Atomistic Study on the Sintering Process and the Strengthening Mechanism of Al-Graphene System
Abstract
:1. Introduction
2. Model Methods
3. Results and Discussion
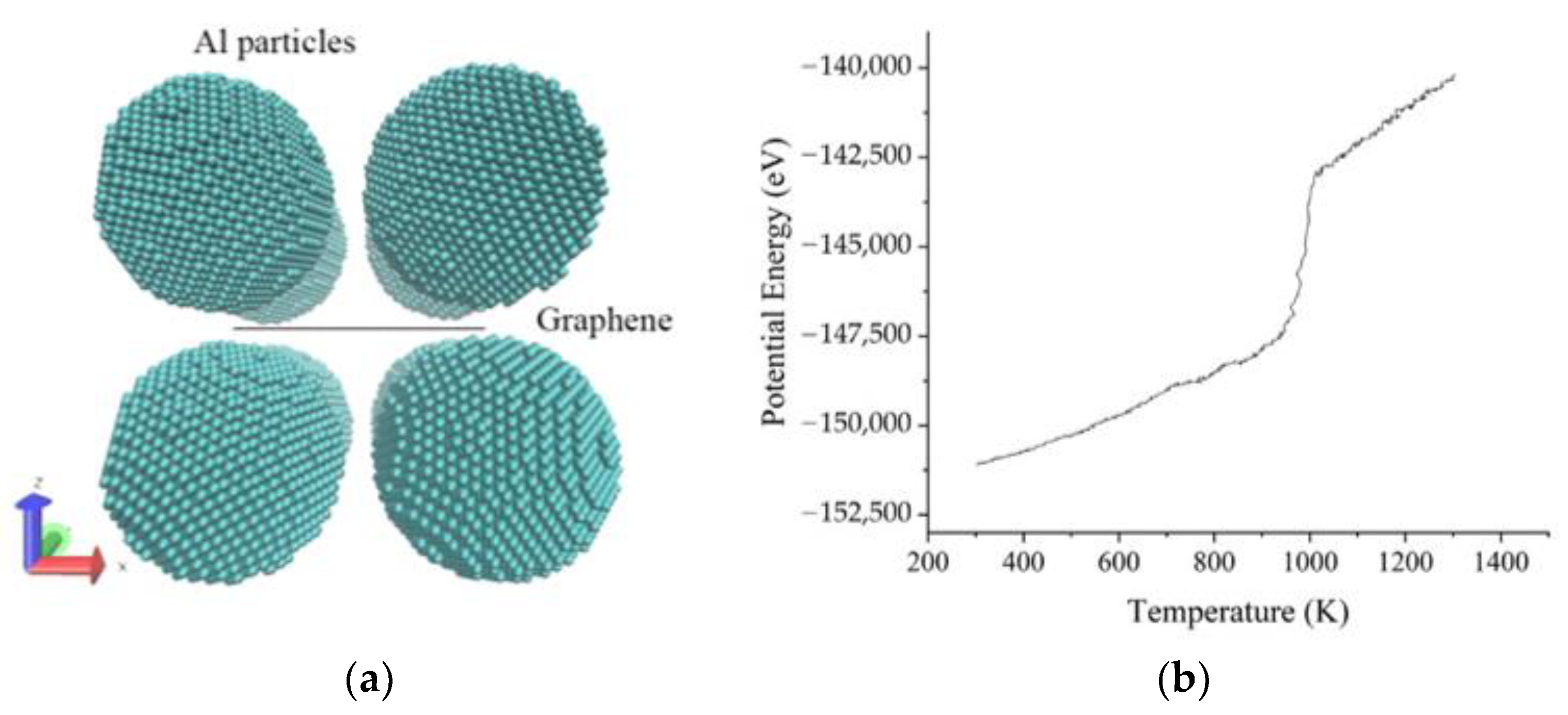
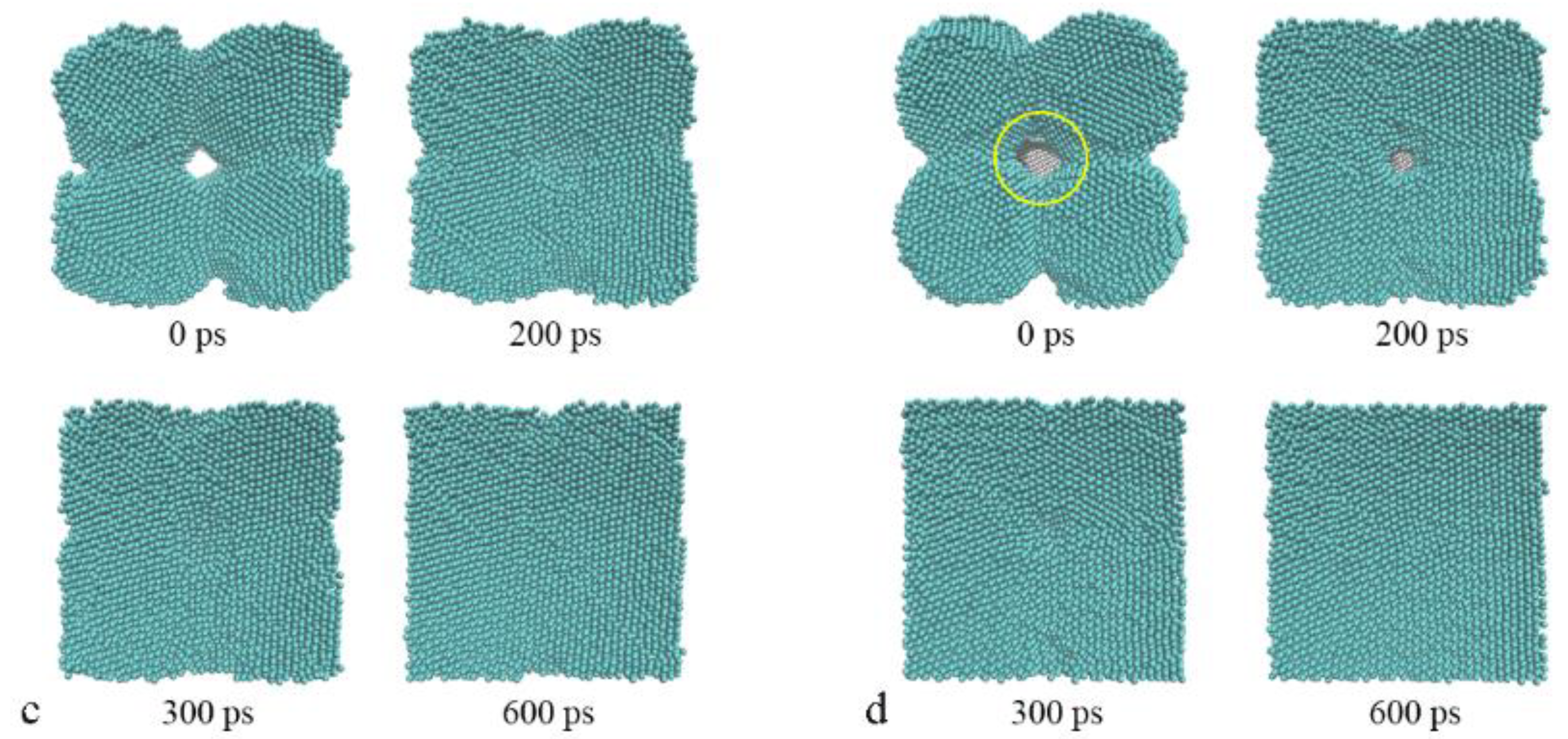
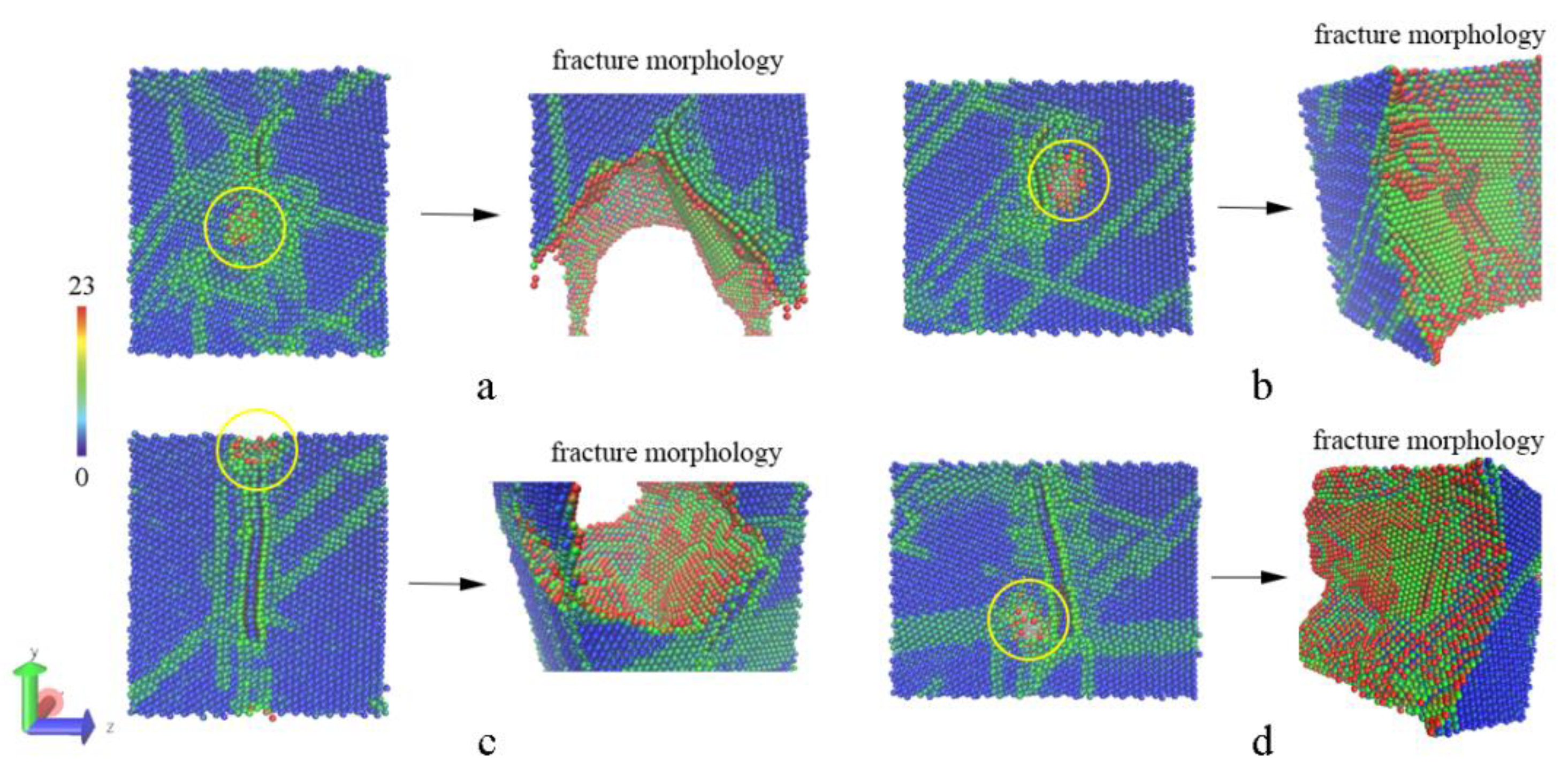
3.1. The Sintering Processes
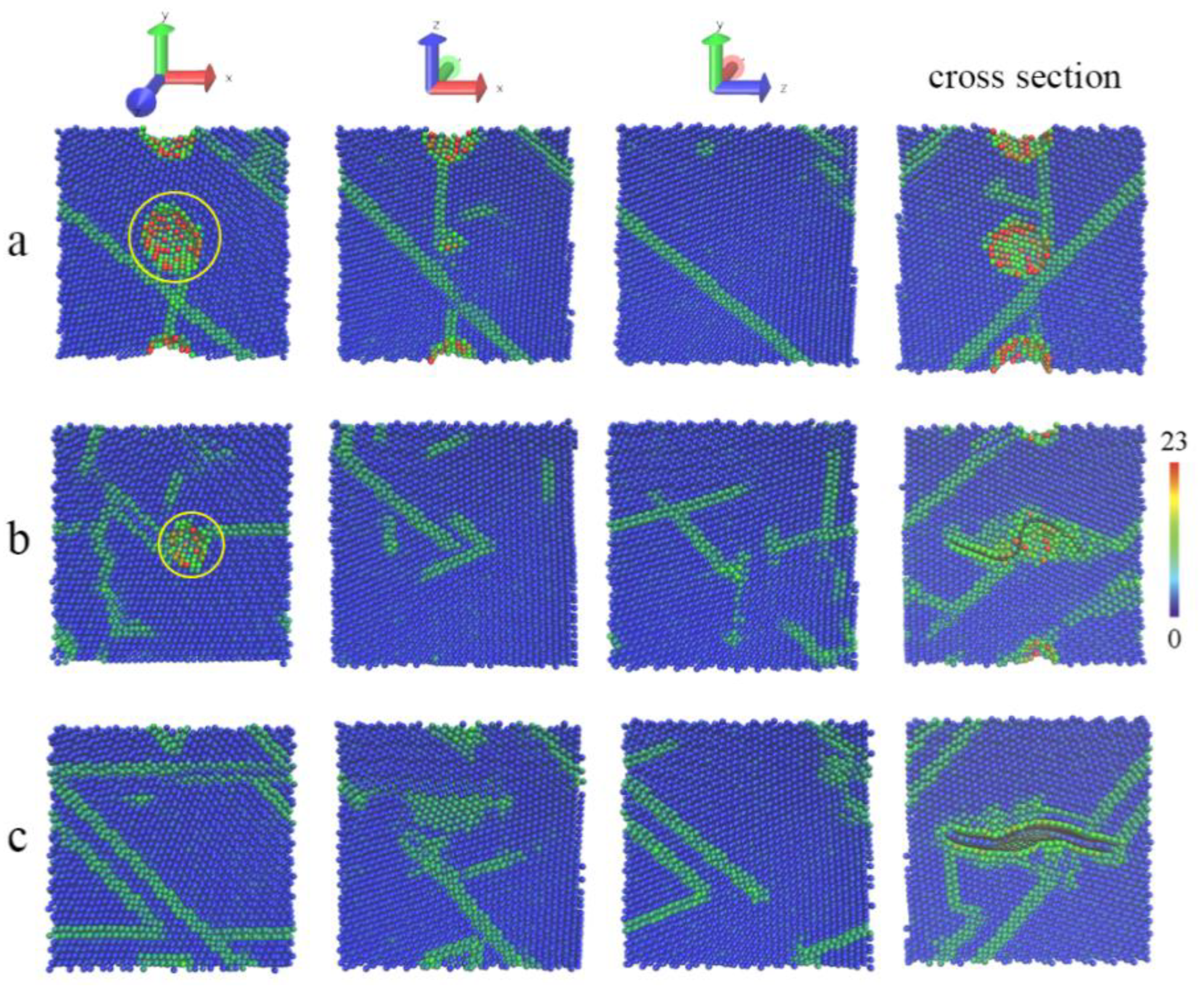
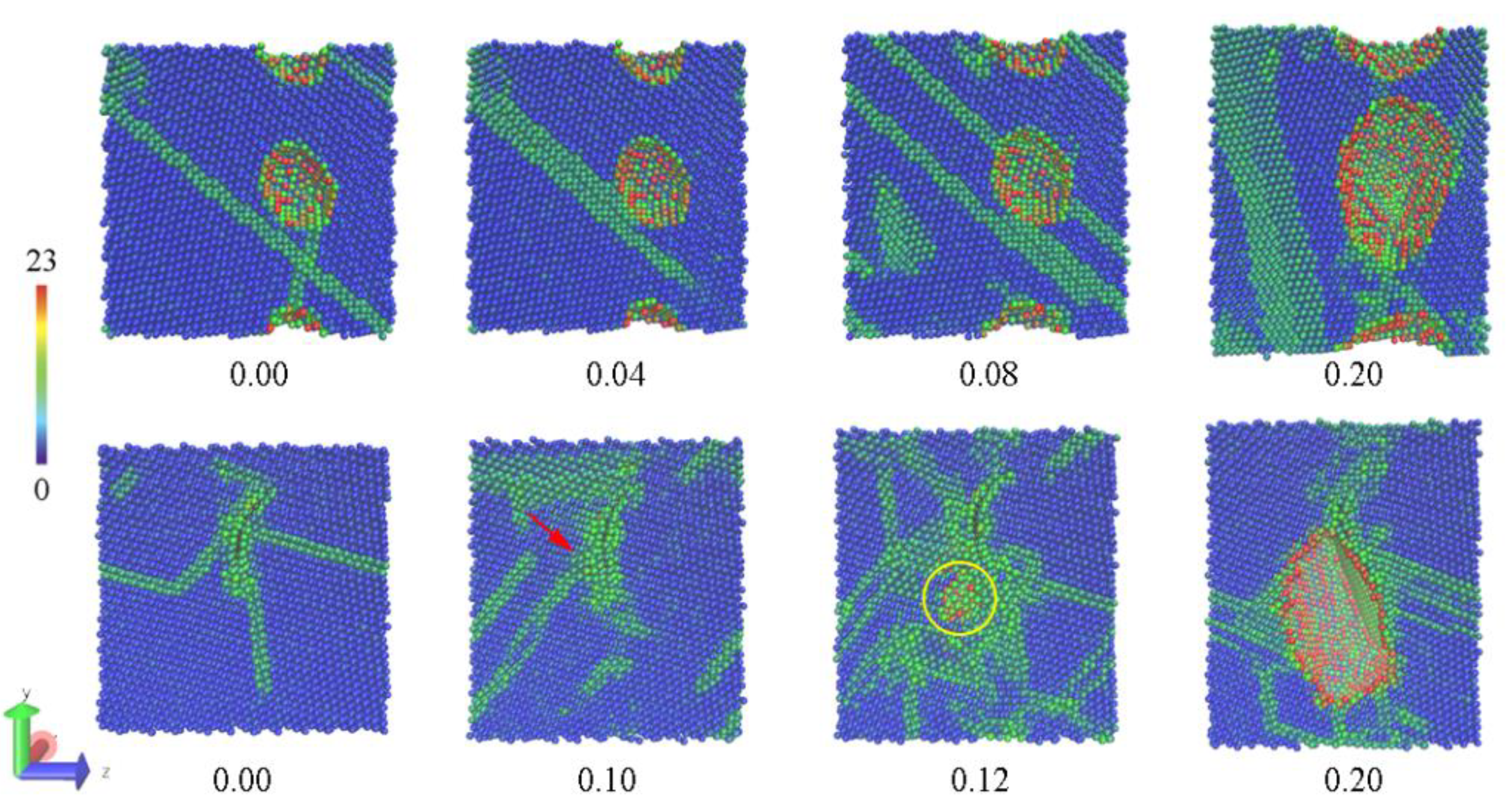
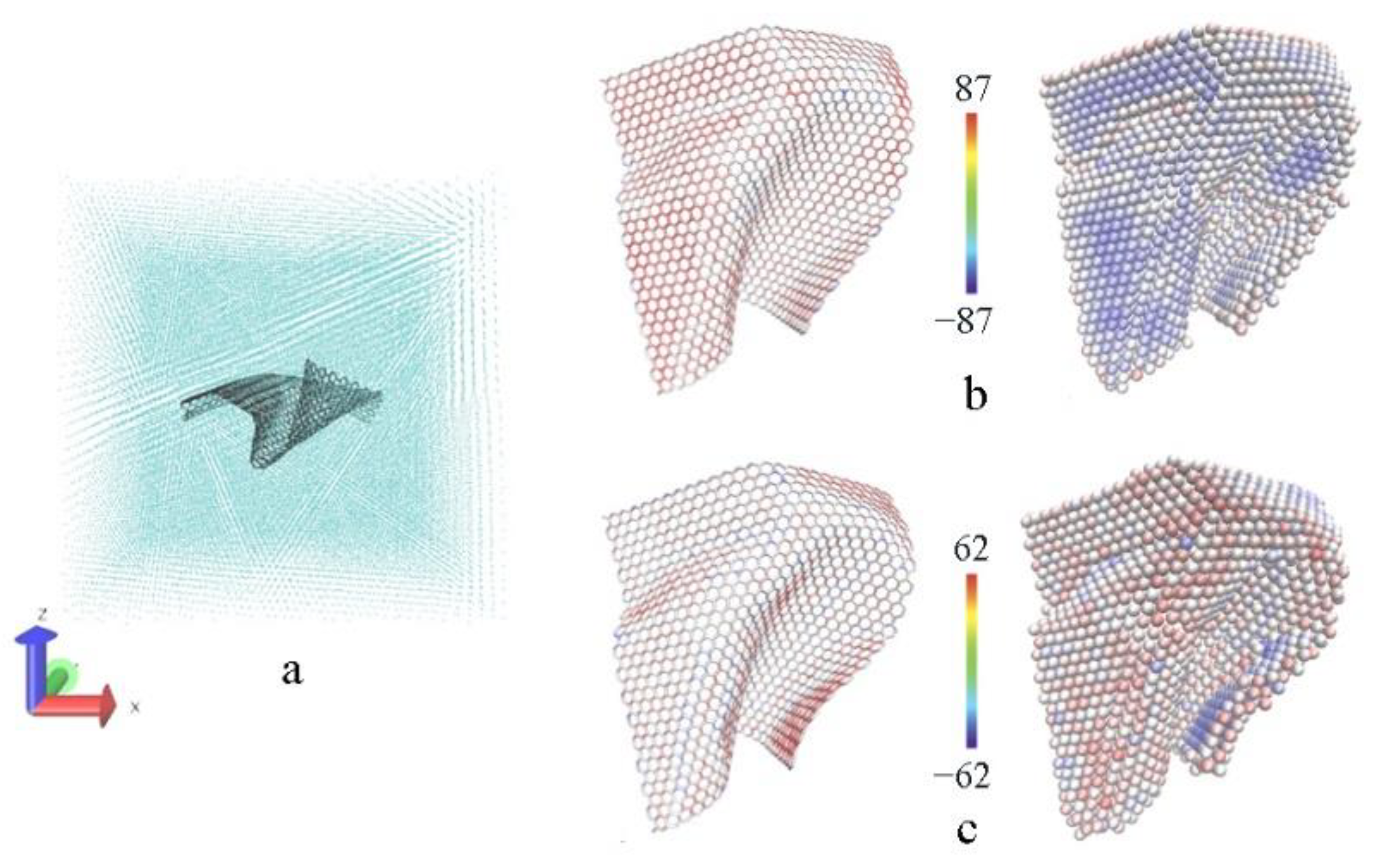
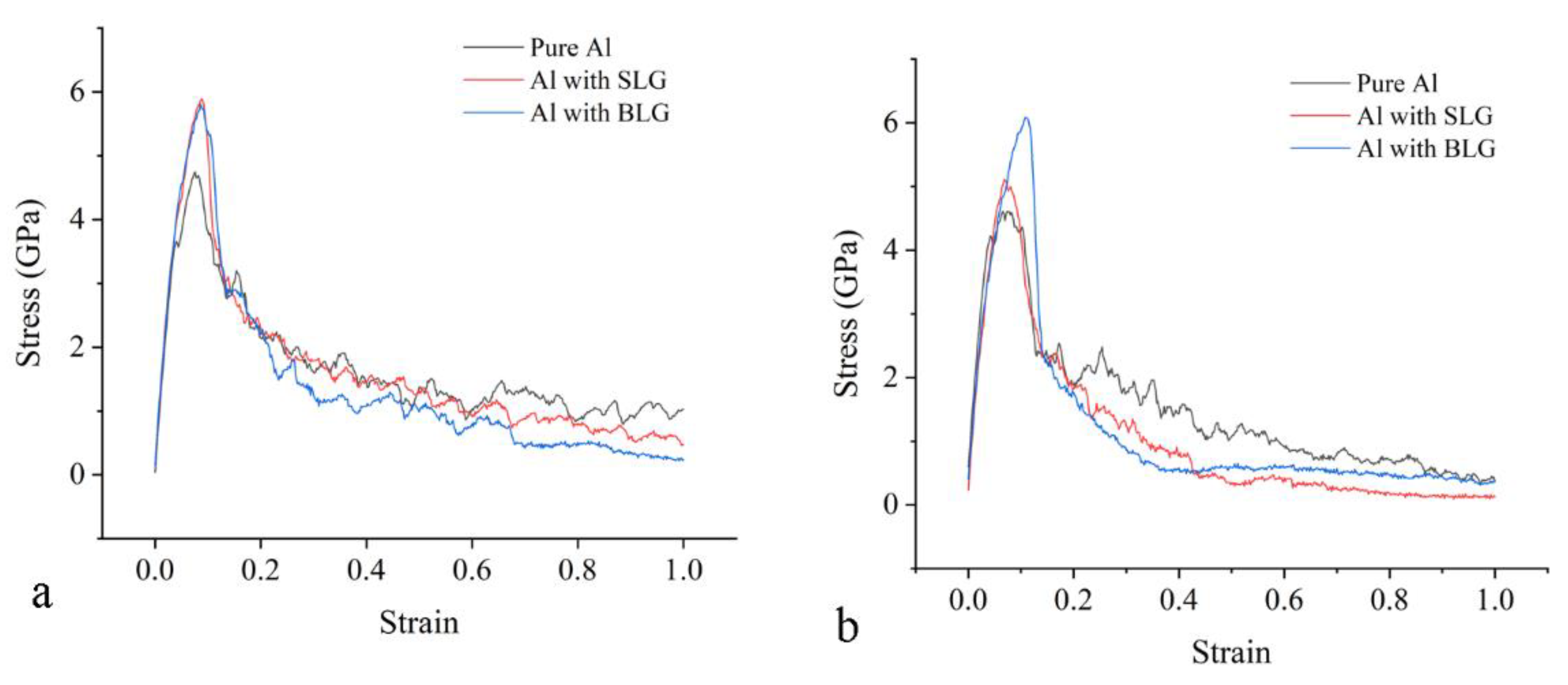
3.2. The Tensile Processes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asgharzadeh, H.; Sedigh, M. Synthesis and mechanical properties of Al matrix composites reinforced with few-layer graphene and graphene oxide. J. Alloys Compd. 2017, 728, 47–62. [Google Scholar] [CrossRef]
- Kumar, S.N.; Keshavamurthy, R.; Haseebuddin, M.R.; Koppad, P.G. Mechanical Properties of Aluminium-Graphene Composite Synthesized by Powder Metallurgy and Hot Extrusion. Trans. Indian Inst. Met. 2017, 70, 605–613. [Google Scholar] [CrossRef]
- Wang, J.Y.; Li, Z.Q.; Fan, G.L.; Pan, H.; Chen, Z.; Zhang, D. Reinforcement with graphene nanosheets in aluminum matrix composites. Scr. Mater. 2012, 66, 594–597. [Google Scholar] [CrossRef] [Green Version]
- Mu, X.N.; Cai, H.N.; Zhang, H.M.; Fan, Q.B.; Wang, F.C.; Zhang, Z.H.; Ge, Y.X.; Shi, R.; Wu, Y.; Wang, Z.; et al. Uniform dispersion and interface analysis of nickel coated graphene nanoflakes/pure titanium matrix composites. Carbon 2018, 137, 146–155. [Google Scholar] [CrossRef]
- Ju, J.-M.; Wang, G.; Sim, K.-H. Facile synthesis of graphene reinforced Al matrix composites with improved dispersion of graphene and enhanced mechanical properties. J. Alloys Compd. 2017, 704, 585–592. [Google Scholar] [CrossRef]
- Li, Z.; Fan, G.; Tan, Z.; Guo, Q.; Xiong, D.; Su, Y.; Li, Z.; Zhang, D. Uniform dispersion of graphene oxide in aluminum powder by direct electrostatic adsorption for fabrication of graphene/aluminum composites. Nanotechnology 2014, 25, 325601. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Xavior, M.A. Fatigue and Wear Behavior of Al6061–Graphene Composites Synthesized by Powder Metallurgy. Trans. Indian Inst. Met. 2015, 69, 415–419. [Google Scholar] [CrossRef]
- Liu, J.; Khan, U.; Coleman, J.; Fernandez, B.; Rodriguez, P.; Naher, S.; Brabazon, D. Graphene oxide and graphene nanosheet reinforced aluminium matrix composites: Powder synthesis and prepared composite characteristics. Mater. Des. 2016, 94, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Tian, W.M.; Li, S.M.; Wang, B.; Chen, X.; Liu, J.H.; Yu, M. Graphene-reinforced aluminum matrix composites prepared by spark plasma sintering. Int. J. Miner. Metall. Mater. 2016, 23, 723–729. [Google Scholar] [CrossRef]
- Shin, S.E.; Choi, H.J.; Shin, J.H.; Bae, D.H. Strengthening behavior of few-layered graphene/aluminum composites. Carbon 2015, 82, 143–151. [Google Scholar] [CrossRef]
- Rashad, M.; Pan, F.; Tang, A.; Asif, M. Effect of Graphene Nanoplatelets addition on mechanical properties of pure aluminum using a semi-powder method. Prog. Nat. Sci. Mater. Int. 2014, 24, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Chen, P.; Sun, W. Monitoring micro-structural evolution during aluminum sintering and understanding the sintering mechanism of aluminum nanoparticles: A molecular dynamics study. J. Mater. Sci. Technol. 2020, 57, 92–100. [Google Scholar] [CrossRef]
- Liu, Z.J.; Cheng, Q.; Wang, Y.; Li, Y.; Zhang, J. Sintering neck growth mechanism of Fe nanoparticles: A molecular dynamics simulation. Chem. Eng. Sci. 2020, 218, 115583. [Google Scholar] [CrossRef]
- Tavakol, M.; Mahnama, M.; Naghdabadi, R. Shock wave sintering of Al/SiC metal matrix nano-composites: A molecular dynamics study. Comput. Mater. Sci. 2016, 125, 255–262. [Google Scholar] [CrossRef]
- Wejrzanowski, T.; Lipecka, J.; Janczak-Rusch, J.; Lewandowska, M. Al-Si/AlN nanomultilayered systems with reduced melting point: Experiments and simulations. Appl. Surf. Sci. 2019, 493, 261–270. [Google Scholar] [CrossRef]
- He, H.P.; Rong, Y.; Zhang, L. Molecular dynamics studies on the sintering and mechanical behaviors of graphene nanoplatelet reinforced aluminum matrix composites. Model. Simul. Mater. Sci. Eng. 2019, 27, 065006. [Google Scholar] [CrossRef]
- Kumar, S. Graphene Engendered aluminium crystal growth and mechanical properties of its composite: An atomistic investigation. Mater. Chem. Phys. 2018, 208, 41–48. [Google Scholar] [CrossRef]
- Kvashnin, D.G.; Ghorbani-Asl, M.; Shtansky, D.V.; Golberg, D.; Krasheninnikov, A.V.; Sorokin, P.B. Mechanical properties and current-carrying capacity of Al reinforced with graohene/BN nanoribbons: A computational study. Nanoscale 2016, 8, 20080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobsen, K.W.; Norskov, J.K.; Puska, M.J. Interatomic interactions in the effective-medium theory. Phys. Rev. B Condens. Matter. 1987, 35, 7423–7442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenner, D.W.; Shenderova, O.A.; Harrison, J.A.; Stuart, S.J.; Ni, B.; Sinnott, S.B. A second-generation reactive empirical bond order (REBO) potential energy expression for hydrocarbons. J. Phys.-Condens. Matter. 2002, 14, 783–802. [Google Scholar] [CrossRef]
- Bai, Q.S.; He, X.; Bai, J.X.; Tong, Z. An atomistic investigation of the effect of strain on frictional properties of suspended graphene. AIP Adv. 2016, 6, 055308. [Google Scholar] [CrossRef]
- Van Wijk, M.M.; Dienwiebel, M.; Frenken, J.W.; Fasolino, A. Superlubric to stick-slip sliding of incommensurate graphene flakes on graphite. Phys. Rev. B 2013, 88, 235423.1–235423.6. [Google Scholar] [CrossRef] [Green Version]
- Peng, P.; Liao, G.; Shi, T.; Tang, Z.; Gao, Y. Molecular dynamic simulations of nanoindentation in aluminum thin film on silicon substrate. Appl. Surf. Sci. 2010, 256, 6284–6290. [Google Scholar] [CrossRef]
- Dandekar, C.R.; Shin, Y.C. Molecular dynamics based cohesive zone law for describing Al–SiC interface mechanics. Compos. Part A 2011, 42, 355–363. [Google Scholar] [CrossRef]
- Alhazaa, A.; Haneklaus, N.; Almutairi, Z. Impulse Pressure-Assisted Diffusion Bonding (IPADB): Review and Outlook. Met. Open Access Metall. J. 2021, 11, 323. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, Y.; Li, N.; Rong, Y.; Xia, H.; Ma, H. Atomistic simulation of Al-graphene thin film growth on polycrystalline Al substrate. Appl. Surf. Sci. 2018, 433, 540–545. [Google Scholar] [CrossRef]
- Pérez-Bustamante, R.; Bolaños-Morales, D.; Bonilla-Martínez, J.; Estrada-Guel, I.; Martínez-Sánchez, R. Microstructural and hardness behavior of graphene-nanoplatelets/aluminum composites synthesized by mechanical alloying. J. Alloys Compd. 2014, 615, S578–S582. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, J.; Yeom, M.S.; Shin, J.W.; Kim, H.; Cui, Y.; Kysar, J.W.; Hone, J.; Jung, Y.; Jeon, S.; et al. Strengthening effect of single-atomic-layer graphene in metal-graphene nanolayered composites. Nat. Commun. 2013, 4, 2114. [Google Scholar] [CrossRef]
- Li, Z.; Guo, Q.; Li, Z.; Fan, G.; Xiong, D.B.; Su, Y.; Zhang, J.; Zhang, D. Enhanced Mechanical Properties of Graphene (Reduced Graphene Oxide)/Aluminum Composites with a Bioinspired Nanolaminated Structure. Nano Lett. 2015, 15, 8077–8083. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Song, C.; Duan, Z.; Fan, G.; Xiong, D.B.; Su, Y.; Zhang, J.; Zhang, D. Molecular dynamics simulations of the shear mechanical properties of graphene/copper composites. Acta Mater. Compos. Sin. 2018, 35, 632–639. [Google Scholar]
- Kumar, S. Graphene engendered 2-D structural morphology of aluminium atoms: Molecular dynamics simulation study. Mater. Chem. Phys. 2017, 202, 329–339. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, W.; Wang, L.; Shi, J.; Sun, L.; Cui, J.; Wang, J. Investigation on mechanical behavior of multilayer graphene reinforced aluminum composites. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 123, 114172. [Google Scholar] [CrossRef]

| Graphene Type | Al Matrix | Graphene Content | The Improvement in Hardness | Research Group |
|---|---|---|---|---|
| Graphene nanoflakes | Al6061 | 0.4 wt% | 8.3% | Kumar et al. [7] |
| Reduced graphene oxide | Al | 0.3 wt% | 32% | Liu et al. [8] |
| Graphene nanoflakes | Al | 0.15 wt% | 43% | Liu et al. [8] |
| Reduced graphene oxide | Al | 0.3 wt% | 17% | Li et al. [6] |
| Graphene oxide | Al7075 | 1 wt% | 15% | Shin et al. [9] |
| Graphene nanoflakes | Al | 0.3 wt% | 11.8% | Rashad et al. [11] |
| Model | Final Volume (nm3) | The Number of Atoms Analyzed by CNA | ||
|---|---|---|---|---|
| Entire System | Al Atoms | f.c.c. Atoms | h.c.p. Atoms | |
| Pure Al | 759.6 | 759.6 | 38,944 | 4606 |
| Al with SLG | 753.4 | 744.8 | 35,216 | 3752 |
| Al with BLG | 759.0 | 739.0 | 31,687 | 6454 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Li, N.; Li, W.; Niu, L.; Li, Z. Atomistic Study on the Sintering Process and the Strengthening Mechanism of Al-Graphene System. Materials 2022, 15, 2644. https://doi.org/10.3390/ma15072644
Zhu Y, Li N, Li W, Niu L, Li Z. Atomistic Study on the Sintering Process and the Strengthening Mechanism of Al-Graphene System. Materials. 2022; 15(7):2644. https://doi.org/10.3390/ma15072644
Chicago/Turabian StyleZhu, Yongchao, Na Li, Wei Li, Liwei Niu, and Zhenghui Li. 2022. "Atomistic Study on the Sintering Process and the Strengthening Mechanism of Al-Graphene System" Materials 15, no. 7: 2644. https://doi.org/10.3390/ma15072644
APA StyleZhu, Y., Li, N., Li, W., Niu, L., & Li, Z. (2022). Atomistic Study on the Sintering Process and the Strengthening Mechanism of Al-Graphene System. Materials, 15(7), 2644. https://doi.org/10.3390/ma15072644






