Organo-Nanocups Assist the Formation of Ultra-Small Palladium Nanoparticle Catalysts for Hydrogen Evolution Reaction
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis
2.3. Characterization
2.4. Catalysis
3. Results and Discussion
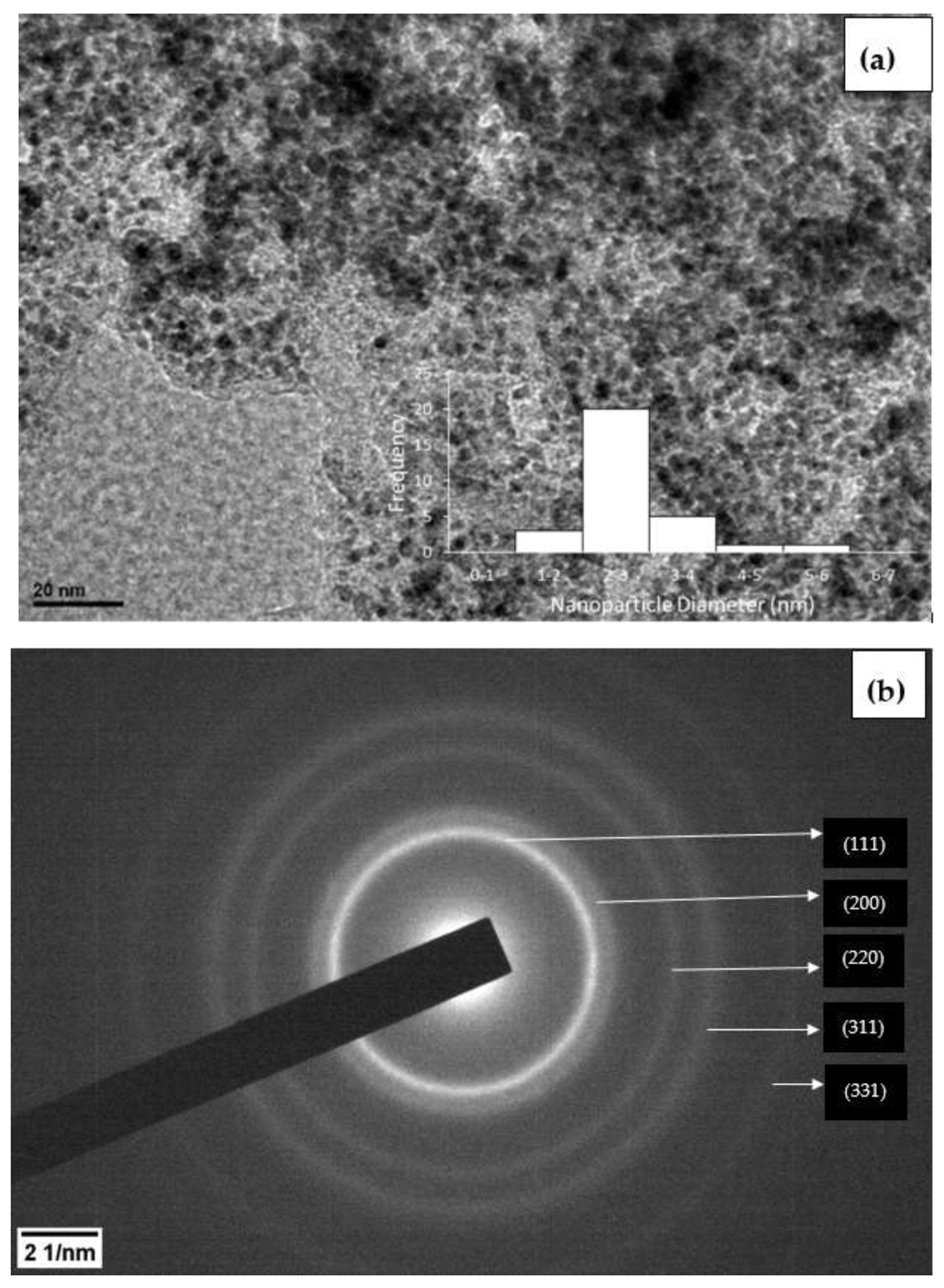
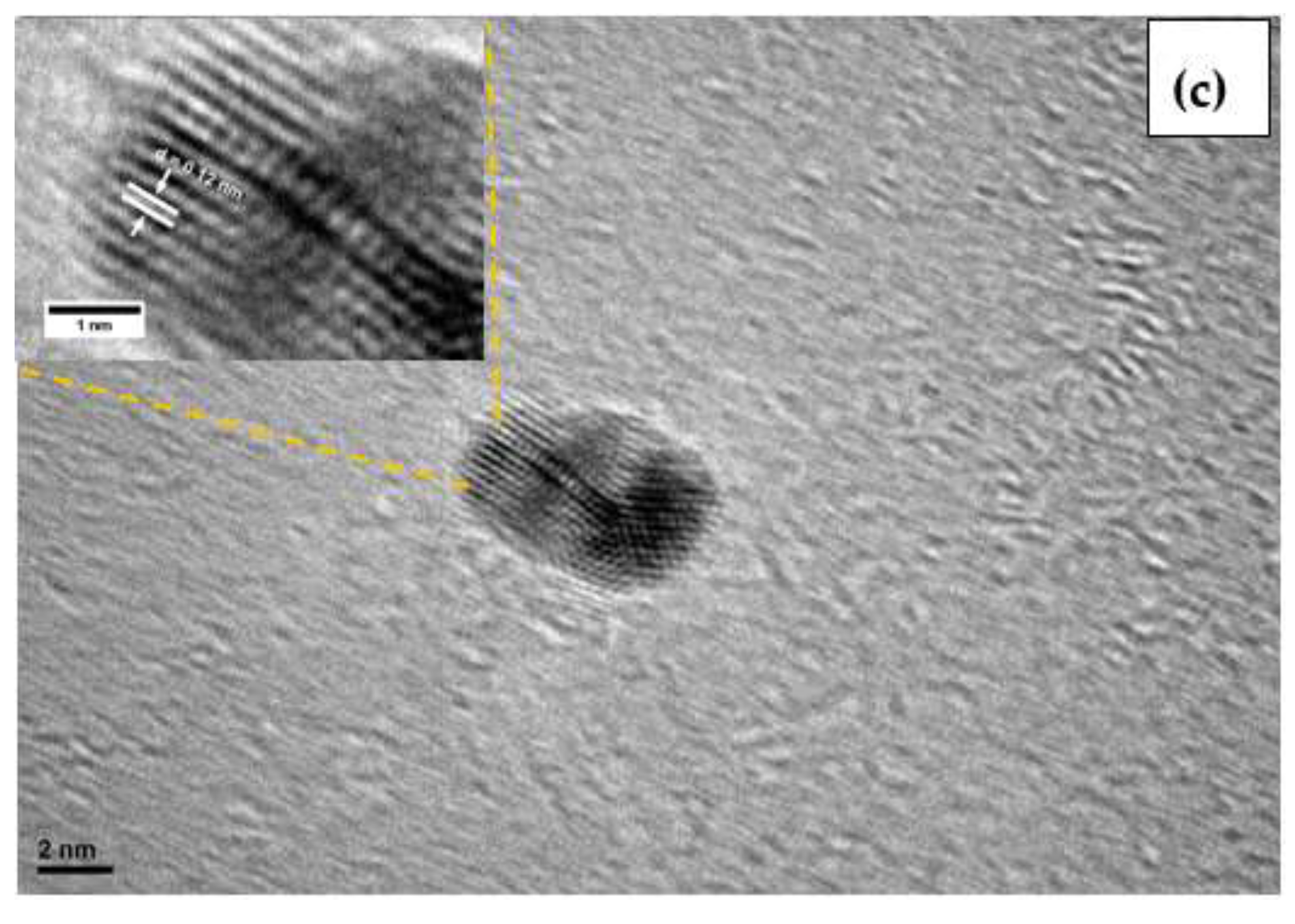
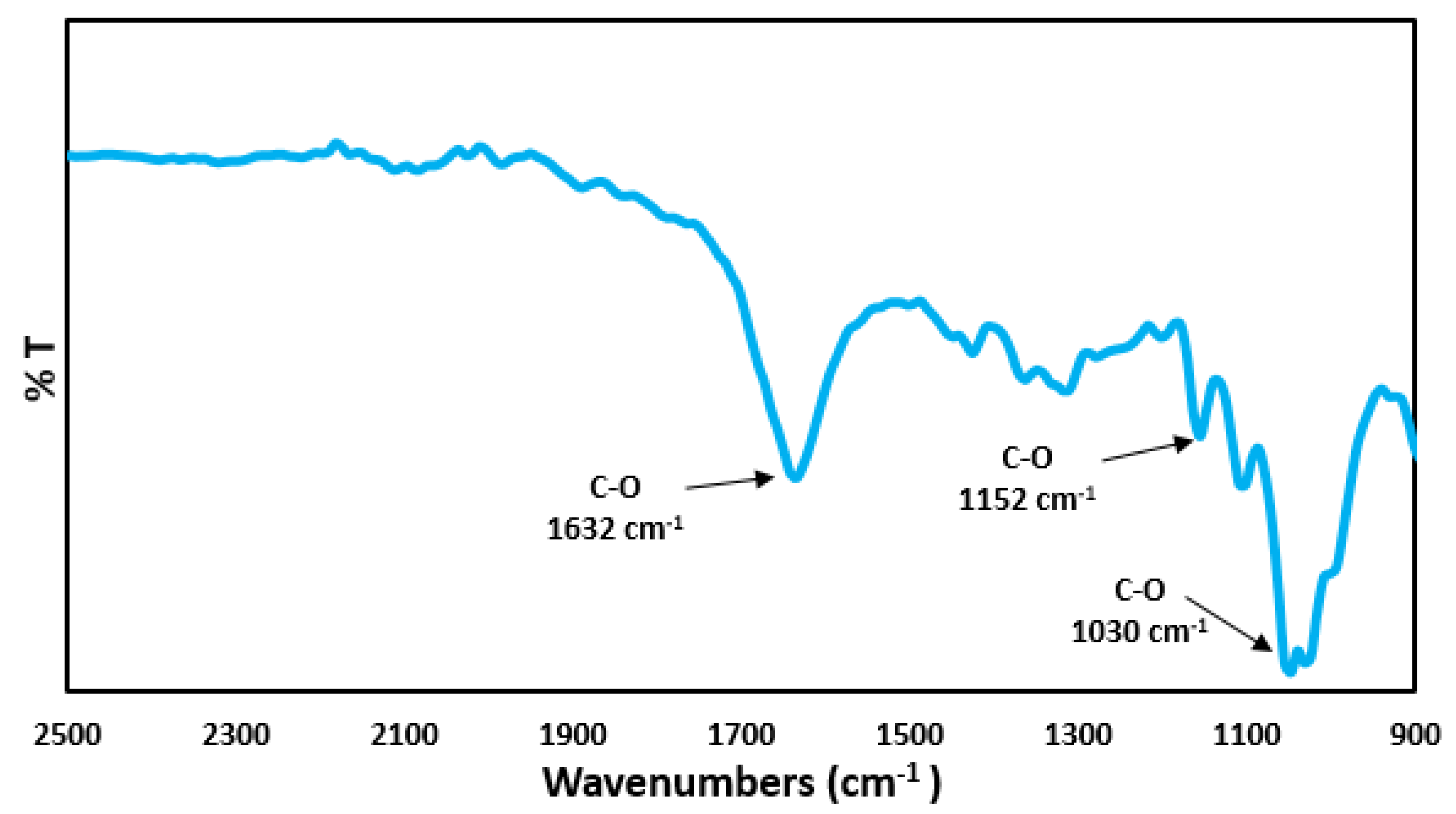
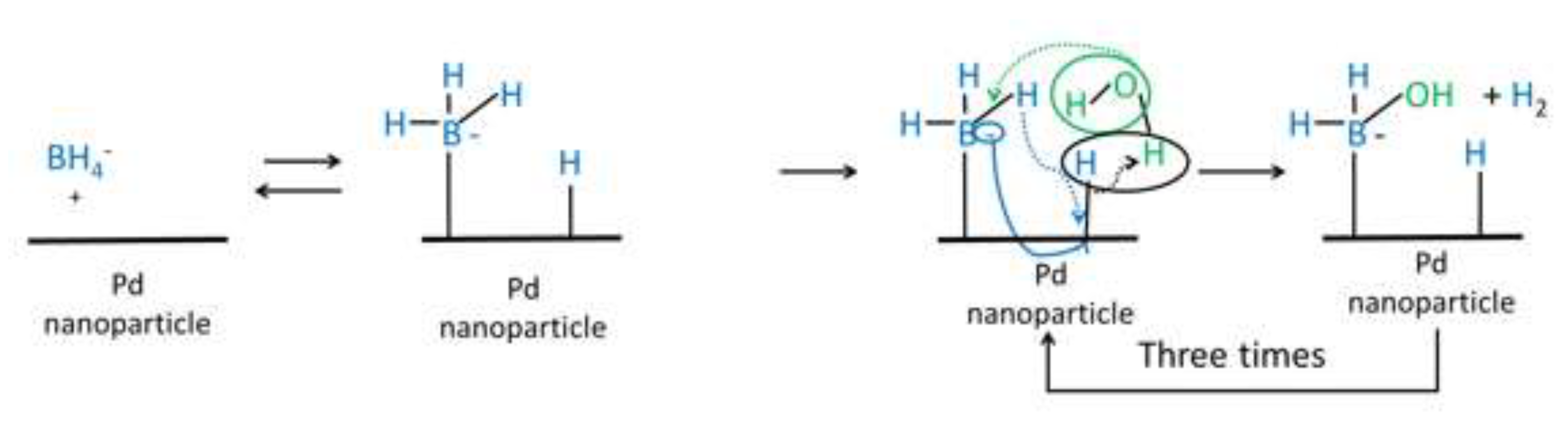
3.1. Characterization
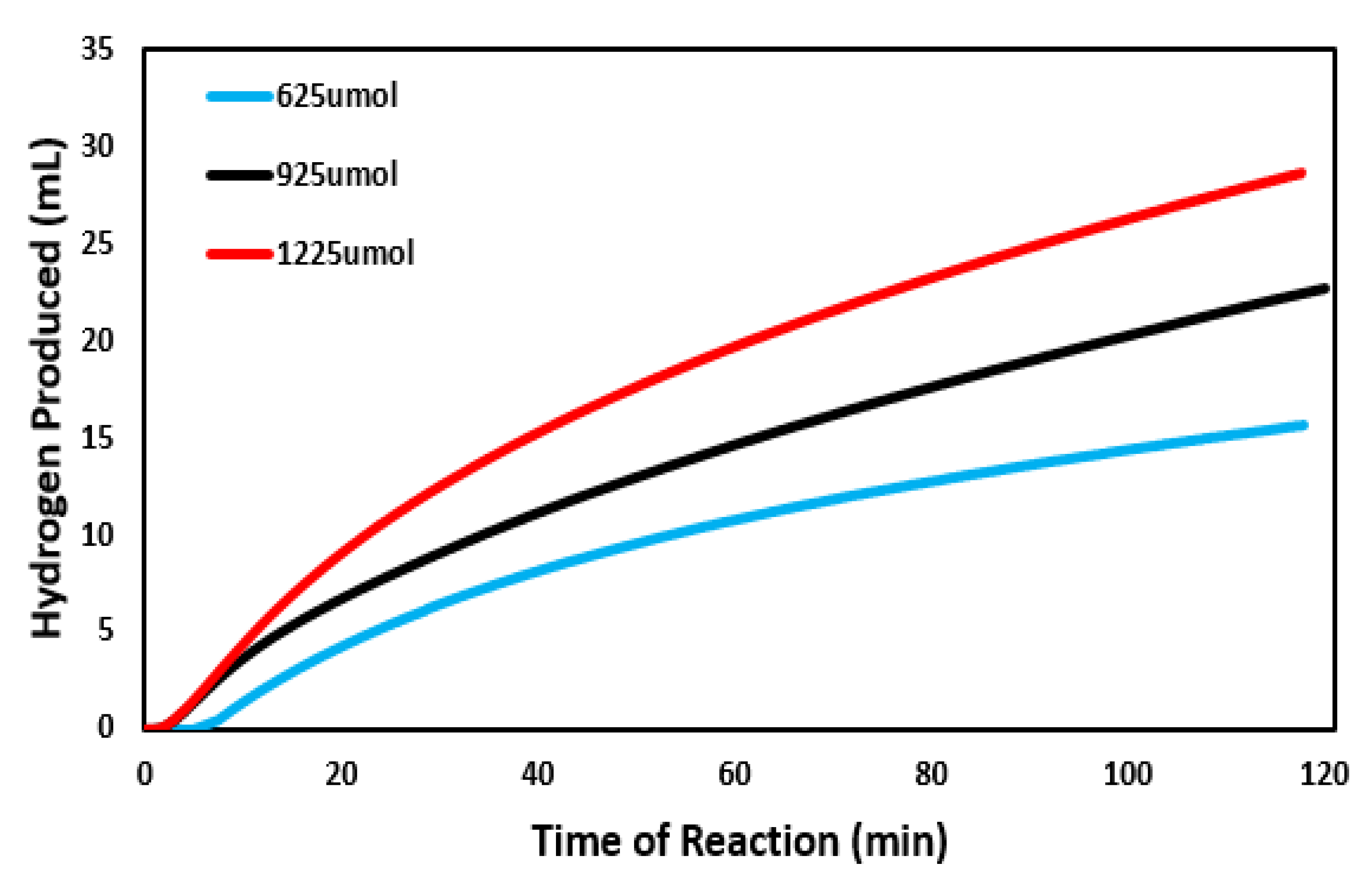
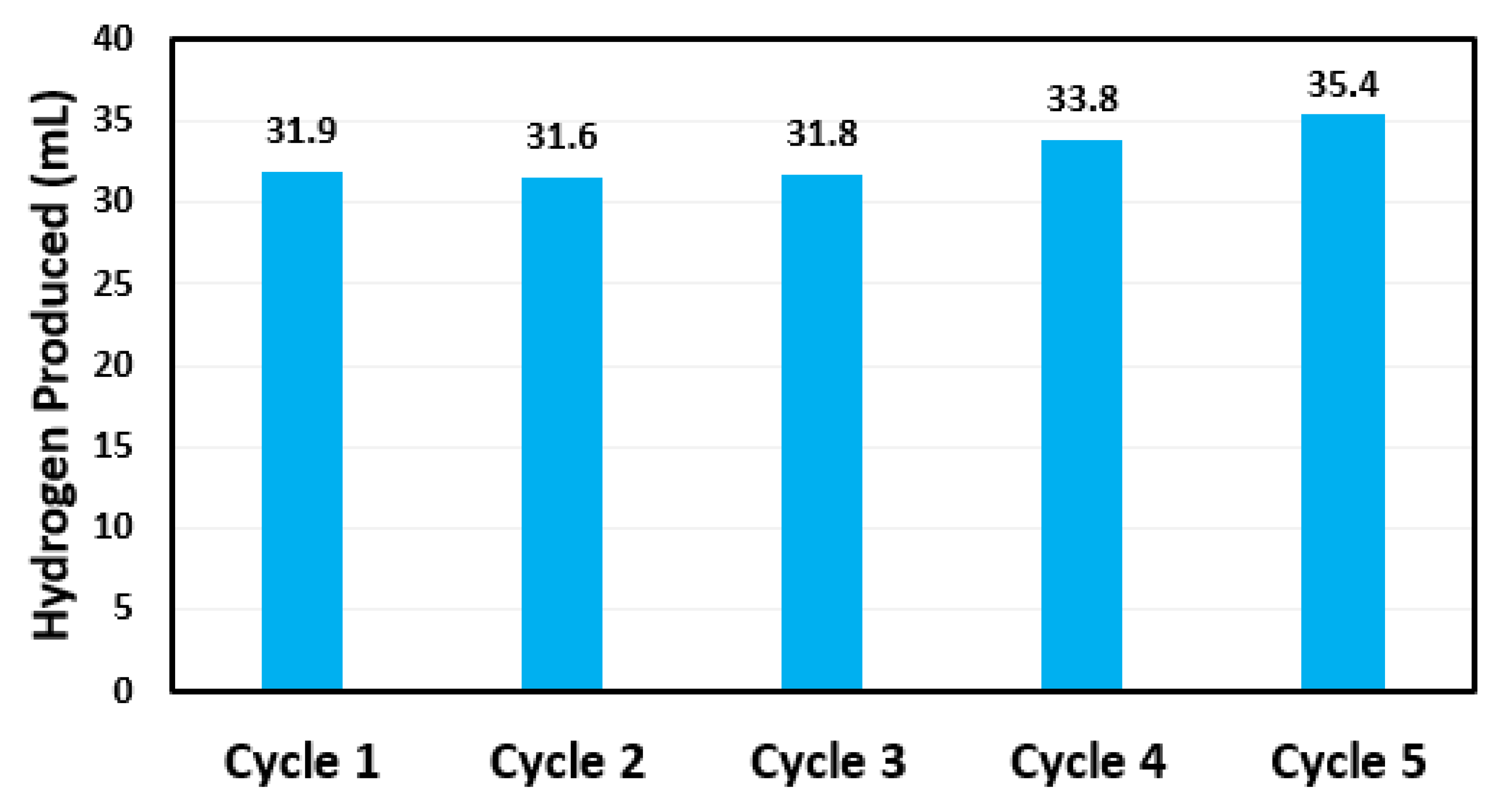
3.2. Concentration Effect on Catalytic Ability
3.3. Effect of pH on Catalytic Ability
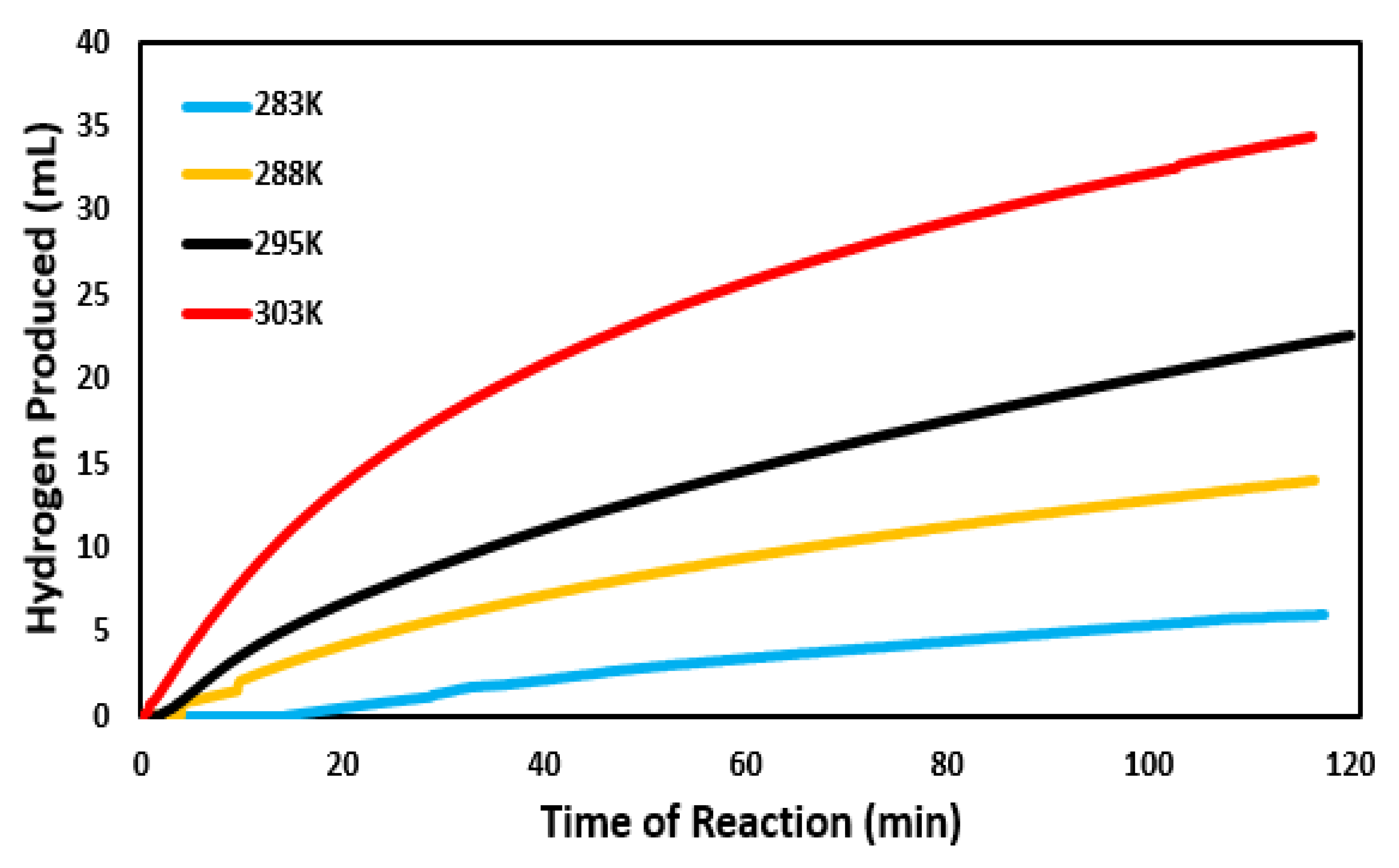
3.4. Activation Energy and Effect of Temperature on Catalytic Ability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahmoud, M.E.; Khalifa, M.; Wakeel, Y.E.; Header, M.; Abdel-Fattah, T.M. Engineered nano-magnetic iron oxide-urea-activated carbon nanolayer sorbent for potential removal of uranium (VI) from aqueous solution. J. Nucl. Mater. 2017, 487, 13–22. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Ahmed, S.B.; Osman, M.M.; Abdel-Fattah, T.M. A novel composite of nanomagnetite-immobilized-baker’s yeast on the surface of activated carbon for magnetic solid phase extraction of Hg(II). Fuel 2015, 139, 614–621. [Google Scholar] [CrossRef]
- Ebrahim, S.; Labeb, M.; Abdel-Fattah, T.; Soliman, M. CdTe quantum dots capped with different stabilizing agents for sensing of ochratoxin a. J. Lumin. 2017, 182, 154–159. [Google Scholar] [CrossRef]
- Dushatinski, T.; Abdel-Fattah, T.M. Carbon nanotube composite mesh films with tunable optoelectronic performance. ECS J. Solid State Sci. Technol. 2015, 4, M1–M5. [Google Scholar] [CrossRef]
- Abdel-Fattah, T.; Wixtrom, A.; Zhang, K.; Baumgart, H. Highly uniform self-assembly gold nanoparticles over high surface area dense ZnO nanorod arrays as novel surface catalysts. ECS J. Solid State Sci. Technol. 2014, 3, M61–M64. [Google Scholar] [CrossRef]
- Saad, L.; Feteha, M.Y.; Ebrahim, S.; Soliman, M.; Abdel-Fattah, T. Dye sensitized solar cell based on polyaniline-carbon nanotubes/graphite composite. ECS J. Solid State Sci. Technol. 2014, 3, M55–M60. [Google Scholar] [CrossRef]
- Abdel-Fattah, T.M.; Wixtrom, A. Catalytic reduction of 4-nitrophenol using gold nanoparticles supported on carbon nanotubes. ECS J. Solid State Sci. Technol. 2014, 3, M18–M20. [Google Scholar] [CrossRef]
- Ebrahim, S.; El-Raey, R.; Hefnawy, A.; Ibrahim, H.; Soliman, M.; Abdel-Fattah, T.M. Electrochemical sensor based on polyaniline nanofibers/single wall carbon nanotubes composite for detection of malathion. Synth. Met. 2014, 190, 13–19. [Google Scholar] [CrossRef]
- Huff, C.; Dushatinski, T.; Barzanjii, A.; Abdel-Fattah, N.; Barzanjii, K.; Abdel-Fattah, T.M. Pretreatment of gold nanoparticle multi-walled carbon nanotube composites for catalytic activity toward hydrogen generation reaction. ECS J. Solid State Sci. Technol. 2017, 6, M69–M71. [Google Scholar] [CrossRef]
- Quach, Q.; Biehler, E.; Elzamzami, A.; Huff, C.; Long, J.M.; Abdel-Fattah, T.M. Catalytic activity of beta-cyclodextrin-gold nanoparticles network in hydrogen evolution reaction. Catalysts 2021, 11, 118. [Google Scholar] [CrossRef]
- Huff, C.; Dushatinski, T.; Abdel-Fattah, T.M. Gold nanoparticle/multi-walled carbon nanotube composite as novel catalyst for hydrogen evolution reactions. Int. J. Hydrog. Energy 2017, 42, 18985–18990. [Google Scholar] [CrossRef]
- Huff, C.; Long, J.; Aboulatta, A.; Heyman, A.; Abdel-Fattah, T. Silver nanoparticle/multi-walled carbon nanotube composite as catalyst for hydrogen production. ECS J. Solid State Sci. Technol. 2017, 6, M115–M118. [Google Scholar] [CrossRef]
- Ye, X.R.; Lin, Y.; Wai, C.M. Decorating catalytic palladium nanoparticles on carbon nanotubes in supercritical carbon dioxide. Chem. Commun. 2003, 642–643. [Google Scholar] [CrossRef]
- Corma, A.; Garcia, H.; Leyva, A. Catalytic activity of palladium supported on single wall carbon nanotubes compared to palladium supported on activated carbon: Study of the Heck and Suzuki couplings, aerobic alcohol oxidation and selective hydrogenation. J. Mol. Catal. A Chem. 2005, 230, 97–105. [Google Scholar] [CrossRef]
- Li, Y.; Hong, X.M.; Collard, D.M.; El-Sayed, M.A. Suzuki cross-coupling reactions catalyzed by palladium nanoparticles in aqueous solution. Org. Lett. 2000, 2, 2385–2388. [Google Scholar] [CrossRef]
- Patel, N.; Patton, B.; Zanchetta, C.; Fernandes, R.; Guella, G.; Kale, A.; Miotello, A. Pd-C poweder and thin film catalysts for hydrogen production by hydrolysis of sodium borohydride. Int. J. Hydrog. Energy 2008, 33, 287–292. [Google Scholar] [CrossRef]
- Pena-Alonso, R.; Sicurelli, A.; Callone, E.; Caturan, G.; Raj, R. A picoscale catalyst for hydrogen generation from NaBH4 for fuel cells. J. Power Sources 2007, 165, 315–323. [Google Scholar] [CrossRef]
- Chandra, M.; Xu, Q. A high-performance hydrogen generation system: Transition metal-catalyzed dissociation and hydrolysis of ammonia-borane. J. Power Sources 2006, 156, 190–194. [Google Scholar] [CrossRef]
- Liu, B.; Li, Z. A review: Hydrogen generation from borohydride hydrolysis reaction. J. Power Sources 2009, 187, 527–534. [Google Scholar] [CrossRef]
- Liu, B.H.; Li, Z.P.; Suda, S. Nickel- and Cobalt-based catalysts for hydrogen generation by hydrolysis of borohydride. J. Alloys Compd. 2006, 415, 288–293. [Google Scholar] [CrossRef]
- Oezkar, S.; Zahmakiran, M. hydrogen generation from hydrolysis of sodium borohydride using Ru (0) nanoclusters as catalysts. J. Alloys Compd. 2005, 404–406, 728–731. [Google Scholar] [CrossRef]
- Hua, D.; Hanxi, Y.; Xinping, A.; Chuasin, C. Hydrogen production from catalytic hydrolysis of sodium borohydride solution using nickel boride catalyst. Int. J. Hydrog. Energy 2003, 28, 1095–1100. [Google Scholar] [CrossRef]
- Schlesinger, H.; Brown, H.; Finholt, A.; Gilbreath, J.; Hoekstra, H.; Hyde, E. Sodium borohydride, its hydrolysis and its use as a reducing agent and in the generation of hydrogen. J. Am. Chem. Soc. 1952, 75, 215–219. [Google Scholar] [CrossRef]
- Ingersoll, J.; Mani, N.; Thenmozhiyal, J.; Muthaiah, A. A Catalytic Hydrolysis of Sodium Borohydride by a Novel Nickel-cobalt-boride Catalyst. J. Power Sources 2007, 173, 450–457. [Google Scholar] [CrossRef]
- Shang, Y.; Chen, R.; Jiang, G. Kinetic Study of NaBH4 Hydrolysis over carbon-supported ruthenium. Int. J. Hydrog. Energy 2008, 33, 6719–6726. [Google Scholar] [CrossRef] [Green Version]
- Levy, A.; Brown, J.B.; Lyons, C.L. Catalyzed hydrolysis of sodium borohydride. Ind. Engg. Chem. 1960, 3, 211–214. [Google Scholar] [CrossRef]
- Daniel, M.; Astruc, D. Gold Nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajadi, S.M.; Honarmand, E.; Maham, M. Preparation of palladium nanoparticles using euphorbia thymifolia L. leaf extract and evaluation of catalytic activity in the ligand-free Stille and Hiyama cross-coupling reactions in water. New J. Chem. 2015, 39, 4745–4752. [Google Scholar] [CrossRef]
- Ramalingam, V.; Raja, S.; Harshavardhan, M. In-situ one step synthesis of polymer functionalized palladium nanoparticles: An efficient anticancer agent against breast cancer. Dalton Trans. 2020, 49, 3510–3518. [Google Scholar] [CrossRef]
- Shaik, M.R.; Ali, Z.J.Q.; Khan, M.; Kuniyil, M.; Assal, M.E.; Alkhathlan, H.Z.; Al-Warthan, A.; Siddiqui, M.R.H.; Khan, M.; Adil, S.F. Green synthesis and characterization of palladium nanoparticles using origanum vulgare L. extract and their catalytic activity. Molecules 2017, 22, 165. [Google Scholar] [CrossRef]
- Szejtli, J. Introduction and General Overview of Cyclodextrin Chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef]
- Chi, T.T.K.; Le, N.T.; Hien, B.T.T.; Trung, D.Q.; Liem, N.Q. Preparation of SERS substrates for the detection of organic molecules at low concentration. Commun. Phys. 2016, 26, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Vorontsov, A.V.; Tsybulya, S.V. Influence of nanoparticles size on XRD Patterns for small Monodisperse nanoparticles of Cu0 and TiO2 anatase. Ind. Eng. Chem. Res. 2018, 57, 2526–2536. [Google Scholar] [CrossRef]
- Navaladian, S.; Viswanathan, B.; Varadarajan, T.K.; Viswanath, R.P. A rapid synthesis of oriented palladium nanoparticles by UV irradiation. Nanoscale Res. Lett. 2009, 4, 181–186. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Kumar, C.S.S.R.; Hormes, J. Synthesis of palladium nanoparticles using a continuous flow polymeric micro reactor. J. Nanosci. Nanotech. 2004, 4, 788–793. [Google Scholar] [CrossRef]
- Mahmood, A.; Ahmad, M.; Sarfraz, R.M.; Minhas, M.U. β-CD based hydrogel microparticulate system to improve the solubility of acyclovir: Optimization through in-vitro, in-vivo and toxicological evaluation. J. Drug Deliv. Sci. Technol. 2016, 36, 75–88. [Google Scholar] [CrossRef]
- Zahmakiran, M.; Ozkar, M. Water dispersible acetate stabilized ruthenium (0) nanoclusters as catalyst for hydrogen generation reaction of sodium borohydride. J. Mol. Catal. 2006, 258, 95–103. [Google Scholar] [CrossRef]
- Metin, O.; Ozkar, S. Hydrogen generation from the hydrolysis of sodium borohydride by using water dispersible, hydrogen phosphate-stabilized nickel (0) nanoclusters as catalyst. Int. J. Hydrog. Energy 2007, 32, 1707–1715. [Google Scholar] [CrossRef]
- Liu, C.H.; Chen, B.H.; Hsueh, C.L.; Ku, J.R.; Jeng, M.S.; Tsau, F. Hydrogen generation from hydrolysis of sodium borohydride using Ni-Ru nanocomposite as catalysts. Int. J. Hydrog. Energy 2009, 34, 2153–2163. [Google Scholar] [CrossRef]
- Chen, B.; Chen, S.; Bandal, H.A.; Appiah-Ntiamoah, R.; Jadhav, A.R.; Kim, H. Cobalt nanoparticles supported on magnetic core-shell structured carbon as a highly efficient catalyst for hydrogen generation from NaBH4 hydrolysis. Int. J. Hydrog. Energy 2018, 43, 9296–9306. [Google Scholar] [CrossRef]
- Chen, Y.; Jin, H. Fabrication of amorphous Co–Cr–B and catalytic sodium borohydride hydrolysis for hydrogen generation. J. Mater. Res. 2020, 35, 281–288. [Google Scholar] [CrossRef]
- Loghmani, M.H.; Shojaei, A.F.; Khakzad, M. Hydrogen generation as a clean energy through hydrolysis of sodium borohydride over Cu-Fe-B nano powders: Effect of polymers and surfactants. Energy 2017, 126, 830–840. [Google Scholar] [CrossRef]
- Hashimi, A.S.; Nohan, M.A.; Chin, S.X.; Khiew, P.S. Copper Nanowires as Highly Efficient and Recyclable Catalyst for Rapid Hydrogen Generation from Hydrolysis of Sodium Borohydride. Nanomaterials 2020, 10, 1153. [Google Scholar] [CrossRef]
- Balbay, A.; Saka, C. Effect of phosphoric acid addition on the hydrogen production from hydrolysis of NaBH4 with Cu based catalyst. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 40, 794–804. [Google Scholar] [CrossRef]
- Huff, C.; Quach, Q.; Long, J.M.; Abdel-Fattah, T.M. Nanocomposite catalyst derived from ultrafine platinum nanoparticles and carbon nanotubes for hydrogen generation. ECS J. Solid State Sci. Technol. 2020, 9, 101008. [Google Scholar] [CrossRef]
- Huff, C.; Biehler, E.; Quach, Q.; Long, J.M.; Abdel-Fattah, T.M. Synthesis of highly dispersive platinum nanoparticles and their application in a hydrogen generation reaction. Colloids Surf. A Physicochem. Eng. Asp. 2020, 610, 125734. [Google Scholar] [CrossRef]
- Huff, C.; Long, J.M.; Abdel-Fattah, T.M. Beta-cyclodextrin-assisted synthesis of silver nanoparticle network and its application in a hydrogen generation reaction. Catalyst 2020, 10, 1014. [Google Scholar] [CrossRef]





| Catalyst | Ea (kJ/mol) | T (K) | Reference |
|---|---|---|---|
| AuNPs | 54.7 | 283-303 | [10] |
| Au/MWCNTs | 21.1 | 273–303 | [11] |
| Ag/MWCNTs | 44.5 | 273–303 | [12] |
| Pd/C | 28.0 | 283–323 | [16] |
| Pt-Pd-CNTs | 19.0 | 302–332 | [17] |
| Ru nanoclusters | 41.0 | 298–318 | [37] |
| Ni nanoclusters | 54.0 | 298–318 | [38] |
| Ni-Ru nanoclusters | 52.7 | 288–348 | [39] |
| Co/Fe3O4@C | 49.2 | 288–328 | [40] |
| Co–Cr–B | 44.5 | 293–333 | [41] |
| Cu-Fe-B | 57 | 285–333 | [42] |
| CuNWs | 42.5 | 298–333 | [43] |
| Cu based catalyst | 61.2 | 293–313 | [44] |
| PtMWCNT | 46.2 | 283–303 | [45] |
| PtNPs | 39.2 | 283–303 | [46] |
| AgNPs | 50.3 | 273–303 | [47] |
| PdNPs | 58.9 | 273–303 | This Work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biehler, E.; Quach, Q.; Huff, C.; Abdel-Fattah, T.M. Organo-Nanocups Assist the Formation of Ultra-Small Palladium Nanoparticle Catalysts for Hydrogen Evolution Reaction. Materials 2022, 15, 2692. https://doi.org/10.3390/ma15072692
Biehler E, Quach Q, Huff C, Abdel-Fattah TM. Organo-Nanocups Assist the Formation of Ultra-Small Palladium Nanoparticle Catalysts for Hydrogen Evolution Reaction. Materials. 2022; 15(7):2692. https://doi.org/10.3390/ma15072692
Chicago/Turabian StyleBiehler, Erik, Qui Quach, Clay Huff, and Tarek M. Abdel-Fattah. 2022. "Organo-Nanocups Assist the Formation of Ultra-Small Palladium Nanoparticle Catalysts for Hydrogen Evolution Reaction" Materials 15, no. 7: 2692. https://doi.org/10.3390/ma15072692
APA StyleBiehler, E., Quach, Q., Huff, C., & Abdel-Fattah, T. M. (2022). Organo-Nanocups Assist the Formation of Ultra-Small Palladium Nanoparticle Catalysts for Hydrogen Evolution Reaction. Materials, 15(7), 2692. https://doi.org/10.3390/ma15072692







