Adsorption of Sodium Diclofenac in Functionalized Palygoskite Clays
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Acid and Organic-Functionalization of Raw Palygorskite
2.3. Characterizations
2.4. Experimental Design
2.5. Kinetics and Equilibrium Models
3. Results and Discussion
3.1. Characterization of the Raw and Functionalized Palygorskite
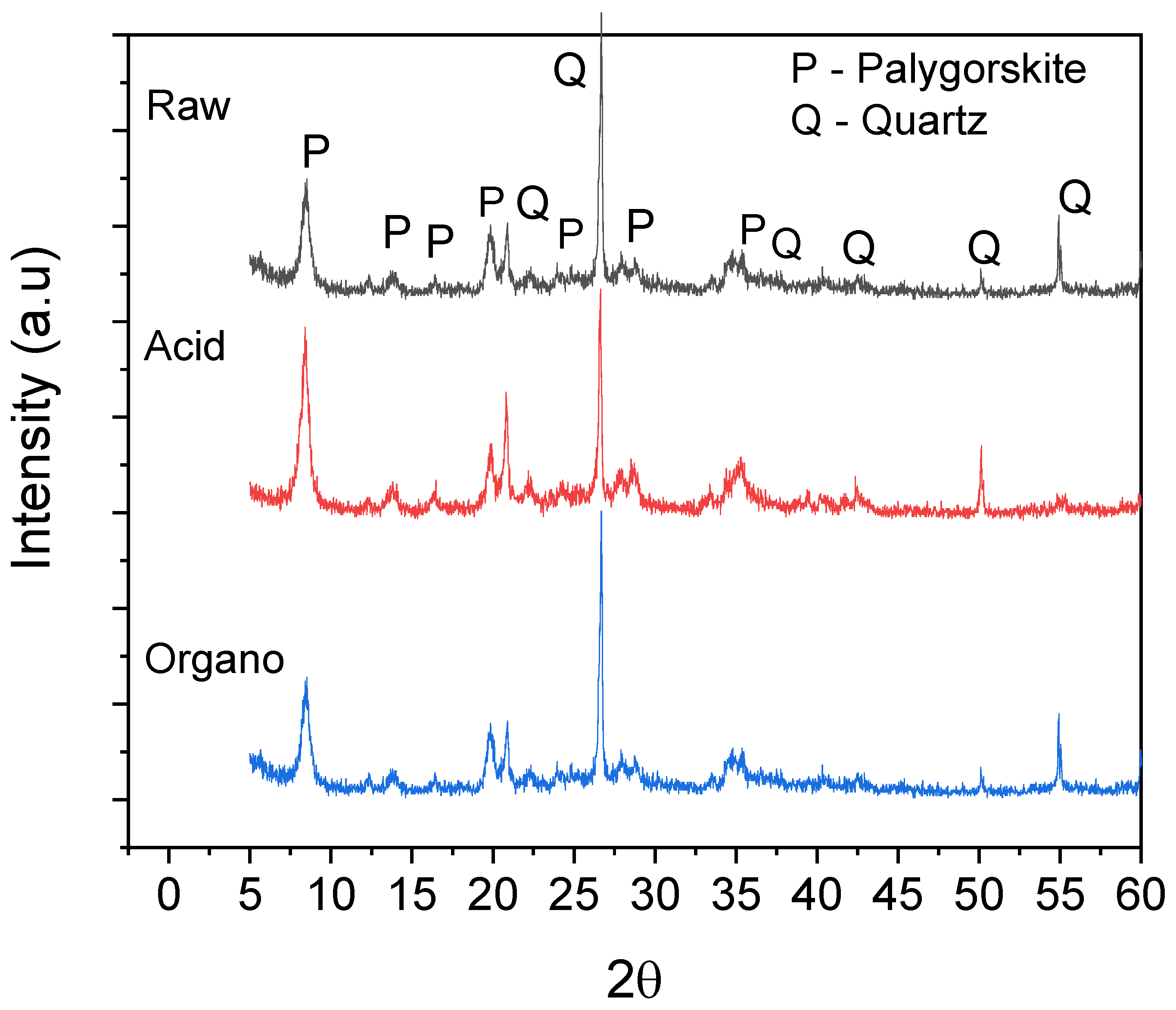
3.1.1. X-ray Diffraction
3.1.2. X-ray Fluorescence Chemical Composition
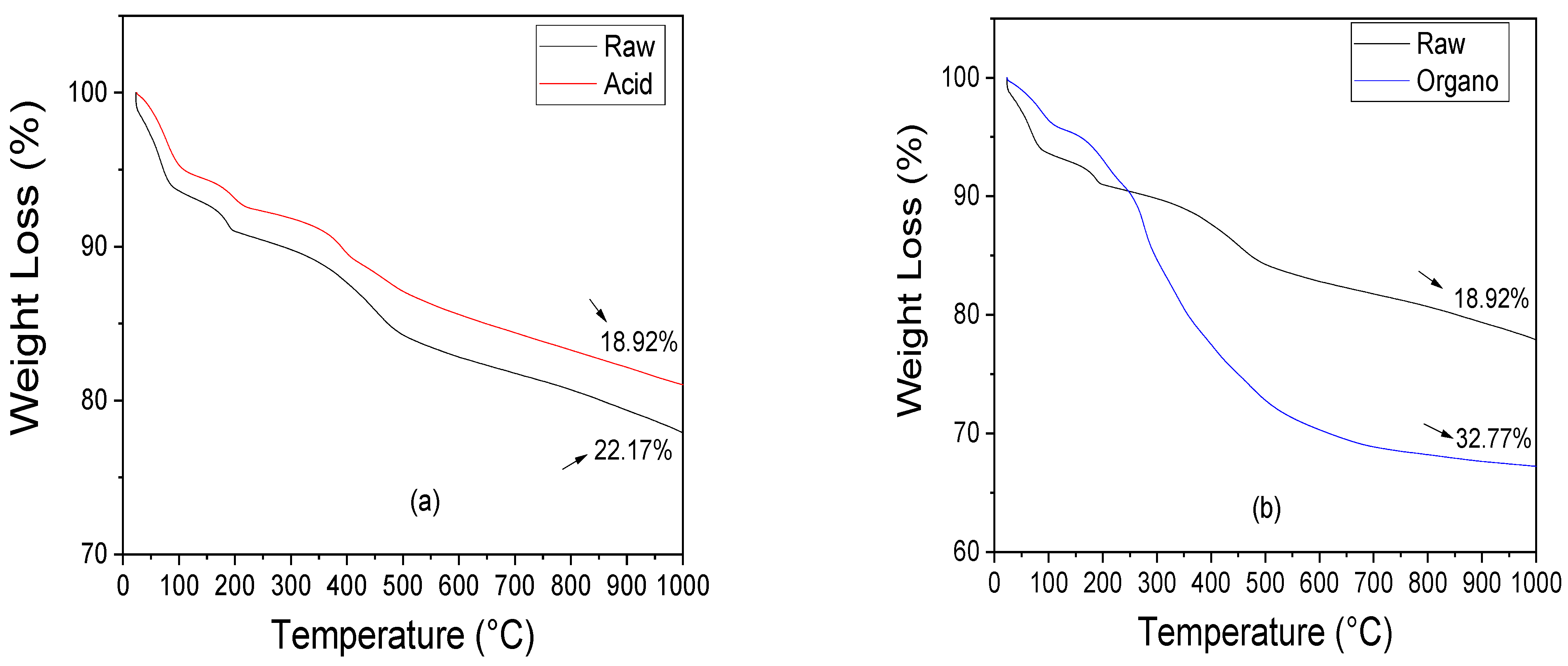
3.1.3. Thermal Behavior
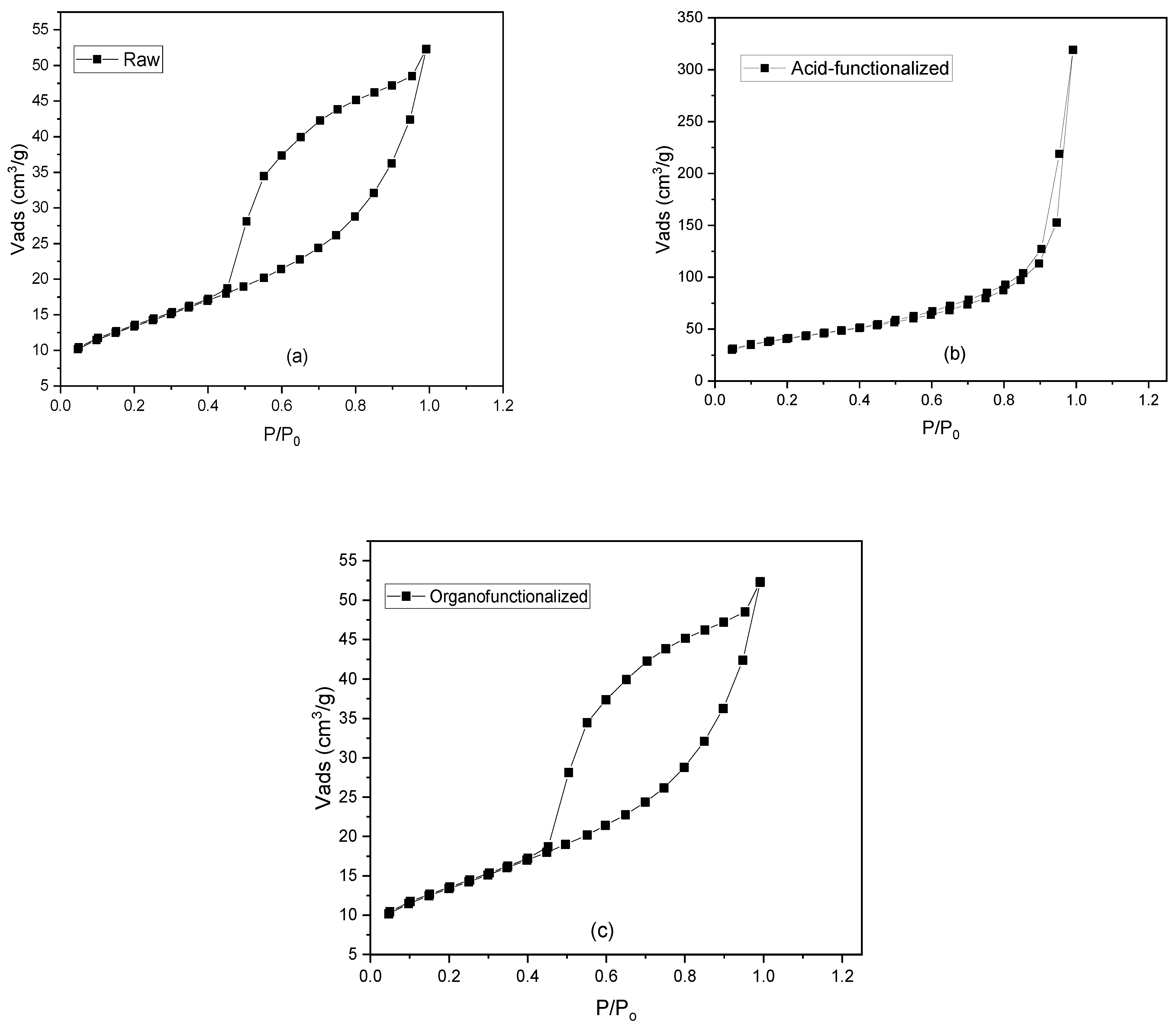
3.1.4. Specific Surface Area by N2 adsorption (BET)
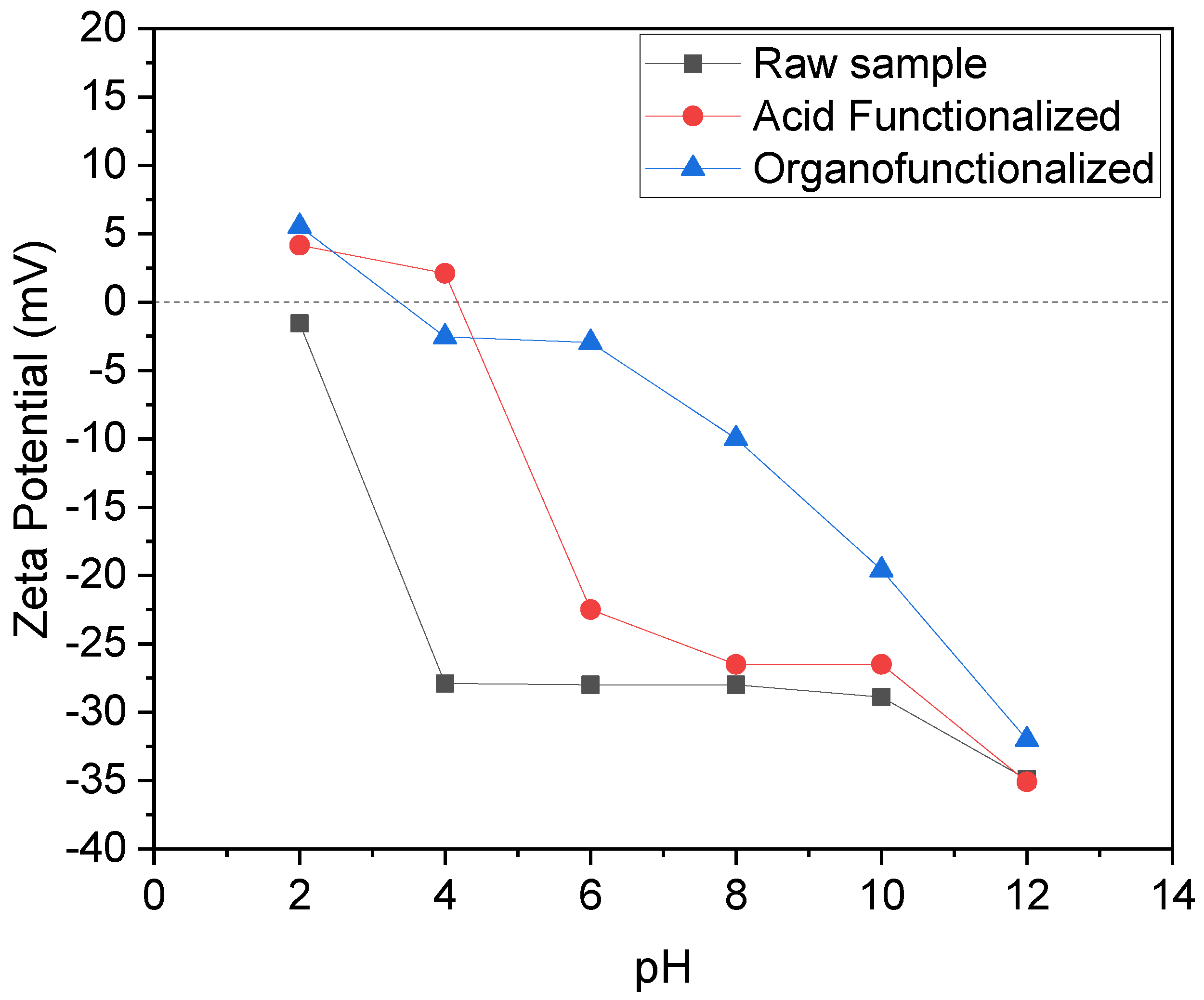
3.1.5. Zeta Potential
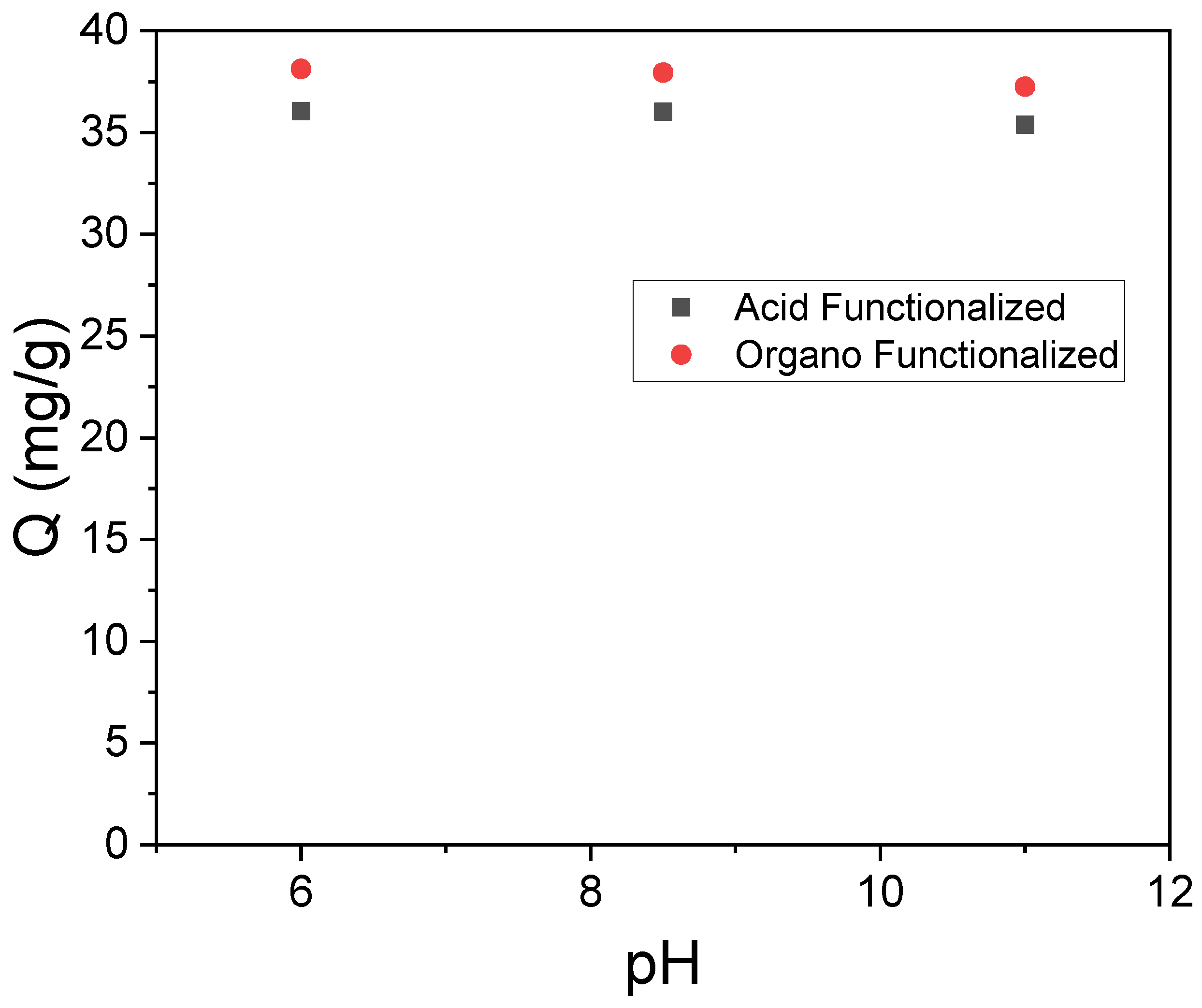
3.2. Influence of pH
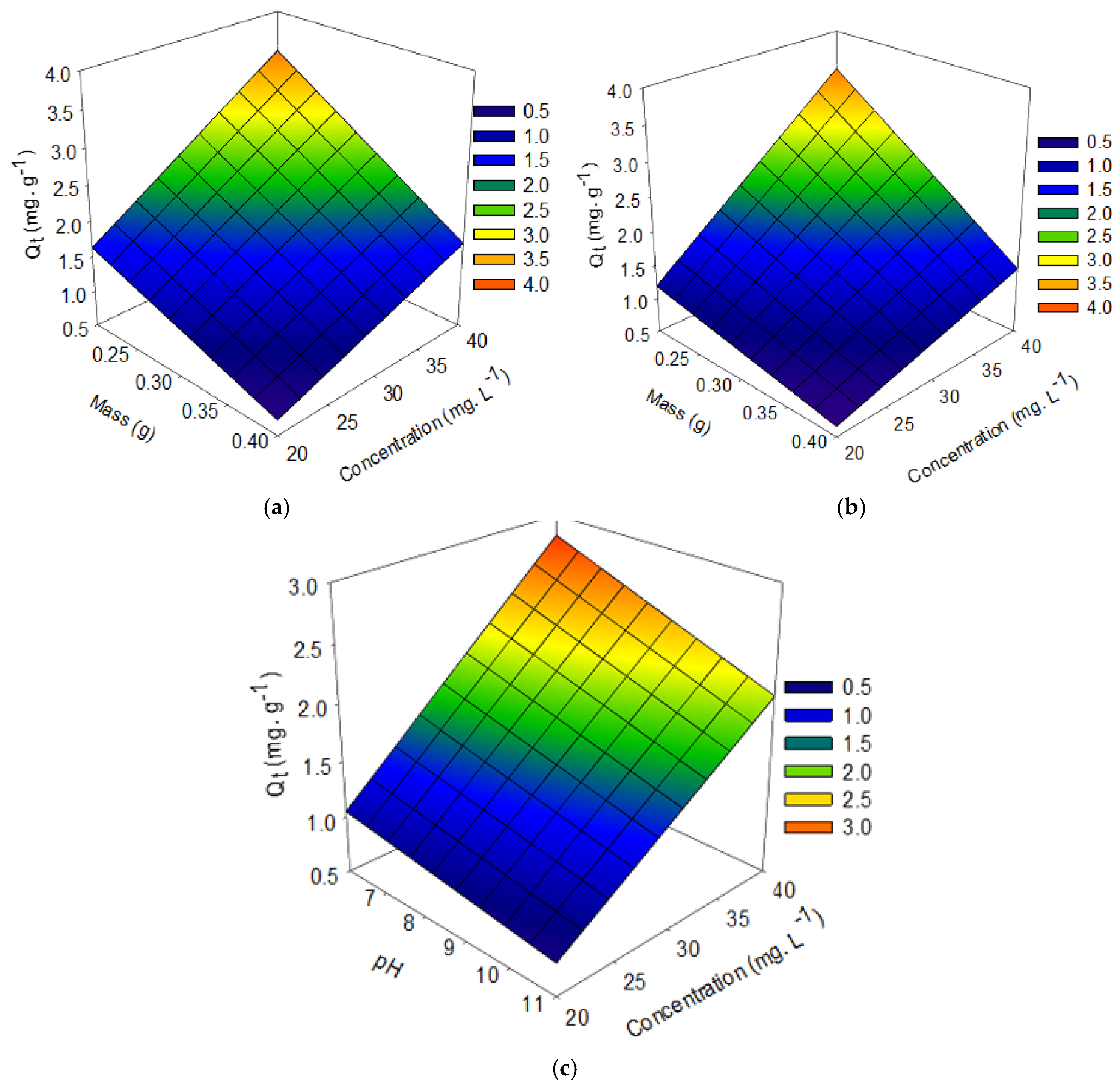
3.3. Experimental Design of the Factorial Type of Functionalized Palygorskite Clay
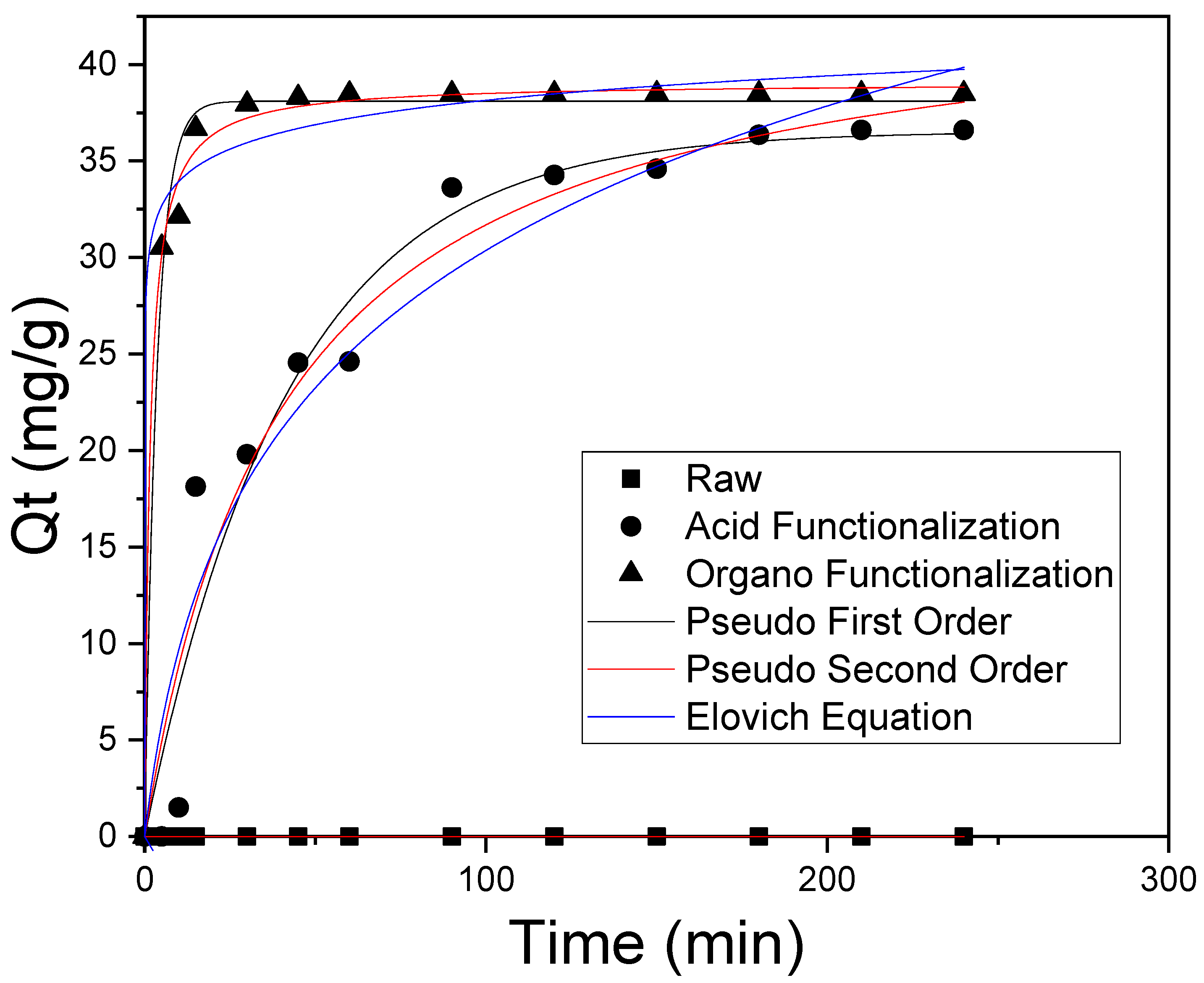
3.4. Adsorption Kinetics
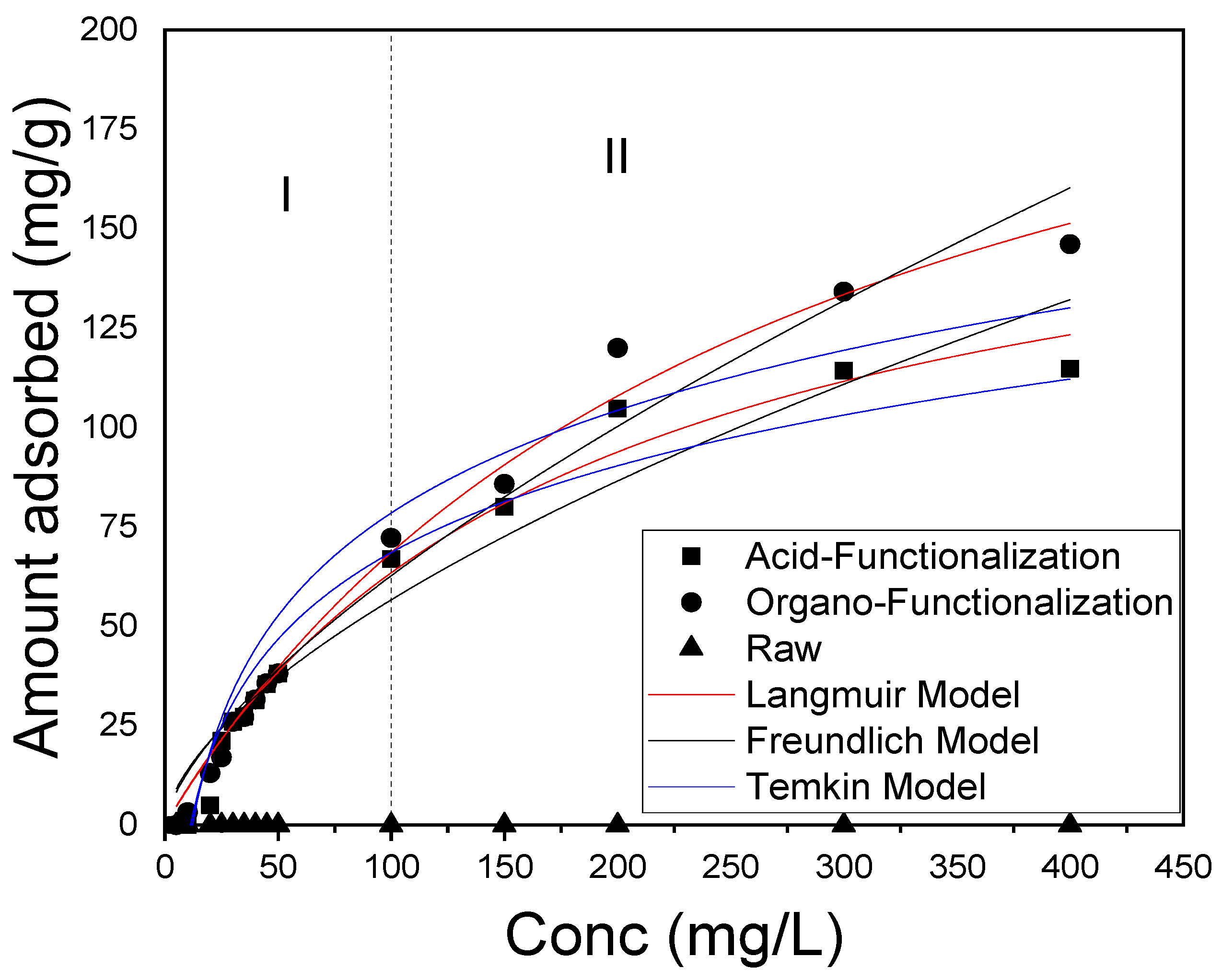
3.5. Adsorption Isotherms
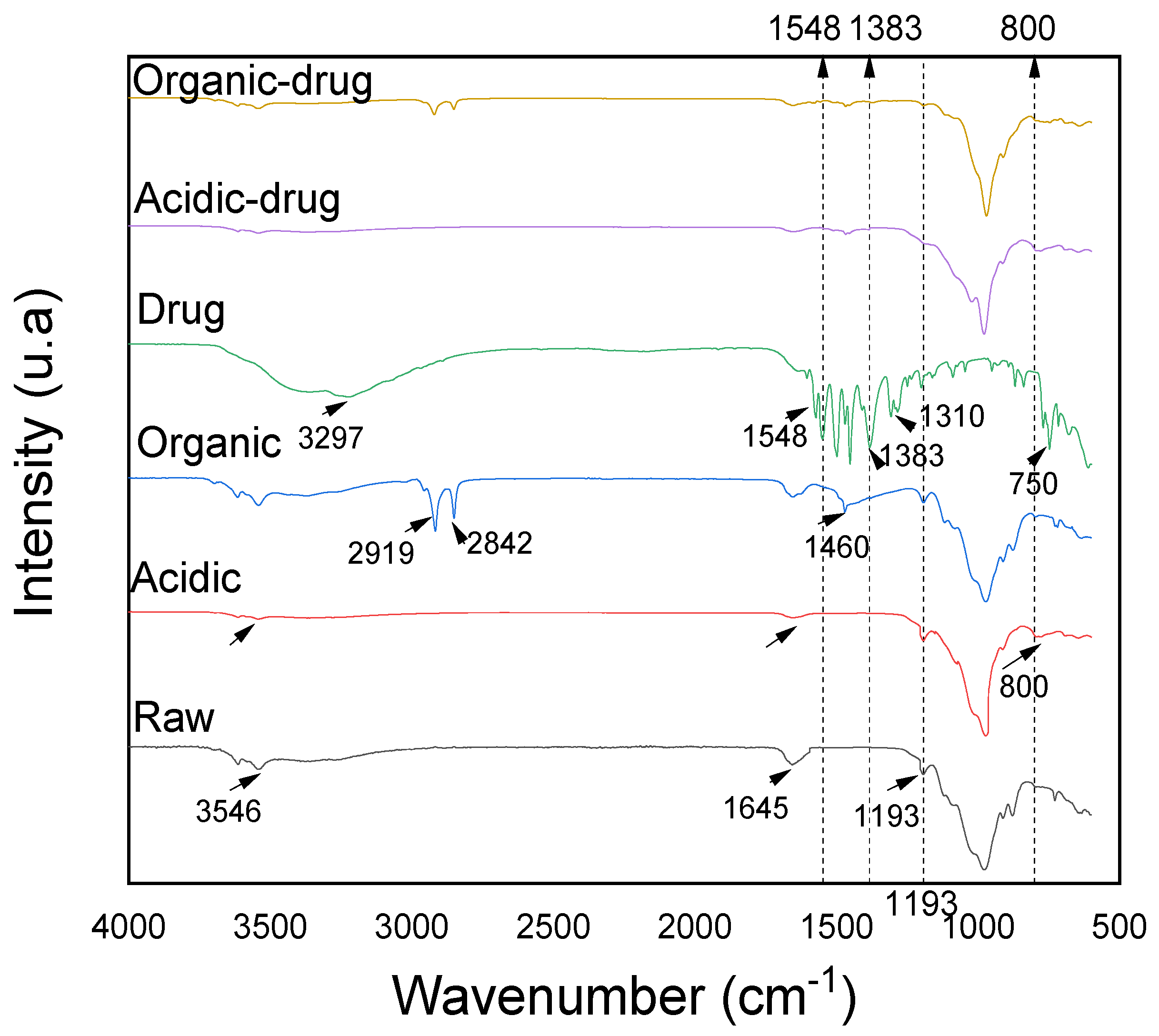
3.6. Characterization after Adsorption by FTIR
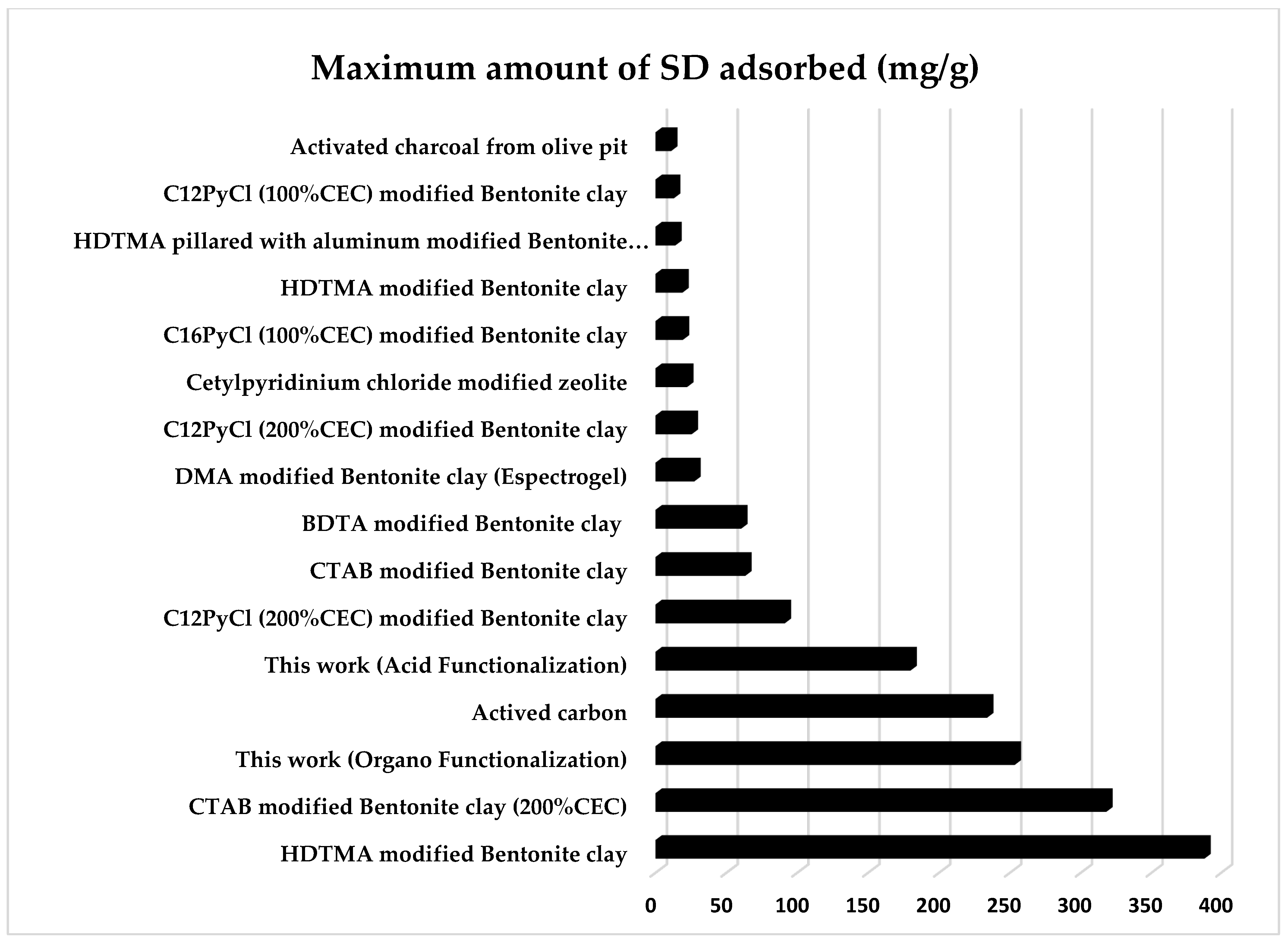
3.7. Comparison with Other Adsorbents of Sodium Diclofenac
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simazaki, D.; Kubota, R.; Suzuki, T.; Akiba, M.; Nishimura, T.; Kunikane, S. Occurrence of selected pharmaceuticals at drinking water purification plants in Japan and implications for human health. Water Res. 2015, 76, 187–200. [Google Scholar] [CrossRef]
- Starling, M.C.V.M.; Amorim, C.C.; Leão, M.M.D. Occurrence, control and fate of contaminants of emerging concern in environmental compartments in Brazil. J. Hazard. Mater. 2019, 372, 17–36. [Google Scholar] [CrossRef] [PubMed]
- Praveena, S.M.; Mohd Rashid, M.Z.; Mohd Nasir, F.A.; Sze Yee, W.; Aris, A.Z. Occurrence and potential human health risk of pharmaceutical residues in drinking water from Putrajaya (Malaysia). Ecotoxicol. Environ. Saf. 2019, 180, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Comber, S.D.W.; Gardner, M.J.; Ellor, B. Seasonal variation of contaminant concentrations in wastewater treatment works effluents and river waters. Environ. Technol. 2020, 41, 2716–2730. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Sui, Q.; Lyu, S.; Zhao, W.; Cao, X.; Wang, J.; Yu, G. Do high levels of PPCPs in landfill leachates influence the water environment in the vicinity of landfills? A case study of the largest landfill in China. Environ. Int. 2020, 135, 105404. [Google Scholar] [CrossRef]
- Biel-Maeso, M.; Baena-Nogueras, R.M.; Corada-Fernández, C.; Lara-Martín, P.A. Occurrence, distribution and environmental risk of pharmaceutically active compounds (PhACs) in coastal and ocean waters from the Gulf of Cadiz (SW Spain). Sci. Total Environ. 2018, 612, 649–659. [Google Scholar] [CrossRef]
- Reichert, G.; Hilgert, S.; Fuchs, S.; Azevedo, J.C.R. Emerging contaminants and antibiotic resistance in the different environmental matrices of Latin America. Environ. Pollut. 2019, 255, 113140. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, T.; Zhang, Y.; Ge, F.; Steel, R.M.; Sun, L. Advances in technologies for pharmaceuticals and personal care products removal. J. Mater. Chem. A 2017, 5, 12001–12014. [Google Scholar] [CrossRef]
- De Oliveira, T.; Guégan, R.; Thiebault, T.; LeMilbeau, C.; Muller, F.; Teixeira, V.; Giovanela, M.; Boussafir, M. Adsorption of diclofenac onto organoclays: Effects of surfactant and environmental (pH and temperature) conditions. J. Hazard. Mater. 2017, 323, 558–566. [Google Scholar] [CrossRef]
- Lima, D.R.S.; Tonucci, M.C.; Libânio, M.; de Aquino, S.F. Fármacos e desreguladores endócrinos em águas Brasileiras: Ocorrência e técnicas de remoção. Eng. Sanit. Ambient. 2017, 22, 1043–1054. [Google Scholar] [CrossRef] [Green Version]
- Montagner, C.C.; Sodré, F.F.; Acayaba, R.D.; Vidal, C.; Campestrini, I.; Locatelli, M.A.; Pescara, I.C.; Albuquerque, A.F.; Umbuzeiro, G.A.; Jardim, W.F. Ten years-snapshot of the occurrence of emerging contaminants in drinking, surface and ground waters and wastewaters from São Paulo State, Brazil. J. Braz. Chem. Soc. 2019, 30, 614–632. [Google Scholar] [CrossRef]
- Scheurell, M.; Franke, S.; Shah, R.M.; Hühnerfuss, H. Occurrence of diclofenac and its metabolites in surface water and effluent samples from Karachi, Pakistan. Chemosphere 2009, 77, 870–876. [Google Scholar] [CrossRef]
- Jia, T.Z.; Lu, J.P.; Cheng, X.Y.; Xia, Q.C.; Cao, X.L.; Wang, Y.; Xing, W.; Sun, S.P. Surface enriched sulfonated polyarylene ether benzonitrile (SPEB) that enhances heavy metal removal from polyacrylonitrile (PAN) thin-film composite nanofiltration membranes. J. Memb. Sci. 2019, 580, 214–223. [Google Scholar] [CrossRef]
- Zangeneh, H.; Zinatizadeh, A.A.L.; Habibi, M.; Akia, M.; Hasnain Isa, M. Photocatalytic oxidation of organic dyes and pollutants in wastewater using different modified titanium dioxides: A comparative review. J. Ind. Eng. Chem. 2015, 26, 1–36. [Google Scholar] [CrossRef]
- Zhou, F.; Cheng, Y.; Gan, L.; Chen, Z.; Megharaj, M.; Naidu, R. Burkholderia vietnamiensis C09V as the functional biomaterial used to remove crystal violet and Cu(II). Ecotoxicol. Environ. Saf. 2014, 105, 1–6. [Google Scholar] [CrossRef]
- Karim, Z.; Mathew, A.P.; Grahn, M.; Mouzon, J.; Oksman, K. Nanoporous membranes with cellulose nanocrystals as functional entity in chitosan: Removal of dyes from water. Carbohydr. Polym. 2014, 112, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Baddouh, A.; El Ibrahimi, B.; Rguitti, M.M.; Mohamed, E.; Hussain, S.; Bazzi, L. Electrochemical removal of methylene bleu dye in aqueous solution using Ti/RuO2–IrO2 and SnO2 electrodes. Sep. Sci. Technol. 2020, 55, 1852–1861. [Google Scholar] [CrossRef]
- França, D.B.; Trigueiro, P.; Silva Filho, E.C.; Fonseca, M.G.; Jaber, M. Monitoring diclofenac adsorption by organophilic alkylpyridinium bentonites. Chemosphere 2020, 242, 125109. [Google Scholar] [CrossRef]
- Haouche, R.; Innocent, C.; Akretche, D.E. Concentration of diclofenac sodium using the nanofiltration combined with laccase degradation from trametes versicolor. Arab. J. Sci. Eng. 2018, 43, 6181–6190. [Google Scholar] [CrossRef]
- Apopei, P.; Orha, C.; Popescu, M.I.; Lazau, C.; Manea, F.; Catrinescu, C.; Teodosiu, C. Diclofenac removal from water by photocatalysis-assisted filtration using activated carbon modified with N-doped TiO2. Process Saf. Environ. Prot. 2020, 138, 324–336. [Google Scholar] [CrossRef]
- Matamoros, V.; Salvadó, V. Evaluation of a coagulation/flocculation-lamellar clarifier and filtration-UV-chlorination reactor for removing emerging contaminants at full-scale wastewater treatment plants in Spain. J. Environ. Manag. 2013, 117, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Deng, S.; Huang, Q.; Nie, Y.; Wang, B.; Huang, J.; Yu, G. Regenerable granular carbon nanotubes/alumina hybrid adsorbents for diclofenac sodium and carbamazepine removal from aqueous solution. Water Res. 2013, 47, 4139–4147. [Google Scholar] [CrossRef] [PubMed]
- Pourzamani, H.; Mengelizadeh, N.; Hajizadeh, Y.; Mohammadi, H. Electrochemical degradation of diclofenac using three-dimensional electrode reactor with multi-walled carbon nanotubes. Environ. Sci. Pollut. Res. 2018, 25, 24746–24763. [Google Scholar] [CrossRef] [PubMed]
- De Queiroga, L.N.F.; França, D.B.; Rodrigues, F.; Santos, I.M.G.; Fonseca, M.G.; Jaber, M. Functionalized bentonites for dye adsorption: Depollution and production of new pigments. J. Environ. Chem. Eng. 2019, 7, 103333. [Google Scholar] [CrossRef]
- Farias, C.K.D.L.; Do Nascimento, K.K.R.; Vieira, F.F.; Almeida, M.M. De ADSORÇÃO de cobre presente em solução aquosa por adsorvente de casca de Laranja seco e modificado com hcl./copper adsorption present in aqueous solution by dry orange shell adsorbent and modified with hcl. Braz. J. Dev. 2020, 6, 102344–102355. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, P.; Wang, Y.; Wen, K.; Su, X.; Zhu, R.; He, H.; Xi, Y. Effect of acid activation of palygorskite on their toluene adsorption behaviors. Appl. Clay Sci. 2018, 159, 60–67. [Google Scholar] [CrossRef]
- Boukhalfa, N.; Djebri, N.; Boutahala, M.; History, A. Remediation of polluted water by diclofenac drug by adsorption technology article info abstract/resume. Alger. J. Environ. Sci. Technol. Ed. 2017, 3, 1–5. [Google Scholar]
- de Andrade, J.R.; Oliveira, M.F.; Canevesi, R.L.S.; Landers, R.; da Silva, M.G.C.; Vieira, M.G.A. Comparative adsorption of diclofenac sodium and losartan potassium in organophilic clay-packed fixed-bed: X-ray photoelectron spectroscopy characterization, experimental tests and theoretical study on DFT-based chemical descriptors. J. Mol. Liq. 2020, 312, 113427. [Google Scholar] [CrossRef]
- De Oliveira, T.; Guégan, R. Coupled organoclay/micelle action for the adsorption of diclofenac. Environ. Sci. Technol. 2016, 50, 10209–10215. [Google Scholar] [CrossRef]
- Ghemit, R.; Makhloufi, A.; Djebri, N.; Flilissa, A.; Zerroual, L.; Boutahala, M. Adsorptive removal of diclofenac and ibuprofen from aqueous solution by organobentonites: Study in single and binary systems. Groundw. Sustain. Dev. 2019, 8, 520–529. [Google Scholar] [CrossRef]
- Maia, G.S.; de Andrade, J.R.; da Silva, M.G.C.; Vieira, M.G.A. Adsorption of diclofenac sodium onto commercial organoclay: Kinetic, equilibrium and thermodynamic study. Powder Technol. 2019, 345, 140–150. [Google Scholar] [CrossRef]
- Martinez-Costa, J.I.; Leyva-Ramos, R.; Padilla-Ortega, E. Sorption of diclofenac from aqueous solution on an organobentonite and adsorption of cadmium on organobentonite saturated with diclofenac. Clays Clay Miner. 2018, 66, 515–528. [Google Scholar] [CrossRef]
- Sun, K.; Shi, Y.; Chen, H.; Wang, X.; Li, Z. Extending surfactant-modified 2:1 clay minerals for the uptake and removal of diclofenac from water. J. Hazard. Mater. 2017, 323, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, A.; Santarén, J.; Esteban-Cubillo, A.; Aparicio, P. Current industrial applications of palygorskite and sepiolite. In Developments in Clay Science, 1st ed.; Elsevier: Oxford, UK, 2011; pp. 281–298. [Google Scholar]
- Krekeler, M.P.S.; Guggenheim, S. Defects in microstructure in palygorskite-sepiolite minerals: A transmission electron microscopy (TEM) study. Appl. Clay Sci. 2008, 39, 98–105. [Google Scholar] [CrossRef]
- Câmara, A.B.F.; Sales, R.V.; Bertolino, L.C.; Furlanetto, R.P.P.; Rodríguez-Castellón, E.; de Carvalho, L.S. Novel application for palygorskite clay mineral: A kinetic and thermodynamic assessment of diesel fuel desulfurization. Adsorption 2020, 26, 267–282. [Google Scholar] [CrossRef]
- Dali Youcef, L.; Belaroui, L.S.; López-Galindo, A. Adsorption of a cationic methylene blue dye on an Algerian palygorskite. Appl. Clay Sci. 2019, 179, 105145. [Google Scholar] [CrossRef]
- Huang, D.; Zheng, Y.; Zhang, Z.; Quan, Q.; Qiang, X. Synergistic effect of hydrophilic palygorskite and hydrophobic zein particles on the properties of chitosan films. Mater. Des. 2020, 185, 108229. [Google Scholar] [CrossRef]
- Poussardin, V.; Paris, M.; Tagnit-Hamou, A.; Deneele, D. Potential for calcination of a palygorskite-bearing argillaceous carbonate. Appl. Clay Sci. 2020, 198, 105846. [Google Scholar] [CrossRef]
- Silva, V.C.; Araújo, M.E.B.; Rodrigues, A.M.; Cartaxo, J.M.; Menezes, R.R.; Neves, G.A. Adsorption behavior of acid-treated Brazilian palygorskite for cationic and anionic dyes removal from the water. Sustainability 2021, 13, 3954. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef]
- Yahia, Y.; García-Villén, F.; Djelad, A.; Belaroui, L.S.; Sanchez-Espejo, R.; Sassi, M.; López-Galindo, A.; Viseras, C. Crosslinked palygorskite-chitosan beads as diclofenac carriers. Appl. Clay Sci. 2019, 180, 105169. [Google Scholar] [CrossRef]
- Wu, J.; Ding, S.; Chen, J.; Zhou, S.; Ding, H. Preparation and drug release properties of chitosan/organomodified palygorskite microspheres. Int. J. Biol. Macromol. 2014, 68, 107–112. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, J.; Wang, A. Preparation and characterization of a novel pH-sensitive chitosan-g-poly (acrylic acid)/attapulgite/sodium alginate composite hydrogel bead for controlled release of diclofenac sodium. Carbohydr. Polym. 2009, 78, 731–737. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, W.; Wu, J.; Wang, A. Effect of attapulgite contents on release behaviors of a pH sensitive carboxymethyl cellulose-g-poly(acrylic acid)/attapulgite/sodium alginate composite hydrogel bead containing diclofenac. J. Appl. Polym. Sci. 2012, 124, 4424–4432. [Google Scholar] [CrossRef]
- Yang, H.; Wang, W.; Zhang, J.; Wang, A. Preparation, characterization, and drug-release behaviors of a pH-sensitive composite hydrogel bead based on guar gum, attapulgite, and sodium alginate. Int. J. Polym. Mater. Polym. Biomater. 2013, 62, 369–376. [Google Scholar] [CrossRef]
- Najafi, H.; Farajfaed, S.; Zolgharnian, S.; Mosavi Mirak, S.H.; Asasian-Kolur, N.; Sharifian, S. A comprehensive study on modified-pillared clays as an adsorbent in wastewater treatment processes. Process Saf. Environ. Prot. 2021, 147, 8–36. [Google Scholar] [CrossRef]
- Farajfaed, S.; Sharifian, S.; Asasian-Kolur, N.; Sillanpää, M. Granular silica pillared clay for levofloxacin and gemifloxacin adsorption from aqueous systems. J. Environ. Chem. Eng. 2021, 9, 106306. [Google Scholar] [CrossRef]
- Xi, R.; Zhang, X.; Jiang, J.; Xu, Q. Application of modified attapulgite clay desulfurizer in gasoline desulfurization. China Pet. Process. Petrochem. Technol. 2014, 16, 63. [Google Scholar]
- Han, J.; Liang, X.; Xu, Y.; Xu, Y. Removal of Cu2+ from aqueous solution by adsorption onto mercapto functionalized palygorskite. J. Ind. Eng. Chem. 2015, 23, 307–315. [Google Scholar] [CrossRef]
- Ghrab, S.; Eloussaief, M.; Lambert, S.; Bouaziz, S.; Benzina, M. Adsorption of terpenic compounds onto organo-palygorskite. Environ. Sci. Pollut. Res. 2017, 25, 18251–18262. [Google Scholar] [CrossRef]
- Fernandes, J.V.; Rodrigues, A.M.; Menezes, R.R.; de Araújo Neves, G. Adsorption of anionic dye on the acid-functionalized bentonite. Materials 2020, 13, 3600. [Google Scholar] [CrossRef]
- Oliveira, R.N.; Acchar, W.; Soares, G.D.A.; Barreto, L.S. The increase of surface area of a Brazilian palygorskite clay activated with sulfuric acid solutions using a factorial design. Mater. Res. 2013, 16, 924–928. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, A.L.; Luna, C.B.B.; de Farias, A.F.F.; de Medeiros, S.A.S.L.; Meneghetti, S.M.P.; Rodrigues, A.M.; de Melo Costa, A.C.F. From disposal to reuse: Production of sustainable fatty acid alkyl esters derived from residual oil using a biphasic magnetic catalyst. Sustainability 2020, 12, 10159. [Google Scholar] [CrossRef]
- Da Silva, A.L.; Farias, A.F.F.; Pontes, J.R.M.; Rodrigues, A.M.; de M. Costa, A.C.F. Synthesis of the ZnO-Ni0.5Zn0.5Fe2O4-Fe2O3 magnetic catalyst in pilot-scale by combustion reaction and its application on the biodiesel production process from oil residual. Arab. J. Chem. 2020, 13, 7665–7679. [Google Scholar] [CrossRef]
- Da Silva Filho, E.B.; Alves, M.C.M.; Da Motta, M.; Oliveira, E.H.D.C.; Brander, W. Study on the use of red mud for removal of dyes from textile effluents. Quim. Nova 2008, 31, 985–989. [Google Scholar] [CrossRef]
- Lagergren, S. About the Theory of so Called Adsorption of Soluble Substances. K. Sven. Vetensk. Handlingar. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Ben-Ali, S.; Jaouali, I.; Souissi-Najar, S.; Ouederni, A. Characterization and adsorption capacity of raw pomegranate peel biosorbent for copper removal. J. Clean. Prod. 2017, 142, 3809–3821. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 2002, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Sahu, O.; Singh, N. Significance of bioadsorption process on textile industry wastewater. In The Impact and Prospects of Green Chemistry for Textile Technology, 1st ed.; Elsevier: New Delhi, India, 2018; pp. 367–416. [Google Scholar]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phy. Chem. 1906, 57, 385–470. [Google Scholar]
- Temkin, M.J.; Pyzhev, V. Recent Modifications to Langmuir isotherms. Acta Physiochim. 1940, 12, 217–225. [Google Scholar]
- García-Rivas, J.; Suárez, M.; García-Romero, E.; Sánchez del Río, M. Presence of oriented fibers in palygorskite powders and its influence on X-Ray diffractograms. Appl. Clay Sci. 2020, 195, 105724. [Google Scholar] [CrossRef]
- Zhuang, G.; Zhang, Z.; Gao, J.; Zhang, X.; Liao, L. Influences of surfactants on the structures and properties of organo-palygorskite in oil-based drilling fluids. Microporous Mesoporous Mater. 2017, 244, 37–46. [Google Scholar] [CrossRef]
- Jiang, J.; Feng, L.; Gu, X.; Qian, Y.; Gu, Y.; Duanmu, C. Synthesis of zeolite A from palygorskite via acid activation. Appl. Clay Sci. 2012, 55, 108–113. [Google Scholar] [CrossRef]
- Cristina De Assis, T.; Cerqueda, M.; Gimenes Souza, C.; Jesus, T.C.; Marçano, G.B.; Mayara, K.; Simões, A.; Rodrigues, P.V.; Paula, R.; Furlanetto, P.; et al. Comparative analysis of palygorskite samples from different occurrences in guadalupe (Piauí, Brazil). J. Aerosp. Technol. Manag. 2019, 11, 62–65. [Google Scholar] [CrossRef]
- Santanna, V.C.; Silva, S.L.; Silva, R.P.; Castro Dantas, T.N. Use of palygorskite as a viscosity enhancer in salted water-based muds: Effect of concentration of palygorskite and salt. Clay Miner. 2020, 55, 48–52. [Google Scholar] [CrossRef]
- Xavier, K.C.M.; Santos, M.S.F.; Osajima, J.A.; Luz, A.B.; Fonseca, M.G.; Silva Filho, E.C. Thermally activated palygorskites as agents to clarify soybean oil. Appl. Clay Sci. 2016, 119, 338–347. [Google Scholar] [CrossRef]
- Wang, K.; Wang, L.; Zhang, Y.; Zhang, Y.; Liang, J. Microstructural evolution and sintering properties of palygorskite nanofibers. Int. J. Appl. Ceram. Technol. 2020, 17, 1833–1842. [Google Scholar] [CrossRef]
- Frinisrasra, N.; Srasra, E. Effect of heating on palygorskite and acid treated palygorskite properties. Electron. Mater. Process. 2008, 44, 43–49. [Google Scholar] [CrossRef]
- de Brito Buriti, B.M.A.; Barsosa, M.E.; da Silva Buriti, J.; de Melo Cartaxo, J.; Ferreira, H.S.; de Araújo Neves, G. Modification of palygorskite with cationic and nonionic surfactants for use in oil-based drilling fluids. J. Therm. Anal. Calorim. 2021, 1–11. [Google Scholar] [CrossRef]
- Pardo-Canales, L.; Essih, S.; Cecilia, J.A.; Domínguez-Maqueda, M.; Olmo-Sánchez, M.I.; Pozo-Rodríguez, M.; Franco, F. Modification of the textural properties of palygorskite through microwave assisted acid treatment. Influence of the octahedral sheet composition. Appl. Clay Sci. 2020, 196, 105745. [Google Scholar] [CrossRef]
- Rehman, A.; Park, M.; Park, S.J. Current progress on the surface chemical modification of carbonaceous materials. Coatings 2019, 9, 103. [Google Scholar] [CrossRef] [Green Version]
- Korichi, S.; Elias, A.; Mefti, A.; Bensmaili, A. The effect of microwave irradiation and conventional acid activation on the textural properties of smectite: Comparative study. Appl. Clay Sci. 2012, 59, 76–83. [Google Scholar] [CrossRef]
- Kooli, F.; Liu, Y.; Al-Faze, R.; Al Suhaimi, A. Effect of acid activation of Saudi local clay mineral on removal properties of basic blue 41 from an aqueous solution. Appl. Clay Sci. 2015, 116, 23–30. [Google Scholar] [CrossRef]
- Andrew Lin, K.Y.; Yang, H.; Lee, W. Der Enhanced removal of diclofenac from water using a zeolitic imidazole framework functionalized with cetyltrimethylammonium bromide (CTAB). RSC Adv. 2015, 5, 81330–81340. [Google Scholar] [CrossRef]
- Chaari, I.; Moussi, B.; Jamoussi, F. Interactions of the dye, C.I. direct orange 34 with natural clay. J. Alloys Compd. 2015, 647, 720–727. [Google Scholar] [CrossRef]
- Duarte Neto, J.F.; Pereira, I.D.S.; Da Silva, V.C.; Ferreira, H.C.; Neves, D.G.A.; Menezes, R.R. Study of equilibrium and kinetic adsorption of rhodamine B onto purified bentonite clays. Ceramica 2018, 64, 598–607. [Google Scholar] [CrossRef]
- Feng, N.; Guo, X.; Liang, S.; Zhu, Y.; Liu, J. Biosorption of heavy metals from aqueous solutions by chemically modified orange peel. J. Hazard. Mater. 2011, 185, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Hamza, W.; Dammak, N.; Hadjltaief, H.B.; Eloussaief, M.; Benzina, M. Sono-assisted adsorption of cristal violet dye onto Tunisian smectite clay: Characterization, kinetics and adsorption isotherms. Ecotoxicol. Environ. Saf. 2018, 163, 365–371. [Google Scholar] [CrossRef]
- Lima, É.C.; Adebayo, M.A.; Machado, F.M. Kinetic and equilibrium models of adsorption. In Carbon Nanostructures, 1st ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 33–69. [Google Scholar]
- Brito, D.F.; Da Silva Filho, E.C.; Fonseca, M.G.; Jaber, M. Organophilic bentonites obtained by microwave heating as adsorbents for anionic dyes. J. Environ. Chem. Eng. 2018, 6, 7080–7090. [Google Scholar] [CrossRef] [Green Version]
- Tian, G.; Wang, W.; Kang, Y.; Wang, A. Palygorskite in sodium sulphide solution via hydrothermal process for enhanced methylene blue adsorption. J. Taiwan Inst. Chem. Eng. 2016, 58, 417–423. [Google Scholar] [CrossRef]
- Suárez, M.; García-Romero, E.; Sánchez Del Río, M.; Martinetto, P.; Dooryhée, E. The effect of the octahedral cations on the dimensions of the palygorskite cell. Clay Miner. 2007, 42, 287–297. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wang, W.; Zhang, J.; Liu, P.; Wang, A. A comparative study about adsorption of natural palygorskite for methylene blue. Chem. Eng. J. 2015, 262, 390–398. [Google Scholar] [CrossRef]
- Mendelovici, E. Infrared study of attapulgite and HCl treated attapulgite. Clays Clay Miner. 1973, 21, 115–119. [Google Scholar] [CrossRef]
- Barrios, M.S.; González, L.V.F.; Rodríguez, M.A.V.; Pozas, J.M.M. Acid activation of a palygorskite with HCl: Development of physico-chemical, textural and surface properties. Appl. Clay Sci. 1995, 10, 247–258. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, Y.; Wang, A. Removal of Cu(II) from aqueous solution by adsorption onto acid-activated palygorskite. J. Hazard. Mater. 2007, 149, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Boudriche, L.; Calvet, R.; Hamdi, B.; Balard, H. Effect of acid treatment on surface properties evolution of attapulgite clay: An application of inverse gas chromatography. Colloids Surf. A Physicochem. Eng. Asp. 2011, 392, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Leite, I.F.; Raposo, C.M.O.; Silva, S.M.L. Structural characterization of Brazilian and imported bentonitic clays: Before and after the process of organophilization for utilization as nanofiller. Ceramica 2008, 54, 303–308. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Lin, Y.; Du, Q.; Zhong, W.; Yang, Y. Preparation and rheology of polyamide-6/attapulgite nanocomposites and studies on their percolated structure. Polymer 2005, 46, 5758–5766. [Google Scholar] [CrossRef]
- Aielo, P.B.; Borges, F.A.; Romeira, K.M.; Miranda, M.C.R.; Arruda, L.B.D.; Paulo, P.N.; Drago, B.D.C.; Herculano, R.D. Evaluation of sodium diclofenac release using natural rubber latex as carrier. Mater. Res. 2014, 17, 146–152. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, T.L.; Vidart, J.M.M.; da Silva, A.C., Jr.; Gimenes, M.L.; Vieira, M.G.A.; da Silva, M.G.C. Evaluation of incorporation of diclofenac sodium in dried sericin-alginate particles prepared by ionic gelation technique. Proc. Chem. Eng. Trans. 2015, 43, 829–834. [Google Scholar]
- Fini, A.; Cavallari, C.; Ospitali, F. Diclofenac salts. V. examples of polymorphism among diclofenac salts with alkyl-hydroxy amines studied by DSC and HSM. Pharmaceutics 2010, 2, 136–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovala-Demertzi, D.; Mentzafos, D.; Terzis, A. Metal complexes of the anti-inflammatory drug sodium [2-[(2,6-dichlorophenyl)amino]phenyl]acetate (diclofenac sodium). Molecular and crystal structure of cadmium diclofenac. Polyhedron 1993, 12, 1361–1370. [Google Scholar] [CrossRef]
- Thanhmingliana; Tiwari, D. Efficient use of hybrid materials in the remediation of aquatic environment contaminated with micro-pollutant diclofenac sodium. Chem. Eng. J. 2015, 263, 364–373. [Google Scholar] [CrossRef]
- Sotelo, J.L.; Ovejero, G.; Rodríguez, A.; Álvarez, S.; Galán, J.; García, J. Competitive adsorption studies of caffeine and diclofenac aqueous solutions by activated carbon. Chem. Eng. J. 2014, 240, 443–453. [Google Scholar] [CrossRef]
- Larous, S.; Meniai, A.H. Adsorption of diclofenac from aqueous solution using activated carbon prepared from olive stones. Int. J. Hydrogen Energy 2016, 41, 10380–10390. [Google Scholar] [CrossRef]


| Design I | Level | ||
|---|---|---|---|
| Independent variables | −1 | 0 | +1 |
| Mass of acid functionalized (g) | 0.2 | 0.3 | 0.4 |
| Concentration of the solution (mg·L−1) | 20 | 30 | 40 |
| solution pH | 6 | 8.5 | 11 |
| Contact time (h) | 4 | 6 | 8 |
| Design II | Level | ||
| Independent variables | −1 | 0 | +1 |
| Mass of organo-functionalized (g) | −1 | 0 | 1 |
| Concentration of the solution (mg·L−1) | 20 | 30 | 40 |
| solution pH | 6 | 8.5 | 11 |
| Contact time (h) | 4 | 6 | 8 |
| Oxides | Raw | Acid | Organic |
|---|---|---|---|
| SiO2 | 61.9 | 76.0 | 62.0 |
| Al2O3 | 18.1 | 11.1 | 18.0 |
| Fe2O3 | 8.6 | 5.9 | 8.1 |
| MgO | 6.5 | 5.0 | 6.5 |
| K2O | 2.8 | 0.6 | 2.7 |
| TiO2 | 0.7 | 0.7 | 0.7 |
| Cl | - | - | 0.8 |
| CaO | 0.5 | - | 0.4 |
| Others | 0.9 | 0.7 | 0.8 |
| Sample | Specific Surface Area (m2/g) |
|---|---|
| Raw | 87.233 |
| Acid | 142.447 |
| Organic | 26.969 |
| Experimental Conditions | Acid | Organic | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Exp. | Mass (g) | Initial Conc. (mg·L−1) | pH | Time (h) | Final Conc. (mg·L−1) | Qt (mg g−1) | Rem (%) | Final Conc. (mg·L−1). | Qt (mg g−1) | Rem (%) |
| 1 | 0.2 | 20 | 6 | 4 | 2.031 | 1.7969 | 98.25 | 2.015 | 1.7985 | 89.93 |
| 2 | 0.4 | 20 | 6 | 4 | 2.808 | 0.8596 | 85.96 | 2.931 | 0.8534 | 85.35 |
| 3 | 0.2 | 40 | 6 | 4 | 2.32 | 3.768 | 94.20 | 5.8 | 3.4200 | 85.50 |
| 4 | 0.4 | 40 | 6 | 4 | 4.009 | 1.7996 | 89.98 | 6.066 | 1.6967 | 84.84 |
| 5 | 0.2 | 20 | 11 | 4 | 4.392 | 1.5608 | 78.04 | 8.698 | 1.1302 | 56.51 |
| 6 | 0.4 | 20 | 11 | 4 | 6.363 | 0.6819 | 68.19 | 8.384 | 0.5808 | 58.08 |
| 7 | 0.2 | 40 | 11 | 4 | 6.661 | 3.3339 | 83.35 | 10.074 | 2.9926 | 74.82 |
| 8 | 0.4 | 40 | 11 | 4 | 6.477 | 1.6762 | 83.81 | 18.827 | 1.0587 | 52.94 |
| 9 | 0.2 | 20 | 6 | 8 | 2.295 | 1.7705 | 88.53 | 2.342 | 1.7658 | 88.29 |
| 10 | 0.4 | 20 | 6 | 8 | 4.388 | 0.7806 | 78.06 | 3.065 | 0.8467 | 84.68 |
| 11 | 0.2 | 40 | 6 | 8 | 5.464 | 3.4536 | 86.34 | 6.251 | 3.3749 | 84.38 |
| 12 | 0.4 | 40 | 6 | 8 | 4.52 | 1.7740 | 88.70 | 5.959 | 1.7020 | 85.10 |
| 13 | 0.2 | 20 | 11 | 8 | 4.378 | 1.5622 | 78.11 | 9.791 | 1.0209 | 51.05 |
| 14 | 0.4 | 20 | 11 | 8 | 6.445 | 0.6778 | 67.78 | 8.262 | 0.5869 | 58.69 |
| 15 | 0.2 | 40 | 11 | 8 | 6.827 | 3.3173 | 82.94 | 10.366 | 2.9634 | 74.09 |
| 16 | 0.4 | 40 | 11 | 8 | 7.039 | 1.6481 | 82.41 | 19.343 | 1.0329 | 51.64 |
| 17 | 0.3 | 30 | 8.5 | 6 | 4.332 | 1.7112 | 85.56 | 4.569 | 1.6954 | 84.77 |
| 18 | 0.3 | 30 | 8.5 | 6 | 3.99 | 1.7340 | 86.70 | 4.225 | 1.7183 | 85.92 |
| 19 | 0.3 | 30 | 8.5 | 6 | 4.002 | 1.7332 | 86.66 | 4.523 | 1.6985 | 84.92 |
| Variation Source | SQ | DF | MS | Fcal | Ftab | R2 |
|---|---|---|---|---|---|---|
| Regression | 15.8543 | 11 | 1.4413 | 318.53 | 3.605 | 0.9949 |
| Residual | 0.0317 | 7 | 0.0045 | |||
| Total | 15.8860 | 18 |
| Variation Source | SQ | DF | QM | Fcal | Ftab | R2 |
|---|---|---|---|---|---|---|
| Regression | 19.0551 | 11 | 1.73228 | 282.99 | 3.605 | 0.9942 |
| Residual | 0.0428 | 7 | 0.00612 | |||
| Total | 19.0979 | 18 |
| (a) | |||
| Terms | Effects | Regression Coefficient | p-Value |
| Mass | −1.3332 | −0.6666 | 0 |
| Concentration | 1.3939 | 0.697 | 0 |
| pH | −0.1931 | −0.0965 | 0.001 |
| Time | −0.0616 | −0.0308 | 0.11 |
| Mass and Conc. interation | −0.4161 | −0.2081 | 0 |
| Mass and pH interation | 0.0606 | 0.0303 | 0.115 |
| Mass and time interation | 0.0274 | 0.0137 | 0.442 |
| Conc. and pH interation | −0.0207 | −0.0104 | 0.557 |
| Conc. and time interation | −0.0401 | −0.0201 | 0.272 |
| pH and time interation | 0.0497 | 0.0249 | 0.183 |
| (b) | |||
| Terms | Effects | Regression Coefficient | p-Value |
| Mass | −1.2624 | −0.6322 | 0 |
| Concentration | 1.5246 | 0.7623 | 0 |
| pH | −0.5106 | −0.2553 | 0 |
| Time | −0.0305 | −0.0153 | 0.461 |
| Mass and Conc. interation | −0.7173 | −0.3591 | 0 |
| Mass and pH interation | 0.0507 | 0.0253 | 0.236 |
| Mass and time interation | 0.0253 | 0.0126 | 0.539 |
| Conc. and pH interation | −0.2431 | −0.1216 | 0 |
| Conc. and time interation | −0.0148 | −0.0074 | 0.716 |
| pH and time interation | −0.0108 | −0.0054 | 0.791 |
| Pseudo-First Order Model | |||||||
| Parameters | Variation Source | SQ | DF | MS | Fcal | Error | R2 (adj) |
| K1 = 0.0264 min−1 | Regression | 9410.097 | 2 | 4705.048 | 738.72 | 1.24 | 0.96 |
| Qef = 36.0295 mg/g | Residual | 63.690 | 10 | 6.36913 | |||
| Total | 9473.788 | 12 | |||||
| Pseudo-Second Order Model | |||||||
| Parameters | Variation Source | SQ | DF | MS | Fcal | Error | R2 (adj) |
| K2 = 0.000729 g·min−1·min−1 | Regression | 9421.694 | 2 | 4710.847 | 904.31 | 1.96 | 0.97 |
| Qef = 42.424 mg/g | Residual | 52.093 | 10 | 5.209 | |||
| Total | 9473.788 | 12 | |||||
| Elovich Model | |||||||
| Parameters | Variation Source | SQ | DF | MS | Fcal | Error | R2 (adj) |
| α = 5.27131 mg/g·min | Regression | 9404.827 | 2 | 4702.414 | 681.9 | 1.51 | 0.95 |
| β = 0.04598 g/mg | Residual | 68.960 | 10 | 6.89605 | |||
| Total | 9473.788 | 12 | |||||
| Pseudo-First Order Model | |||||||
| Parameters | Variation Source | SQ | DF | MS | Fcal | Error | R2 (adj) |
| K1 = 0.27775 min−1 | Regression | 16,561.380 | 2 | 8280.69 | 4933.21 | 0.41 | 0.985 |
| Qef = 38.09673 mg/g | Residual | 18.464 | 11 | 1.67856 | |||
| Total | 16,579.842 | 13 | |||||
| Pseudo-Second Order Model | |||||||
| Parameters | Variation Source | SQ | DF | MS | Fcal | Error | R2 (adj) |
| K2 = 0.01722 g·min−1·min−1 | Regression | 16,573.664 | 2 | 8286.83 | 14,753.23 | 0.28 | 0.995 |
| Qef = 39. 06836 mg/g | Residual | 6.178 | 11 | 0.56 | |||
| Total | 16,579.842 | 13 | |||||
| Elovich Model | |||||||
| Parameters | Variation Source | SQ | DF | MS | Fcal | Error | R2 (adj) |
| α = 5.107 mg/g·min | Regression | 16,556.29 | 2 | 8278.146 | 3866.58 | 1.84 | 0.981 |
| β = 0.23802 g/mg | Residual | 23.55 | 11 | 2.14 | |||
| Total | 16,579.84 | 13 | |||||
| Langmuir Model | |||||||
| Parameters | Variation Source | SQ | DF | MS | Fcal | Error | R2 (adj) |
| KL = 0.00545 | Regression | 53,210.65 | 2 | 26,605.33 | 655.69 | 0.91 | 0.97795 |
| Qmax = 179.87849 | Residual | 486.91 | 12 | 40.5761 | |||
| Total | 53,697.57 | 14 | |||||
| Freundlich Model | |||||||
| Parameters | Variation Source | SQ | DF | MS | Fcal | Error | R2 (adj) |
| KF = 3.40006 | Regression | 52,332.94 | 2 | 26,166.47 | 230.096 | 1.07 | 0.93891 |
| N = 1.63677 | Residual | 1364.63 | 12 | 113.72 | |||
| Total | 53,697.57 | 14 | |||||
| Temkin Model | |||||||
| Parameters | Variation Source | SQ | DF | MS | Fcal | Error | R2 (adj) |
| αt = 0.08844 | Regression | 20,587.27 | 1 | 20,587.27 | 164.793 | 0.65 | 0.93212 |
| βt = 0.03178 | Residual | 1499.134 | 12 | 124.927 | |||
| Total | 22,086.41 | 13 | |||||
| Langmuir Model | |||||||
| Parameters | Variation Source | SQ | DF | MS | Fcal | Error | R2 (adj) |
| KL = 0.00371 | Regression | 71,632.444 | 2 | 35,816.222 | 1373.097 | 0.49 | 0.99 |
| Qmax = 253.34452 | Residual | 313.011 | 12 | 26.084 | |||
| Total | 71,945.455 | 14 | |||||
| Freundlich Model | |||||||
| Parameters | Variation Source | SQ | DF | MS | Fcal | Error | R2 (adj) |
| KF = 2.78045 | Regression | 70,937.93 | 2 | 35,468.97 | 422.45 | 0.71 | 0.965 |
| N = 1.47776 | Residual | 1007.52 | 12 | 83.96 | |||
| Total | 71,945.455 | 14 | |||||
| Temkin Model | |||||||
| Parameters | Variation Source | SQ | DF | MS | Fcal | Error | R2 (adj) |
| αt = 0.08236 | Regression | 28,839.644 | 1 | 28,839.644 | 119.194 | 0.65 | 0.908 |
| βt = 0.02685 | Residual | 2903.45 | 12 | 241.95 | |||
| Total | 31,743.095 | 13 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, M.U.; Rodrigues, A.M.; Araujo, M.E.B.; Menezes, R.R.; Neves, G.A.; Lira, H.L. Adsorption of Sodium Diclofenac in Functionalized Palygoskite Clays. Materials 2022, 15, 2708. https://doi.org/10.3390/ma15082708
Sousa MU, Rodrigues AM, Araujo MEB, Menezes RR, Neves GA, Lira HL. Adsorption of Sodium Diclofenac in Functionalized Palygoskite Clays. Materials. 2022; 15(8):2708. https://doi.org/10.3390/ma15082708
Chicago/Turabian StyleSousa, Matheus Urtiga, Alisson Mendes Rodrigues, Maria Eduarda Barbosa Araujo, Romualdo Rodrigues Menezes, Gelmires Araújo Neves, and Hélio Lucena Lira. 2022. "Adsorption of Sodium Diclofenac in Functionalized Palygoskite Clays" Materials 15, no. 8: 2708. https://doi.org/10.3390/ma15082708









