. Acta Biomater. 2019, 91, 1–23. [Google Scholar] [CrossRef]
- Dutta, S.R.; Passi, D.; Singh, P.; Bhuibhar, A. Ceramic and non-ceramic hydroxyapatite as a bone graft material: A brief review. Ir. J. Med. Sci. 2015, 184, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Sommerfeldt, D.W.; Rubin, C.T. Biology of bone and how it orchestrates the form and function of the skeleton. Eur. Spine J. 2001, 10 (Suppl. S2), S86–S95. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Best, S.M.; Bonfield, W.; Brooks, R.A.; Rushton, N.; Jayasinghe, S.N.; Edirisinghe, M.J. In vitro assessment of the biological response to nano-sized hydroxyapatite. J. Mater. Sci. Mater. Med. 2004, 15, 441–445. [Google Scholar] [CrossRef]
- Lebourg, M.; Suay Anton, J.; Gomez Ribelles, J.L. Hybrid structure in PCL-HAp scaffold resulting from biomimetic apatite growth. J. Mater. Sci. Mater. Med. 2010, 21, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Guda, T.; Walker, J.A.; Singleton, B.M.; Hernandez, J.W.; Son, J.S.; Kim, S.G.; Oh, D.S.; Appleford, M.R.; Ong, J.L.; Wenke, J.C. Guided bone regeneration in long-bone defects with a structural hydroxyapatite graft and collagen membrane. Tissue Eng. Part. A 2013, 19, 1879–1888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.K.; Shin, M.; Kim, B.; Park, J.W.; Lee, H. A visible light-curable yet visible wavelength-transparent resin for stereolithography 3D printing. NPG Asia Mater. 2018, 10, 82–89. [Google Scholar] [CrossRef] [Green Version]
- Bandyopadhyay, A.; Mitra, I.; Bose, S. 3D Printing for Bone Regeneration. Curr. Osteoporos. Rep. 2020, 18, 505–514. [Google Scholar] [CrossRef]
- Kim, K.; Yeatts, A.; Dean, D.; Fisher, J.P. Stereolithographic bone scaffold design parameters: Osteogenic differentiation and signal expression. Tissue Eng. Part. B Rev. 2010, 16, 523–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidleithner, C.; Malferarri, S.; Palgrave, R.; Bomze, D.; Schwentenwein, M.; Kalaskar, D.M. Application of high resolution DLP stereolithography for fabrication of tricalcium phosphate scaffolds for bone regeneration. Biomed. Mater. 2019, 14, 045018. [Google Scholar] [CrossRef]
- Guillotin, B.; Souquet, A.; Catros, S.; Duocastella, M.; Pippenger, B.; Bellance, S.; Bareille, R.; Remy, M.; Bordenave, L.; Amedee, J.; et al. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials 2010, 31, 7250–7256. [Google Scholar] [CrossRef] [PubMed]
- Obregon, F.; Vaquette, C.; Ivanovski, S.; Hutmacher, D.W.; Bertassoni, L.E. Three-Dimensional Bioprinting for Regenerative Dentistry and Craniofacial Tissue Engineering. J. Dent. Res. 2015, 94, 143S–152S. [Google Scholar] [CrossRef]
- Guvendiren, M.; Molde, J.; Soares, R.M.; Kohn, J. Designing Biomaterials for 3D Printing. ACS Biomater. Sci. Eng. 2016, 2, 1679–1693. [Google Scholar] [CrossRef]
- Seunarine, K.; Gadegaard, N.; Tormen, M.; Meredith, D.O.; Riehle, M.O.; Wilkinson, C.D. 3D polymer scaffolds for tissue engineering. Nanomedicine 2006, 1, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Goh, B.T.; Teh, L.Y.; Tan, D.B.; Zhang, Z.; Teoh, S.H. Novel 3D polycaprolactone scaffold for ridge preservation—A pilot randomised controlled clinical trial. Clin. Oral. Implant. Res. 2015, 26, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Rasperini, G.; Pilipchuk, S.P.; Flanagan, C.L.; Park, C.H.; Pagni, G.; Hollister, S.J.; Giannobile, W.V. 3D-printed Bioresorbable Scaffold for Periodontal Repair. J. Dent. Res. 2015, 94, 153S–157S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, M.D.J.; Marnie, C.; Tricco, A.C.; Pollock, D.; Munn, Z.; Alexander, L.; McInerney, P.; Godfrey, C.M.; Khalil, H. Updated methodological guidance for the conduct of scoping reviews. JBI Evid. Synth. 2020, 18, 2119–2126. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [Green Version]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Fiorellini, J.P.; Norton, M.R.; Luan, K.W.; Kim, D.M.; Wada, K.; Sarmiento, H.L. Alveolar Ridge Augmentation with Three-Dimensional Printed Hydroxyapatite Devices: A Preclinical Study. Int. J. Periodontics Restor. Dent. 2018, 38, 389–394. [Google Scholar] [CrossRef] [Green Version]
- Lopez, C.D.; Diaz-Siso, J.R.; Witek, L.; Bekisz, J.M.; Cronstein, B.N.; Torroni, A.; Flores, R.L.; Rodriguez, E.D.; Coelho, P.G. Three dimensionally printed bioactive ceramic scaffold osseoconduction across critical-sized mandibular defects. J. Surg. Res. 2018, 223, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Lopez, C.D.; Coelho, P.G.; Witek, L.; Torroni, A.; Greenberg, M.I.; Cuadrado, D.L.; Guarino, A.M.; Bekisz, J.M.; Cronstein, B.N.; Flores, R.L. Regeneration of a Pediatric Alveolar Cleft Model Using Three-Dimensionally Printed Bioceramic Scaffolds and Osteogenic Agents: Comparison of Dipyridamole and rhBMP-2. Plast. Reconstr. Surg. 2019, 144, 358–370. [Google Scholar] [CrossRef]
- Shen, C.; Wang, M.M.; Witek, L.; Tovar, N.; Cronstein, B.N.; Torroni, A.; Flores, R.L.; Coelho, P.G. Transforming the Degradation Rate of beta-tricalcium Phosphate Bone Replacement Using 3-Dimensional Printing. Ann. Plast. Surg. 2021, 87, e153–e162. [Google Scholar] [CrossRef]
- Chang, P.C.; Luo, H.T.; Lin, Z.J.; Tai, W.C.; Chang, C.H.; Chang, Y.C.; Cochran, D.L.; Chen, M.H. Regeneration of critical-sized mandibular defect using a 3D-printed hydroxyapatite-based scaffold: An exploratory study. J. Periodontol. 2021, 92, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.C.; Lin, Z.J.; Luo, H.T.; Tu, C.C.; Tai, W.C.; Chang, C.H.; Chang, Y.C. Degradable RGD-Functionalized 3D-Printed Scaffold Promotes Osteogenesis. J. Dent. Res. 2021, 100, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Yang, B.E.; Hong, S.J.; Choi, H.G.; Byeon, S.J.; Lim, H.K.; Chung, S.M.; Lee, J.H.; Byun, S.H. Bone Regeneration Capability of 3D Printed Ceramic Scaffolds. Int. J. Mol. Sci. 2020, 21, 4837. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Park, T.H.; Ryu, J.Y.; Kim, D.K.; Oh, E.J.; Kim, H.M.; Shim, J.H.; Yun, W.S.; Huh, J.B.; Moon, S.H.; et al. Osteogenesis of 3D-Printed PCL/TCP/bdECM Scaffold Using Adipose-Derived Stem Cells Aggregates; An Experimental Study in the Canine Mandible. Int. J. Mol. Sci. 2021, 22, 5409. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Sun, M.; Zhang, F.; Liu, A.; He, Y.; Fu, J.; Yang, X.; Wang, H.; Gou, Z. Custom Repair of Mandibular Bone Defects with 3D Printed Bioceramic Scaffolds. J. Dent. Res. 2018, 97, 68–76. [Google Scholar] [CrossRef] [Green Version]
- Carrel, J.P.; Wiskott, A.; Scherrer, S.; Durual, S. Large Bone Vertical Augmentation Using a Three-Dimensional Printed TCP/HA Bone Graft: A Pilot Study in Dog Mandible. Clin. Implant. Dent. Relat. Res. 2016, 18, 1183–1192. [Google Scholar] [CrossRef] [Green Version]
- Szpalski, C.; Nguyen, P.D.; Cretiu Vasiliu, C.E.; Chesnoiu-Matei, I.; Ricci, J.L.; Clark, E.; Smay, J.E.; Warren, S.M. Bony engineering using time-release porous scaffolds to provide sustained growth factor delivery. J. Craniofac. Surg. 2012, 23, 638–644. [Google Scholar] [CrossRef]
- Bal, Z.; Kaito, T.; Korkusuz, F.; Yoshikawa, H. Bone regeneration with hydroxyapatite-based biomaterials. Emergent Mater. 2020, 3, 521–544. [Google Scholar] [CrossRef]
- Sun, F.; Zhou, H.; Lee, J. Various preparation methods of highly porous hydroxyapatite/polymer nanoscale biocomposites for bone regeneration. Acta Biomater. 2011, 7, 3813–3828. [Google Scholar] [CrossRef]
- Asa’ad, F.; Pagni, G.; Pilipchuk, S.P.; Gianni, A.B.; Giannobile, W.V.; Rasperini, G. 3D-Printed Scaffolds and Biomaterials: Review of Alveolar Bone Augmentation and Periodontal Regeneration Applications. Int. J. Dent. 2016, 2016, 1239842. [Google Scholar] [CrossRef] [Green Version]
- Donnaloja, F.; Jacchetti, E.; Soncini, M.; Raimondi, M.T. Natural and Synthetic Polymers for Bone Scaffolds Optimization. Polymers 2020, 12, 905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ezati, M.; Safavipour, H.; Houshmand, B.; Faghihi, S. Development of a PCL/gelatin/chitosan/beta-TCP electrospun composite for guided bone regeneration. Prog. Biomater. 2018, 7, 225–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shim, J.H.; Won, J.Y.; Park, J.H.; Bae, J.H.; Ahn, G.; Kim, C.H.; Lim, D.H.; Cho, D.W.; Yun, W.S.; Bae, E.B.; et al. Effects of 3D-Printed Polycaprolactone/beta-Tricalcium Phosphate Membranes on Guided Bone Regeneration. Int. J. Mol. Sci. 2017, 18, 899. [Google Scholar] [CrossRef] [Green Version]
- Mirtchi, A.A.; Lemaitre, J.; Terao, N. Calcium phosphate cements: Study of the beta-tricalcium phosphate--monocalcium phosphate system. Biomaterials 1989, 10, 475–480. [Google Scholar] [CrossRef]
- Hwang, K.S.; Choi, J.W.; Kim, J.H.; Chung, H.Y.; Jin, S.; Shim, J.H.; Yun, W.S.; Jeong, C.M.; Huh, J.B. Comparative Efficacies of Collagen-Based 3D Printed PCL/PLGA/beta-TCP Composite Block Bone Grafts and Biphasic Calcium Phosphate Bone Substitute for Bone Regeneration. Materials 2017, 10, 421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohner, M.; Santoni, B.L.G.; Döbelin, N. β-tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef]
- Owen, G.R.; Dard, M.; Larjava, H. Hydoxyapatite/beta-tricalcium phosphate biphasic ceramics as regenerative material for the repair of complex bone defects. J. Biomed. Mater. Res. B Appl Biomater. 2018, 106, 2493–2512. [Google Scholar] [CrossRef]
- Petrovic, V.; Zivkovic, P.; Petrovic, D.; Stefanovic, V. Craniofacial bone tissue engineering. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, e1–e9. [Google Scholar] [CrossRef]
- Ramay, H.R.; Zhang, M. Biphasic calcium phosphate nanocomposite porous scaffolds for load-bearing bone tissue engineering. Biomaterials 2004, 25, 5171–5180. [Google Scholar] [CrossRef]
- Schwarz, F.; Herten, M.; Ferrari, D.; Wieland, M.; Schmitz, L.; Engelhardt, E.; Becker, J. Guided bone regeneration at dehiscence-type defects using biphasic hydroxyapatite + beta tricalcium phosphate (Bone Ceramic) or a collagen-coated natural bone mineral (BioOss Collagen): An immunohistochemical study in dogs. Int. J. Oral Maxillofac. Surg. 2007, 36, 1198–1206. [Google Scholar] [CrossRef]
- Sager, M.; Ferrari, D.; Wieland, M.; Dard, M.; Becker, J.; Schwarz, F. Immunohistochemical characterization of wound healing at two different bone graft substitutes. Int. J. Oral Maxillofac. Surg. 2012, 41, 657–666. [Google Scholar] [CrossRef]
- Nery, E.B.; LeGeros, R.Z.; Lynch, K.L.; Lee, K. Tissue response to biphasic calcium phosphate ceramic with different ratios of HA/beta TCP in periodontal osseous defects. J. Periodontol. 1992, 63, 729–735. [Google Scholar] [CrossRef]
- TenHuisen, K.S.; Brown, P.W. Formation of calcium-deficient hydroxyapatite from alpha-tricalcium phosphate. Biomaterials 1998, 19, 2209–2217. [Google Scholar] [CrossRef]
- Zhao, J.; Shen, G.; Liu, C.; Wang, S.; Zhang, W.; Zhang, X.; Zhang, X.; Ye, D.; Wei, J.; Zhang, Z.; et al. Enhanced healing of rat calvarial defects with sulfated chitosan-coated calcium-deficient hydroxyapatite/bone morphogenetic protein 2 scaffolds. Tissue Eng. Part. A 2012, 18, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Su, J.; Wei, J.; Kong, H.; Liu, C. Biocompatibility and osteogenicity of degradable Ca-deficient hydroxyapatite scaffolds from calcium phosphate cement for bone tissue engineering. Acta Biomater. 2009, 5, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Sethuraman, S.; Nair, L.S.; El-Amin, S.; Nguyen, M.T.; Greish, Y.E.; Bender, J.D.; Brown, P.W.; Allcock, H.R.; Laurencin, C.T. Novel low temperature setting nanocrystalline calcium phosphate cements for bone repair: Osteoblast cellular response and gene expression studies. J. Biomed. Mater. Res. A 2007, 82, 884–891. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Claudia, L.; Reichert, J.; Berner, A.; Chen, F.; Fratzl, P.; Schantz, J.-T.; Hutmacher, D.W. Bone tissue engineering: From bench to bedside. Mater. Today 2012, 15, 430–435. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, J.P.; Hollinger, J.O. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin. Orthop. Relat. Res. 1986, 205, 299–308. [Google Scholar] [CrossRef]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Muller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef] [PubMed]
- Malhan, D.; Muelke, M.; Rosch, S.; Schaefer, A.B.; Merboth, F.; Weisweiler, D.; Heiss, C.; Arganda-Carreras, I.; El Khassawna, T. An Optimized Approach to Perform Bone Histomorphometry. Front. Endocrinol. 2018, 9, 666. [Google Scholar] [CrossRef]
- He, T.; Cao, C.; Xu, Z.; Li, G.; Cao, H.; Liu, X.; Zhang, C.; Dong, Y. A comparison of micro-CT and histomorphometry for evaluation of osseointegration of PEO-coated titanium implants in a rat model. Sci. Rep. 2017, 7, 16270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basyuni, S.; Ferro, A.; Santhanam, V.; Birch, M.; McCaskie, A. Systematic scoping review of mandibular bone tissue engineering. Br. J. Oral Maxillofac. Surg. 2020, 58, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Azais, T.; Robin, M.; Vallee, A.; Catania, C.; Legriel, P.; Pehau-Arnaudet, G.; Babonneau, F.; Giraud-Guille, M.M.; Nassif, N. The predominant role of collagen in the nucleation, growth, structure and orientation of bone apatite. Nat. Mater. 2012, 11, 724–733. [Google Scholar] [CrossRef]
- Geiger, F.; Bertram, H.; Berger, I.; Lorenz, H.; Wall, O.; Eckhardt, C.; Simank, H.G.; Richter, W. Vascular endothelial growth factor gene-activated matrix (VEGF165-GAM) enhances osteogenesis and angiogenesis in large segmental bone defects. J. Bone Miner. Res. 2005, 20, 2028–2035. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, S.; Li, Y.; Katz, M.S.; Elango, N. Translational regulation is a control point in RUNX2/Cbfa1 gene expression. Biochem Biophys Res. Commun. 2001, 289, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Wancket, L.M. Animal Models for Evaluation of Bone Implants and Devices: Comparative Bone Structure and Common Model Uses. Vet. Pathol. 2015, 52, 842–850. [Google Scholar] [CrossRef] [Green Version]
- Moran, C.J.; Ramesh, A.; Brama, P.A.; O’Byrne, J.M.; O’Brien, F.J.; Levingstone, T.J. The benefits and limitations of animal models for translational research in cartilage repair. J. Exp. Orthop. 2016, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Pearce, A.I.; Richards, R.G.; Milz, S.; Schneider, E.; Pearce, S.G. Animal models for implant biomaterial research in bone: A review. Eur. Cell Mater. 2007, 13, 1–10. [Google Scholar] [CrossRef]
- Ribitsch, I.; Baptista, P.M.; Lange-Consiglio, A.; Melotti, L.; Patruno, M.; Jenner, F.; Schnabl-Feichter, E.; Dutton, L.C.; Connolly, D.J.; van Steenbeek, F.G.; et al. Large Animal Models in Regenerative Medicine and Tissue Engineering: To Do or Not to Do. Front. Bioeng. Biotechnol. 2020, 8, 972. [Google Scholar] [CrossRef]
- Shamsuddin, S.A.; Ramli, R.; Razali, M.; Baharin, B.; Sulaiman, S.; Hwei Ng, M.; Low, C.K.; Jabar, M.N.A.; Nordin, R.; Yahaya, N. Guided bone regeneration using autologous plasma, bone marrow cells and β-TCP/HA granules for experimental alveolar ridge reconstruction in Macaca fascicularis. J. Biomater. Tissue Eng. 2017, 7, 111–118. [Google Scholar] [CrossRef]
- Brierly, G.I.; Tredinnick, S.; Lynham, A.; Woodruff, M.A. Critical Sized Mandibular Defect Regeneration in Preclinical In Vivo Models. Curr. Mol. Bio Rep. 2016, 2, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Omar, N.I.; Baharin, B.; Lau, S.F.; Ibrahim, N.; Mohd, N.; Ahmad Fauzi, A.; Muhammad, N.; Fernandez, N.M. The Influence of Ficus deltoidea in Preserving Alveolar Bone in Ovariectomized Rats. Vet. Med. Int. 2020, 2020, 8862489. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, S. Translational research challenges: Finding the right animal models. J. Investig. Med. 2012, 60, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Lorbach, O.; Baums, M.H.; Kostuj, T.; Pauly, S.; Scheibel, M.; Carr, A.; Zargar, N.; Saccomanno, M.F.; Milano, G. Advances in biology and mechanics of rotator cuff repair. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 530–541. [Google Scholar] [CrossRef]


| Author | 3D Material (Test) | Used Supplement | 3D Printing Technique | Assessment Methods | Main Findings |
|---|---|---|---|---|---|
| Carrel et al., 2016 [46] | α-TCP and micro-crystalline/ CDHA (OsteoFlux) | - | Extrusion | Histology, Histopathology, Histomorphometry | New bone growth above its natural bed up to 4.5 mm |
| Fiorellini et al., 2018 [37] | HA (TheriRidge) | - | Digital light processing (DLP) | Histopathology, Histomorphometry | 3D-printed blocks exhibit new bone growth adjacent to and within the graft. The amount of bone ingrowth and the presence of osteoid were slightly higher in the blocks without a screw |
| Kim et al., 2020 [43] | HA/TCP (6:4 ratio) (Genoss) | - | Digital light processing (DLP) stereolithography | Micro-CT, Histomorphometry | Total amount of new bone formation higher in 3D HA scaffold than particle-type substitute |
| Chang et al., 2021 [41] | 90 wt.% HA/10 wt.% 82:18 PLGA | - | Micro- extrusion | Gene expression, Micro-CT, Histology | Allow direct bone apposition and facilitate new bone formation compared to the control group |
| Chang et al., 2021 [42] | 90 wt.% HA/10 wt.% 82:18 PLGA | RGD-functionalized alginate matrix (RAM) | Micro- extrusion | Gene expression, Micro-CT, Histology | Adding oxidized RAM with osteoid-like stiffness induces bone formation and facilitates the synthesis of collagen, angiogenesis and osteogenesis |
| Author | 3D Material (Test) | Cells Seeding | 3D-Printing Technique | Assessment Methods | Main Findings |
|---|---|---|---|---|---|
| Lopez et al., 2018 [38] | β-TCP | - | Direct writing | Micro-CT, Histology | β-TCP scaffolds able to repair critical segmental mandibular defects to levels similar to native bone |
| Shao et al., 2018 [45] | β-TCP | - | Direct writing | Micro-CT, Histology, Histomorphometry | β-TCP had lowest new bone formation compared to other materials (CSi, CSi-Mg10 and Bred) |
| Lopez et al., 2019 [39] | β-TCP (coated with DIPY or rhBMP-2) | - | Direct writing with additive manufacturing | Micro-CT, Histology | Both β-TCP scaffolds with DIPY or rhBMP-2 able to regenerate vascularized bone in skeletally immature alveolar bone defects |
| Lee et al., 2021 [44] | PCL/β-TCP (coated with bdECM) | ADSCs | Fused deposition | Micro-CT, Histology, Gene expression, Protein expression | PCL/TCP coated with bdECM and ADSC aggregates increased mandibular ossification |
| Shen et al., 2021 [40] | β-TCP (coated with DIPY) | - | Direct writing | Micro-CT, Histology | β-TCP/DIPY scaffolds accelerate degradation rate and replacement of β-TCP with vascularized bone |
| Author | Animal Model | Total No of Defects | Sex | Age | Weight | Defect Size | Time of Sacrifice |
|---|---|---|---|---|---|---|---|
| Carrel et al., 2016 [46] | Beagle dogs | 4 | Male | 18 months | 16 kg | - | 8 weeks |
| Fiorellini et al., 2018 [37] | Canines | 32 | Male | NR | NR | 8 × 5 mm | 16, 26 weeks |
| Kim et al., 2020 [43] | Beagle dogs | 48 | Male | 22–26 weeks | 10–12 kg | 7 × 3 × 5 mm3 | 4, 8 weeks |
| Chang et al., 2021 [41] | Sprague Dawley rats | 28 | Male | NR | 250–300 g | 4 mm (diameter) | 1, 4 weeks |
| Chang et al., 2021 [42] | Sprague Dawley rats | 60 | Male | NR | 250–300 g | 4 mm (diameter) | 1, 4 weeks |
| Lopez et al., 2018 [38] | NZ white rabbits | 8 | NR | NR | ~3.5 kg | 12 mm | 8 weeks |
| Shao et al., 2018 [45] | NZ white rabbits | 64 | Male | NR | 2.8 ± 0.2 kg | 10 × 6 × 4 mm3 | 8, 16 weeks |
| Lopez et al., 2019 [39] | NZ white rabbits | 24 | NR | NR | NR | 3.5 × 3.5 mm | 8 weeks |
| Lee et al., 2021 [44] | Beagle dogs | 10 | NR | 36 months | NR | - | 8 weeks |
| Shen et al., 2021 [40] | NZ white rabbits | 22 | NR | 1 month | NR | 3.5 × 3.5 mm | 2, 6, 8 and 18 months |
| Author | 3D-Printed Scaffolds (Test) | Additional Features to 3D-Printed Scaffolds | Porosity/Pore Size | Pre-Intervention | Intervention | Additional Material to Cover/Fix 3D-Printed Scaffolds |
|---|---|---|---|---|---|---|
| Carrel et al., 2016 [46] | α-TCP and microcrystalline/CDHA (OsteoFlux) | Regular porosity and forms an interconnected network, scaffold’s macro-porosity 40% to 50% | Total porosity 50% to 65% | Extraction of mandibular first premolar to the first molar (both sides) | Guided bone regeneration | Collagen membrane |
| Fiorellini et al., 2018 [37] | HA (TheriRidge) | Macro-channel blocks with through and through mesial to distal channel (1.4 × 1.6 mm) or microchannel blocks with through and through buccal to lingual channel (20–50 μm) | NR | Extraction of mandibular first premolar to the first molar (both sides) | Alveolar ridge augmentation | Fixation screw |
| Kim et al., 2020 [43] | HA/TCP (6:4 ratio) (Genoss) | - | NR | Extraction of mandibular first premolar to the first molar (both sides) | Guided bone regeneration | Collagen membrane and fixation pins |
| Chang et al., 2021 [41] | 90 wt.% HA/10 wt.% 82:18 PLGA | Orthogonal pores | Pore size 400 × 400 μm Mean pore size of 0.420 ± 0.028 × 0.328 ± 0.005 mm2 | - | Regeneration of mandibular critical-sized defects | - |
| Chang et al., 2021 [42] | 90 wt.% HA/10 wt.% 82:18 PLGA | Interconnected orthogonal pores with lid (6 mm diameter) to hold main body for the scaffold | Total porosity 37.78% ± 2.30% Pore size 400 × 400 μm Mean pore size 0.426 ± 0.041× 0.368 ± 0.015 mm2 | - | Regeneration of mandibular critical-sized defects | - |
| Lopez et al., 2018 [38] | β-TCP | - | Pore spacing 330 μm | - | Regeneration of mandibular critical-sized defects | Plate and screws |
| Shao et al., 2018 [45] | β-TCP | - | Total porosity 57.3% ± 4.4% Pore size 302 ± 17.2 × 261 ± 12.9 μm | - | Regeneration of alveolar bone defect | - |
| Lopez et al., 2019 [39] | β-TCP (coated with DIPY or rhBMP-2) | - | Pore spacing 330 μm | - | Regeneration of alveolar bone defect | - |
| Lee et al., 2021 [44] | PCL/β-TCP (coated with bdECM) | 4 holes, diameter 1 mm | NR | Extraction of mandibular first premolar to the first molar (left side) | Mandibular reconstruction | Plate and screws |
| Shen et al., 2021 [40] | β-TCP (coated with DIPY) | - | Pore spacing 500 μm | - | Regeneration of alveolar bone defect | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd, N.; Razali, M.; Ghazali, M.J.; Abu Kasim, N.H. 3D-Printed Hydroxyapatite and Tricalcium Phosphates-Based Scaffolds for Alveolar Bone Regeneration in Animal Models: A Scoping Review. Materials 2022, 15, 2621. https://doi.org/10.3390/ma15072621
Mohd N, Razali M, Ghazali MJ, Abu Kasim NH. 3D-Printed Hydroxyapatite and Tricalcium Phosphates-Based Scaffolds for Alveolar Bone Regeneration in Animal Models: A Scoping Review. Materials. 2022; 15(7):2621. https://doi.org/10.3390/ma15072621
Chicago/Turabian StyleMohd, Nurulhuda, Masfueh Razali, Mariyam Jameelah Ghazali, and Noor Hayaty Abu Kasim. 2022. "3D-Printed Hydroxyapatite and Tricalcium Phosphates-Based Scaffolds for Alveolar Bone Regeneration in Animal Models: A Scoping Review" Materials 15, no. 7: 2621. https://doi.org/10.3390/ma15072621







