Influence of Different Parameters on the Performance of Alkali-Activated Slag/Fly Ash Composite System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Slag
2.1.2. Fly Ash
2.1.3. Alkaline Activator
2.2. Test Preparation
2.2.1. Matching Ratio Design
2.2.2. Configuration of Alkaline Activator Solution
2.3. Test Preparation
2.3.1. Fluidity Test
2.3.2. Coagulation Time Test
2.3.3. Flexural and Compressive Strength Testing
2.3.4. SEM Observation
2.3.5. FTIR Spectroscopy Test
3. Results
3.1. The Effect of Each Parameter on the Flow Degree
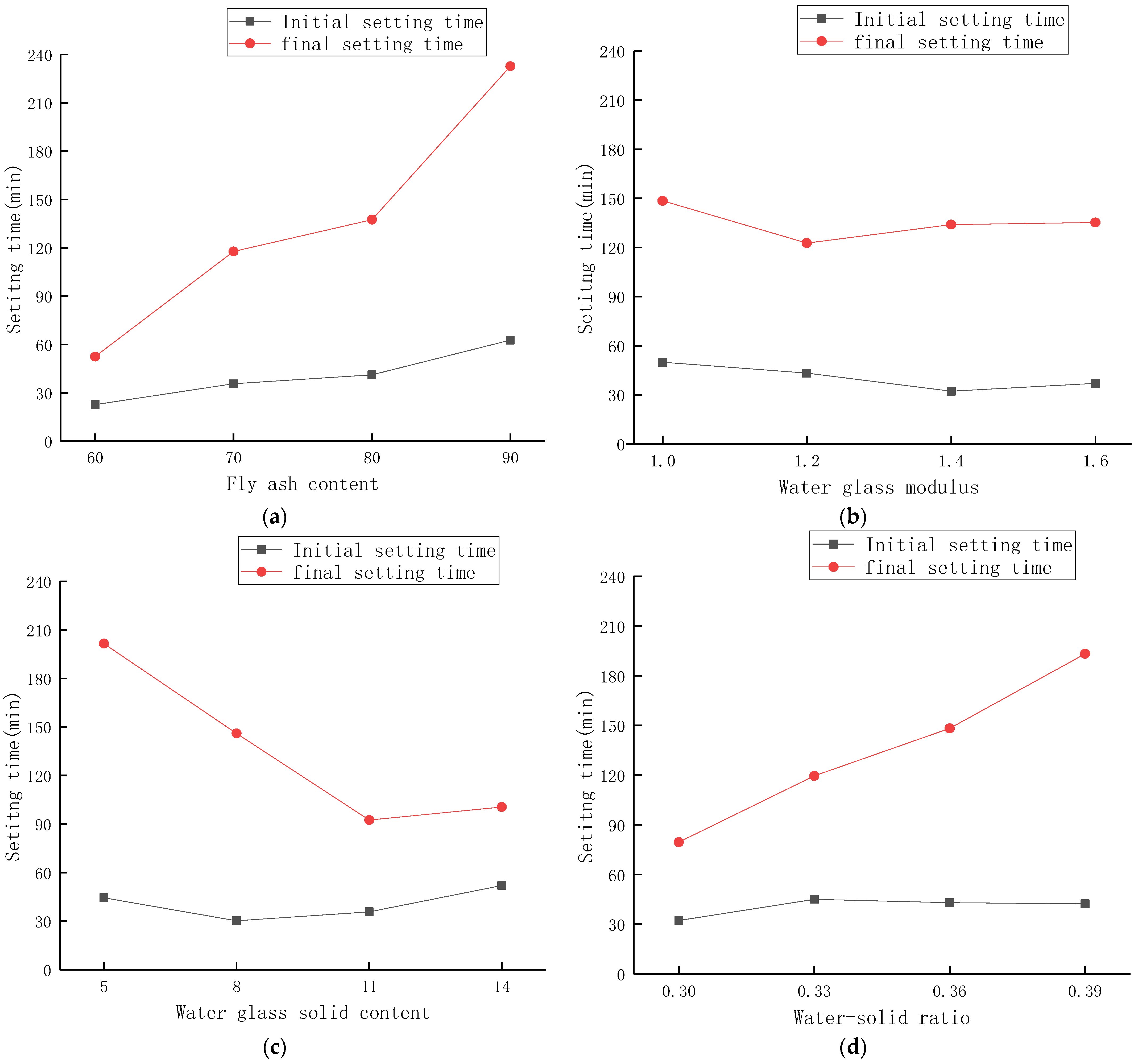
3.2. The Effect of Each Parameter on the Setting Time
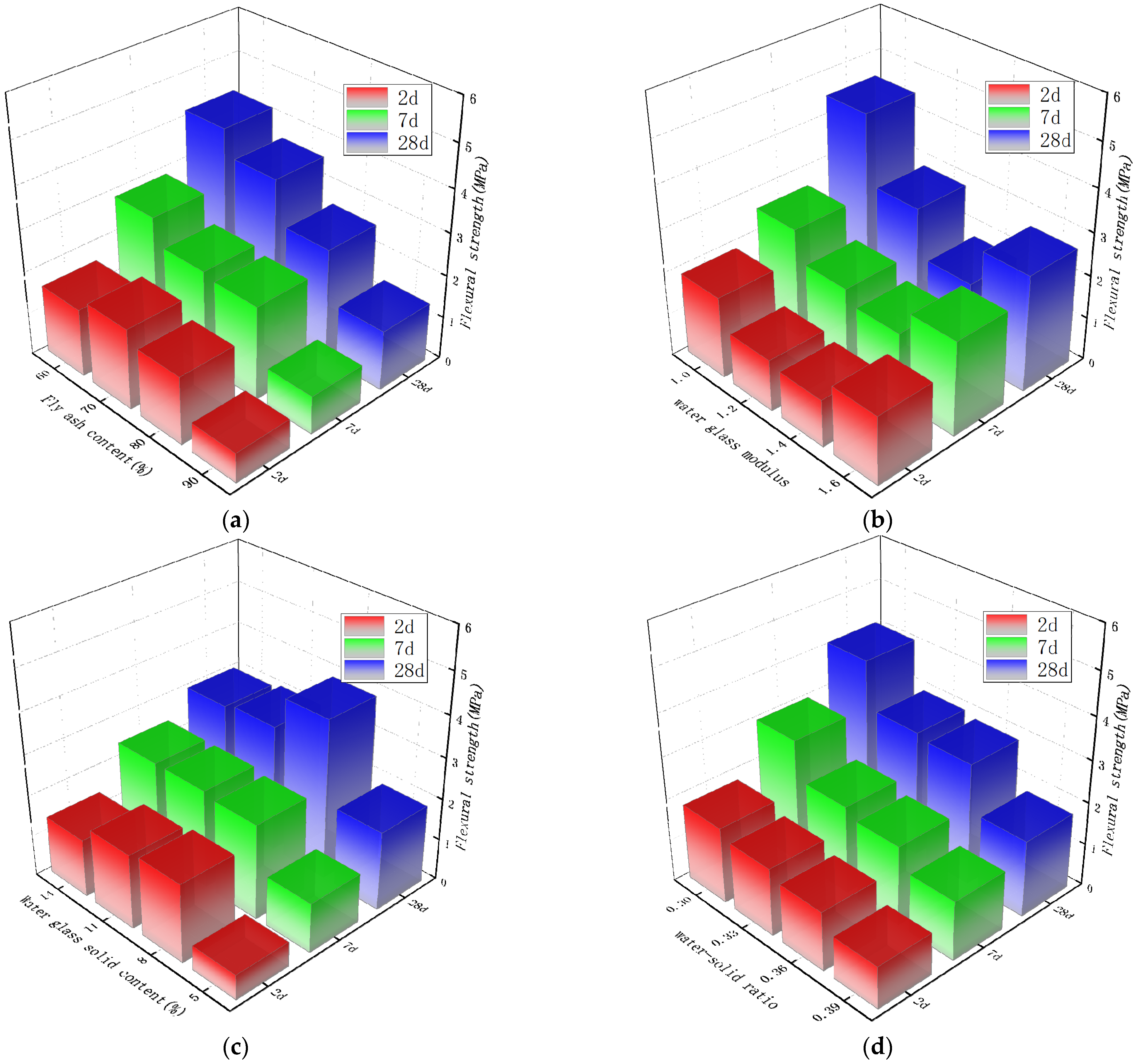
3.3. The Influence of Each Parameter on the Flexural Strength
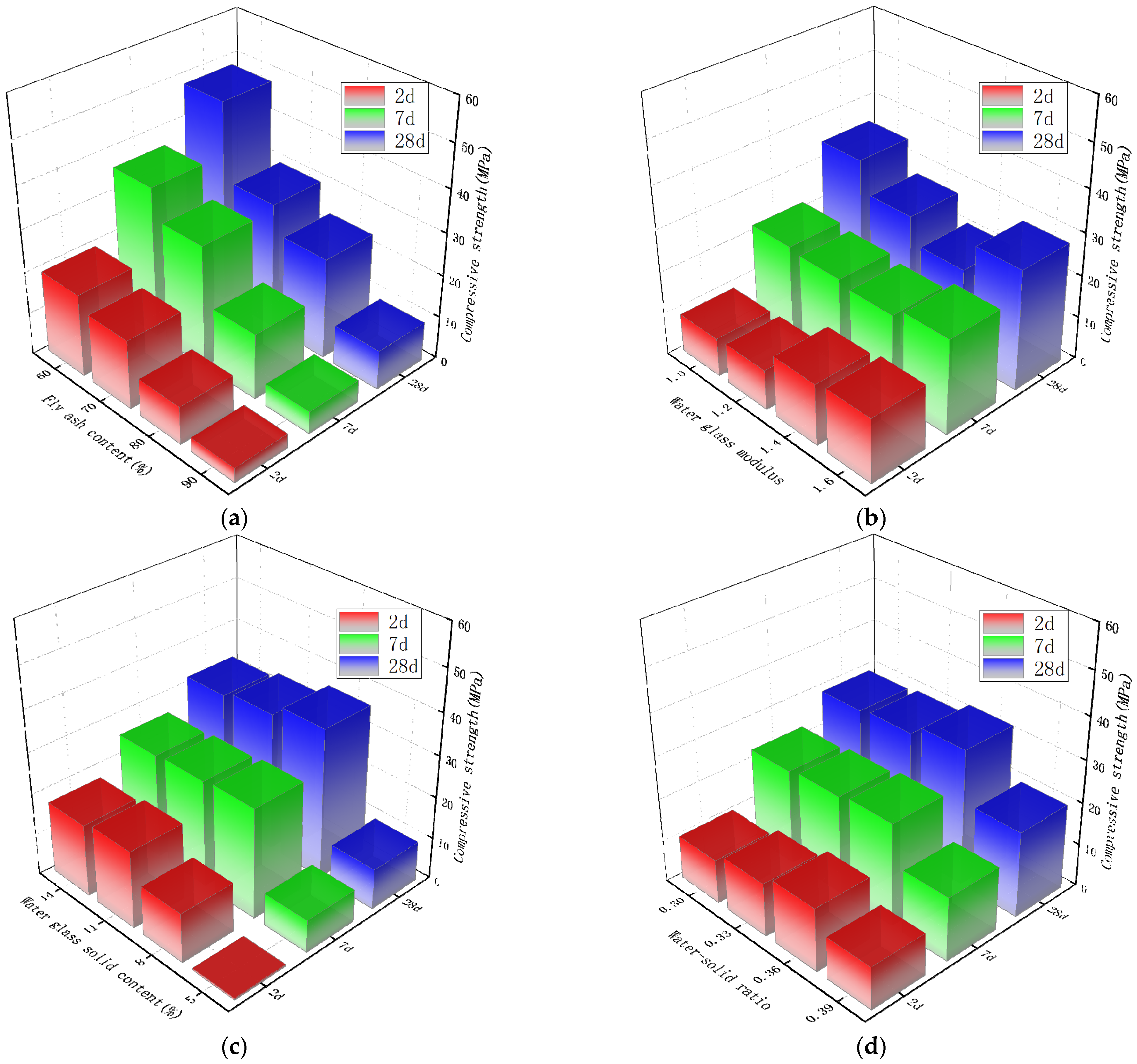
3.4. The Influence of Each Parameter on the Compressive Strength
3.5. SEM Image Analysis
3.6. FTIR Analysis
4. Conclusions
- (1)
- The order of significance of the influence of the four parameters on the fluidity of the material is: water glass solid content > water–solid ratio > fly ash content > water glass modulus. That is, the change in the solid content of water glass has the greatest influence on fluidity. The amount of water glass solid content determines whether the amount of SiO2 and Na2O involved in the alkali-activated reaction is sufficient. Controlling the water glass solid content is the key to ensuring the fluidity of the material.
- (2)
- The change in fly ash content had the greatest influence on the setting time, and the setting time gradually became longer with the increase in fly ash content. The content of fly ash affects the content of Ca2+ in the material, which in turn affects the formation of multiple condensation nuclei in the initial process of depolymerization of silico-alumina raw materials. This eventually leads to a change in setting time. A key control for adjusting the setting time of the material is the fly ash content.
- (3)
- In the early stage of an alkali-activated reaction, the amount of alkali will affect the reaction rate. More C-S-H gel or C-A-S-H gel is formed, which directly affects the early strength of the material. Controlling the solid content of water glass is the primary factor for adjusting the early strength of the material. With the growth of age, a large amount of Ca2+ participates in the reaction to form the main solid phase that improves the strength of the material. The mechanical strength of the material at 7 d and 28 d age are most affected by the content of fly ash. Controlling the solid content of water glass is the primary factor for adjusting the mid- and late-stage strength of the material.The resulting gel product increases with the addition of slag and alkali activator, resulting in a very dense structure and increased strength of the material. When the amount of silica in the material is too high, the phenomenon of lower macroscopic mechanical properties occurs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Izumi, Y.; Iizuka, A.; Ho, H.-J. Calculation of greenhouse gas emissions for a carbon recycling system using mineral carbon capture and utilization technology in the cement industry. J. Clean. Prod. 2021, 312, 127618. [Google Scholar] [CrossRef]
- Ostovari, H.; Müller, L.; Skocek, J.; Bardow, A. From Unavoidable CO2 Source to CO2 Sink? A Cement Industry Based on CO2 Mineralization. Environ. Sci. Technol. 2021, 55, 5212–5223. [Google Scholar] [CrossRef] [PubMed]
- Benhelal, E.; Shamsaei, E.; Rashid, M.I. Challenges against CO2 abatement strategies in cement industry: A review. J. Environ. Sci. 2020, 104, 84–101. [Google Scholar] [CrossRef] [PubMed]
- Provis, J.L.; Bernal, S.A. Geopolymers and related alkali-activated materials. Annu. Rev. Mater. Res. 2014, 44, 299–327. [Google Scholar] [CrossRef]
- Li, X.; Qin, D.; Hu, Y.; Ahmad, W.; Ahmad, A.; Aslam, F.; Joyklad, P. A systematic review of waste materials in cement-based composites for construction applications. J. Build. Eng. 2022, 45, 103447. [Google Scholar] [CrossRef]
- Pardo Alvarez, N.S.; López Castaño, D.J.; Rico Pérez, M.A. Inclusión de concretos sostenibles en el cumplimiento de la Resolución 0472 de 2017 y la disminución de emisiones del sector constructor colombiano: Análisis de materiales. Rev. Logos Cienc. Tecnol. 2022, 14, 76–85. [Google Scholar] [CrossRef]
- Wang, H.; Wu, H.; Xing, Z.; Wang, R.; Dai, S. The Effect of Various Si/Al, Na/Al Molar Ratios and Free Water on Micromorphology and Macro-Strength of Metakaolin-Based Geopolymer. Materials 2021, 14, 3845. [Google Scholar] [CrossRef] [PubMed]
- Ramos, F.J.H.T.V.; Marques, M.d.F.V.; Rodrigues, J.G.P.; de Oliveira Aguiar, V.; da Luz, F.S.; de Azevedo, A.R.G.; Monteiro, S.N. Development of novel geopolymeric foam composites coated with polylactic acid to remove heavy metals from contaminated water. Case Stud. Constr. Mater. 2022, 16, e00795. [Google Scholar] [CrossRef]
- Davidovits, J.; Comrie, D.C.; Paterson, J.H.; Ritcey, D.J. Geopolymeric concretes for environmental protection. Concr. Int. 1990, 12, 30–40. [Google Scholar]
- Davidovits, J. Geopolymers: Inorganic polymeric new materials. J. Therm. Anal. Calorim. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Zannerni, G.M.; Fattah, K.P.; Al-Tamimi, A.K. Ambient-cured geopolymer concrete with single alkali activator. Sustain. Mater. Technol. 2020, 23, e00131. [Google Scholar] [CrossRef]
- Arbi, K.; Nedeljkovic, M.; Zuo, Y.; Ye, G. A review on the durability of alkali-activated fly ash/slag systems: Advances, issues, and perspectives. Ind. Eng. Chem. Res. 2016, 55, 5439–5453. [Google Scholar] [CrossRef] [Green Version]
- Hojati, M.; Radlińska, A. Shrinkage and strength development of alkali-activated fly ash-slag binary cements. Constr. Build. Mater. 2017, 150, 808–816. [Google Scholar] [CrossRef]
- Hu, X.; Shi, C.; Shi, Z.; Zhang, L. Compressive strength, pore structure and chloride transport properties of alkali-activated slag/fly ash mortars. Cem. Concr. Compos. 2019, 104, 103392. [Google Scholar] [CrossRef]
- Castillo, H.; Collado, H.; Droguett, T.; Sánchez, S.; Vesely, M.; Garrido, P.; Palma, S. Factors Affecting the Compressive Strength of Geopolymers: A Review. Minerals 2021, 11, 1317. [Google Scholar] [CrossRef]
- Zheng, W.; Zou, M.; Wang, Y. Literature review of alkali-activated cementitious materials. J. Build. Struct. 2019, 40, 28–39. [Google Scholar]
- Dai, X.; Aydin, S.; Yardimci, M.Y.; Lesage, K.; De Schutter, G. Influence of water to binder ratio on the rheology and structural Build-up of Alkali-Activated Slag/Fly ash mixtures. Constr. Build. Mater. 2020, 264, 120253. [Google Scholar] [CrossRef]
- Ma, X. Resarch on Preparation and Performance of New Type High Performance Lightweight Aggregate Concrete Based on Inorganic Polymer Cement; Central South University: Changsha, China, 2012. (In Chinese) [Google Scholar]
- Han, F.; Wu, L. Comprehensive Utilization of Fly Ash. In Industrial Solid Waste Recycling in Western China; Springer: Singapore, 2019; pp. 207–304. [Google Scholar]
- Jin, L.; Huang, G.; Li, Y.; Zhang, X.; Ji, Y.; Xu, Z. Positive Influence of Liquid Sodium Silicate on the Setting Time, Polymerization, and Strength Development Mechanism of MSWI Bottom Ash Alkali-Activated Mortars. Materials 2021, 14, 1927. [Google Scholar] [CrossRef]
- Sun, B.; Sun, Y.; Ye, G.; De Schutter, G. A mix design methodology of slag and fly ash-based alkali-activated paste. Cem. Concr. Compos. 2022, 126, 104368. [Google Scholar] [CrossRef]
- Yang, D.; Pang, L.; Song, D.; Lu, M.; Wang, J.; Guan, Z. Reaction Mechanism of Fly Ash in Alkali-Activated Slaf/Fly Ash System. Bull. Chin. Ceram. Soc. 2021, 40, 3005–3011. [Google Scholar]
- Samarakoon, M.; Ranjith, P. Upcycling of cementitious wastes in one-part alkaline cement binders. In Proceedings of the IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2021; p. 012002. [Google Scholar]
- Li, S.; Sha, F.; Liu, R.; Li, W.; Li, Z.; Wang, G. Properties of cement-based grouts with high amounts of ground granulated blast-furnace slag and fly ash. J. Mater. Civ. Eng. 2017, 29, 04017219. [Google Scholar] [CrossRef]
- Chen, K. Design and Analysis of Experiments; Tsinghua University Press: Beijing, China, 1996. [Google Scholar]
- General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China. Ground Granulated Blast Furnace Slag Used for Cement, Mortar and Concrete; General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2017.
- General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China. Fly Ash Used for Cement and Concrete; General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2017.
- General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China. Sodium Silicate for Industrial Use; General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2008.
- Yang, T.; Zhu, H.; Zhang, Z.; Gao, X.; Zhang, C.; Wu, Q. Effect of fly ash microsphere on the rheology and microstructure of alkali-activated fly ash/slag pastes. Cem. Concr. Res. 2018, 109, 198–207. [Google Scholar] [CrossRef]
- Yu, S. Study on the Physical and Mechanical Propertiesof Alkali Activated Slag-Fly Ash Gel; Hubei University of Technology: Wuhan, China, 2020. (In Chinese) [Google Scholar]
- Gao, X.; Yu, Q.; Brouwers, H. Reaction kinetics, gel character and strength of ambient temperature cured alkali activated slag–fly ash blends. Constr. Build. Mater. 2015, 80, 105–115. [Google Scholar] [CrossRef] [Green Version]
- Peng, X.; Yang, T.; Wang, K.; Meng, X. Preparation of Geopolymeric Concrete and Its Application to Rapid Repair of Cement Concrete Pavement. J. Southwest Jiaotong Univ. 2011, 46, 6. [Google Scholar]
- Han, T. Analysis of Influencing actors on Geopolymerization Process of Akali-Activated Fly Ash; Changsha University of Science & Technology: Changsha, China, 2016. (In Chinese) [Google Scholar]
- General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China. Determination of Fluidity of Cement Mortar; General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2005.
- General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China. Test Methods for Water Consumption, Setting Time and Stability of Cement Standard Consistency; General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2011.
- State Bureau of Quality and Technical Supervision. Cement Mortar Strength Test Method (ISO Method); State Bureau of Quality and Technical Supervision: Beijing, China, 1999.
- National Development and Reform Commission of the People’s Republic of China. 40mm × 40mm Cement Compression Fixture; National Development and Reform Commission of the People’s Republic of China: Beijing, China, 2005.
- Tay, Y.W.D.; Qian, Y.; Tan, M.J. Printability region for 3D concrete printing using slump and slump flow test. Compos. Part B Eng. 2019, 174, 106968. [Google Scholar] [CrossRef]
- Yip, C.K.; Lukey, G.C.; Provis, J.L.; Van Deventer, J.S. Effect of calcium silicate sources on geopolymerisation. Cem. Concr. Res. 2008, 38, 554–564. [Google Scholar] [CrossRef]
- Lloyd, R.R.; Provis, J.L.; van Deventer, J.S. Microscopy and microanalysis of inorganic polymer cements. 2: The gel binder. J. Mater. Sci. 2009, 44, 620–631. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P.K. Effect of GGBFS on setting, workability and early strength properties of fly ash geopolymer concrete cured in ambient condition. Constr. Build. Mater. 2014, 66, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Lu, T.; Liang, X.; Dong, H.; Ye, G. Mechanisms of autogenous shrinkage of alkali-activated slag and fly ash pastes. Cem. Concr. Res. 2020, 135, 106107. [Google Scholar] [CrossRef]
- Xiao, S.; Ma, Y.; Liu, R.; Hu, J.; Yin, S. Effect of raw materials on the early age properties of alkali-activated fly ash/slag. Concrete 2019, 89–95. [Google Scholar]


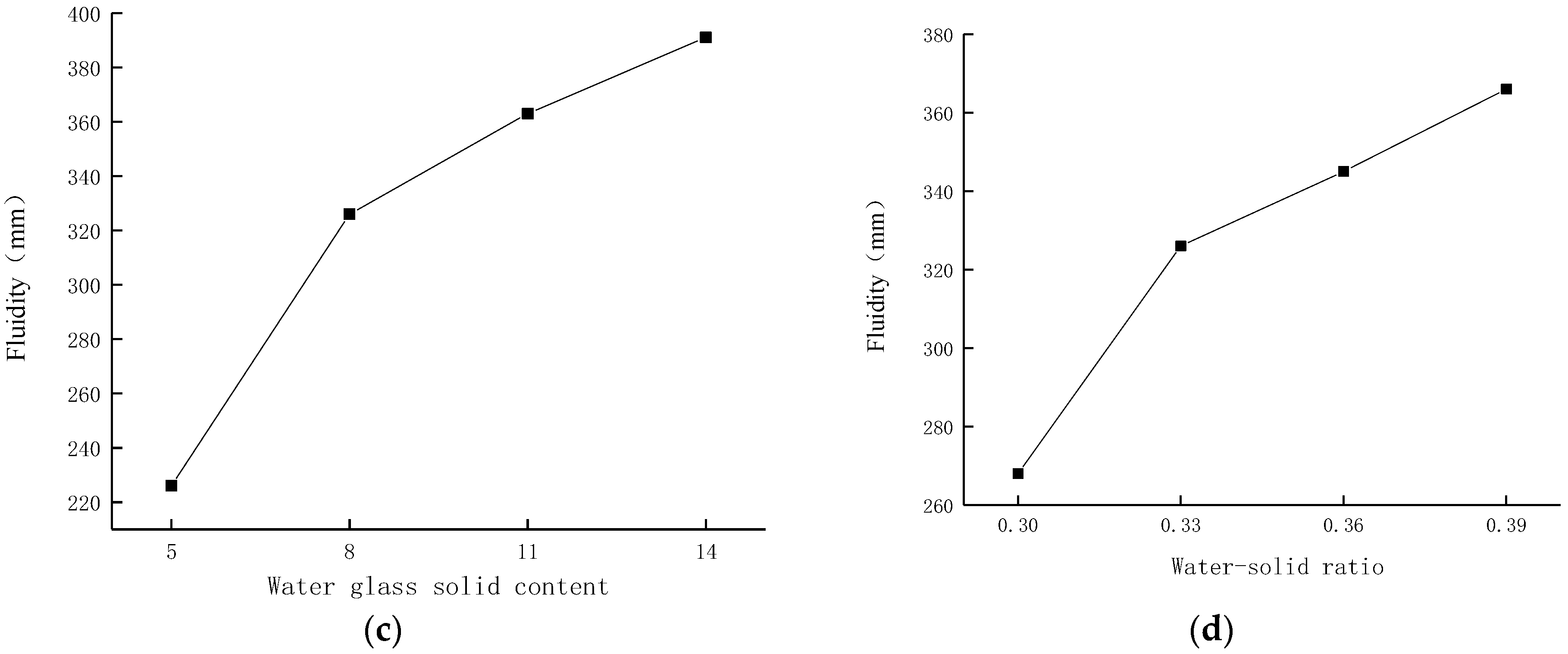
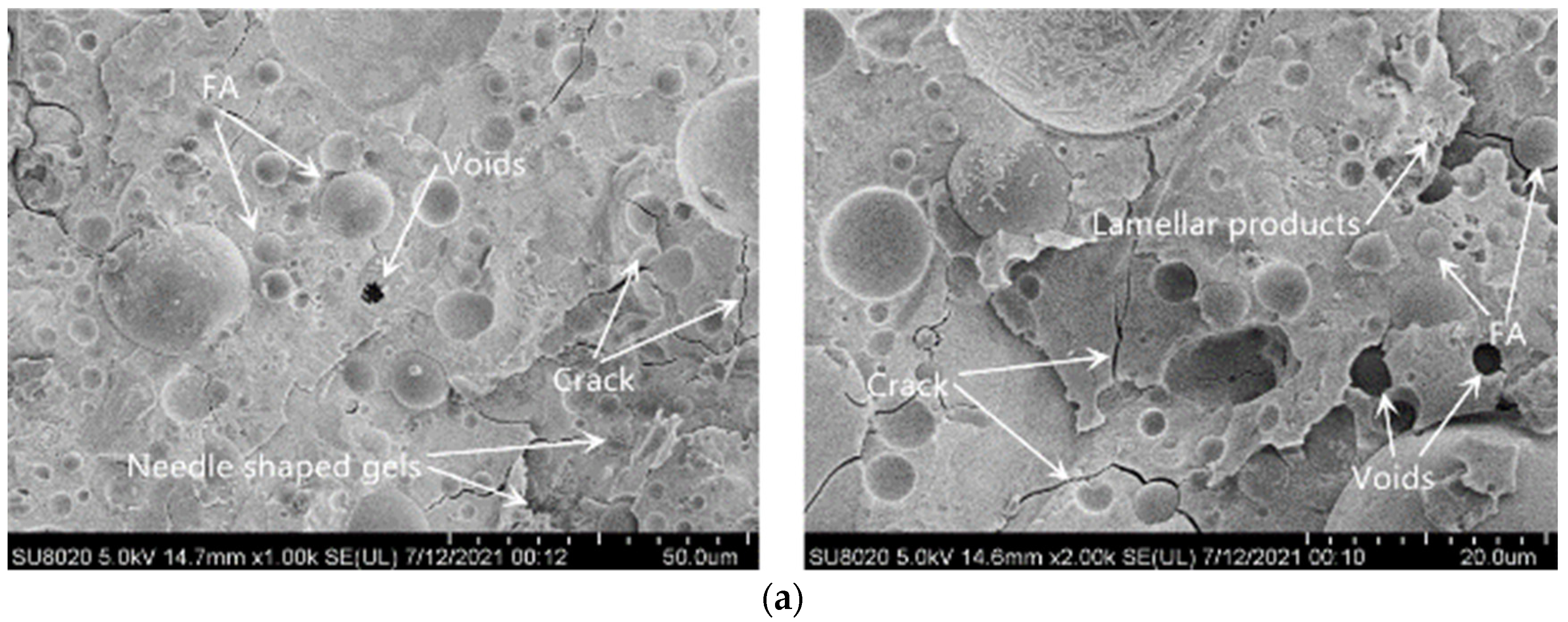
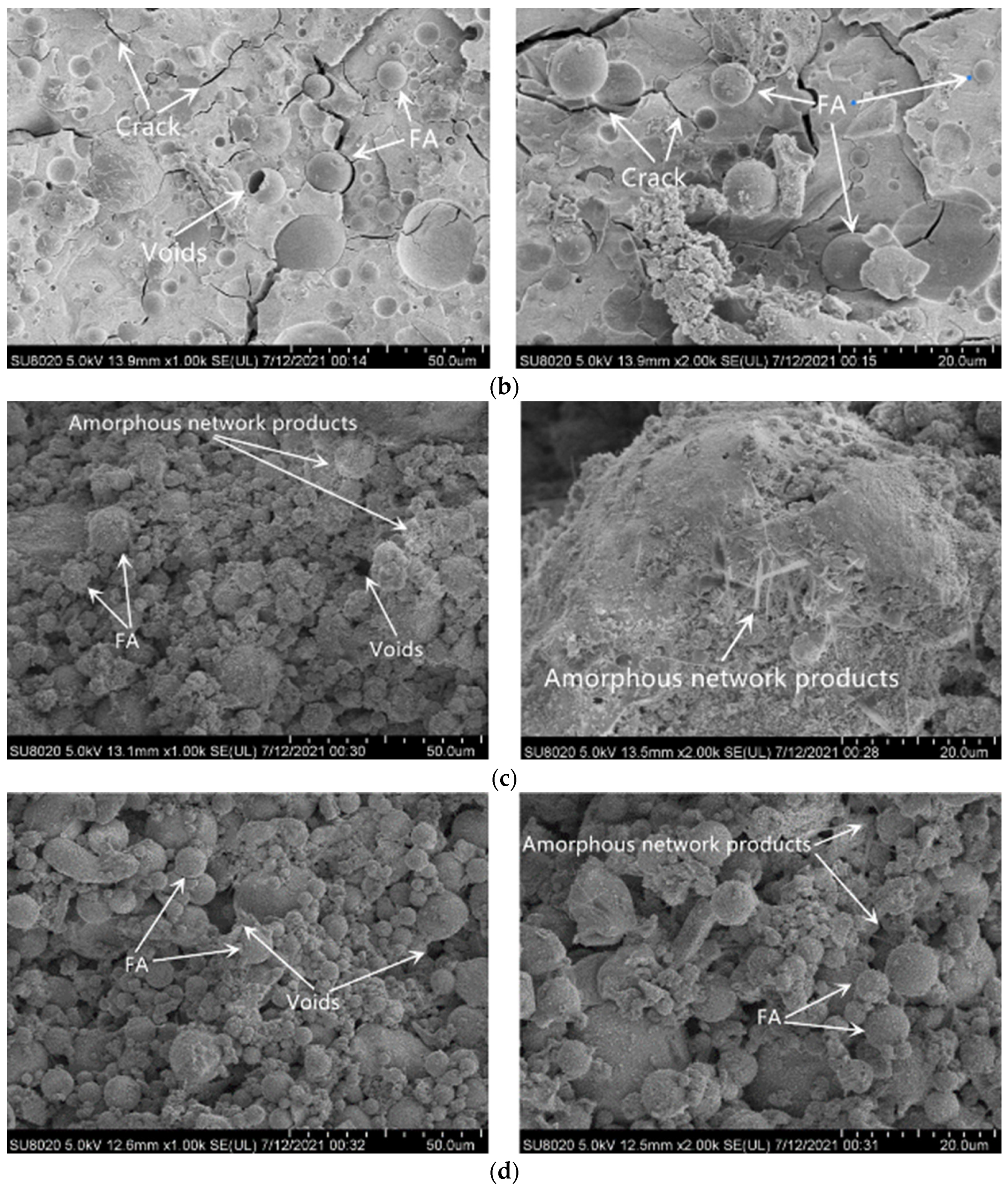
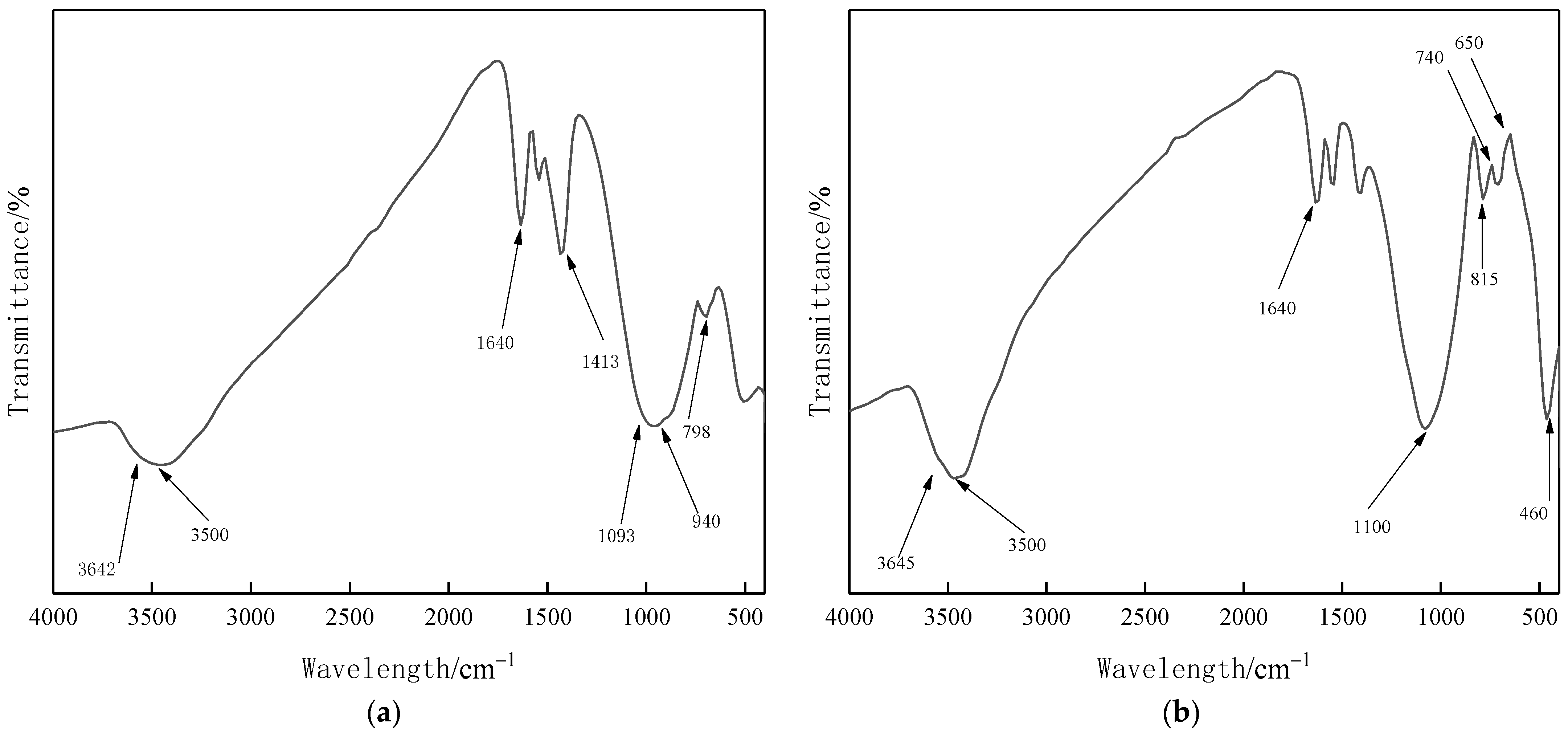
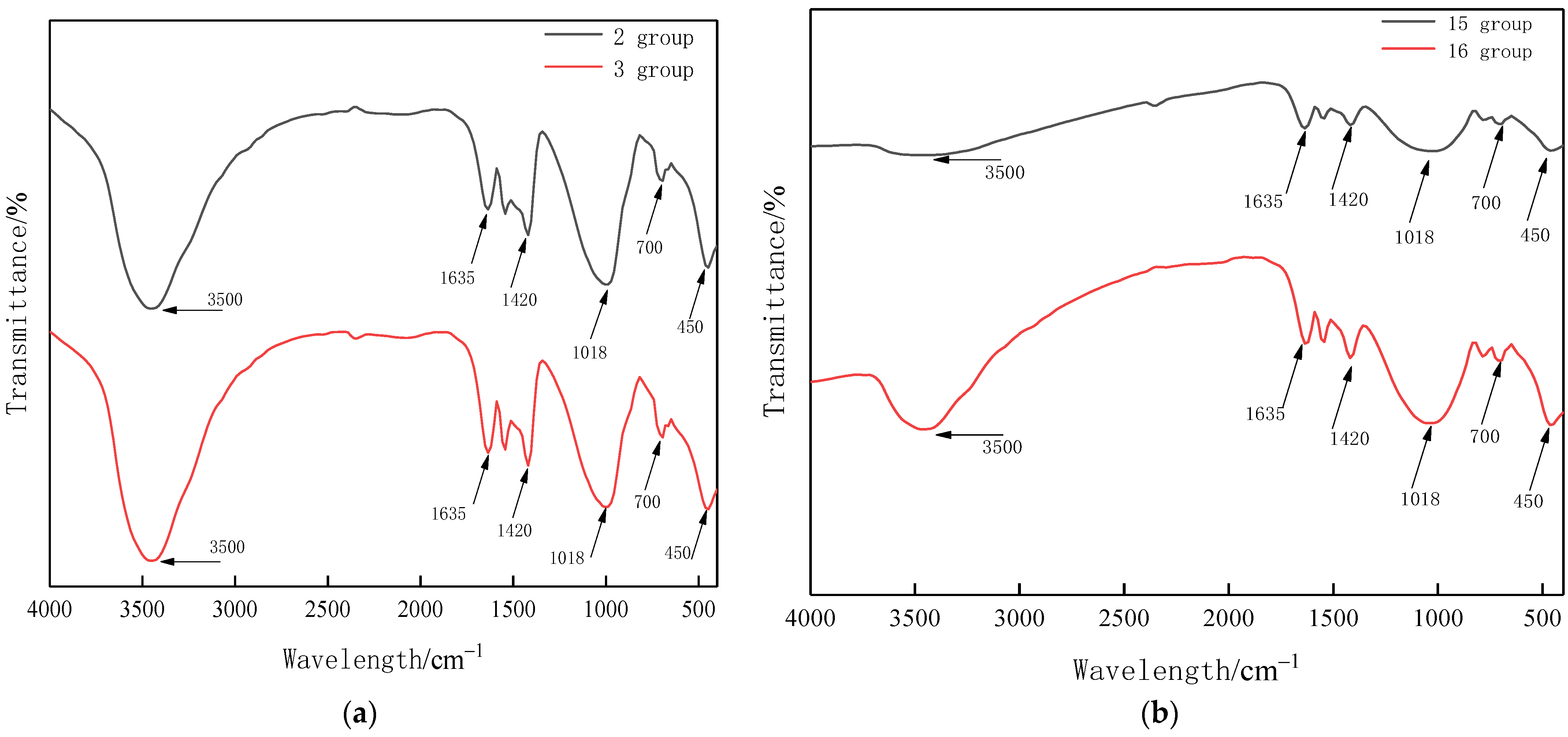
| Test Items | Unit | Standard Indicator | Test Result |
|---|---|---|---|
| Density | g/cm3 | ≥2.8 | 2.8 |
| Specific surface area | m2/kg | ≥350 | 350 |
| 7 d activity index | % | ≥75 | 76 |
| 28 d activity index | % | ≥95 | 96 |
| Mobility ratio | % | ≥90 | 90 |
| Moisture content (mass fraction) | % | ≤1.0 | 1.0 |
| Sulfur trioxide (mass fraction) | % | ≤4.0 | 4.0 |
| Chloride (mass fraction) | % | ≤0.06 | 0.02 |
| Loss on ignition (mass fraction) | % | ≤3.0 | 3.0 |
| Test Items | Unit | Standard Indicator | Test Result |
|---|---|---|---|
| Fineness | % | ≤12.0 | 10.1 |
| Water demand | % | ≤95 | 92 |
| Loss on ignition | % | ≤5.0 | 2.2 |
| Water content | % | ≤1.0 | 0.1 |
| Density | g/cm3 | ≤3.0 | 2.3 |
| Sulfur trioxide | % | ≤4.0 | 1.2 |
| Intensity activity index | % | ≥70.0 | 75.9 |
| Test Items | Unit | Standard Indicator | Test Result |
|---|---|---|---|
| Exterior | -- | Liquid sodium silicate is a slightly colored translucent viscous liquid | Slightly colored translucent viscous liquid |
| Baume degree (20 °C) | Be | 49–51 | 50 |
| Iron (Fe) content | % | ≤0.02 | 0.01 |
| Water-insoluble content | % | ≤0.20 | 0.06 |
| Density (20 °C) | g/cm3 | 1.52–1.59 | 1.53 |
| Sodium oxide (Na2O) content | % | ≥12.8 | 13.73 |
| Silicon dioxide (SiO2) content | % | ≥29.2 | 32.35 |
| Modulus (M) | -- | 2.2–2.5 | 2.43 |
| Level | (A) Fly Ash Content | (B) Water Glass Modulus | (C) Water Glass Solid Content | (D) Water–Solid Ratio |
|---|---|---|---|---|
| 1 | 60% | 1.0 | 5% | 0.30 |
| 2 | 70% | 1.2 | 8% | 0.33 |
| 3 | 80% | 1.4 | 11% | 0.36 |
| 4 | 90% | 1.6 | 14% | 0.39 |
| Level | (A) Fly Ash Content | (B) Water Glass Modulus | (C) Water Glass Solid Content | (D) Water–Solid Ratio |
|---|---|---|---|---|
| 1 | 60% | 1.0 | 5% | 0.30 |
| 2 | 60% | 1.2 | 8% | 0.33 |
| 3 | 60% | 1.4 | 11% | 0.36 |
| 4 | 60% | 1.6 | 14% | 0.39 |
| 5 | 70% | 1.0 | 8% | 0.36 |
| 6 | 70% | 1.2 | 5% | 0.39 |
| 7 | 70% | 1.4 | 14% | 0.30 |
| 8 | 70% | 1.6 | 11% | 0.33 |
| 9 | 80% | 1.0 | 11% | 0.39 |
| 10 | 80% | 1.2 | 14% | 0.36 |
| 11 | 80% | 1.4 | 5% | 0.33 |
| 12 | 80% | 1.6 | 8% | 0.30 |
| 13 | 90% | 1.0 | 14% | 0.33 |
| 14 | 90% | 1.2 | 11% | 0.30 |
| 15 | 90% | 1.4 | 8% | 0.39 |
| 16 | 90% | 1.6 | 5% | 0.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Jia, Y.; Liu, J. Influence of Different Parameters on the Performance of Alkali-Activated Slag/Fly Ash Composite System. Materials 2022, 15, 2714. https://doi.org/10.3390/ma15082714
Zhang Z, Jia Y, Liu J. Influence of Different Parameters on the Performance of Alkali-Activated Slag/Fly Ash Composite System. Materials. 2022; 15(8):2714. https://doi.org/10.3390/ma15082714
Chicago/Turabian StyleZhang, Zhipeng, Yanmin Jia, and Jinliang Liu. 2022. "Influence of Different Parameters on the Performance of Alkali-Activated Slag/Fly Ash Composite System" Materials 15, no. 8: 2714. https://doi.org/10.3390/ma15082714
APA StyleZhang, Z., Jia, Y., & Liu, J. (2022). Influence of Different Parameters on the Performance of Alkali-Activated Slag/Fly Ash Composite System. Materials, 15(8), 2714. https://doi.org/10.3390/ma15082714





