Enhanced Mechanical and Durability Properties of Cement Mortar by Using Alumina Nanocoating on Carbon Nanofibers
Abstract
:1. Introduction
2. Experimental Program
2.1. Materials
2.2. Synthesis of Al2O3 Coating on CNFs
2.3. Fabrication of Mortars
2.4. Characterization Techniques
2.4.1. Surface Characterizations
2.4.2. Thermogravimetric Analysis
2.4.3. X-ray Diffraction Analysis
2.4.4. Fluidity and Compressive Strength Tests
2.4.5. Freeze–Thaw Tests
2.4.6. Drying Shrinkage Tests
3. Results and Discussion
3.1. Transmission Electron Microscopy (TEM)
3.2. Microstructure Analysis
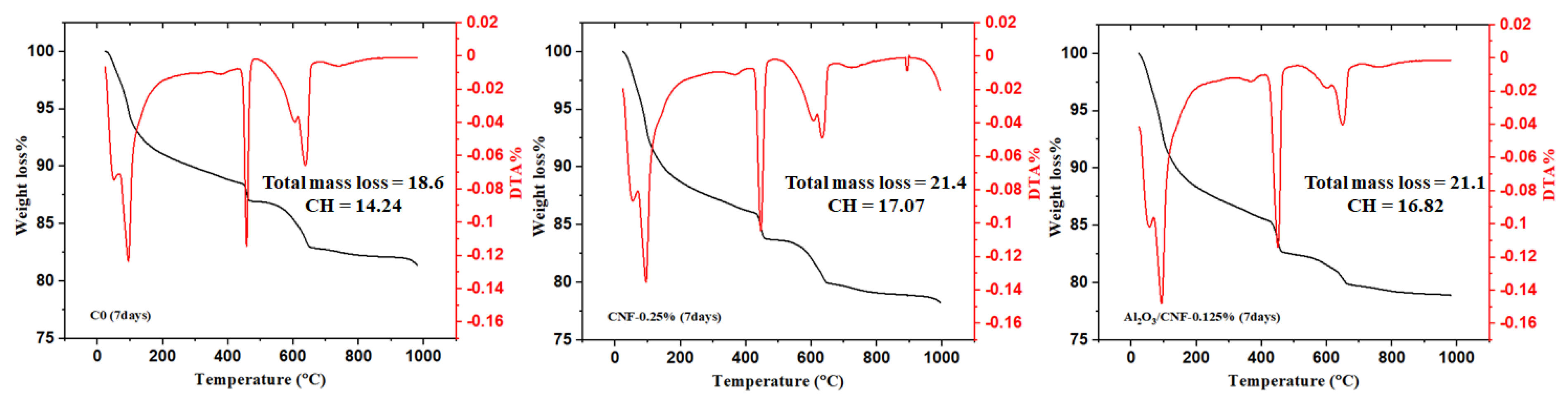
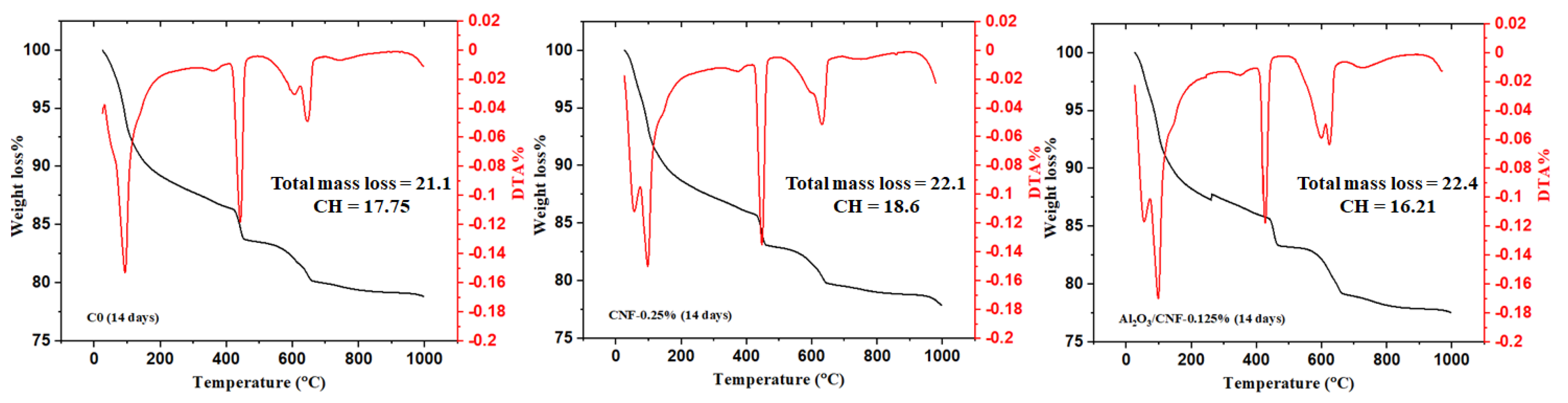
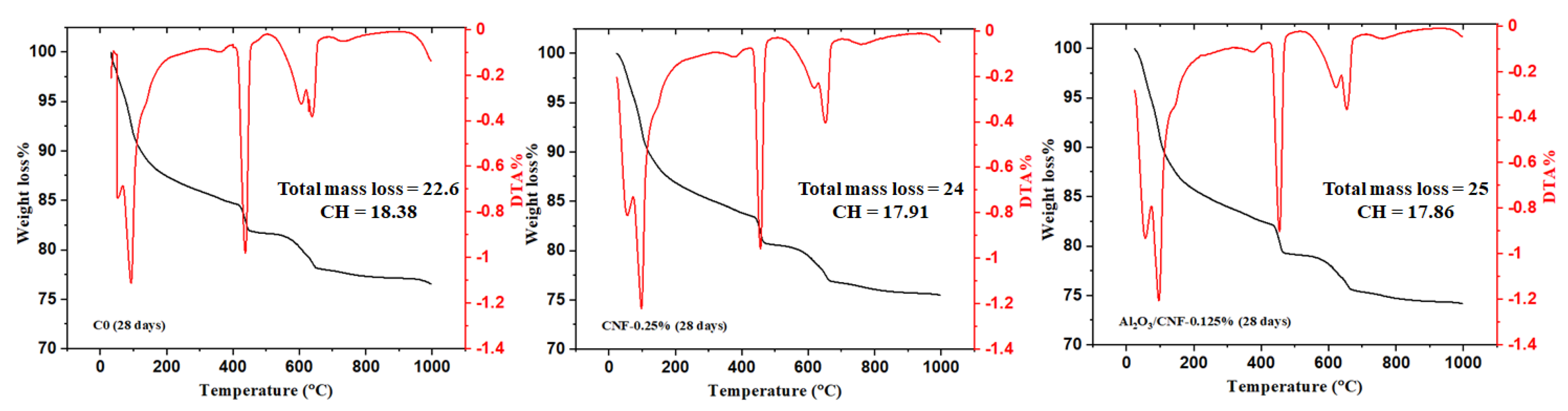
3.3. Cement Hydration by Thermal Analysis
3.4. X-ray Diffraction (XRD)
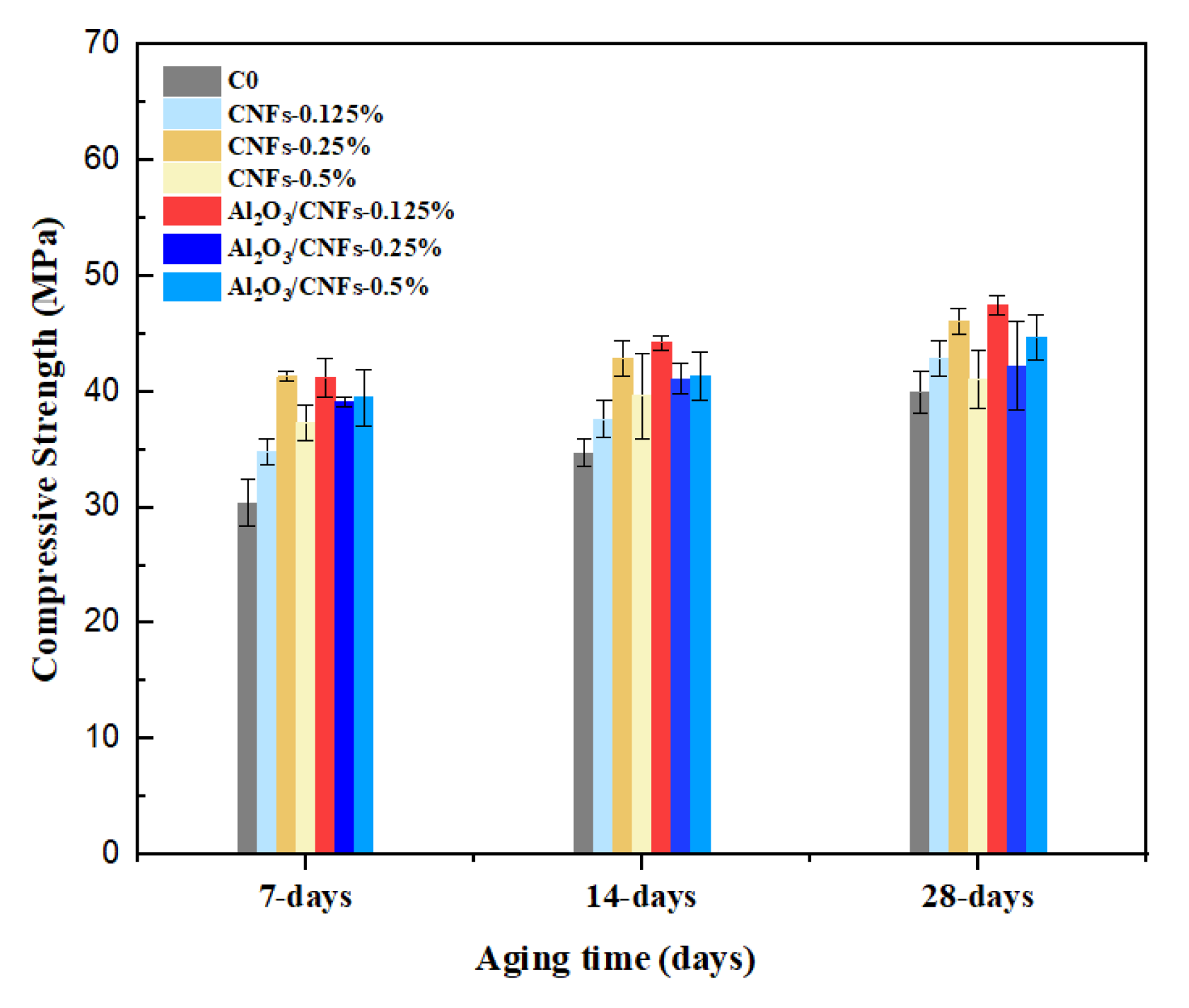
3.5. Compressive Strength
3.6. Fluidity
3.7. Freeze–Thaw Effects
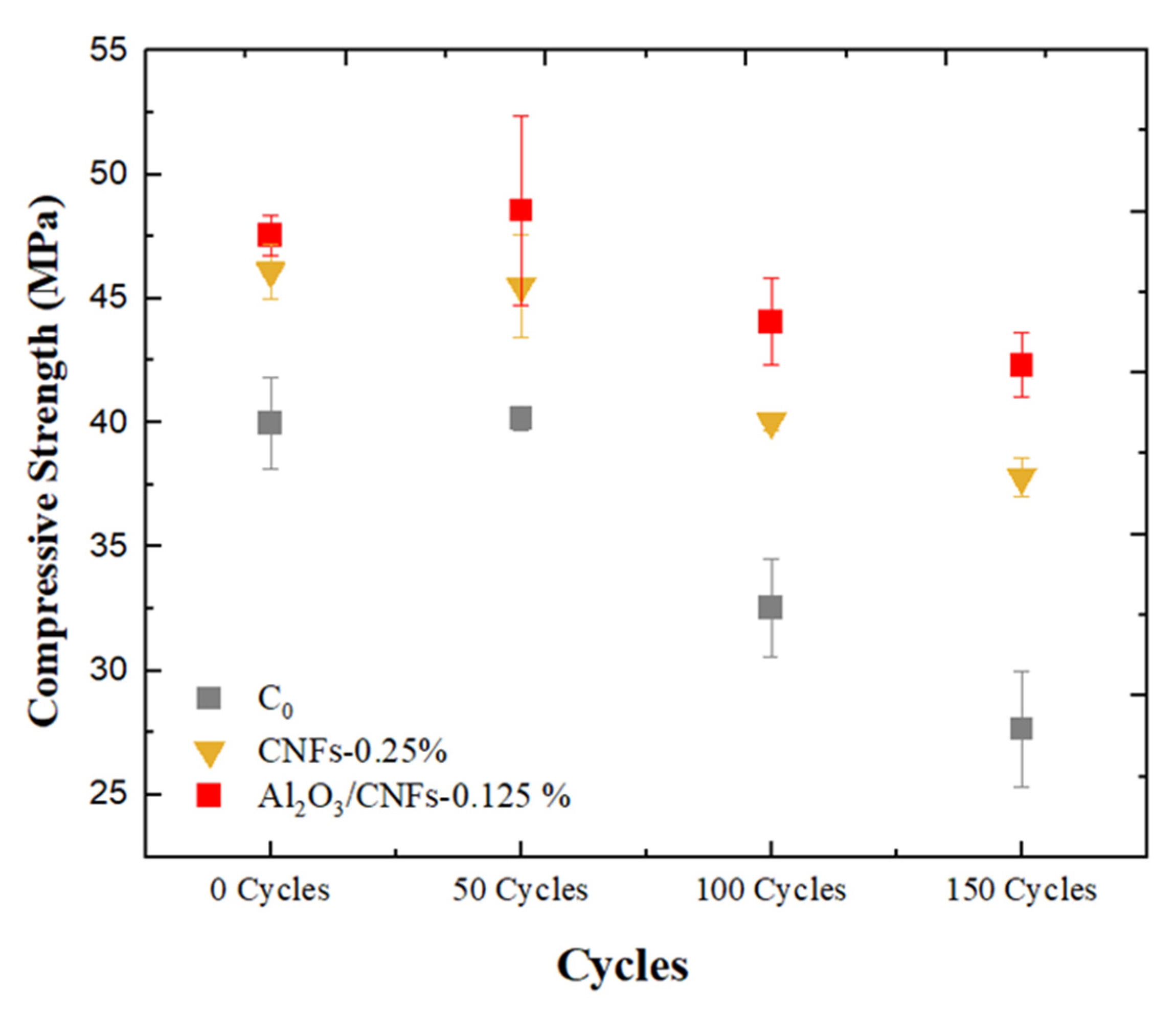
3.7.1. Compressive Strength
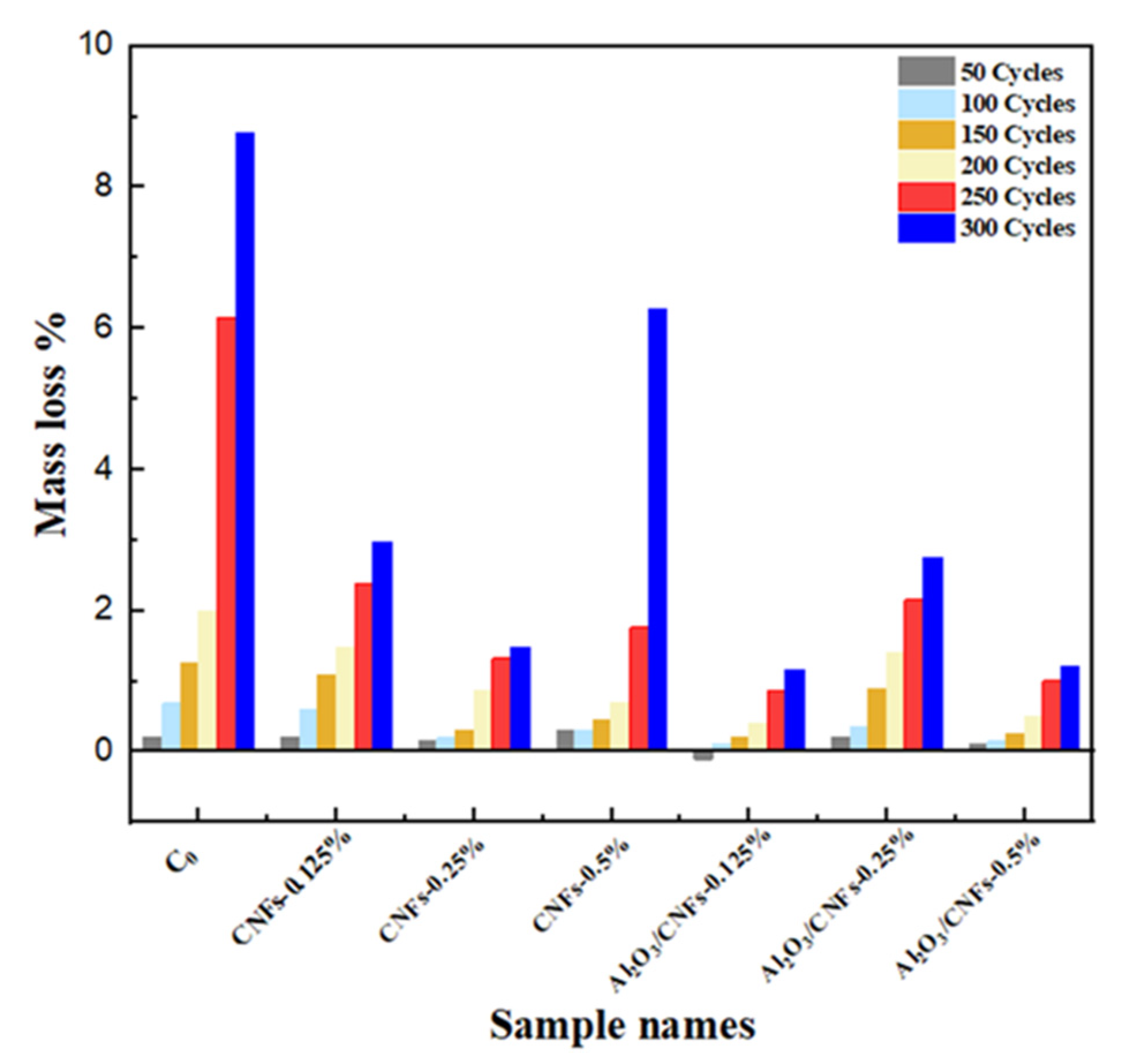
3.7.2. Mass Loss
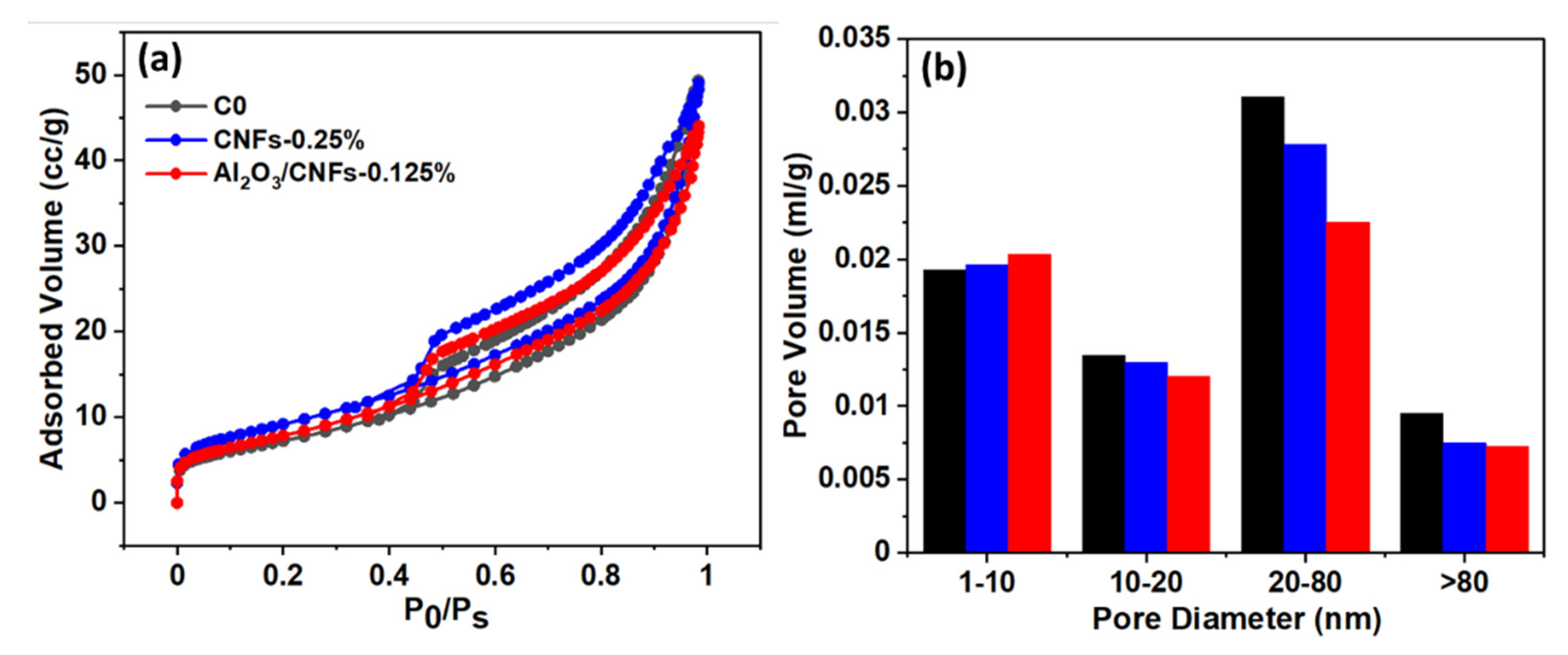
3.7.3. Pore Structure
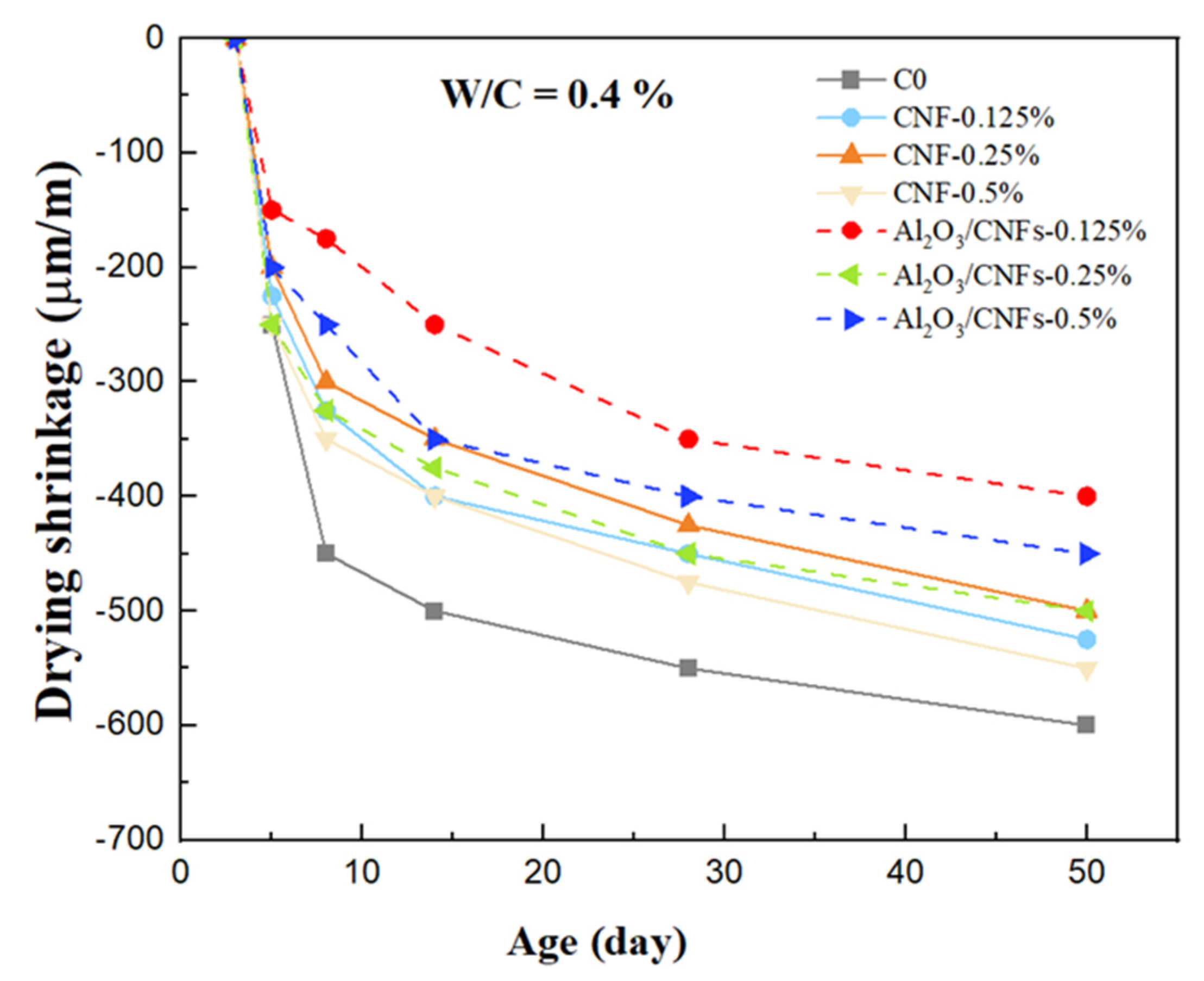
3.8. Drying Shrinkage
4. Limitations of the Study
5. Conclusions
- The TEM images revealed a well-coated layer on the CNF surfaces.
- The SEM results show the nanomaterials were well-distributed in the mortar microstructure.
- The SEM images indicate the formation of the hydration products gels such as CH, C-S-H, and ettringite. In the case of the Al2O3/CNFs-mortar composite, most of the hydrated products were well-distributed C-S-H and were identified from their amorphous appearance, while CNFs-mortar composites showed mostly ettringite and hexagonal CH.
- With increasing curing age, all samples experienced an increase in their compressive strength. The compressive strength for samples that contained Al2O3/CNFs demonstrated progressive improvements compared to other samples evaluated in this research. Relative to control specimens, the addition of Al2O3/CNFs-0.125% increased the strength by 36%, 27%, and 19% at the age of 7, 14, and 28 days, respectively. The alumina porous layer improved the binding between the mortar matrix and the CNFs’ surfaces and altered the hydration process.
- TGA-DTA tests showed all CNFs and Al2O3/CNF composites exhibited higher degrees of hydration than the control sample. Decreasing calcium hydroxide was observed at an age of 28 days with the addition of the nanomaterials, with a significant effect for the case of coated CNFs. This could be due to the nucleation effect, which produced a good distribution of the gel hydration of C-S-H inside the mortar matrix, which restricts the growth of the CH. Additionally, this may be due to the pozzolanic effect of the Al2O3 layer. There might be some minor overlapping in the signal from CNFs burning and CH diminishing.
- The XRD measurement at the age of 28 days focused on the intensity of two cementitious moieties: the C2S and C3S to be consumed in the hydration reaction. Al2O3/CNFs-0.125% showed the lowest intensity of these two compounds, which indicates that they increased the dissolution of C3S and C2S, and thus more C-S-H was produced. In addition, XRD data focused on the intensity of CH, as it showed lower intensity in the case of CNFs-0.25% and Al2O3/CNFs 0.125% composites. These results are in good agreement with TGA and EDS analyses findings.
- The addition of Al2O3/CNFs-0.125% and Al2O3/CNFs-0.5% showed a significant low mass loss of about 1% after 300 freeze–thaw cycles, while the reference sample and CNFs-0.25 showed a mass loss of 9% and 3%, respectively.
- The compressive strength degradation was the lowest in the case of the Al2O3/CNFs-0.125% sample after 150 freeze–thaw cycles at about 14%, while the control and CNFs-0.25% samples showed a degradation of about 45 and 22%, respectively. Such results were attributed to the highly dense microstructure, which was later confirmed by the BET measurements. The BET and BJH tests exhibited low gas adsorption and more refinement in the capillary pores.
- Incorporation of the optimum amounts of CNFs (0.25%) and Al2O3/CNFs (0.125%) led to a decrease in the drying shrinkage due to increasing the stiffness and density of these composites. In addition, the hydrophilicity of the alumina layer explained the significant drying shrinkage mitigation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ateş, A. Mechanical properties of sandy soils reinforced with cement and randomly distributed glass fibers (GRC). Compos. Part B Eng. 2016, 96, 295–304. [Google Scholar] [CrossRef]
- Balaguru, P.N.; Shah, S.P. Fiber-Reinforced Cement Composites; McGraw-Hill: New York, NY, USA, 1992. [Google Scholar]
- Bentur, A.; Mindess, S. Fibre Reinforced Cementitious Composites; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Banthia, N.; Sappakittipakorn, M. Toughness enhancement in steel fiber reinforced concrete through fiber hybridization. Cem. Concr. Res. 2007, 37, 1366–1372. [Google Scholar] [CrossRef]
- Pigeon, M. Durability of Concrete in Cold Climates; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- del Carmen Camacho, M.; Galao, O.; Baeza, F.J.; Zornoza, E.; Garcés, P. Mechanical properties and durability of CNT cement composites. Mater. Des. 2014, 7, 1640–1651. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Ballesta, C.; Galao, Ó.; Baeza, F.J.; Zornoza, E.; Garcés, P. Durability and Mechanical Properties of CNT Cement Composites; Springer International Publishing: Cham, Switzerland, 2019; pp. 31–41. [Google Scholar]
- Nivethitha, D.; Srividhya, S.; Dharmar, S. Review on mechanical properties of cement mortar enhanced with nanoparticles. Int. J. Sci. Res. Dev. News R D News 2016, 5, 913–916. [Google Scholar]
- Wang, T.; Xu, J.; Meng, B.; Peng, G. Experimental study on the effect of carbon nanofiber content on the durability of concrete. Constr. Build. Mater. 2020, 250, 118891. [Google Scholar] [CrossRef]
- Chuah, S.; Pan, Z.; Sanjayan, J.G.; Wang, C.M.; Duan, W.H. Nano reinforced cement and concrete composites and new perspective from graphene oxide. Constr. Build. Mater. 2014, 73, 113–124. [Google Scholar] [CrossRef]
- Wang, F.; Kong, X.; Jiang, L.; Wang, D. The acceleration mechanism of nano-C-S-H particles on OPC hydration. Constr. Build. Mater. 2020, 249, 118734. [Google Scholar] [CrossRef]
- Ebrahimi, K.; Daiezadeh, M.J.; Zakertabrizi, M.; Zahmatkesh, F.; Korayem, A.H. A review of the impact of micro- and nanoparticles on freeze-thaw durability of hardened concrete: Mechanism perspective. Constr. Build. Mater. 2018, 186, 1105–1113. [Google Scholar] [CrossRef]
- Kumar, S.; Kolay, P.; Malla, S.; Mishra, S. Effect of Multiwalled Carbon Nanotube in Cement Composite on Mechanical Strength and Freeze-Thaw Susceptibility. Adv. Civ. Eng. Mater. 2015, 4, 257–274. [Google Scholar] [CrossRef]
- Lee, H.; Jeong, S.; Cho, S.; Chung, W. Enhanced bonding behavior of multi-walled carbon nanotube cement composites and reinforcing bars. Compos. Struct. 2020, 243, 112201. [Google Scholar] [CrossRef]
- Al-Rub, R.K.A.; Ashour, A.I.; Tyson, B.M. On the aspect ratio effect of multi-walled carbon nanotube reinforcements on the mechanical properties of cementitious nanocomposites. Constr. Build. Mater. 2012, 35, 647–655. [Google Scholar] [CrossRef]
- Cwirzen, A.; Habermehl-Cwirzen, K. The effect of carbon nano-and microfibers on strength and residual cumulative strain of mortars subjected to freeze-thaw cycles. J. Adv. Concr. Technol. 2013, 11, 80–88. [Google Scholar] [CrossRef] [Green Version]
- Metaxa, Z.S.; Konsta-Gdoutos, M.S.; Shah, S.P. Carbon Nanofiber–Reinforced Cement-Based Materials. Transp. Res. Rec. J. Transp. Res. Board 2010, 2142, 114–118. [Google Scholar] [CrossRef]
- Shi, T.; Li, Z.; Guo, J.; Gong, H.; Gu, C. Research progress on CNTs/CNFs-modified cement-based composites—A review. Constr. Build. Mater. 2019, 202, 290–307. [Google Scholar] [CrossRef]
- Givi, A.N.; Rashid, S.A.; Aziz, F.N.A.; Salleh, M.A.M. Experimental investigation of the size effects of SiO2 nano-particles on the mechanical properties of binary blended concrete. Compos. Part B Eng. 2010, 41, 673–677. [Google Scholar] [CrossRef]
- Reches, Y. Nanoparticles as concrete additives: Review and perspectives. Constr. Build. Mater. 2018, 175, 483–495. [Google Scholar] [CrossRef]
- Behfarnia, K.; Salemi, N. The effects of nano-silica and nano-alumina on frost resistance of normal concrete. Constr. Build. Mater. 2013, 48, 580–584. [Google Scholar] [CrossRef]
- El-Gamal, S.M.A.; Abo-El-Enein, S.A.; El-Hosiny, F.I.; Amin, M.S.; Ramadan, M. Thermal resistance, microstructure and mechanical properties of type I Portland cement pastes containing low-cost nanoparticles. J. Therm. Anal. 2018, 131, 949–968. [Google Scholar] [CrossRef]
- Nazari, A.; Riahi, S.; Riahi, S.; Shamekhi, S.F.; Khademno, A. Influence of Al2O3 nanoparticles on the compressive strength and workability of blended concrete. J. Am. Sci. 2010, 6, 6–9. [Google Scholar]
- Muzenski, S.; Flores-Vivian, I.; Sobolev, K. Ultra-high strength cement-based composites designed with aluminum oxide nano-fibers. Constr. Build. Mater. 2019, 220, 177–186. [Google Scholar] [CrossRef]
- Fu, X.; Lu, W.; Chung, D.D.L. Ozone treatment of carbon fiber for reinforcing cement. Carbon 1998, 36, 1337–1345. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, Y.; Yuan, L.; Xu, D.; Du, P.; Hou, P.; Zhou, Z.; Cheng, X.; Liu, S.; Wang, Y. Effect of nano-silica on chemical and volume shrinkage of cement-based composites. Constr. Build. Mater. 2020, 247, 118529. [Google Scholar] [CrossRef]
- Abdel-Gawwad, H.A.; Mohammed, M.S.; Alomayri, T. Single and dual effects of magnesia and alumina nano-particles on strength and drying shrinkage of alkali activated slag. Constr. Build. Mater. 2019, 228, 116827. [Google Scholar] [CrossRef]
- Sanchez, F.; Ince, C. Microstructure and macroscopic properties of hybrid carbon nanofiber/silica fume cement composites. Compos. Sci. Technol. 2009, 69, 1310–1318. [Google Scholar] [CrossRef]
- Yazdanbakhsh, A.; Grasley, Z. Utilization of Silica Fume to Stabilize the Dispersion of Carbon Nanofilaments in Cement Paste. J. Mater. Civ. Eng. 2014, 26, 06014010. [Google Scholar] [CrossRef]
- Stynoski, P.; Mondal, P.; Marsh, C. Effects of silica additives on fracture properties of carbon nanotube and carbon fiber reinforced Portland cement mortar. Cem. Concr. Compos. 2015, 55, 232–240. [Google Scholar] [CrossRef]
- Sikora, P.; Elrahman, M.A.; Chung, S.-Y.; Cendrowski, K.; Mijowska, E.; Stephan, D. Mechanical and microstructural properties of cement pastes containing carbon nanotubes and carbon nanotube-silica core-shell structures, exposed to elevated temperature. Cem. Concr. Compos. 2019, 95, 193–204. [Google Scholar] [CrossRef]
- Garg, M.; Das, C.S.; Gupta, R. Use of silica particles to improve dispersion of -COOH CNTs/carbon fibers to produce HyFRCC. Constr. Build. Mater. 2020, 250, 118777. [Google Scholar] [CrossRef]
- Kim, H.-K.; Nam, I.; Lee, H. Enhanced effect of carbon nanotube on mechanical and electrical properties of cement composites by incorporation of silica fume. Compos. Struct. 2014, 107, 60–69. [Google Scholar] [CrossRef]
- Dong, S.; Wang, D.; Ashour, A.; Han, B.; Ou, J. Nickel plated carbon nanotubes reinforcing concrete composites: From nano/micro structures to macro mechanical properties. Compos. Part A: Appl. Sci. Manuf. 2021, 141, 106228. [Google Scholar] [CrossRef]
- Jasim, A.M.; He, X.; White, T.A.; Xing, Y. Nano-layer deposition of metal oxides via a condensed water film. Commun. Mater. 2020, 1, 9. [Google Scholar]
- ASTM C1437-13. Standard Test Method for Flow of Hydraulic Cement Mortar. ASTM: West Conshohocken, PA, USA, 2013.
- ASTM C109/C109M. Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or (50-mm) Cube Specimens). ASTM: West Conshohocken, PA, USA, 1999; Volume 2.
- ASTM C666–03. Standard Test Method for Resistance of Concrete to Rapid Freezing and Thawing. American Society for Testing Materials International: West Conshohocken, PA, USA, 2008.
- ASTM C596-18. Standard Test Method for Drying Shrinkage of Mortar Containing Hydraulic Cement. ASTM: West Conshohocken, PA, USA, 2018.
- ASTM C490-07. Standard Practice for Use of Apparatus for the Determination of Length Change of Hardened Cement Paste, Mortar, and Concrete. ASTM: West Conshohocken, PA, USA, 2011.
- da Silva Andrade, D.; da Silva Rêgo, J.H.; Morais, P.C.; de Mendonça Lopes, A.N.; Rojas, M.F. Investigation of C-S-H in ternary cement pastes containing nanosilica and highly-reactive supplementary cementitious materials (SCMs): Microstructure and strength. Constr. Build. Mater. 2019, 198, 445–455. [Google Scholar] [CrossRef]
- Al Qader, H.J.N. Enhancing the Mechanical and Durability Properties of Cement Mortars by using Alumina Nanocoating on Carbon Nanofibers. Master Thesis, University of Missouri, Columbia, MO, USA, 2021. [Google Scholar]
- Kumar, S.; Rath, T.; Mahaling, R.N.; Das, C.K. Processing and characterization of carbon nanofiber/syndiotactic polystyrene composites in the absence and presence of liquid crystalline polymer. Compos. Part A Appl. Sci. Manuf. 2007, 38, 1304–1317. [Google Scholar] [CrossRef]
- Deboucha, W.; Leklou, N.; Khelidj, A.; Oudjit, M.N. Hydration development of mineral additives blended cement using thermogravimetric analysis (TGA): Methodology of calculating the degree of hydration. Constr. Build. Mater. 2017, 146, 687–701. [Google Scholar] [CrossRef]
- Makar, J.M.; Chan, G.W. Growth of Cement Hydration Products on Single-Walled Carbon Nanotubes. J. Am. Ceram. Soc. 2009, 92, 1303–1310. [Google Scholar] [CrossRef]
- Tafesse, M.; Kim, H.-K. The role of carbon nanotube on hydration kinetics and shrinkage of cement composite. Compos. Part B: Eng. 2019, 169, 55–64. [Google Scholar] [CrossRef]
- Mahinroosta, M.; Allahverdi, A. A Scoping Review on Integrating Inorganic Nanomaterials into Cement Composites. Adv. Civ. Eng. Mater. 2019, 8, 526–553. [Google Scholar] [CrossRef]
- Fehervari, A.; MacLeod, A.J.N.; Garcez, E.O.; Aldridge, L.; Gates, W.P.; Yang, Y.; Collins, F. On the mechanisms for improved strengths of carbon nanofiber-enriched mortars. Cem. Concr. Res. 2020, 136, 106178. [Google Scholar] [CrossRef]
- Peyvandi, A.; Soroushian, P.; Abdol, N.; Balachandra, A.M. Surface-modified graphite nanomaterials for improved reinforcement efficiency in cementitious paste. Carbon 2013, 63, 175–186. [Google Scholar] [CrossRef]
- Li, G.Y.; Wang, P.M.; Zhao, X. Mechanical behavior and microstructure of cement composites incorporating surface-treated multi-walled carbon nanotubes. Carbon 2005, 43, 1239–1245. [Google Scholar] [CrossRef]
- Bharj, J. Experimental study on compressive strength of cement-CNT composite paste. Indian J. Pure Appl. Phys. 2015, 52, 35–38. [Google Scholar]
- Gao, D.; Sturm, M.; Mo, Y. Electrical resistance of carbon-nanofiber concrete. Smart Mater. Struct. 2009, 18, 095039. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Lin, S.; Yan, J.; Du, S. Effects of nano-SiO2 coated multi-walled carbon nanotubes on mechanical properties of cement-based composites. Constr. Build. Mater. 2021, 281, 122577. [Google Scholar] [CrossRef]
- Liu, J.; Suh, H.; Jee, H.; Xu, J.; Nezhad, E.Z.; Choi, C.-S.; Bae, S. Synergistic effect of carbon nanotube/TiO2 nanotube multi-scale reinforcement on the mechanical properties and hydration process of portland cement paste. Constr. Build. Mater. 2021, 293, 123447. [Google Scholar] [CrossRef]
- Shafaei, D.; Yang, S.; Berlouis, L.; Minto, J. Multiscale pore structure analysis of nano titanium dioxide cement mortar composite. Mater. Today Commun. 2020, 22, 100779. [Google Scholar] [CrossRef]
- Vardharajula, S.; Ali, S.Z.; Tiwari, P.M.; Eroğlu, E.; Vig, K.; Dennis, V.A.; Singh, S.R. Functionalized carbon nanotubes: Biomedical applications. Int. J. Nanomed. 2012, 7, 5361. [Google Scholar]
- Meng, W.; Khayat, K.H. Effect of graphite nanoplatelets and carbon nanofibers on rheology, hydration, shrinkage, mechanical properties, and microstructure of UHPC. Cem. Concr. Res. 2018, 105, 64–71. [Google Scholar] [CrossRef]
- Engbert, A.; Plank, J. Impact of sand and filler materials on the hydration behavior of calcium aluminate cement. J. Am. Ceram. Soc. 2021, 104, 1067–1075. [Google Scholar] [CrossRef]
- Reches, Y.; Thomson, K.; Helbing, M.; Kosson, D.S.; Sanchez, F. Agglomeration and reactivity of nanoparticles of SiO2, TiO2, Al2O3, Fe2O3, and clays in cement pastes and effects on compressive strength at ambient and elevated temperatures. Constr. Build. Mater. 2018, 167, 860–873. [Google Scholar] [CrossRef]
- Young, J. Physical mechanisms and their mathematical descriptions. In Mathematical Modelling of Creep and Shrinkage of Concrete; Bazant, Z.P., Ed.; Wiley: Chichester, UK, 1988; pp. 63–98. [Google Scholar]
- Hawreen, A.; Bogas, J.; Dias, A.P.S. On the mechanical and shrinkage behavior of cement mortars reinforced with carbon nanotubes. Constr. Build. Mater. 2018, 168, 459–470. [Google Scholar] [CrossRef]
- Liu, X.; Fang, T.; Zuo, J. Effect of Nano-Materials on Autogenous Shrinkage Properties of Cement Based Materials. Symmetry 2019, 11, 1144. [Google Scholar] [CrossRef] [Green Version]






| Sample Designation | Water-to-Cement Ratio (w/c) | Sand-to-Cement Ratio (s/c) | Mix Proportion (% Weight) | Fluidity (mm) | CNF Content (%wt) * | ||
|---|---|---|---|---|---|---|---|
| CNFs | Al2O3/CNFs | Superplasticizer | |||||
| C0 | 0.35 | 2 | - | - | 0.1 | 133 | - |
| CNF-0.125 | 0.35 | 2 | 0.125 | - | 0.1 | 156 | 100 |
| CNF-0.25 | 0.35 | 2 | 0.25 | - | 0.2 | 158 | 100 |
| CNF-0.5 | 0.35 | 2 | 0.5 | - | 0.4 | 164 | 100 |
| Al2O3/CNF-0.125 | 0.35 | 2 | - | 0.125 | 0.1 | 151 | 58 |
| Al2O3/CNF-0.25 | 0.35 | 2 | - | 0.25 | 0.2 | 150 | 58 |
| Al2O3/CNF-0.5 | 0.35 | 2 | - | 0.5 | 0.4 | 161 | 58 |
| Property | Enhancement in Properties | Material Concentration | Reference |
|---|---|---|---|
| Compressive strength | 36% | 0.125% Al2O3/CNFs | This study |
| 19% | 0.5 wt.% MWCNTs | Li et al. [50] | |
| 22% | 0.1 wt.% MWCNTs | Bharj et al. [51] | |
| 42.7% | 0.16 wt.% CNFs | Gao et al. [52] | |
| 25.4% | 0.5 wt.% SiO2/MWCNTs | Shaojie et al. [53] | |
| 20% | 0.125 wt.% MWCNTs/NSs | Pawel et al. [31] | |
| 40% | 0.5 wt.% CNT/TNT | Liu et al. [54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Qader, H.; Jasim, A.M.; Salim, H.; Xing, Y.; Stalla, D. Enhanced Mechanical and Durability Properties of Cement Mortar by Using Alumina Nanocoating on Carbon Nanofibers. Materials 2022, 15, 2768. https://doi.org/10.3390/ma15082768
Al Qader H, Jasim AM, Salim H, Xing Y, Stalla D. Enhanced Mechanical and Durability Properties of Cement Mortar by Using Alumina Nanocoating on Carbon Nanofibers. Materials. 2022; 15(8):2768. https://doi.org/10.3390/ma15082768
Chicago/Turabian StyleAl Qader, Huda, Ahmed M. Jasim, Hani Salim, Yangchuan Xing, and David Stalla. 2022. "Enhanced Mechanical and Durability Properties of Cement Mortar by Using Alumina Nanocoating on Carbon Nanofibers" Materials 15, no. 8: 2768. https://doi.org/10.3390/ma15082768
APA StyleAl Qader, H., Jasim, A. M., Salim, H., Xing, Y., & Stalla, D. (2022). Enhanced Mechanical and Durability Properties of Cement Mortar by Using Alumina Nanocoating on Carbon Nanofibers. Materials, 15(8), 2768. https://doi.org/10.3390/ma15082768






