A pH-Gated Functionalized Hollow Mesoporous Silica Delivery System for Photodynamic Sterilization in Staphylococcus aureus Biofilm
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Regents
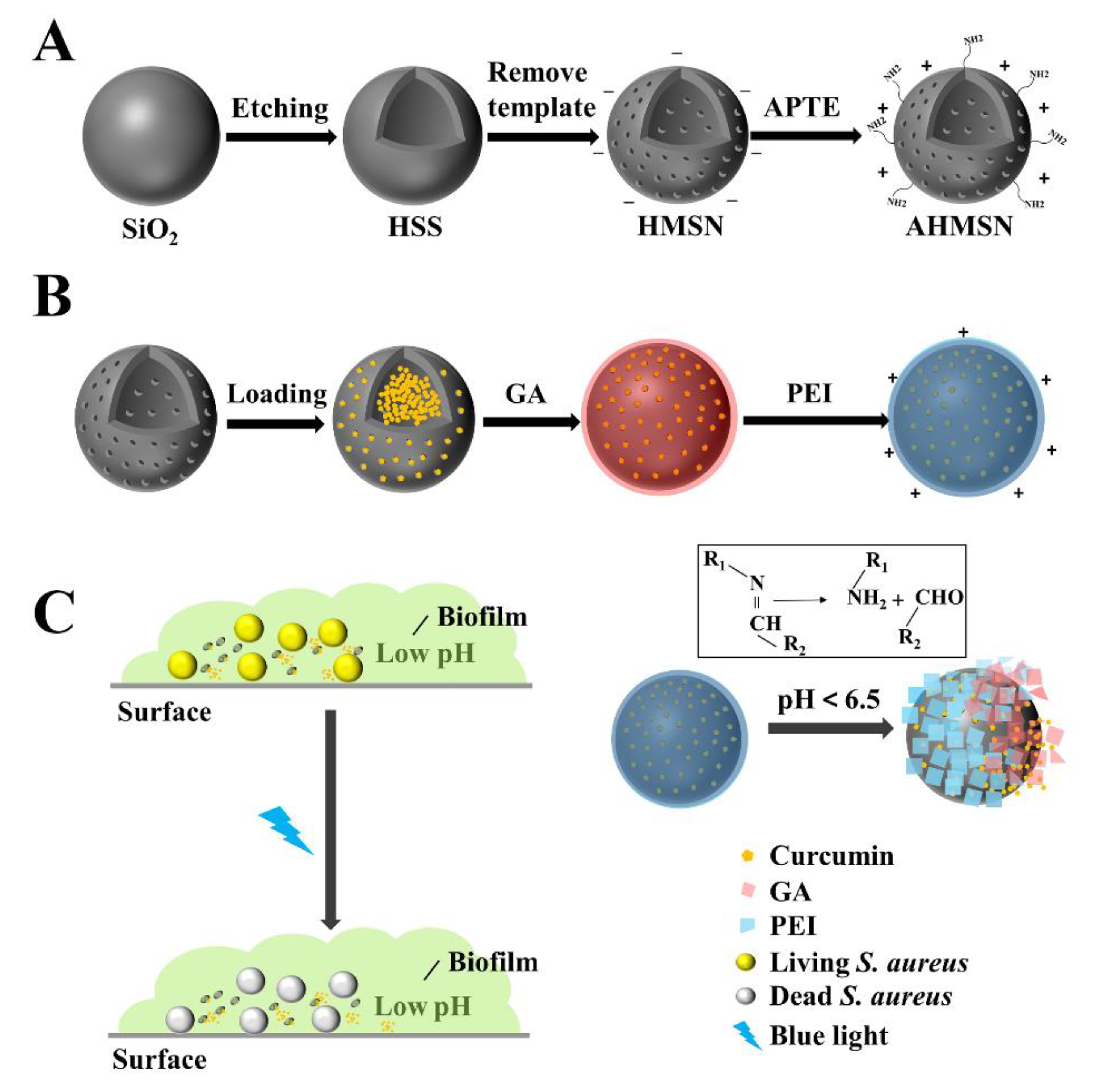
2.2. Synthesis of Functionalized Hollow Mesoporous Silica Nanoparticles Loaded with Curcumin (AHMSN@GA@PEI@Cur)
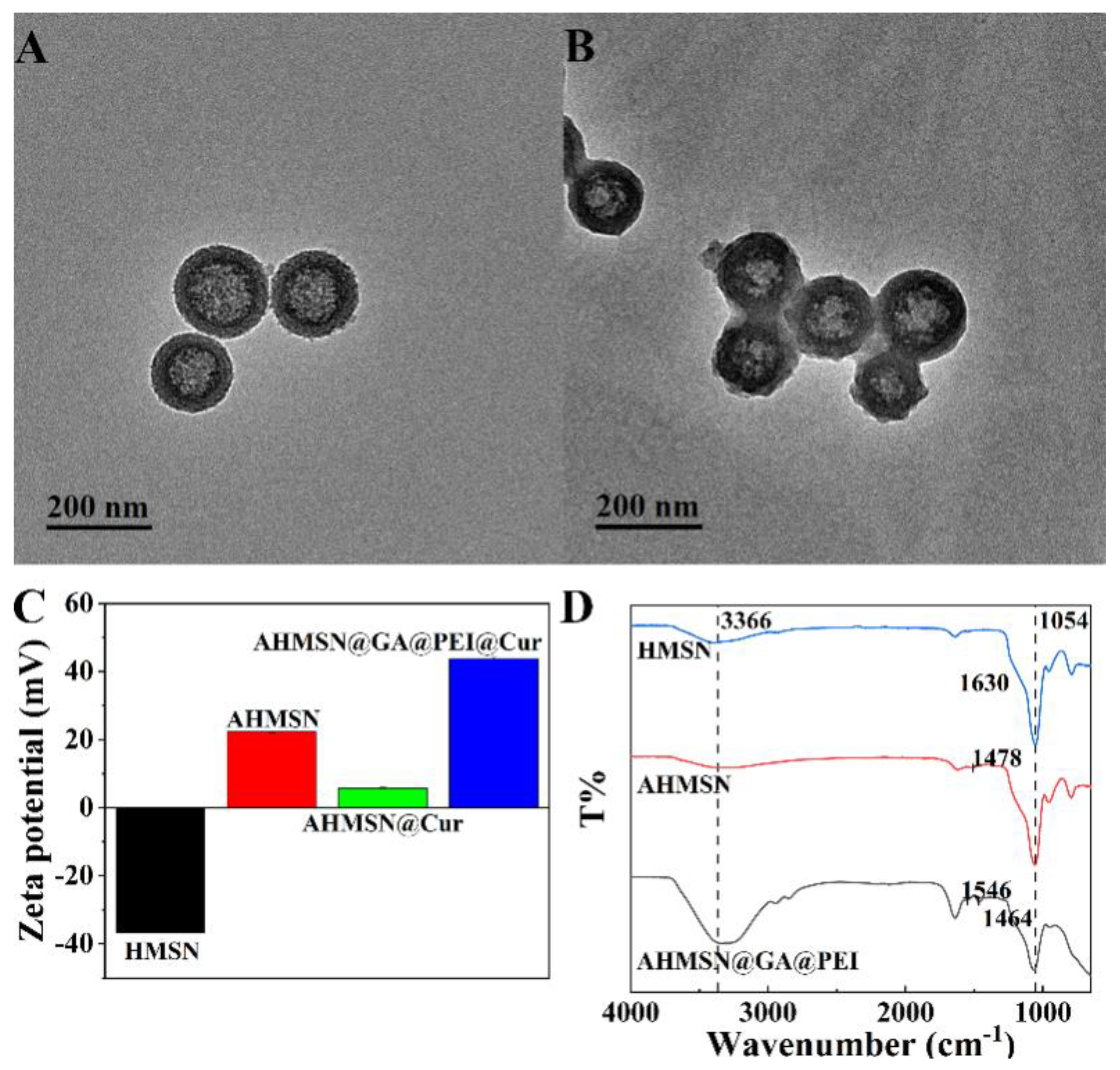
2.3. Characterization of the Nanomaterials
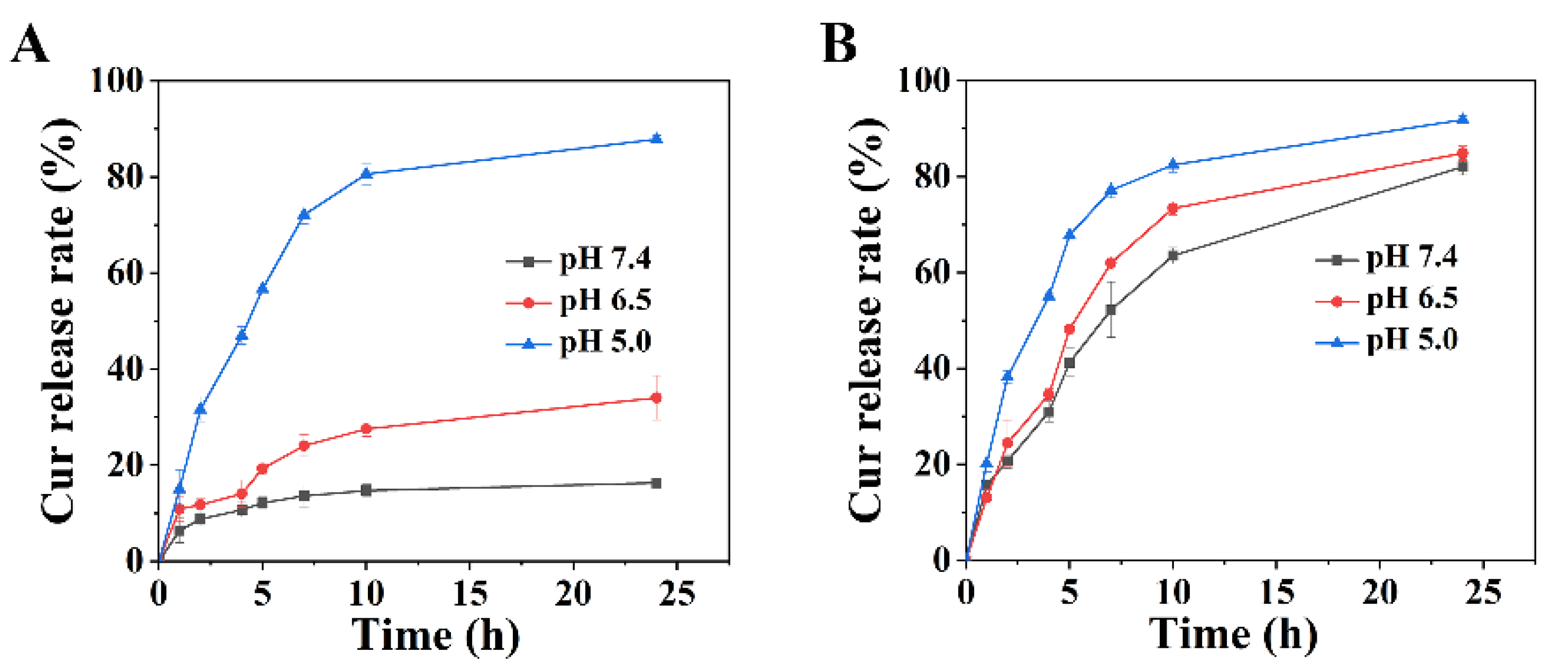
2.4. pH-Triggered Release In Vitro
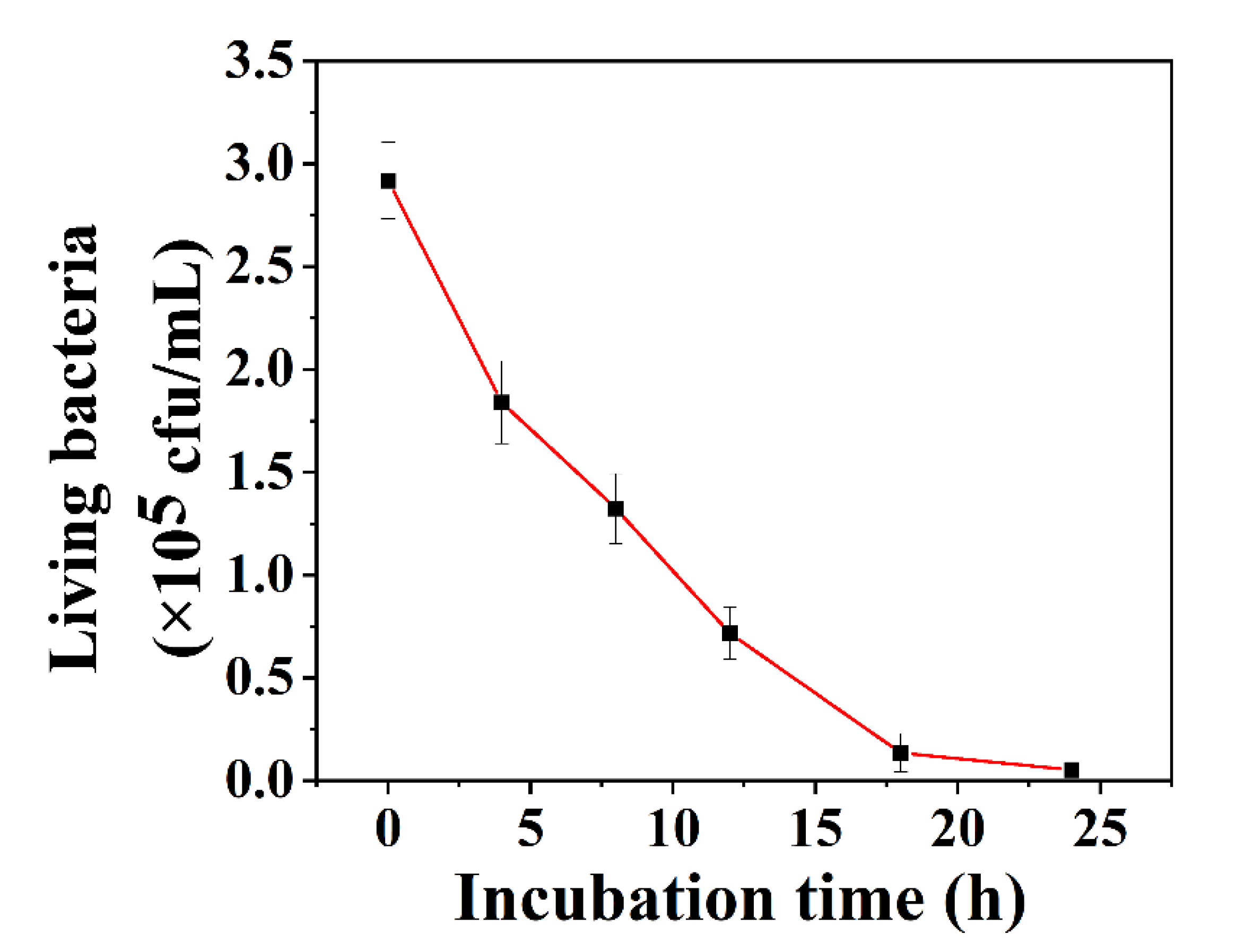
2.5. Preparation of S. aureus Biofilm
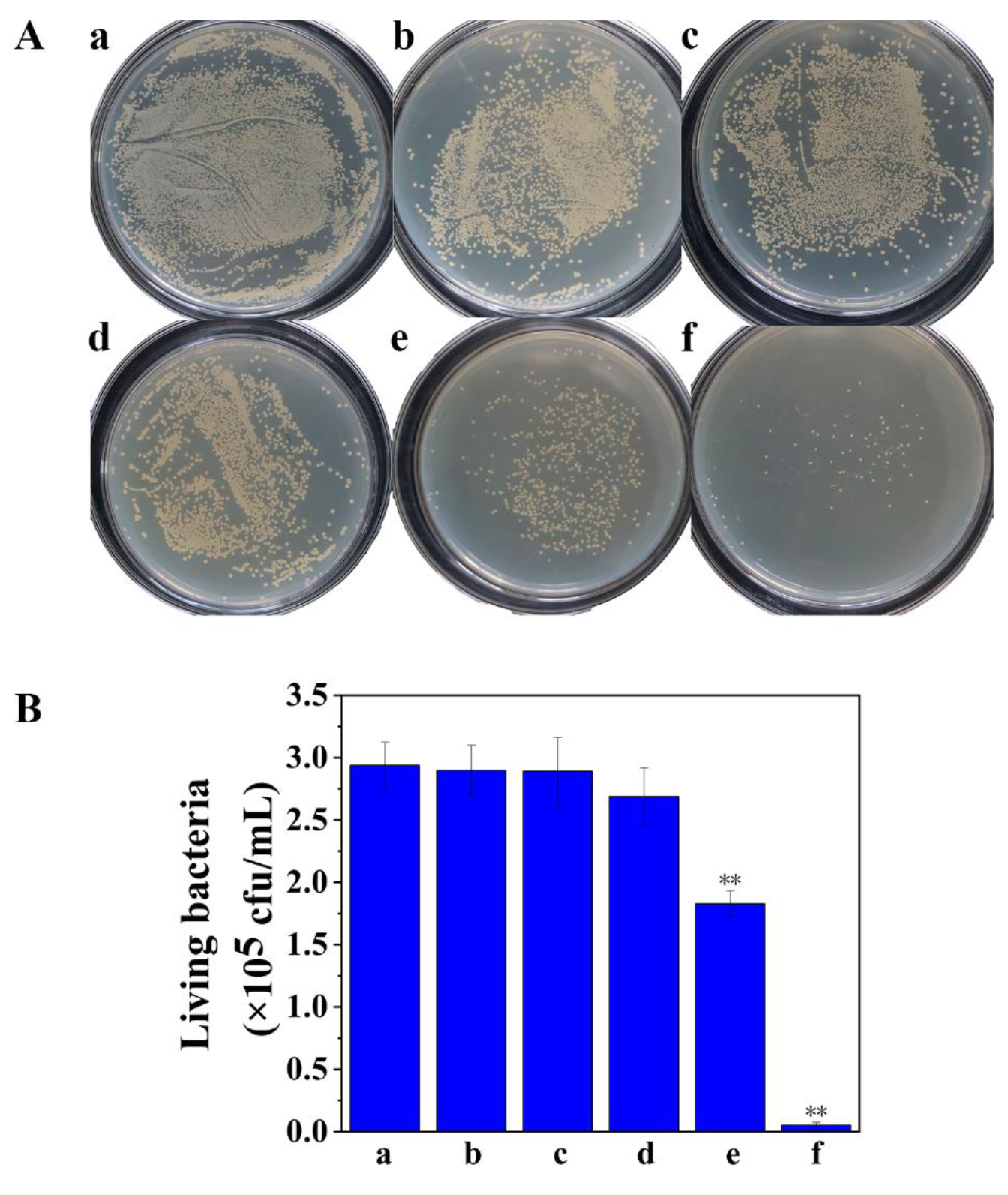
2.6. Photodynamic Treatment of Biofilm-Associated S. aureus and the Evaluation of the Sterilization Effect
3. Results
3.1. The Design of the Nano-Delivery System That Can Kill Biofilm-Associated Bacteria
3.2. Characterization of the Nanomaterials
3.3. pH-Responsive Release of Curcumin In Vitro
3.4. Treatment of Biofilm-Associated S. aureus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shahbazi, M.-A.; Ferreira, M.; Santos, H.A. Landing a lethal blow on bacterial infections: An emerging advance of nanodots for wound healing acceleration. Nanomedicine 2019, 14, 2269–2272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uruén, C.; Chopo-Escuin, G.; Tommassen, J.; Mainar-Jaime, R.C.; Arenas, J. Biofilms as Promoters of Bacterial Antibiotic Re-sistance and Tolerance. Antibiotics 2021, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, Y.; Wu, H.; Song, Z.; Høiby, N.; Molin, S.; Givskov, M. Combating biofilms. FEMS Immunol. Med. Microbiol. 2012, 65, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Forier, K.; Raemdonck, K.; De Smedt, S.; Demeester, J.; Coenye, T.; Braeckmans, K. Lipid and polymer nanoparticles for drug delivery to bacterial biofilms. J. Control. Release 2014, 190, 7–23. [Google Scholar] [CrossRef] [Green Version]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, X.; Yang, W.; Zhang, Y.; Ye, Z.; Hu, S.; Ye, C.; Li, Y.; Lan, Y.; Shen, J.; et al. In vitro antimicrobial effects and mechanism of air plasma-activated water on Staphylococcus aureus biofilm. Plasma Process. Polym. 2020, 17, e1900270. [Google Scholar] [CrossRef]
- Schulze, A.; Mitterer, F.; Pombo, J.P.; Schild, S. Biofilms by bacterial human pathogens: Clinical relevance—Development, composition and regulation—Therapeutical strategies. Microb. Cell 2021, 8, 28–56. [Google Scholar] [CrossRef]
- Kamiya, M.; Mori, T.; Nomura, M.; Inagaki, T.; Nonogaki, T.; Nagatsu, A.; Yamagishi, Y.; Mikamo, H.; Ikeda, Y. Tradescantia pallida extract inhibits biofilm formation in Pseudomonas aeruginosa. Nagoya J. Med. Sci. 2019, 81, 439–452. [Google Scholar] [CrossRef]
- Hu, X.; Huang, Y.-Y.; Wang, Y.; Wang, X.; Hamblin, M.R. Antimicrobial Photodynamic Therapy to Control Clinically Relevant Biofilm Infections. Front. Microbiol. 2018, 9, 1299. [Google Scholar] [CrossRef] [Green Version]
- Verderosa, A.D.; Totsika, M.; Fairfull-Smith, K.E. Bacterial Biofilm Eradication Agents: A Current Review. Front. Chem. 2019, 7, 824. [Google Scholar] [CrossRef] [Green Version]
- Fulaz, S.; Hiebner, D.; Barros, C.H.N.; Devlin, H.; Vitale, S.; Quinn, L.; Casey, E. Ratiometric Imaging of the In Situ pH Distribution of Biofilms by Use of Fluorescent Mesoporous Silica Nanosensors. ACS Appl. Mater. Interfaces 2019, 11, 32679–32688. [Google Scholar] [CrossRef] [PubMed]
- Lebeaux, D.; Ghigo, J.M.; Beloin, C. Biofilm-Related Infections: Bridging the Gap between Clinical Management and Funda-mental Aspects of Recalcitrance toward Antibiotics. Microbiol. Mol. Biol. R. 2014, 78, 510–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavitt, T.B.; Carlisle, J.G.; Dodds, A.R.; Faulkner, R.A.; Garfield, T.C.; Ghebranious, V.N.; Hendley, P.R.; Henry, E.B.; Holt, C.J.; Lowe, J.R.; et al. Thermodynamic Surface Analyses to Inform Biofilm Resistance. iScience 2020, 23, 101702. [Google Scholar] [CrossRef] [PubMed]
- Gold, K.; Slay, B.; Knackstedt, M.; Gaharwar, A.K. Antimicrobial Activity of Metal and Metal-Oxide Based Nanoparticles. Adv. Ther. 2018, 1, 1700033. [Google Scholar] [CrossRef]
- Paladini, F.; Pollini, M. Antimicrobial silver nanoparticles for wound healing application: Progress and future trends. Materials 2019, 12, 2540. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Gao, Y.; Chen, Y.; Liu, L.; Mo, A.; Peng, Q. Nanomaterials-based photothermal therapy and its potentials in anti-bacterial treatment. J. Control. Release 2020, 328, 251–262. [Google Scholar] [CrossRef]
- Baptista, P.V.; McCusker, M.P.; Carvalho, A.; Ferreira, D.A.; Mohan, N.M.; Martins, M.; Fernandes, A.R. Nano-Strategies to Fight Multidrug Resistant Bacteria-“A Battle of the Titans”. Front. Microbiol. 2018, 9, 1441. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Shi, L.; Su, L.; van der Mei, H.C.; Jutte, P.C.; Ren, Y.; Busscher, H.J. Nanotechnology-based antimicrobials and delivery sys-tems for biofilm-infection control. Chem. Soc. Rev. 2018, 48, 428–446. [Google Scholar] [CrossRef]
- Fulaz, S.; Vitale, S.; Quinn, L.; Casey, E. Nanoparticle—Biofilm Interactions: The Role of the EPS Matrix. Trends Microbiol. 2019, 27, 915–926. [Google Scholar] [CrossRef]
- Li, Y.; Chi, Y.-Q.; Yu, C.-H.; Xie, Y.; Xia, M.-Y.; Zhang, C.-L.; Han, X.; Peng, Q. Drug-free and non-crosslinked chitosan scaffolds with efficient antibacterial activity against both Gram-negative and Gram-positive bacteria. Carbohydr. Polym. 2020, 241, 116386. [Google Scholar] [CrossRef]
- Qiu, Y.; Wu, Y.; Lu, B.; Zhu, G.; Gong, T.; Wang, R.; Peng, Q.; Li, Y. Inhibition of methicillin-resistant Staphylococcus aureus (MRSA) biofilm by cationic poly (D, L-lactide-co-glycolide) nanoparticles. Biofouling 2020, 36, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Aznar, E.; Oroval, M.; Pascual, L.; Murguía, J.R.; Martínez-Máñez, R.; Sancenón, F. Gated Materials for On-Command Release of Guest Molecules. Chem. Rev. 2016, 116, 561–718. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Zhao, X.; Fang, W.; Chen, C.; Zheng, N. Self-templating synthesis of hollow mesoporous silica and their applications in catalysis and drug delivery. Nanoscale 2013, 5, 2205–2218. [Google Scholar] [CrossRef]
- Fang, X.; Chen, C.; Liu, Z.; Liu, P.; Zheng, N. A cationic surfactant assisted selective etching strategy to hollow meso-porous silica spheres. Nanoscale 2011, 3, 1632–1639. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Carmona, M.; Gun’ko, Y.K.; Vallet-Regí, M. Mesoporous Silica Materials as Drug Delivery: “The Nightmare” of Bac-terial Infection. Pharmaceutics 2018, 10, 279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Carmona, M.; Izquierdo-Barba, I.; Colilla, M.; Vallet-Regí, M. Concanavalin A-targeted mesoporous silica nanoparticles for infection treatment. Acta Biomater. 2019, 96, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Stanton, M.M.; Park, B.-W.; Vilela, D.; Bente, K.; Faivre, D.; Sitti, M.; Sánchez, S. Magnetotactic Bacteria Powered Biohybrids Target E. coli Biofilms. ACS Nano 2017, 11, 9968–9978. [Google Scholar] [CrossRef]
- González, B.; Colilla, M.; Díez, J.; Pedraza, D.; Guembe, M.; Izquierdo-Barba, I.; Vallet-Regí, M. Mesoporous silica nanoparticles decorated with polycationic dendrimers for infection treatment. Acta Biomater. 2018, 68, 261–271. [Google Scholar] [CrossRef]
- Xu, C.; He, Y.; Li, Z.; Nor, Y.A.; Ye, Q. Nanoengineered hollow mesoporous silica nanoparticles for the delivery of antimi-crobial proteins into biofilms. J. Mater. Chem B 2018, 6, 1899–1902. [Google Scholar] [CrossRef]
- Martin-Illana, A.; Cazorla-Luna, R.; Notario-Pérez, F.; Ruiz-Caro, R.; Bedoya, L.M.; Veiga-Ochoa, M.D.; Rubio, J.; Tamayo, A. Amino Functionalized Micro-Mesoporous Hybrid Particles for the Sustained Release of the Antiretroviral Drug T enofovir. Materials 2020, 13, 3494. [Google Scholar] [CrossRef]
- Xu, C.; Shan, Y.; Bilal, M.; Xu, B.; Cao, L.; Huang, Q. Copper ions chelated mesoporous silica nanoparticles via dopamine chemistry for controlled pesticide release regulated by coordination bonding. Chem. Eng. J. 2020, 395, 125093. [Google Scholar] [CrossRef]
- Lee, N.K.; Park, S.S.; Ha, C.S. pH-Sensitive Drug Delivery System Based on Mesoporous Silica Modified with Poly-L-Lysine (PLL) as a Gatekeeper. J. Nanosci. Nanotechnol. 2020, 20, 6925–6934. [Google Scholar] [CrossRef] [PubMed]
- Zago, L.H.D.P.; de Annunzio, S.R.; de Oliveira, K.T.; Barbugli, P.A.; Valdes, B.R.; Feres, M.; Fontana, C.R. Antimicrobial photodynamic therapy against metronidazole-resistant dental plaque bactéria. J. Photochem. Photobiol. B Biol. 2020, 209, 111903. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanzadeh, R.; Assadian, H.; Chiniforush, N.; Parker, S.; Pourakbari, B.; Ehsani, B.; Alikhani, M.Y.; Bahador, A. Modulation of virulence in Enter-ococcus faecalis cells surviving antimicrobial photodynamic inactivation with reduced graphene oxide-curcumin: An ex vivo biofilm model. Photodiagnosis Photodyn. Ther. 2020, 29, 101643. [Google Scholar] [CrossRef] [PubMed]
- Eslami, L.M.; Vatanpour, M.; Aminzadeh, N.; Mehrvarzfar, P.; Taheri, S. The comparison of intracanal medicaments, diode laser and photodynamic therapy on removing the biofilm of Enterococcus faecalis and Candida albicans in the root canal system (ex-vivo study). Photodiagnosis Photodyn. Ther. 2019, 26, 157–161. [Google Scholar] [CrossRef]
- Rani, D.J.; Mala, R.; Mohan, P.; Keerthana, R.; Prasath, N.H.; Celsia, A.S.R. Chitosan nanoparticle-mediated delivery of curcumin and phycocyanin for photodynamic therapy against biofilm forming bacteria. Mater. Express 2020, 10, 1854–1870. [Google Scholar] [CrossRef]
- Zikmundova, M.; Vereshaka, M.; Kolarova, K.; Pajorova, J.; Svorcik, V.; Bacakova, L. Effects of Bacterial Nanocellulose Loaded withEffects of Bacterial Nanocellulose Loaded withHuman Dermal Fibroblasts. Materials 2020, 13, 4759. [Google Scholar] [CrossRef]
- Lo, T.H.; Wu, Z.Y.; Chen, S.Y.; Meng, F.Y.; Chou, P.T.; Wang, C.M.; Lin, H.M. Curcumin-loaded mesoporous silica nanoparticles with dual-imaging and temperature control inhibits the infection of Zika virus. Microporous Mesoporous Mater. 2021, 314, 110886. [Google Scholar] [CrossRef]
- Dias, L.D.; Blanco, K.C.; Mfouo-Tynga, I.S.; Inada, N.M.; Bagnato, V.S. Curcumin as a photosensitizer: From molecular structure to recent advances in antimicrobial photodynamic therapy. J. Photochem. Photobiol. C Photochem. Rev. 2020, 45, 100384. [Google Scholar] [CrossRef]
- RS, P.; Mal, A.; Valvi, S.K.; Srivastava, R.; De, A.; Bandyopadhyaya, R. Noninvasive Preclinical Evaluation of Targeted Nanoparticles for the Delivery of Curcumin in Treating Pancreatic Cancer. ACS Appl. Bio Mater. 2020, 3, 4643–4654. [Google Scholar] [CrossRef]
- Chen, W.; Cheng, C.-A.; Cosco, E.D.; Ramakrishnan, S.; Lingg, J.G.P.; Bruns, O.T.; Zink, J.I.; Sletten, E.M. Shortwave Infrared Imaging with J-Aggregates Stabilized in Hollow Mesoporous Silica Nanoparticles. J. Am. Chem. Soc. 2019, 141, 12475–12480. [Google Scholar] [CrossRef] [PubMed]
- Golestaneh, D.; Varshosaz, J. Enhancement in Biological Activity of L-Asparginase by its Conjugation on Silica Nanoparticles. Recent Pat. Nanotechnol. 2018, 12, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Kaziem, A.; Zhang, Y.; Xiao, Y.; He, S.; Li, J. A hollow mesoporous silica and poly (diacetone acrylamide) composite with sustained-release and adhesion properties. Microporous Mesoporous Mater. 2018, 255, 15–22. [Google Scholar] [CrossRef]
- Majoul, N.; Aouida, S.; Bessaïs, B. Progress of porous silicon APTES-functionalization by FTIR investigations. Appl. Surf. Sci. 2015, 331, 388–391. [Google Scholar] [CrossRef]
- Peng, X.; Liu, P.; Pang, B.; Yao, Y.; Wang, J.; Zhang, K. Facile fabrication of pH-responsive nanoparticles from cellulose derivatives via Schiff base formation for controlled release. Carbohydr. Polym. 2019, 216, 113–118. [Google Scholar] [CrossRef]
- Cai, L.; Zhu, P.; Huan, F.; Wang, J.; Zhou, L.; Jiang, H.; Ji, M.; Chen, J. Toxicity-attenuated mesoporous silica Schiff-base bonded anti-cancer drug complexes for chemotherapy of drug resistant cancer. Colloids Surf. B. 2021, 205, 111839. [Google Scholar] [CrossRef]
- Liu, F.; Ni, A.S.Y.; Lim, Y.; Mohanram, H.; Bhattacharjya, S.; Xing, B. Lipopolysaccharide Neutralizing Peptide—Porphyrin Conjugates for Effective Photoinactivation and Intracellular Imaging of Gram-Negative Bacteria Strains. Bioconj. Chem. 2012, 23, 1639–1647. [Google Scholar] [CrossRef]
- Ding, T.; Gao, Y.; Sun, K.; Wang, Y.; Liu, J.; Tian, S. Effect of curcumin—Mediated photodynamic therapy on methicillin—Resistant Staphylococcus aureus and biofilm in vitro. Chin. J. Exp. Surg. 2019, 36, 1092–1094. [Google Scholar]
- Lavaee, F.; Motamedifar, M.; Rafiee, G. The effect of photodynamic therapy by gold nanoparticles on Streptococcus mutans and biofilm formation: An in vitro study. Lasers Med. Sci. 2021, 37, 1717–1725. [Google Scholar] [CrossRef]
- De Paula Ribeiro, I.; Pinto, J.G.; Souza, B.M.N.; Miñán, A.G.; Ferreira-Strixino, J. Antimicrobial photodynamic therapy with curcumin on methicillin-resistant Staphylococcus aureus biofilm. Photodiagnosis Photodyn. Ther. 2022, 37, 102729. [Google Scholar] [CrossRef]
- Wei, J.; Cao, X.; Qian, J.; Liu, Z.; Wang, X.; Su, Q.; Wang, Y.; Xie, R.; Li, X. Evaluation of antimicrobial peptide LL-37 for treatment of Staphylococcus aureus biofilm on titanium plate. Medicine 2021, 100, e27426. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Wang, X.; Zhang, P.; Zhang, M.; Kou, M.; Shi, C.; Peng, X.; Wang, X. Control of Foodborne Staphylococcus aureus by Shikonin, a Natural Extract. Foods 2021, 10, 2954. [Google Scholar] [CrossRef] [PubMed]
- Jee, S.-C.; Kim, M.; Sung, J.-S.; Kadam, A.A. Efficient Biofilms Eradication by Enzymatic-Cocktail of Pancreatic Protease Type-I and Bacterial α-Amylase. Polymers 2020, 12, 3032. [Google Scholar] [CrossRef] [PubMed]
| Nanomaterials | Z-Ave (nm) | PDI |
|---|---|---|
| MSN | 186.03 | 0.038 |
| AHMSN | 200.70 | 0.150 |
| AHMSN@GA@PEI | 366.63 | 0.264 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, N.; Cai, R.; Zhang, Y.; Wang, X.; Zhou, N. A pH-Gated Functionalized Hollow Mesoporous Silica Delivery System for Photodynamic Sterilization in Staphylococcus aureus Biofilm. Materials 2022, 15, 2815. https://doi.org/10.3390/ma15082815
Zhao N, Cai R, Zhang Y, Wang X, Zhou N. A pH-Gated Functionalized Hollow Mesoporous Silica Delivery System for Photodynamic Sterilization in Staphylococcus aureus Biofilm. Materials. 2022; 15(8):2815. https://doi.org/10.3390/ma15082815
Chicago/Turabian StyleZhao, Nanxin, Rongfeng Cai, Yuting Zhang, Xiaoli Wang, and Nandi Zhou. 2022. "A pH-Gated Functionalized Hollow Mesoporous Silica Delivery System for Photodynamic Sterilization in Staphylococcus aureus Biofilm" Materials 15, no. 8: 2815. https://doi.org/10.3390/ma15082815







